Using Cerebral Metabolites to Guide Precision Medicine for Subarachnoid Hemorrhage: Lactate and Pyruvate
Abstract
1. Introduction
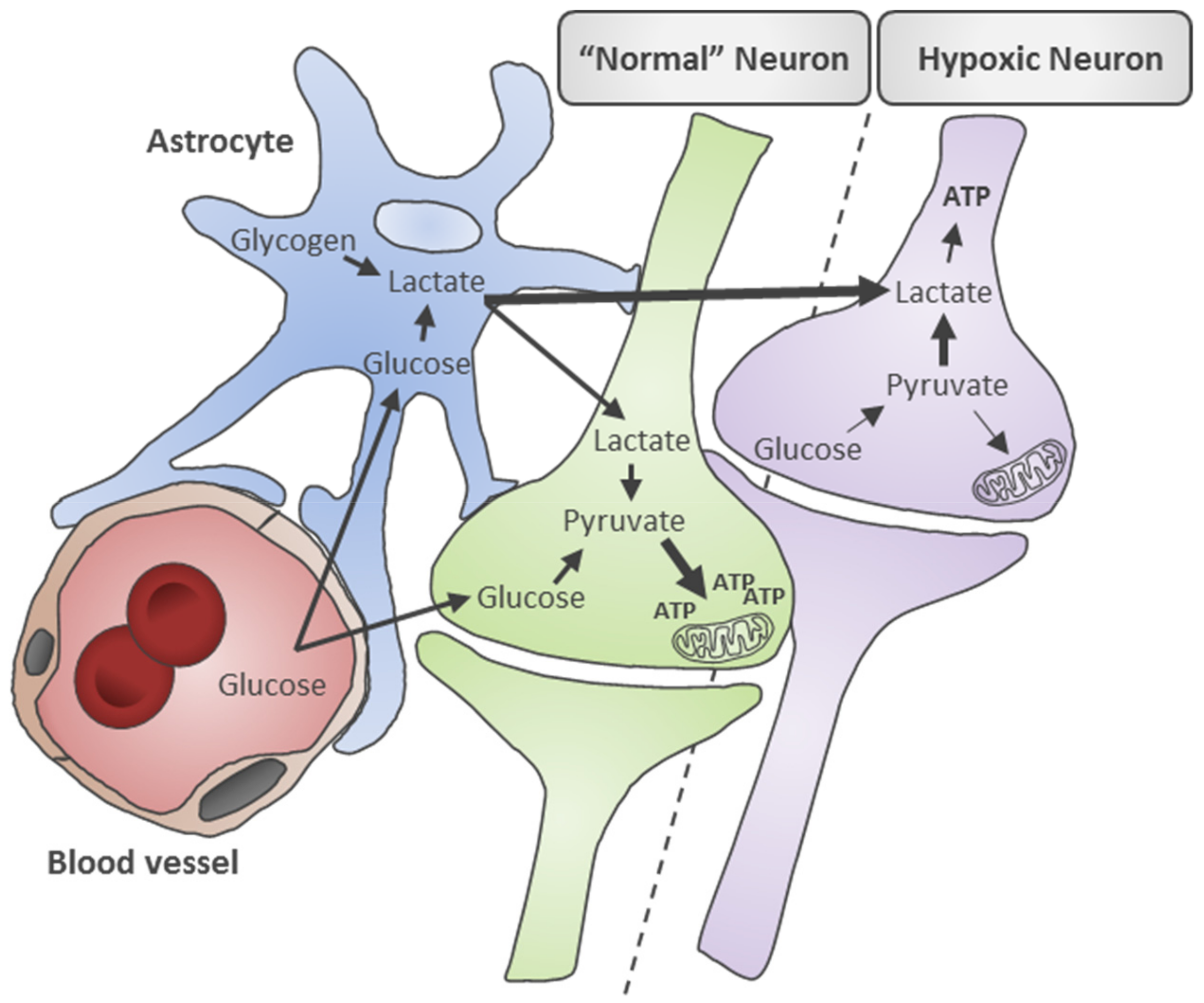
2. Metabolic Mechanisms Underlying Disturbed Energy Metabolism in SAH
3. Lactate and Pyruvate after SAH: Predictors of Clinical Events and Outcomes?
4. Brain Hemodynamics and Lactate and Pyruvate
5. Association of Lactate and Pyruvate with Secondary Complications and Events
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wijdicks, E.F.; Kallmes, D.F.; Manno, E.M.; Fulgham, J.R.; Piepgras, D.G. Subarachnoid hemorrhage: Neurointensive care and aneurysm repair. Mayo Clin. Proc. 2005, 80, 550–559. [Google Scholar] [CrossRef] [PubMed]
- Johnston, S.C.; Selvin, S.; Gress, D.R. The burden, trends, and demographics of mortality from subarachnoid hemorrhage. Neurology 1998, 50, 1413–1418. [Google Scholar] [CrossRef] [PubMed]
- Van Gijn, J.; Kerr, R.S.; Rinkel, G.J. Subarachnoid haemorrhage. Lancet 2007, 369, 306–318. [Google Scholar] [CrossRef]
- Ritzenthaler, T.; Gobert, F.; Dailler, F. “Vasospasm mimic” after aneurysmal subarachnoid hemorrhage. World Neurosurg. 2019, 124, 295–297. [Google Scholar] [CrossRef]
- Broderick, J.P.; Brott, T.G.; Duldner, J.E.; Tomsick, T.; Leach, A. Initial and recurrent bleeding are the major causes of death following subarachnoid hemorrhage. Stroke 1994, 25, 1342–1347. [Google Scholar] [CrossRef]
- Huhtakangas, J.; Lehto, H.; Seppa, K.; Kivisaari, R.; Niemela, M.; Hernesniemi, J.; Lehecka, M. Long-Term Excess Mortality After Aneurysmal Subarachnoid Hemorrhage: Patients With Multiple Aneurysms at Risk. Stroke 2015, 46, 1813–1818. [Google Scholar] [CrossRef]
- Macdonald, R.L. Delayed neurological deterioration after subarachnoid haemorrhage. Nat. Rev. Neurol. 2014, 10, 44–58. [Google Scholar] [CrossRef]
- Geraghty, J.R.; Davis, J.L.; Testai, F.D. Neuroinflammation and Microvascular Dysfunction After Experimental Subarachnoid Hemorrhage: Emerging Components of Early Brain Injury Related to Outcome. Neurocrit. Care 2019, 31, 373–389. [Google Scholar] [CrossRef]
- Chyatte, D.; Sundt, T.M. Cerebral Vasospasm After Subarachnoid Hemorrhage. Mayo Clin. Proc. 1984, 59, 498–505. [Google Scholar] [CrossRef][Green Version]
- Pluta, R.M.; Hansen-Schwartz, J.; Dreier, J.; Vajkoczy, P.; Macdonald, R.L.; Nishizawa, S.; Kasuya, H.; Wellman, G.; Keller, E.; Zauner, A. Cerebral vasospasm following subarachnoid hemorrhage: Time for a new world of thought. Neurol. Res. 2009, 31, 151–158. [Google Scholar] [CrossRef]
- Van Lieshout, J.H.; Dibue-Adjei, M.; Cornelius, J.F.; Slotty, P.J.; Schneider, T.; Restin, T.; Boogaarts, H.D.; Steiger, H.J.; Petridis, A.K.; Kamp, M.A. An introduction to the pathophysiology of aneurysmal subarachnoid hemorrhage. Neurosurg. Rev. 2018, 41, 917–930. [Google Scholar] [CrossRef] [PubMed]
