Subnormothermic Machine Perfusion of Steatotic Livers Results in Increased Energy Charge at the Cost of Anti-Oxidant Capacity Compared to Normothermic Perfusion
Abstract
1. Introduction
2. Results
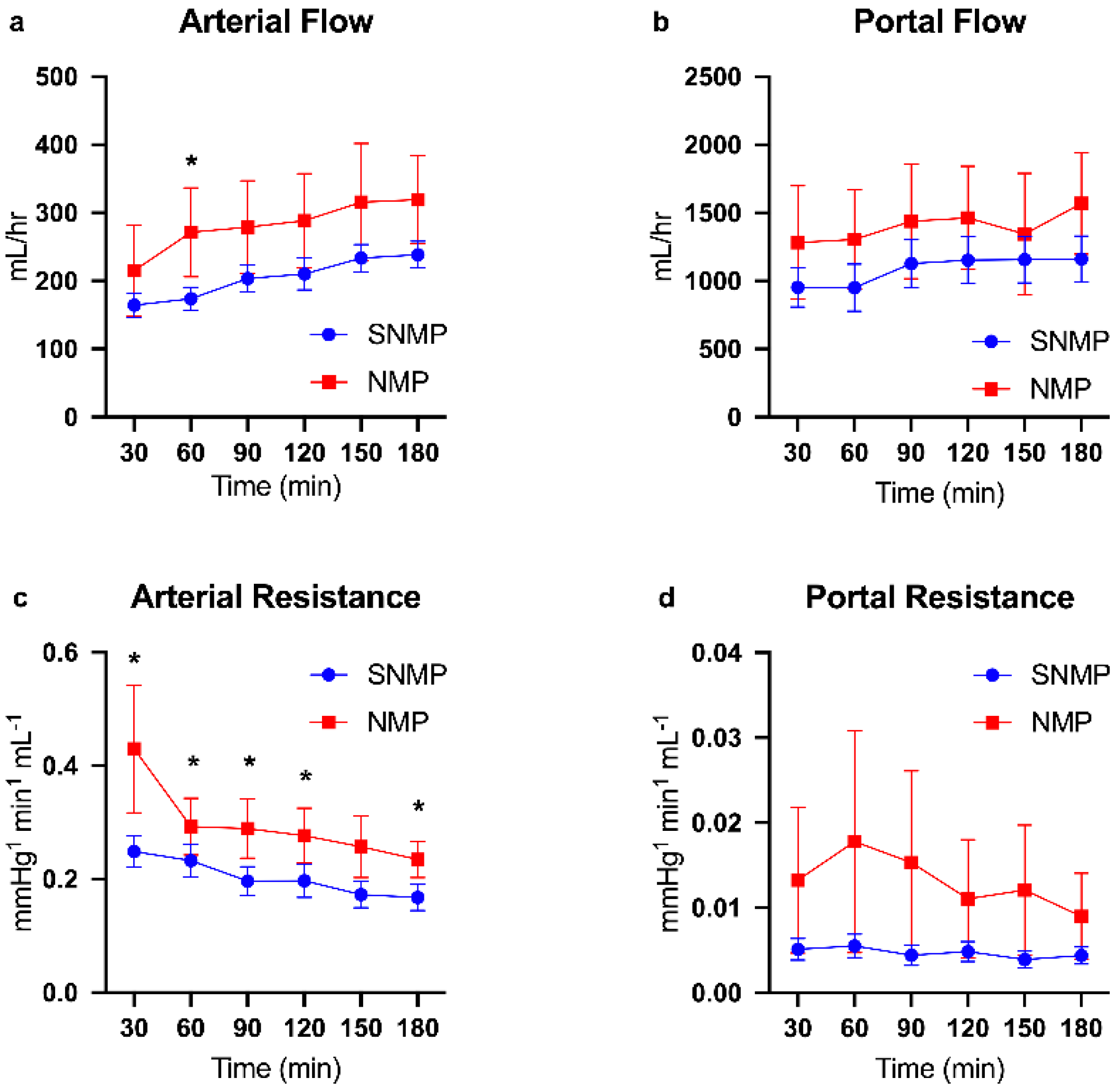
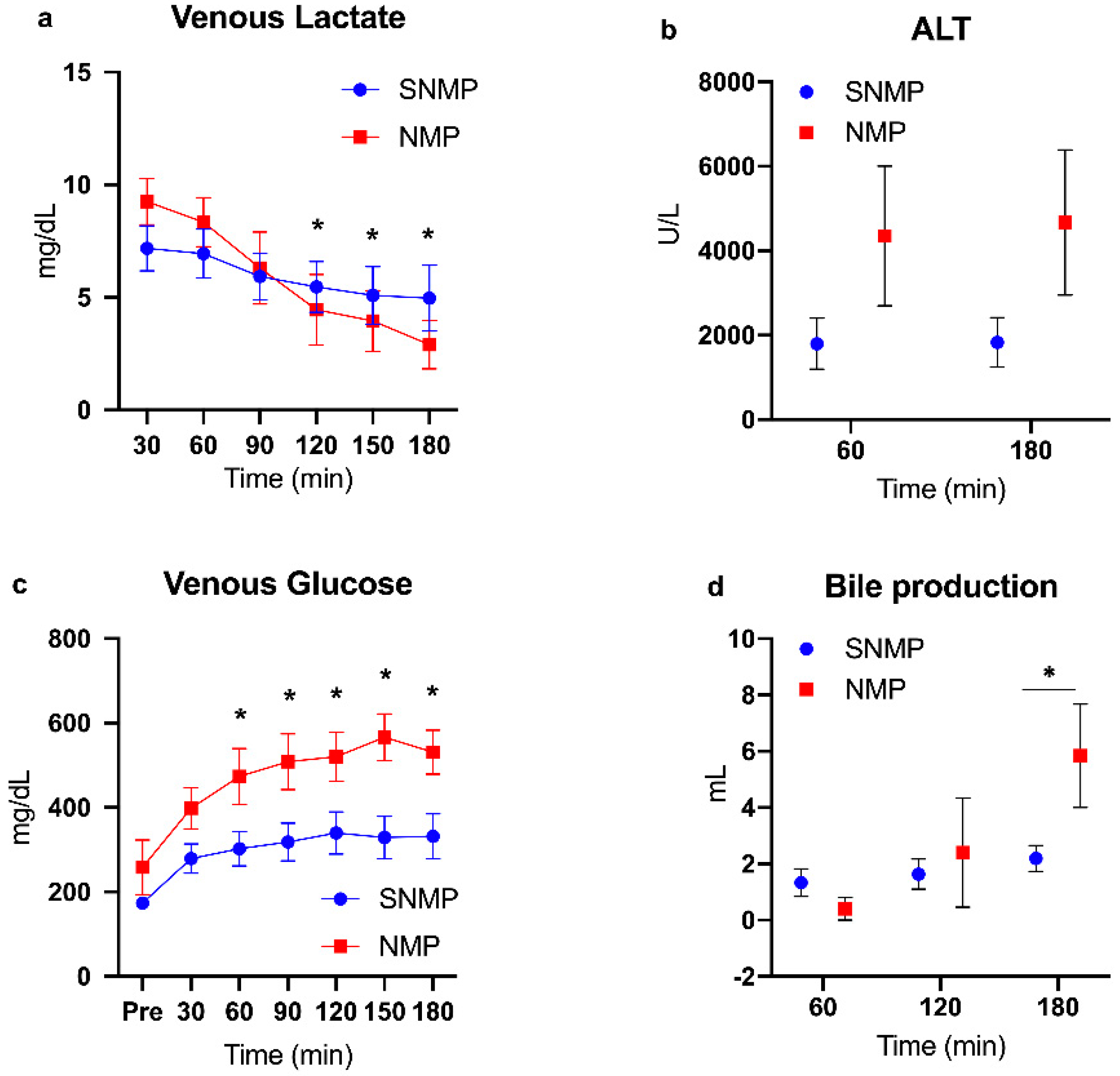
2.1. Functional Recovery during Perfusion
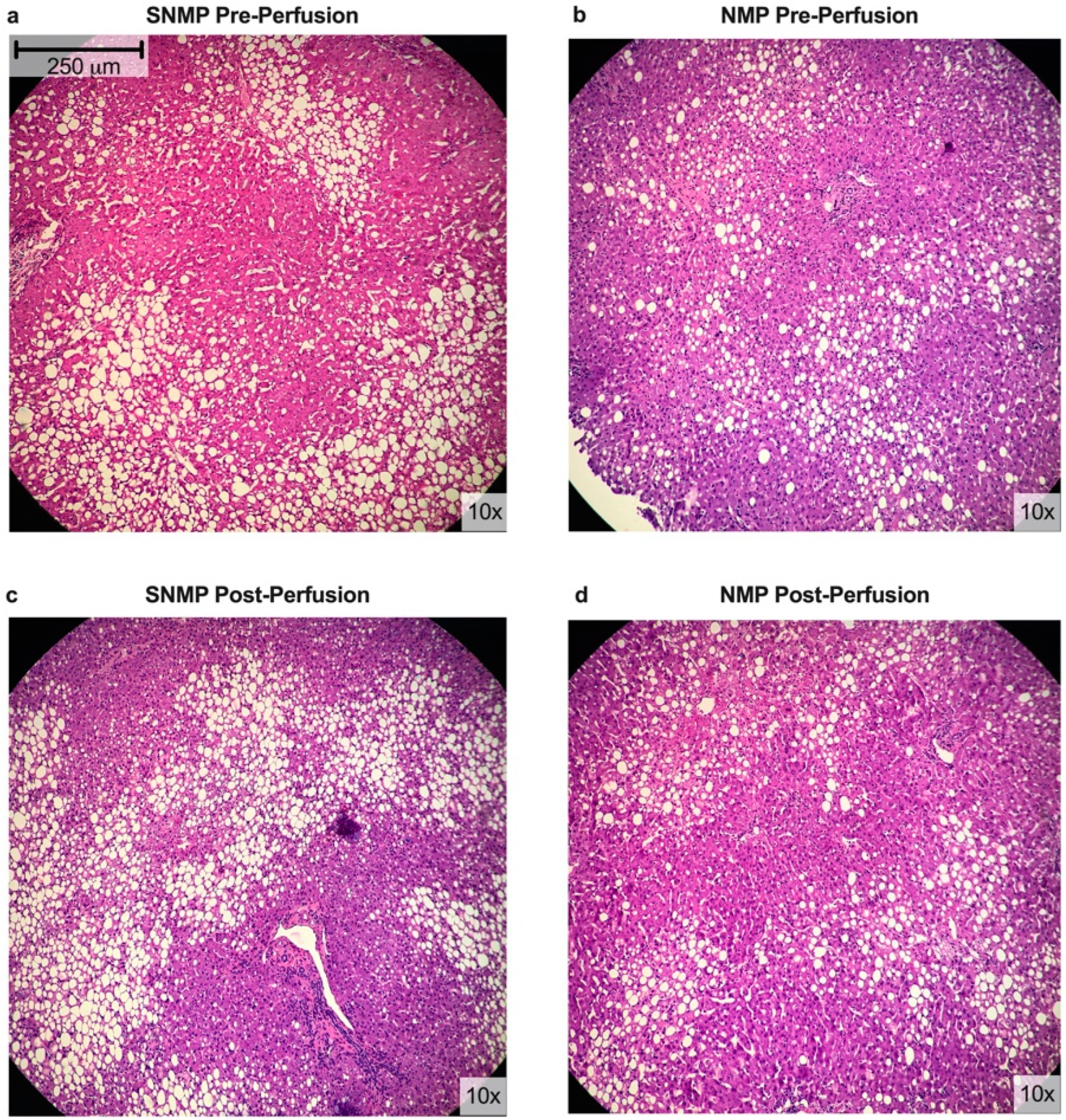
2.2. Histologic Assessment
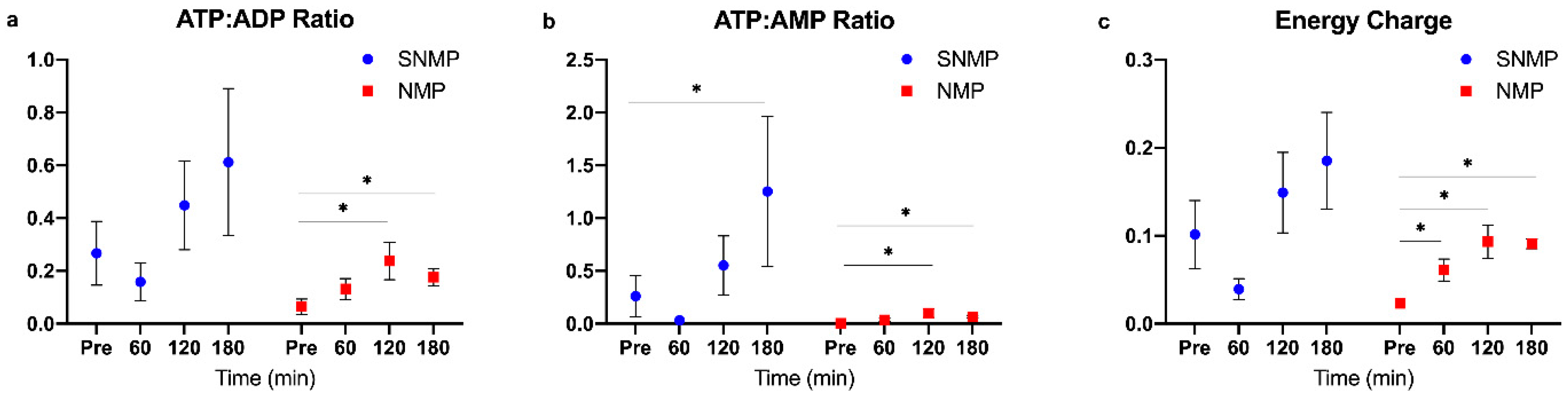
2.3. Targeted Energy Cofactor Analysis
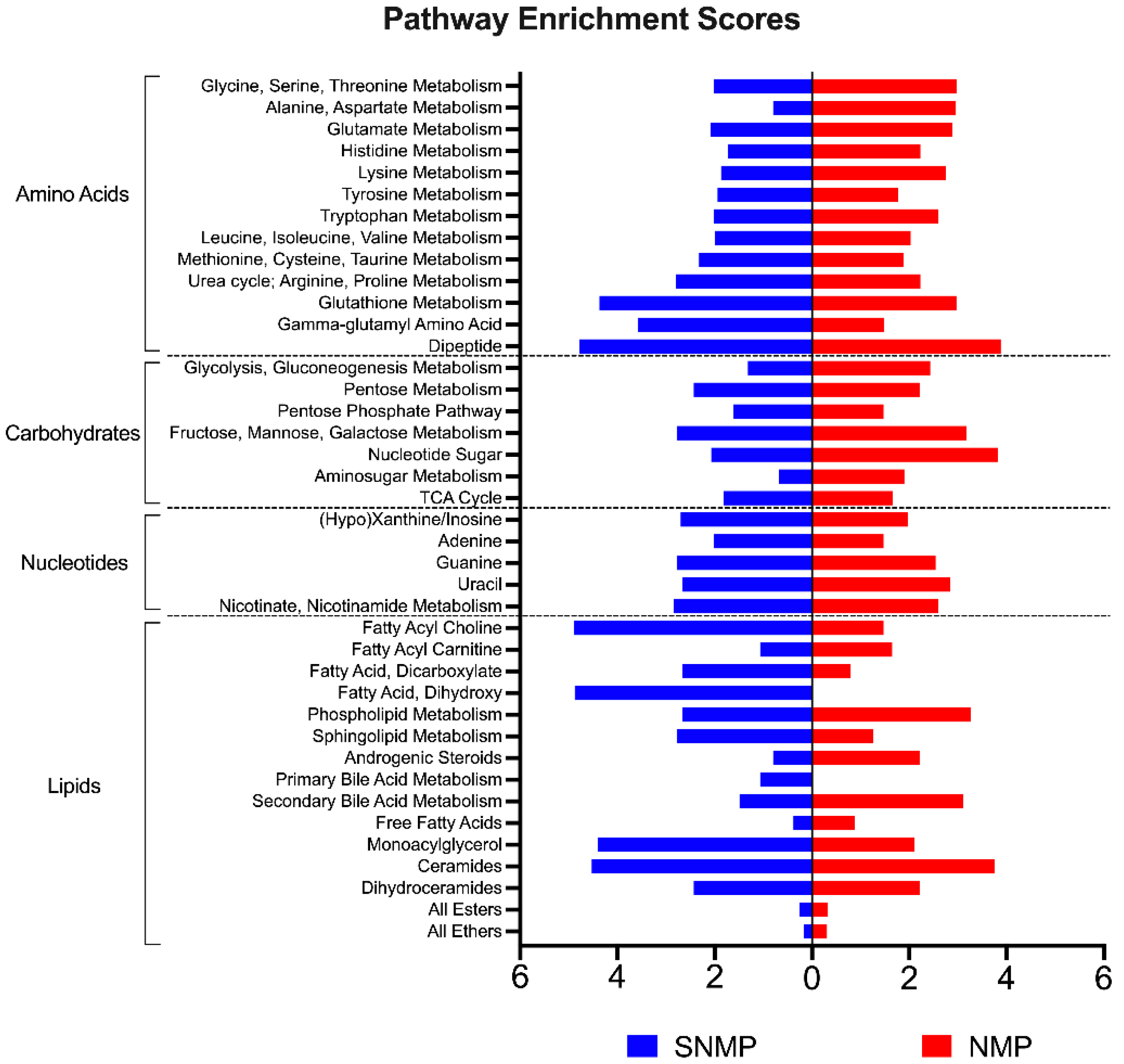
2.4. Untargeted Metabolomic Analysis
2.4.1. Data Reduction Methods
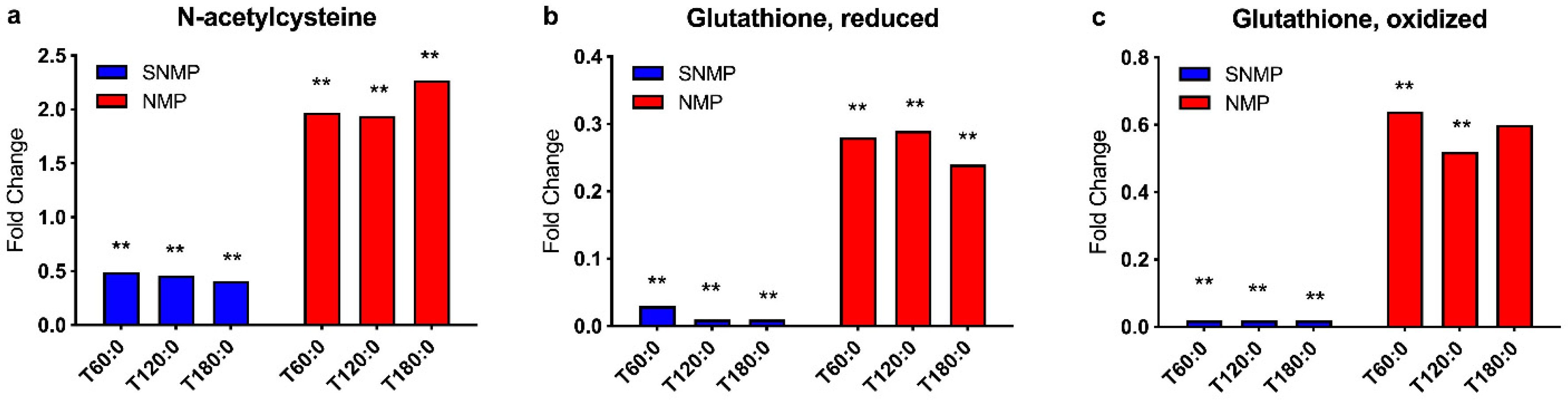
2.4.2. Anti-Oxidative Capacity
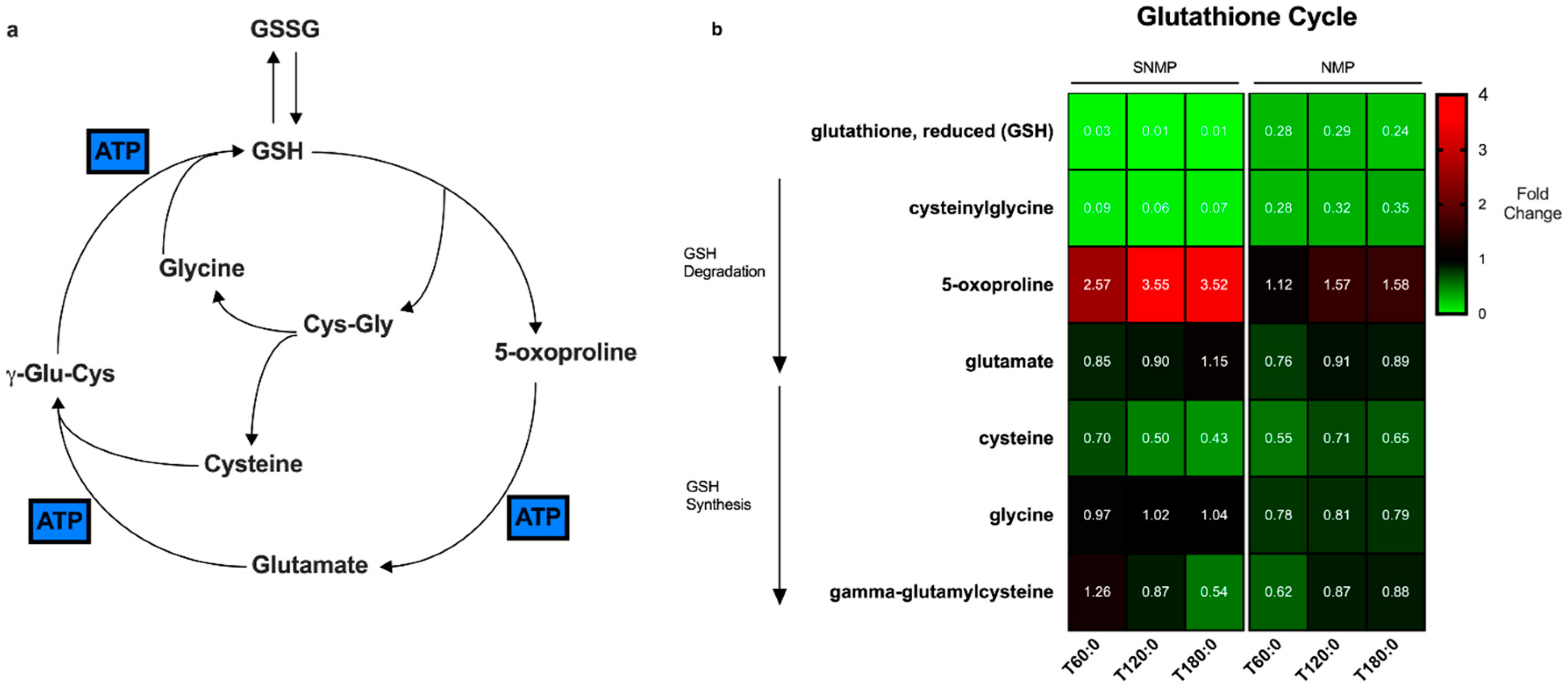
2.4.3. Glutathione Cycle
2.4.4. Carbohydrate Metabolism
2.4.5. Lipid Metabolism
3. Discussion
4. Materials and Methods
4.1. Donor Livers
4.2. Procurement and Preparation of Liver Grafts
4.3. Machine Perfusion
4.4. Evaluation of Hepatic Injury and Function
4.5. Histological Assessment
4.6. Targeted Metabolomics Analysis—Energy Cofactors
4.7. Untargeted Metabolomic and Lipidomic Analysis
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nasralla, D.; Coussios, C.C.; Mergental, H.; Akhtar, M.Z.; Butler, A.J.; Ceresa, C.D.L.; Chiocchia, V.; Dutton, S.J.; Garcia-Valdecasas, J.C.; Heaton, N.; et al. A randomized trial of normothermic preservation in liver transplantation. Nature 2018, 557, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Laing, R.W.; Mergental, H.; Yap, C.; Kirkham, A.; Whilku, M.; Barton, D.; Curbishley, S.; Boteon, Y.L.; Neil, D.A.; Hubscher, S.G.; et al. Viability testing and transplantation of marginal livers (VITTAL) using normothermic machine perfusion: Study protocol for an open-label, non-randomised, prospective, single-arm trial. BMJ Open 2017, 7, e017733. [Google Scholar] [PubMed]
