Metabolomics Approach Reveals the Effects of Breed and Feed on the Composition of Chicken Eggs
Abstract
1. Introduction
2. Results
2.1. Egg Traits
2.2. Egg Metabolite Traits
3. Discussion
4. Materials and Methods
4.1. Study Animals
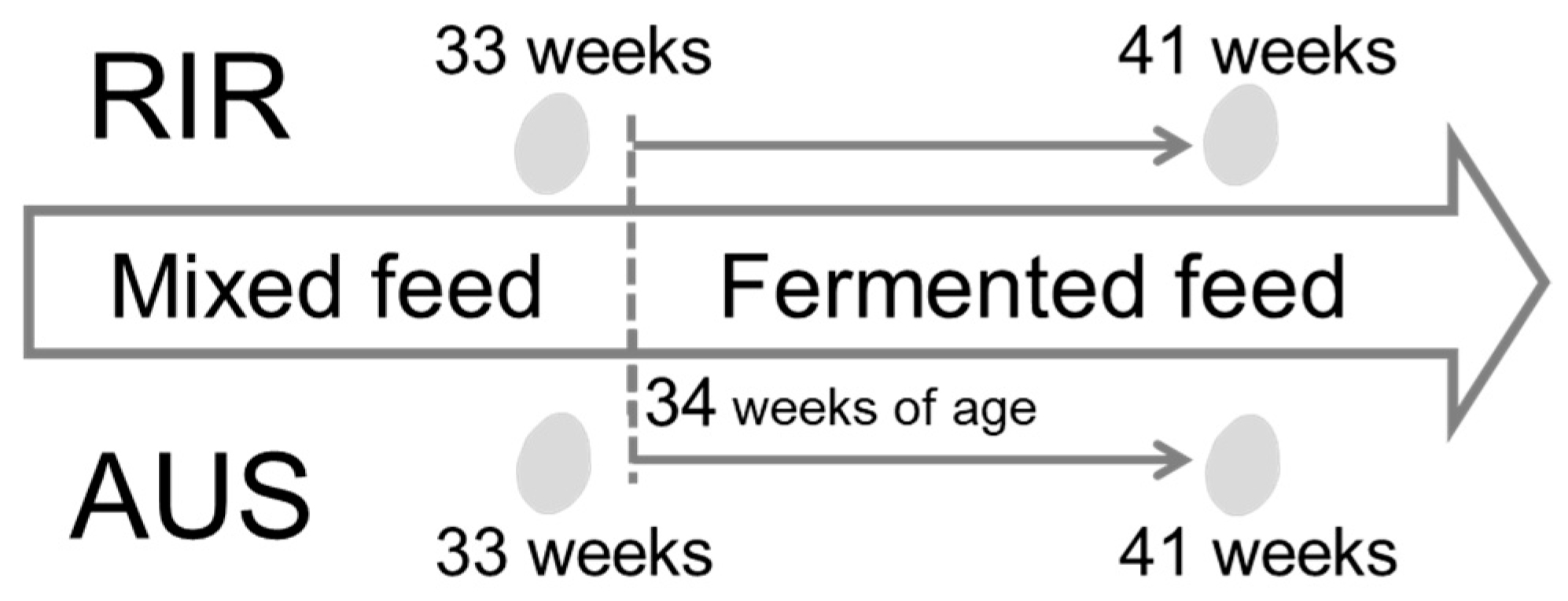
4.2. Experimental Conditions and Sampling
4.3. Measuring Egg Properties
4.4. Metabolomic Analysis of Egg Yolk and Albumen
4.5. Statistical Methods
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- FAOSTAT. 2019. Available online: http://www.fao.org/faostat/en/#home (accessed on 20 September 2019).
- Goto, T.; Fernandes, A.F.A.; Tsudzuki, M.; Rosa, G.J.M. Causal phenotypic networks for egg traits in an F2 chicken population. Mol. Genet. Genom. 2019, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hunger Map. 2018. Available online: https://www.wfp.org/content/2018-hunger-map (accessed on 20 September 2019).