- Vergouwen, M.D.; Vermeulen, M.; van Gijn, J.; Rinkel, G.J.; Wijdicks, E.F.; Muizelaar, J.P.; Mendelow, A.D.; Juvela, S.; Yonas, H.; Terbrugge, K.G.; et al. Definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as an outcome event in clinical trials and observational studies: Proposal of a multidisciplinary research group. Stroke 2010, 41, 2391–2395. [Google Scholar] [CrossRef] [PubMed]
- Dreier, J.P. The role of spreading depression, spreading depolarization and spreading ischemia in neurological disease. Nat. Med. 2011, 17, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Sjoberg, R.L.; Bergenheim, T.; Moren, L.; Antti, H.; Lindgren, C.; Naredi, S.; Lindvall, P. Blood Metabolomic Predictors of 1-Year Outcome in Subarachnoid Hemorrhage. Neurocrit. Care 2015, 23, 225–232. [Google Scholar] [CrossRef]
- Lu, A.Y.; Damisah, E.C.; Winkler, E.A.; Grant, R.A.; Eid, T.; Bulsara, K.R. Cerebrospinal fluid untargeted metabolomic profiling of aneurysmal subarachnoid hemorrhage: An exploratory study. Br. J. Neurosurg. 2018, 32, 637–641. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.C.; Wang, R.; Xu, M.M.; Jing, X.R.; Ji, Y.A.; Sun, R.B.; Na, S.J.; Liu, T.; Ding, X.S.; Sun, C.Y.; et al. Aneurysmal Subarachnoid Hemorrhage Onset Alters Pyruvate Metabolism in Poor-Grade Patients and Clinical Outcome Depends on More: A Cerebrospinal Fluid Metabolomic Study. ACS Chem. Neurosci. 2019, 10, 1660–1667. [Google Scholar] [CrossRef] [PubMed]
- De Lima Oliveira, M.; Kairalla, A.C.; Fonoff, E.T.; Martinez, R.C.R.; Teixeira, M.J.; Bor-Seng-Shu, E. Cerebral Microdialysis in Traumatic Brain Injury and Subarachnoid Hemorrhage: State of the Art. Neurocrit. Care 2014, 21, 152–162. [Google Scholar] [CrossRef]
- Marcoux, J.; McArthur, D.A.; Miller, C.; Glenn, T.C.; Villablanca, P.; Martin, N.A.; Hovda, D.A.; Alger, J.R.; Vespa, P.M. Persistent metabolic crisis as measured by elevated cerebral microdialysis lactate-pyruvate ratio predicts chronic frontal lobe brain atrophy after traumatic brain injury. Crit. Care Med. 2008, 36, 2871–2877. [Google Scholar] [CrossRef]
- Woitzik, J.; Pinczolits, A.; Hecht, N.; Sandow, N.; Scheel, M.; Drenckhahn, C.; Dreier, J.P.; Vajkoczy, P. Excitotoxicity and metabolic changes in association with infarct progression. Stroke 2014, 45, 1183–1185. [Google Scholar] [CrossRef]
- Albanese, M.; Zagaglia, S.; Landi, D.; Boffa, L.; Nicoletti, C.G.; Marciani, M.G.; Mandolesi, G.; Marfia, G.A.; Buttari, F.; Mori, F.; et al. Cerebrospinal fluid lactate is associated with multiple sclerosis disease progression. J. Neuroinflamm. 2016, 13, 36. [Google Scholar] [CrossRef]
- Pellerin, L.; Magistretti, P.J. Glutamate uptake into astrocytes stimulates aerobic glycolysis: A mechanism coupling neuronal activity to glucose utilization. Proc. Natl. Acad. Sci. USA 1994, 91, 10625–10629. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.M.; Ransom, B.R. Astrocyte glycogen and brain energy metabolism. Glia 2007, 55, 1263–1271. [Google Scholar] [CrossRef] [PubMed]