- Matton, A.P.M.; Burlage, L.C.; van Rijn, R.; de Vries, Y.; Karangwa, S.A.; Nijsten, M.W.; Gouw, A.S.H.; Wiersema-Buist, J.; Adelmeijer, J.; Westerkamp, A.C.; et al. Normothermic machine perfusion of donor livers without the need for human blood products. Liver Transplant. 2018, 24, 528–538. [Google Scholar] [CrossRef]
- Mergental, H.; Perera, M.T.; Laing, R.W.; Muiesan, P.; Isaac, J.R.; Smith, A.; Stephenson, B.T.; Cilliers, H.; Neil, D.A.; Hubscher, S.G.; et al. Transplantation of declined liver allografts following normothermic Ex-Situ evaluation. Am. J. Transplant. 2016, 16, 3235–3245. [Google Scholar] [CrossRef]
- Bruinsma, B.G.; Sridharan, G.V.; Weeder, P.D.; Avruch, J.H.; Saeidi, N.; Ozer, S.; Geerts, S.; Porte, R.J.; Heger, M.; van Gulik, T.M.; et al. Metabolic profiling during ex vivo machine perfusion of the human liver. Sci. Rep. 2016, 6, 22415. [Google Scholar] [CrossRef] [PubMed]
- Bruinsma, B.G.; Yeh, H.; Ozer, S.; Martins, P.N.; Farmer, A.; Wu, W.; Saeidi, N.; Op den Dries, S.; Berendsen, T.A.; Smith, R.N.; et al. Subnormothermic machine perfusion for ex vivo preservation and recovery of the human liver for transplantation. Am. J. Transplant. 2014, 14, 1400–1409. [Google Scholar] [CrossRef]
- Spetzler, V.N.; Goldaracena, N.; Echiverri, J.; Kaths, J.M.; Louis, K.S.; Adeyi, O.A.; Yip, P.M.; Grant, D.R.; Selzner, N.; Selzner, M. Subnormothermic ex vivo liver perfusion is a safe alternative to cold static storage for preserving standard criteria grafts. Liver Transplant. 2016, 22, 111–119. [Google Scholar] [CrossRef]