- Kralik, G.; Kralik, Z. Poultry products enriched with nutricines have beneficial effects on human health. Med. Glas. 2017, 14, 1–7. [Google Scholar] [CrossRef]
- Laudodio, V.; Lorusso, V.; Lastella, N.M.B.; Dhama, K.; Karthik, K.; Tiwari, R.; Alam, G.M.; Tufarelli, V. Enhancement of nutraceutical value of table eggs through poultry feeding strategies. Int. J. Pharm. 2015, 11, 201–212. [Google Scholar] [CrossRef]
- Rajasekaran, A.; Kalaivani, M. Designer foods and their benefits: A review. J. Food Sci. Technol. 2013, 50, 1–16. [Google Scholar] [CrossRef]
- Zaheer, K. An updated review on chicken eggs: Production, consumption, management aspects and nutritional benefits to human health. Food Nutr. Sci. 2015, 6, 1208–1220. [Google Scholar] [CrossRef]
- Alagawany, M.; Farag, M.R.; Dhama, K.; Patra, A. Nutritional significance and health benefits of designer eggs. Worlds Poult. Sci. J. 2018, 74, 317–330. [Google Scholar] [CrossRef]
- Roberts, J. Factors affecting egg internal quality and egg shell quality in laying hens. J. Poult. Sci. 2004, 41, 161–177. [Google Scholar] [CrossRef]
- Wilson, P.B. Recent advances in avian egg science: A review. Poult. Sci. 2017, 96, 3747–3754. [Google Scholar] [CrossRef] [PubMed]
- Goto, T.; Tsudzuki, M. Genetic mapping of quantitative trait loci for egg production and egg quality traits in chickens: A review. J. Poult. Sci. 2017, 54, 0160121. [Google Scholar] [CrossRef]
- Wolc, A.; White, I.M.S.; Hill, W.G.; Olori, V.E. Inheritance of hatchability in broiler chickens and its relationship to egg quality traits. Poult. Sci. 2010, 89, 2334–2340. [Google Scholar] [CrossRef]
- Wolc, A.; Arango, J.; Settar, P.; O’Sullivan, N.P.; Olori, V.E.; White, I.M.S.; Hill, W.G.; Dekkers, J.C.M. Genetic parameters of egg defects and egg quality in layer chickens. Poult. Sci. 2012, 91, 1292–1298. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.C.; Ning, Z.H.; Xu, G.Y.; Hou, Z.C.; Yang, N. Heritabilities and genetic and phenotypic correlations of egg quality traits in brown-egg dwarf layers. Poult. Sci. 2005, 84, 1209–1213. [Google Scholar] [CrossRef] [PubMed]
- Fraeye, I.; Bruneel, C.; Lemahieu, C.; Buyse, J.; Muylaert, K.; Foubert, I. Dietary enrichment of eggs with omega-3 fatty acids: A review. Food Res. Int. 2012, 48, 961–969. [Google Scholar] [CrossRef]
- Surai, P.F.; Sparks, N.H.C. Designer eggs: From improvement of egg composition to functional food. Trends. Food Sci. Technol. 2001, 12, 7–16. [Google Scholar] [CrossRef]
- Yin, J.D.; Shang, X.G.; Li, D.F.; Wang, F.L.; Guan, Y.F.; Wang, Z.Y. Effects of dietary conjugated linoleic acid on the fatty acid profile and cholesterol content of egg yolks from different breeds of layers. Poult. Sci. 2008, 87, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Mori, H.; Takaya, M.; Nishimura, K.; Goto, T. Breed and feed affect amino acid contents of egg yolk and eggshell color in chickens. Poult. Sci. 2019. [Google Scholar] [CrossRef]
- Weckwerth, W. Metabolomics in systems biology. Annu. Rev. Plant Biol. 2003, 54, 669–689. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, W.J.; Koal, T.; Wang, Y.; Kohl, M.; Enot, D.P.; Deigner, H.P. Targeted metabolomics for biomarker discovery. Angew. Chem. Int. Ed. Engl. 2010, 26, 5426–5445. [Google Scholar] [CrossRef]