- Pellerin, L.; Magistretti, P.J. Food for thought: Challenging the dogmas. J. Cereb. Blood Flow Metab. 2003, 23, 1282–1286. [Google Scholar] [CrossRef] [PubMed]
- Alberini, C.M.; Cruz, E.; Descalzi, G.; Bessières, B.; Gao, V. Astrocyte glycogen and lactate: New insights into learning and memory mechanisms. Glia 2018, 66, 1244–1262. [Google Scholar] [CrossRef]
- Larach, D.B.; Kofke, W.A.; Le Roux, P. Potential non-hypoxic/ischemic causes of increased cerebral interstitial fluid lactate/pyruvate ratio: A review of available literature. Neurocrit. Care 2011, 15, 609–622. [Google Scholar] [CrossRef]
- Goodman, J.C.; Robertson, C.S. Microdialysis: Is it ready for prime time? Curr. Opin. Crit. Care 2009, 15, 110. [Google Scholar] [CrossRef]
- Cesarini, K.G.; Enblad, P.; Ronne-Engstrom, E.; Marklund, N.; Salci, K.; Nilsson, P.; Hardemark, H.G.; Hillered, L.; Persson, L. Early cerebral hyperglycolysis after subarachnoid haemorrhage correlates with favourable outcome. Acta Neurochir. 2002, 144, 1121–1131. [Google Scholar] [CrossRef]
- Oddo, M.; Levine, J.M.; Frangos, S.; Maloney-Wilensky, E.; Carrera, E.; Daniel, R.T.; Levivier, M.; Magistretti, P.J.; LeRoux, P.D. Brain lactate metabolism in humans with subarachnoid hemorrhage. Stroke 2012, 43, 1418–1421. [Google Scholar] [CrossRef]
- Jacobsen, A.; Nielsen, T.H.; Nilsson, O.; Schalen, W.; Nordstrom, C.H. Bedside diagnosis of mitochondrial dysfunction in aneurysmal subarachnoid hemorrhage. Acta Neurol. Scand. 2014, 130, 156–163. [Google Scholar] [CrossRef]
- Aisiku, I.P.; Chen, P.R.; Truong, H.; Monsivais, D.R.; Edlow, J. Admission serum lactate predicts mortality in aneurysmal subarachnoid hemorrhage. Am. J. Emerg. Med. 2016, 34, 708–712. [Google Scholar] [CrossRef]
- Poblete, R.A.; Cen, S.Y.; Zheng, L.; Emanuel, B.A. Serum lactic acid following aneurysmal subarachnoid hemorrhage is a marker of disease severity but is not associated with hospital outcomes. Front. Neurol. 2018, 9, 593. [Google Scholar] [CrossRef] [PubMed]
- Sarrafzadeh, A.; Haux, D.; Kuchler, I.; Lanksch, W.R.; Unterberg, A.W. Poor-grade aneurysmal subarachnoid hemorrhage: Relationship of cerebral metabolism to outcome. J. Neurosurg. 2004, 100, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Staub, F.; Graf, R.; Gabel, P.; Kochling, M.; Klug, N.; Heiss, W.D. Multiple interstitial substances measured by microdialysis in patients with subarachnoid hemorrhage. Neurosurgery 2000, 47, 1106–1115. [Google Scholar] [CrossRef]
- Sarrafzadeh, A.S.; Sakowitz, O.W.; Kiening, K.L.; Benndorf, G.; Lanksch, W.R.; Unterberg, A.W. Bedside microdialysis: A tool to monitor cerebral metabolism in subarachnoid hemorrhage patients? Crit. Care Med. 2002, 30, 1062–1070. [Google Scholar] [CrossRef]