- Kron, P.; Schlegel, A.; Mancina, L.; Clavien, P.A.; Dutkowski, P. Hypothermic oxygenated perfusion (HOPE) for fatty liver grafts in rats and humans. J. Hepatol. 2017. [Google Scholar] [CrossRef]
- Schlegel, A.; Muller, X.; Kalisvaart, M.; Muellhaupt, B.; Perera, M.; Isaac, J.R.; Clavien, P.A.; Muiesan, P.; Dutkowski, P. Outcomes of DCD liver transplantation using organs treated by hypothermic oxygenated perfusion before implantation. J. Hepatol. 2019, 70, 50–57. [Google Scholar] [CrossRef]
- Boteon, Y.L.; Attard, J.; Boteon, A.; Wallace, L.; Reynolds, G.; Hubscher, S.; Mirza, D.F.; Mergental, H.; Bhogal, R.H.; Afford, S.C. Manipulation of lipid metabolism during normothermic machine perfusion: Effect of defatting therapies on donor liver functional recovery. Liver Transplant. 2019, 25, 1007–1022. [Google Scholar] [CrossRef]
- Dutkowski, P.; Guarrera, J.V.; de Jonge, J.; Martins, P.N.; Porte, R.J.; Clavien, P.A. Evolving Trends in machine perfusion for liver transplantation. Gastroenterology 2019, 156, 1542–1547. [Google Scholar] [CrossRef] [PubMed]
- Minor, T.; Efferz, P.; Fox, M.; Wohlschlaeger, J.; Luer, B. Controlled oxygenated rewarming of cold stored liver grafts by thermally graduated machine perfusion prior to reperfusion. Am. J. Transplant. 2013, 13, 1450–1460. [Google Scholar] [CrossRef] [PubMed]
- de Vries, Y.; Matton, A.P.M.; Nijsten, M.W.N.; Werner, M.J.M.; van den Berg, A.P.; de Boer, M.T.; Buis, C.I.; Fujiyoshi, M.; de Kleine, R.H.J.; van Leeuwen, O.B.; et al. Pretransplant sequential hypo- and normothermic machine perfusion of suboptimal livers donated after circulatory death using a hemoglobin-based oxygen carrier perfusion solution. Am. J. Transplant. 2019, 19, 1202–1211. [Google Scholar] [CrossRef] [PubMed]