- Li, B.; He, X.; Jia, W.; Li, H. Novel applications of metabolomics in personalized medicine: A mini-review. Molecules 2017, 22, 1173. [Google Scholar] [CrossRef]
- Wishart, D.S. Metabolomics: Applications to food science and nutrition research. Trends Food Sci. Technol. 2008, 19, 482–493. [Google Scholar] [CrossRef]
- Cubero-Leon, E.; Peñalver, R.; Maquet, A. Review on metabolomics for food authentication. Food Res. Int. 2014, 60, 95–107. [Google Scholar] [CrossRef]
- Bundy, J.G.; Davey, M.P.; Viant, M.R. Environmental metabolomics: A critical review and future perspectives. Metabolomics 2009, 5, 3. [Google Scholar] [CrossRef]
- Macel, M.; Van Dam, N.M.; Keurentjes, J.J. Metabolomics: The chemistry between ecology and genetics. Mol. Ecol. Resour. 2010, 10, 583–593. [Google Scholar] [CrossRef] [PubMed]
- Goto, T.; Kubota, Y.; Toyoda, A. Plasma and liver metabolic profiles in mice subjected to subchronic and mild social defeat stress. J. Proteome Res. 2015, 14, 1025–1032. [Google Scholar] [CrossRef] [PubMed]
- Goto, T.; Tomonaga, S.; Toyoda, A. Effects of diet quality and psychosocial stress on the metabolic profiles of mice. J. Proteome Res. 2017, 16, 1857–1867. [Google Scholar] [CrossRef] [PubMed]
- Goldansaz, S.A.; Guo, A.C.; Sajed, T.; Steele, M.A.; Plastow, G.S.; Wishart, D.S. Livestock metabolomics and the livestock metabolome: A systematic review. PLoS ONE 2017, 12, e0177675. [Google Scholar] [CrossRef] [PubMed]
- Takemoto, S.; Tomonaga, S.; Funaba, M.; Matsui, T. Effect of long-distance transportation on serum metabolic profiles of steer calves. Anim. Sci. J. 2017, 88, 1970–1978. [Google Scholar] [CrossRef]
- Tomonaga, S.; Okuyama, H.; Tachibana, T.; Makino, R. Effects of high ambient temperature on plasma metabolomic profiles in chicks. Anim. Sci. J. 2018, 89, 448–455. [Google Scholar] [CrossRef]
- Wishart, D.S.; Feunang, Y.D.; Marcu, A.; Guo, A.C.; Liang, K.; Vázquez-Fresno, R.; Sajed, T.; Johnson, D.; Li, C.; Karu, N.; et al. HMDB 4.0: The human metabolome database for 2018. Nucleic Acid Res. 2017, 46, D608–D617. [Google Scholar] [CrossRef]
- Goto, T.; Ishikawa, A.; Nishibori, M.; Tsudzuki, M. A longitudinal quantitative trait locus mapping of chicken growth traits. Mol. Genet. Genom. 2019, 294, 243–252. [Google Scholar] [CrossRef]
- Goto, T.; Ishikawa, A.; Yoshida, M.; Goto, N.; Umino, T.; Nishibori, M.; Tsudzuki, M. Quantitative trait loci mapping for external egg traits in F2 chickens. J. Poult. Sci. 2014, 51, 118–129. [Google Scholar] [CrossRef]
- Goto, T.; Shiraishi, J.I.; Bungo, T.; Tsudzuki, M. Characteristics of egg-related traits in the Onagadori (Japanese Extremely Long Tail) breed of chickens. J. Poult. Sci. 2015, 52, 81–87. [Google Scholar] [CrossRef]
- Sirri, F.; Zampiga, M.; Berardinelli, A.; Meluzzi, A. Variability and interaction of some egg physical and eggshell quality attributes during the entire laying hen cycle. Poult. Sci. 2018, 97, 1818–1823. [Google Scholar] [CrossRef] [PubMed]
- Grembecka, M. Sugar alcohols—their role in the modern world of sweeteners: A review. Eur. Food Res. Technol. 2015, 241, 1–14. [Google Scholar] [CrossRef]