- Patet, C.; Quintard, H.; Zerlauth, J.B.; Maibach, T.; Carteron, L.; Suys, T.; Bouzat, P.; Bervini, D.; Levivier, M.; Daniel, R.T.; et al. Bedside cerebral microdialysis monitoring of delayed cerebral hypoperfusion in comatose patients with poor grade aneurysmal subarachnoid haemorrhage. J. Neurol. Neurosurg. Psychiatry 2017, 88, 332–338. [Google Scholar] [CrossRef]
- Radolf, S.; Smoll, N.; Drenckhahn, C.; Dreier, J.P.; Vajkoczy, P.; Sarrafzadeh, A.S. Cerebral Lactate Correlates with Early Onset Pneumonia after Aneurysmal SAH. Transl. Stroke Res. 2014, 5, 278–285. [Google Scholar] [CrossRef][Green Version]
- Schiefecker, A.J.; Dietmann, A.; Beer, R.; Pfausler, B.; Lackner, P.; Kofler, M.; Fischer, M.; Broessner, G.; Sohm, F.; Mulino, M.; et al. Neuroinflammation is Associated with Brain Extracellular TAU-Protein Release After Spontaneous Subarachnoid Hemorrhage. Curr. Drug Targets 2017, 18, 1408–1416. [Google Scholar] [CrossRef]
- Zanier, E.R.; Zoerle, T.; Fiorini, M.; Longhi, L.; Cracco, L.; Bersano, A.; Branca, V.; Benedetti, M.D.; De Simoni, M.G.; Monaco, S.; et al. Heart-fatty acid-binding and tau proteins relate to brain injury severity and long-term outcome in subarachnoid haemorrhage patients. Br. J. Anaesth. 2013, 111, 424–432. [Google Scholar] [CrossRef]
- Helbok, R.; Schiefecker, A.; Delazer, M.; Beer, R.; Bodner, T.; Pfausler, B.; Benke, T.; Lackner, P.; Fischer, M.; Sohm, F. Cerebral tau is elevated after aneurysmal subarachnoid haemorrhage and associated with brain metabolic distress and poor functional and cognitive long-term outcome. J. Neurol. Neurosurg. Psychiatry 2015, 86, 79–86. [Google Scholar] [CrossRef]
- Van Donkelaar, C.E.; Dijkland, S.A.; van den Bergh, W.M.; Bakker, J.; Dippel, D.W.; Nijsten, M.W.; van der Jagt, M. Early Circulating Lactate and Glucose Levels After Aneurysmal Subarachnoid Hemorrhage Correlate With Poor Outcome and Delayed Cerebral Ischemia: A Two-Center Cohort Study. Crit. Care Med. 2016, 44, 966–972. [Google Scholar] [CrossRef]
- Okazaki, T.; Hifumi, T.; Kawakita, K.; Shishido, H.; Ogawa, D.; Okauchi, M.; Shindo, A.; Kawanishi, M.; Inoue, S.; Tamiya, T.; et al. Serial blood lactate measurements and its prognostic significance in intensive care unit management of aneurysmal subarachnoid hemorrhage patients. J. Crit. Care 2017, 41, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Lindgren, C.; Koskinen, L.O.; Ssozi, R.; Naredi, S. Cerebrospinal fluid lactate and neurological outcome after subarachnoid haemorrhage. J. Clin. Neurosci. Off. J. Neurosurg. Soc. Australas. 2019, 60, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Leen, W.G.; Willemsen, M.A.; Wevers, R.A.; Verbeek, M.M. Cerebrospinal fluid glucose and lactate: Age-specific reference values and implications for clinical practice. PLoS ONE 2012, 7, e42745. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Nakajima, K.; Maeda, M. Long-term monitoring of CSF lactate levels and lactate/pyruvate ratios following subarachnoid haemorrhage. Acta Neurochir. 1993, 125, 20–26. [Google Scholar] [CrossRef]