- Bachhawat, A.K.; Yadav, S. The glutathione cycle: Glutathione metabolism beyond the gamma-glutamyl cycle. IUBMB Life 2018, 70, 585–592. [Google Scholar] [CrossRef]
- Alva, N.; Panisello-Rosello, A.; Flores, M.; Rosello-Catafau, J.; Carbonell, T. Ubiquitin-proteasome system and oxidative stress in liver transplantation. World J. Gastroenterol. 2018, 24, 3521–3530. [Google Scholar] [CrossRef]
- McLaren, A.J.; Friend, P.J. Trends in organ preservation. Transpl. Int. 2003, 16, 701–708. [Google Scholar] [CrossRef]
- Shi, Z.Z.; Habib, G.M.; Rhead, W.J.; Gahl, W.A.; He, X.; Sazer, S.; Lieberman, M.W. Mutations in the glutathione synthetase gene cause 5-oxoprolinuria. Nat. Genet. 1996, 14, 361–365. [Google Scholar] [CrossRef]
- Grezzana Filho Tde, J.; Corso, C.O.; Zanotelli, M.L.; Marroni, C.A.; Brandao, A.B.; Schlindwein, E.; Leipnitz, I.; Meine, M.H.; Fleck, A., Jr.; Hoppen, R.; et al. Liver glutathione depletion after preservation and reperfusion in human liver transplantation. Acta Cir. Bras. 2006, 21, 223–229. [Google Scholar] [CrossRef][Green Version]
- Grezzana, T.J.; Corso, C.O.; Zanotelli, M.L.; Marroni, C.A.; Brandao, A.B.; Schlindwein, E.; Leipnitz, I.; Meine, M.H.; Fleck, A., Jr.; Cassal, A.; et al. Oxidative stress, hepatocellular integrity, and hepatic function after initial reperfusion in human hepatic transplantation. Transplant. Proc. 2004, 36, 843–845. [Google Scholar] [CrossRef]
- Boteon, Y.L.; Laing, R.W.; Schlegel, A.; Wallace, L.; Smith, A.; Attard, J.; Bhogal, R.H.; Neil, D.A.H.; Hubscher, S.; Perera, M.; et al. Combined Hypothermic and Normothermic Machine Perfusion Improves Functional Recovery of Extended Criteria Donor Livers. Liver Transplant. 2018, 24, 1699–1715. [Google Scholar] [CrossRef]