- Wisselink, H.W.; Weusthuis, R.A.; Eggink, G.; Hugenholtz, J.; Grobben, G. Mannitol production by lactic acid bacteria: A review. Int. Dairy J. 2002, 12, 151–161. [Google Scholar] [CrossRef]
- Bernt, W.O.; Borzelleca, J.F.; Flamm, G.; Munro, I.C. Erythritol: A review of biological and toxicological studies. Regul. Toxicol. Pharm. 1996, 24, S191–S197. [Google Scholar] [CrossRef] [PubMed]
- Malcicka, M.; Visser, B.; Ellers, J. An evolutionary perspective on linoleic acid synthesis in animals. Evol. Biol. 2018, 45, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Abedi, E.; Sahari, M.A. Long-chain polyunsaturated fatty acid sources and evaluation of their nutritional and functional properties. Food Sci. Nutr. 2014, 2, 443–463. [Google Scholar] [CrossRef] [PubMed]
- Stanislaus, A.; Guo, K.; Li, L. Development of an isotope labeling ultra-high performance liquid chromatography mass spectrometric method for quantification of acylglycines in human urine. Anal. Chim. Acta 2012, 750, 161–172. [Google Scholar] [CrossRef]
- Čermáková, M.; Pelantová, H.; Neprašová, B.; Šedivá, B.; Maletínská, L.; Kuneš, J.; Tomášová, P.; Železná, B.; Kuzma, M. Metabolomic study of obesity and its treatment with palmitoylated prolactin-releasing peptide analog in spontaneously hypertensive and normotensive rats. J. Proteome Res. 2019, 18, 1735–1750. [Google Scholar] [CrossRef]
- Lai, Z.; Tsugawa, H.; Wohlgemuth, G.; Mehta, S.; Mueller, M.; Zheng, Y.; Ogiwara, A.; Meissen, J.; Showalter, M.; Takeuchi, K.; et al. Identifying metabolites by integrating metabolome databases with mass spectrometry cheminformatics. Nat. Methods 2018, 15, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Tsugawa, H.; Kanazawa, M.; Ogiwara, A.; Arita, M. MRMPROBS suite for metabolomics using large-scale MRM assays. Bioinformatics 2014, 30, 2379–2380. [Google Scholar] [CrossRef] [PubMed]
- Olejnik, S.; Algina, J. Generalized eta and omega squared statistics: Measures of effect size for some common research designs. Psychol. Methods 2003, 4, 434–447. [Google Scholar] [CrossRef] [PubMed]
- Franz, V.H.; Loftus, G.R. Standard errors and confidence intervals in within-subjects designs: Generalizing Loftus and Masson (1994) and avoiding the biases of alternative accounts. Psychon. Bull. Rev. 2012, 19, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Nikiforuk, A.; Potasiewicz, A.; Kos, T.; Popik, P. The combination of memantine and galantamine improves cognition in rats: The synergistic role of the α7 nicotinic acetylcholine and NMDA receptors. Behav. Brain Res. 2016, 313, 214–218. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.R-project.org/ (accessed on 20 September 2019).
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B 1995, 57, 298–300. [Google Scholar] [CrossRef]
- McClay, J.L.; Adkins, D.E.; Vunck, S.A.; Batman, A.M.; Vann, R.E.; Clark, S.L.; Beardsley, P.M.; van den Oord, E.J. Large-scale neurochemical metabolomics analysis identifies multiple compounds associated with methamphetamine exposure. Metabolomics 2013, 9, 392–402. [Google Scholar] [CrossRef]
- Pedersen, H.K.; Gudmundsdottir, V.; Nielsen, H.B.; Hyotylainen, T.; Nielsen, T.; Jensen, B.A.; Forslund, K.; Hildebrand, F.; Prifti, E.; Falony, G.; et al. Human gut microbes impact host serum metabolome and insulin sensitivity. Nature 2016, 535, 376–381. [Google Scholar] [CrossRef] [PubMed]