- Renfrow, J.J.; Frey, C.D.; Arnel, M.; Wolfe, S.Q.; McLouth, C.; Datar, S. Utility of cerebrospinal fluid lactate in aneurysmal subarachnoid hemorrhage. Surg. Neurol. Int. 2018, 9, 155. [Google Scholar] [CrossRef]
- Gjedde, A.; Hansen, A.J.; Quistorff, B. Blood-brain glucose transfer in spreading depression. J. Neurochem. 1981, 37, 807–812. [Google Scholar] [CrossRef]
- Lauritzen, M.; Diemer, N.H. Uncoupling of cerebral blood flow and metabolism after single episode of cortical spreading depression in the rat brain. Brain Res. 1986, 370, 405–408. [Google Scholar] [CrossRef]
- Csiba, L.; Paschen, W.; Mies, G. Regional changes in tissue pH and glucose content during cortical spreading depression in rat brain. Brain Res. 1985, 336, 167–170. [Google Scholar] [CrossRef]
- Rogers, M.L.; Leong, C.L.; Gowers, S.A.; Samper, I.C.; Jewell, S.L.; Khan, A.; McCarthy, L.; Pahl, C.; Tolias, C.M.; Walsh, D.C.; et al. Simultaneous monitoring of potassium, glucose and lactate during spreading depolarization in the injured human brain—Proof of principle of a novel real-time neurochemical analysis system, continuous online microdialysis. J. Cereb. Blood Flow Metab. 2017, 37, 1883–1895. [Google Scholar] [CrossRef]
- Cesak, T.; Adamkov, J.; Habalova, J.; Poczos, P.; Kanta, M.; Bartos, M.; Hosszu, T. The relationship between intracranial pressure and lactate/pyruvate ratio in patients with subarachnoid haemorrhage. Bratisl. Lek. Listy 2018, 119, 139–142. [Google Scholar] [CrossRef]
- Papadopoulos, D.; Filippidis, A.; Krommidas, G.; Vretzakis, G.; Paterakis, K.; Komnos, A.; Fountas, K.N. Regional cerebral blood flow and cellular environment in subarachnoid hemorrhage: A thermal doppler flowmetry and microdialysis study. Neurol. I Neurochir. Pol. 2017, 51, 66–71. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rostami, E.; Engquist, H.; Howells, T.; Johnson, U.; Ronne-Engstrom, E.; Nilsson, P.; Hillered, L.; Lewen, A.; Enblad, P. Early low cerebral blood flow and high cerebral lactate: Prediction of delayed cerebral ischemia in subarachnoid hemorrhage. J. Neurosurg. 2018, 128, 1762–1770. [Google Scholar] [CrossRef] [PubMed]
- Rostami, E.; Engquist, H.; Howells, T.; Ronne-Engstrom, E.; Nilsson, P.; Hillered, L.T.; Lewen, A.; Enblad, P. The Correlation between Cerebral Blood Flow Measured by Bedside Xenon-CT and Brain Chemistry Monitored by Microdialysis in the Acute Phase following Subarachnoid Hemorrhage. Front. Neurol. 2017, 8, 369. [Google Scholar] [CrossRef] [PubMed]