- von Horn, C.; Baba, H.A.; Hannaert, P.; Hauet, T.; Leuvenink, H.; Paul, A.; Minor, T.; COPE Consortium Partners. Controlled oxygenated rewarming up to normothermia for pretransplant reconditioning of liver grafts. Clin. Transplant. 2017, 31, e13101. [Google Scholar] [CrossRef] [PubMed]
- Hoyer, D.P.; Mathe, Z.; Gallinat, A.; Canbay, A.C.; Treckmann, J.W.; Rauen, U.; Paul, A.; Minor, T. Controlled oxygenated rewarming of cold stored livers prior to transplantation: First clinical application of a new concept. Transplantation 2016, 100, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Sacco, R.; Eggenhoffner, R.; Giacomelli, L. Glutathione in the treatment of liver diseases: Insights from clinical practice. Minerva Gastroenterol. Dietol. 2016, 62, 316–324. [Google Scholar] [PubMed]
- Sun, Y.; Pu, L.Y.; Lu, L.; Wang, X.H.; Zhang, F.; Rao, J.H. N-acetylcysteine attenuates reactive-oxygen-species-mediated endoplasmic reticulum stress during liver ischemia-reperfusion injury. World J. Gastroenterol. 2014, 20, 15289–15298. [Google Scholar] [CrossRef]
- Vilar-Gomez, E.; Vuppalanchi, R.; Gawrieh, S.; Ghabril, M.; Saxena, R.; Cummings, O.W.; Chalasani, N. Vitamin E improves transplant-free survival and hepatic decompensation among patients with nonalcoholic steatohepatitis and advanced fibrosis. Hepatology 2018. [Google Scholar] [CrossRef]
- Garcia-Ruiz, C.; Mato, J.M.; Vance, D.; Kaplowitz, N.; Fernandez-Checa, J.C. Acid sphingomyelinase-ceramide system in steatohepatitis: A novel target regulating multiple pathways. J. Hepatol. 2015, 62, 219–233. [Google Scholar] [CrossRef]
- Kurek, K.; Piotrowska, D.M.; Wiesiolek-Kurek, P.; Lukaszuk, B.; Chabowski, A.; Gorski, J.; Zendzian-Piotrowska, M. Inhibition of ceramide de novo synthesis reduces liver lipid accumulation in rats with nonalcoholic fatty liver disease. Liver Int. 2014, 34, 1074–1083. [Google Scholar] [CrossRef]
- Ussher, J.R.; Koves, T.R.; Cadete, V.J.; Zhang, L.; Jaswal, J.S.; Swyrd, S.J.; Lopaschuk, D.G.; Proctor, S.D.; Keung, W.; Muoio, D.M.; et al. Inhibition of de novo ceramide synthesis reverses diet-induced insulin resistance and enhances whole-body oxygen consumption. Diabetes 2010, 59, 2453–2464. [Google Scholar] [CrossRef]
- Imig, J.D. Epoxyeicosatrienoic acids and 20-hydroxyeicosatetraenoic acid on endothelial and vascular function. Adv. Pharmacol. 2016, 77, 105–141. [Google Scholar]
- Imig, J.D.; Hammock, B.D. Soluble epoxide hydrolase as a therapeutic target for cardiovascular diseases. Nat. Rev. Drug Discov. 2009, 8, 794–805. [Google Scholar] [CrossRef]
- Tripathi, N.; Paliwal, S.; Sharma, S.; Verma, K.; Gururani, R.; Tiwari, A.; Verma, A.; Chauhan, M.; Singh, A.; Kumar, D.; et al. Discovery of novel soluble epoxide hydrolase inhibitors as potent vasodilators. Sci. Rep. 2018, 8, 14604. [Google Scholar] [CrossRef] [PubMed]
- Farrell, G.C.; Teoh, N.C.; McCuskey, R.S. Hepatic microcirculation in fatty liver disease. Anat. Rec. Hoboken 2008, 291, 684–692. [Google Scholar] [CrossRef] [PubMed]
- Laing, R.W.; Bhogal, R.H.; Wallace, L.; Boteon, Y.; Neil, D.A.H.; Smith, A.; Stephenson, B.T.F.; Schlegel, A.; Hubscher, S.G.; Mirza, D.F.; et al. The use of an acellular oxygen carrier in a human liver model of normothermic machine perfusion. Transplantation 2017, 101, 2746–2756. [Google Scholar] [CrossRef] [PubMed]