- Zetterling, M.; Hillered, L.; Samuelsson, C.; Karlsson, T.; Enblad, P.; Ronne-Engstrom, E. Temporal patterns of interstitial pyruvate and amino acids after subarachnoid haemorrhage are related to the level of consciousness—A clinical microdialysis study. Acta Neurochir. 2009, 151, 771–780; discussion 780. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.C.; Tang, S.C.; Lee, J.E.; Jeng, J.S.; Lai, D.M.; Huang, S.J.; Hsieh, S.T.; Tu, Y.K. Intrathecal lactate predicting hydrocephalus after aneurysmal subarachnoid hemorrhage. J. Surg. Res. 2015, 199, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Satoh, E.; Tagami, T.; Watanabe, A.; Matsumoto, G.; Suzuki, G.; Onda, H.; Fuse, A.; Gemma, A.; Yokota, H. Association between serum lactate levels and early neurogenic pulmonary edema after nontraumatic subarachnoid hemorrhage. J. Nippon Med Sch. 2014, 81, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Gordon, G.R.; Howarth, C.; MacVicar, B.A. Bidirectional Control of Blood Flow by Astrocytes: A Role for Tissue Oxygen and Other Metabolic Factors. Adv. Exp. Med. Biol. 2016, 903, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Hein, T.W.; Xu, W.; Kuo, L. Dilation of retinal arterioles in response to lactate: Role of nitric oxide, guanylyl cyclase, and ATP-sensitive potassium channels. Investig. Ophthalmol. Vis. Sci. 2006, 47, 693–699. [Google Scholar] [CrossRef]
- Yamanishi, S.; Katsumura, K.; Kobayashi, T.; Puro, D.G. Extracellular lactate as a dynamic vasoactive signal in the rat retinal microvasculature. Am. J. Physiol. Heart Circ. Physiol. 2006, 290, 925–934. [Google Scholar] [CrossRef]
- Gordon, G.R.; Choi, H.B.; Rungta, R.L.; Ellis-Davies, G.C.; MacVicar, B.A. Brain metabolism dictates the polarity of astrocyte control over arterioles. Nature 2008, 456, 745. [Google Scholar] [CrossRef]
- Samuelsson, C.; Howells, T.; Kumlien, E.; Enblad, P.; Hillered, L.; Ronne-Engstrom, E. Relationship between intracranial hemodynamics and microdialysis markers of energy metabolism and glutamate-glutamine turnover in patients with subarachnoid hemorrhage. Clinical article. J. Neurosurg. 2009, 111, 910–915. [Google Scholar] [CrossRef] [PubMed]
- Nagel, A.; Graetz, D.; Schink, T.; Frieler, K.; Sakowitz, O.; Vajkoczy, P.; Sarrafzadeh, A. Relevance of intracranial hypertension for cerebral metabolism in aneurysmal subarachnoid hemorrhage. Clinical article. J. Neurosurg. 2009, 111, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Izawa, I.; Korosue, K.; Hamano, S.; Nagao, Y.; Tamaki, N.; Matsumoto, S. Hydrocephalus and vasospasm after subarachnoid hemorrhage from ruptured intracranial aneurysms. Neurol. Surg. 1988, 16, 487–492. [Google Scholar]
- Nam, K.-H.; Hamm, I.-S.; Kang, D.-H.; Park, J.; Kim, Y.-S. Risk of shunt dependent hydrocephalus after treatment of ruptured intracranial aneurysms: Surgical clipping versus endovascular coiling according to fisher grading system. J. Korean Neurosurg. Soc. 2010, 48, 313. [Google Scholar] [CrossRef]
- Dorai, Z.; Hynan, L.S.; Kopitnik, T.A.; Samson, D. Factors related to hydrocephalus after aneurysmal subarachnoid hemorrhage. Neurosurgery 2003, 52, 763–771. [Google Scholar] [CrossRef]
- Kahn, J.M.; Caldwell, E.C.; Deem, S.; Newell, D.W.; Heckbert, S.R.; Rubenfeld, G.D. Acute lung injury in patients with subarachnoid hemorrhage: Incidence, risk factors, and outcome. Crit. Care Med. 2006, 34, 196–202. [Google Scholar] [CrossRef]