- Watson, C.J.E.; Kosmoliaptsis, V.; Pley, C.; Randle, L.; Fear, C.; Crick, K.; Gimson, A.E.; Allison, M.; Upponi, S.; Brais, R.; et al. Observations on the ex situ perfusion of livers for transplantation. Am. J. Transplant. 2018, 18, 2005–2020. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, A.; Muller, X.; Dutkowski, P. Hypothermic liver perfusion. Curr. Opin. Organ. Transplant. 2017, 22, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Faitot, F.; Besch, C.; Battini, S.; Ruhland, E.; Onea, M.; Addeo, P.; Woehl-Jaegle, M.L.; Ellero, B.; Bachellier, P.; Namer, I.J. Impact of real-time metabolomics in liver transplantation: Graft evaluation and donor-recipient matching. J. Hepatol. 2018, 68, 699–706. [Google Scholar] [CrossRef]
- Karimian, N.; Matton, A.P.; Westerkamp, A.C.; Burlage, L.C.; Op den Dries, S.; Leuvenink, H.G.; Lisman, T.; Uygun, K.; Markmann, J.F.; Porte, R.J. Ex Situ normothermic machine perfusion of donor livers. J. Vis. Exp. 2015, e52688. [Google Scholar] [CrossRef]
- Berendsen, T.A.; Bruinsma, B.G.; Lee, J.; D’Andrea, V.; Liu, Q.; Izamis, M.L.; Uygun, K.; Yarmush, M.L. A simplified subnormothermic machine perfusion system restores ischemically damaged liver grafts in a rat model of orthotopic liver transplantation. Transplant. Res. 2012, 1, 6. [Google Scholar] [CrossRef][Green Version]
- Yuan, M.; Breitkopf, S.B.; Yang, X.; Asara, J.M. A positive/negative ion-switching, targeted mass spectrometry-based metabolomics platform for bodily fluids, cells, and fresh and fixed tissue. Nat. Protoc. 2012, 7, 872–881. [Google Scholar] [CrossRef]
| Steatotic SNMP | Steatotic NMP | |
|---|---|---|
| N = 9 | N = 5 | |
| Age (years) | 44.9 (22–60) | 48.4 (37–55) |
| Gender (female) | 2 | 1 |
| BMI (kg/m2) | 29.4 (22.7–38) | 32.6 (25.8–37.9) |
| Type of Donor Liver | ||
| DCD | 5 | 2 |