| Study Design | Sample Size | Age, y (±SD or Range) * | Sex | Sample (Method) | Relevant Results | Ref. |
|---|---|---|---|---|---|---|
| R | 46 | 61.0 ± 10.7 | 18 M 28 F | CSF (LP and cisternal drain) | Elevated CSF pyruvate concentration is strongly associated with poor grade SAH (WFNS ≥ III). | [16] |
| R | 55 | 55 ± 12 | 15 M 40 F | ISF (CMD) | Biochemical patterns of mitochondrial dysfunction (LPR > 30) in 29 patients, and cerebral ischemia (LPR > 30 and > 40) in 10 patients, including 6 patients who also demonstrated periods of mitochondrial dysfunction. | [29] |
| R | 249 | 55 ± 11 | 102 M 47 F | Serum | Elevated admission serum lactate is predictive of mortality (3.5 ± 2.5 mmol/L vs. 2.2 ± 1.6 mmol/L). | [30] |
| R | 105 | 59 ± 13 | 34 M 71 F | Serum | Early (24 h from admission), serum lactate elevation > 2.2 mmol/L (mean of 2.91 mmol/L) did not independently predict patient mortality and discharge (adjusted odds for Hunt and Hess scale, GCS, age and DCI). | [31] |
| P | 20 | 60 (51–64) | 2 M 18 F | ISF (CMD) | Elevated lactate levels and high LPR (51 ± 36) is correlated with delayed cerebral hypoperfusion (< 32.5 mL/100 g/min) in comatose patients with SAH. | [35] |
| P | 18 | 52 ± 10.7 | 8 M 10 F | ISF (CMD) | Early (day 3) interstitial lactate levels are elevated in patients with bacterial pneumonia (median, 6.82 mmol/L) compared to those without pneumonia (median, 2.90 mmol/L). | [36] |
| R | 285 | 55 (47–65) | 96 M 189 F | Serum | Elevated serum lactate levels (≥ 2.1 mmol/L) in first 24 h after SAH are associated with increased risk of DCI and poor outcomes (mRS of 4–6 at 3 months). | [40] |
| R | 145 | 62 ± 16.3 | 44 M 101 F | Serum | Serum lactate of > 1.1 mmol/L after 48 h of admission is the most accurate predictor of unfavorable neurological outcomes in terms of mRS at discharge. | [41] |
| R | 33 | 61 (26–77) | 11 M 22 F | CSF (EVD) | No association between elevated CSF lactate > 2.1 mmol/L (at 0–240 h post SAH measurement) and impaired circulation and clinical outcomes. However age (≥ 61 years) and coiling for treatment are significantly correlated with elevated lactate levels. | [42] |
| P | 20 | 60 (32–83) | 9 M 11 F | CSF (EVD) | CSF lactate in the first 12 days after SAH and an increased LPR on days 5–7 correlated with onset of cerebral vasospasm. | [44] |
| R | 51 | 55 (44–64) | 18 M 33F | CSF (EVD) | Elevated CSF lactate level (median, 3.2 mmol/L) within 10 days post-SAH correlates with intraventricular hemorrhage and unfavorable outcomes at discharge. | [45] |
| P | 15 | N/A | N/A | ISF (CMD) | Interstitial LPR > 30 is associated with decreased cerebral perfusion, but not with increased ICP of greater than 20 mmHg. | [50] |
| R | 21 | 48 ± 15.9 | 14 M 7 F | ISF (CMD) | Elevated LPR (50.01 ± 24.79) correlates with increased mortality. Survivors had elevated lactate values (8.52 mmol/L vs. 5.89 mmol/L) compared to non survivors. | [51] |
| R | 30 | 58.9 (28–84) | 5 M 25 F | ISF (CMD) | Low CBF (< 28 mL/100 g/min), elevated ISF lactate (4.8 ± 2.2 mmol/L), and elevated LPR (32 ± 16) are early warning signs (day 0–3) of DCI before any clinical symptoms appear. | [52] |
| R | 30 | 58.9 (28–84) | 5 M 25 F | ISF (CMD) | Blood flow measurements and CMD sample monitoring on days 0–3 after onset of SAH showed elevated lactate levels 3.9 ± 2 mmol/L and low regional CBF in territory of the impending ischemia. | [53] |
| P | 19 | 55 (46–73) | 6 M 13 F | ISF (CMD) | Interstitial pyruvate levels vary with level of consciousness. Between 84 and 132 h after SAH, conscious SAH individuals had normal levels (159–196 µM) but in unconscious SAH patients, pyruvate levels remained low (102–131 µM). | [54] |
| R | 28 | 55.4 | 13 M 15 F | CSF (intrathecal and intraventricular) | Patients with modified Fisher grades 3 and 4 have elevated intrathecal CSF lactate (> 5.5 mmol/L) on day 7 post SAH and is predictive of poor neurological outcomes and hydrocephalus requiring a shunt. | [55] |
| R | 140 | 48 (47.3–65.5) (patients who developed NPE only) | 48 M 92 F | Serum | Increase serum lactate levels (54.0 mg/dl), within one hour after SAH are associated with early onset of neurogenic pulmonary edema (NPE). | [56] |
| P | 10 | 52.2 ± 5.0 | 7 M 3 W | ISF (CMD) | 10 fold increase in lactate levels (baseline levels 100–200 µmol/L) along with increase in excitatory amino acids in SAH showed correlation with poor outcomes at the 3 months (GOS 1 to 3). | [33] |
| P | 22 | 56 (47–68) | 7 M 15 F | ISF (CMD) | High CMD tau protein was positively correlated with elevated levels of lactate (> 4 mmol/L) and also positively correlated with pyruvate, LPR, and poor functional outcome (mRS ≥ 4) 12 months after SAH after adjusting for disease severity and age. | [39] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zahra, K.; Gopal, N.; Freeman, W.D.; Turnbull, M.T. Using Cerebral Metabolites to Guide Precision Medicine for Subarachnoid Hemorrhage: Lactate and Pyruvate. Metabolites 2019, 9, 245. https://doi.org/10.3390/metabo9110245
Zahra K, Gopal N, Freeman WD, Turnbull MT. Using Cerebral Metabolites to Guide Precision Medicine for Subarachnoid Hemorrhage: Lactate and Pyruvate. Metabolites. 2019; 9(11):245. https://doi.org/10.3390/metabo9110245
Chicago/Turabian StyleZahra, Kaneez, Neethu Gopal, William D. Freeman, and Marion T. Turnbull. 2019. "Using Cerebral Metabolites to Guide Precision Medicine for Subarachnoid Hemorrhage: Lactate and Pyruvate" Metabolites 9, no. 11: 245. https://doi.org/10.3390/metabo9110245
APA StyleZahra, K., Gopal, N., Freeman, W. D., & Turnbull, M. T. (2019). Using Cerebral Metabolites to Guide Precision Medicine for Subarachnoid Hemorrhage: Lactate and Pyruvate. Metabolites, 9(11), 245. https://doi.org/10.3390/metabo9110245