| DBD | 4 | 3 |
| WIT (min) | 35.3 (17–48) | 27 * |
| CIT (min) | 554 (304–808) | 735 (463–935) |
| Weight of Liver (kg) | 2.3 (1.5–3.1) | 2.3 (2.0–2.8) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karimian, N.; Raigani, S.; Huang, V.; Nagpal, S.; Hafiz, E.O.A.; Beijert, I.; Mahboub, P.; Porte, R.J.; Uygun, K.; Yarmush, M.; et al. Subnormothermic Machine Perfusion of Steatotic Livers Results in Increased Energy Charge at the Cost of Anti-Oxidant Capacity Compared to Normothermic Perfusion. Metabolites 2019, 9, 246. https://doi.org/10.3390/metabo9110246
Karimian N, Raigani S, Huang V, Nagpal S, Hafiz EOA, Beijert I, Mahboub P, Porte RJ, Uygun K, Yarmush M, et al. Subnormothermic Machine Perfusion of Steatotic Livers Results in Increased Energy Charge at the Cost of Anti-Oxidant Capacity Compared to Normothermic Perfusion. Metabolites. 2019; 9(11):246. https://doi.org/10.3390/metabo9110246
Chicago/Turabian StyleKarimian, Negin, Siavash Raigani, Viola Huang, Sonal Nagpal, Ehab O. A. Hafiz, Irene Beijert, Paria Mahboub, Robert J. Porte, Korkut Uygun, Martin Yarmush, and et al. 2019. "Subnormothermic Machine Perfusion of Steatotic Livers Results in Increased Energy Charge at the Cost of Anti-Oxidant Capacity Compared to Normothermic Perfusion" Metabolites 9, no. 11: 246. https://doi.org/10.3390/metabo9110246
APA StyleKarimian, N., Raigani, S., Huang, V., Nagpal, S., Hafiz, E. O. A., Beijert, I., Mahboub, P., Porte, R. J., Uygun, K., Yarmush, M., & Yeh, H. (2019). Subnormothermic Machine Perfusion of Steatotic Livers Results in Increased Energy Charge at the Cost of Anti-Oxidant Capacity Compared to Normothermic Perfusion. Metabolites, 9(11), 246. https://doi.org/10.3390/metabo9110246