| Traits | Rhode Island Red (RIR) | Australorp (AUS) | P-Value from ANOVA Mixed Design | ||||
|---|---|---|---|---|---|---|---|
| Mixed | Fermented | Mixed | Fermented | Main-Effect | Interaction-Effect | ||
| 33 Weeks | 41 Weeks | 33 Weeks | 41 Weeks | Breed | Feed | Breed × Feed | |
| Egg weight (g) | 54.2 ± 2.0 | 55.5 ± 2.5 | 52.6 ± 4.7 | 56.7 ± 4.9 | 0.068 | 0.573 | 0.918 |
| Length of long axis of the egg (mm) | 56.2 ± 2.4 | 57.8 ± 1.0 | 54.8 ± 2.1 | 57.8 ± 1.6 | 0.067 | 0.421 | 0.563 |
| Length of short axis of the egg (mm) | 42.0 ± 0.9 | 41.8 ± 1.0 | 41.4 ± 1.4 | 41.2 ± 1.2 | 0.065 | 0.974 | 0.953 |
| Yolk weight (g) | 15.5 ± 0.5 | 17.0 ± 1.2 | 13.4 ± 1.1 | 15.5 ± 1.1 | 0.015 | 0.287 | 0.826 |
| Eggshell weight (g) | 6.0 ± 0.4 | 6.7 ± 0.5 | 6.8 ± 0.8 | 6.3 ± 1.4 | 0.019 | 0.657 | 0.186 |
| Albumen weight (g) | 29.7 ± 1.7 | 29.5 ± 2.0 | 29.5 ± 3.0 | 31.1 ± 2.7 | 0.360 | 0.404 | 0.971 |
| Eggshell thickness (mm) | 0.38 ± 0.01 | 0.40 ± 0.05 | 0.42 ± 0.03 | 0.38 ± 0.03 | 0.227 | 0.725 | 0.667 |
| Eggshell color L * | 62.5 ± 5.0 | 66.3 ± 5.4 | 68.5 ± 2.0 | 75.5 ± 1.5 | 0.030 | 0.705 | 0.950 |
| Eggshell color a * | 14.3 ± 2.9 | 12.2 ± 2.6 | 9.5 ± 1.3 | 6.3 ± 0.7 | 0.006 | 0.376 | 0.600 |
| Eggshell color b * | 22.3 ± 3.5 | 20.6 ± 3.0 | 15.2 ± 1.8 | 12.5 ± 2.1 | 0.003 | 0.453 | 0.886 |
| Metabolite | HMDB 1 | Relative Area (Mean ± Standard Deviation (SD)) | Mixed-Design ANOVA | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RIR | RIR | AUS | AUS | Breed | ||||||||||||
| Mixed | Fermented | Mixed | Fermented | P-Value | Q-Value | |||||||||||
| Albumen_Ribitol | HMDB0000508 | −0.17 | ± | 0.65 | 0.59 | ± | 0.49 | −0.84 | ± | 1.27 | 0.42 | ± | 0.59 | 0.0005 | 0.0628 | * |
| Metabolite | HMDB 1 | Relative Area (Mean ± SD) | Mixed Design ANOVA | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RIR | RIR | AUS | AUS | Feed | ||||||||||||
| Mixed | Fermented | Mixed | Fermented | P-Value | Q-Value | |||||||||||
| Yolk_Urea | HMDB0000294 | 0.47 | ± | 0.22 | −0.78 | ± | 0.56 | 0.81 | ± | 1.24 | −0.50 | ± | 0.42 | 0.002 | 0.085 | * |
| Yolk_Threitol | HMDB0004136 | −0.84 | ± | 0.27 | 0.89 | ± | 0.33 | −1.02 | ± | 0.13 | 0.97 | ± | 0.37 | 0.001 | 0.082 | * |
| Yolk_Erythritol | HMDB0002994 | −0.77 | ± | 0.34 | 0.93 | ± | 0.45 | −1.05 | ± | 0.09 | 0.89 | ± | 0.39 | 0.001 | 0.075 | * |
| Albumen_Isoleucine | HMDB0000172 | 0.26 | ± | 0.77 | −0.89 | ± | 0.63 | 1.20 | ± | 0.41 | −0.57 | ± | 0.39 | 0.001 | 0.020 | * |
| Albumen_Dihydrouracil | HMDB0000076 | −0.57 | ± | 0.93 | 0.69 | ± | 0.93 | −0.77 | ± | 0.48 | 0.65 | ± | 0.43 | 0.000 | 0.001 | * |
| Albumen_Erythritol | HMDB0002994 | −0.67 | ± | 0.20 | 1.13 | ± | 0.72 | −1.04 | ± | 0.12 | 0.58 | ± | 0.30 | 0.001 | 0.017 | * |
| Albumen_Linoleic acid | HMDB0000673 | −0.02 | ± | 0.88 | 0.81 | ± | 1.34 | −0.75 | ± | 0.32 | −0.04 | ± | 0.25 | 0.000 | 0.008 | * |
| Albumen_4-Hydroxyphenyllactic acid | HMDB0000755 | 0.41 | ± | 0.79 | −1.05 | ± | 0.52 | 1.05 | ± | 0.43 | −0.40 | ± | 0.57 | 0.007 | 0.091 | * |
| Albumen_Alanine | HMDB0000161 | 0.54 | ± | 1.29 | −0.75 | ± | 0.39 | 0.85 | ± | 0.55 | −0.64 | ± | 0.18 | 0.007 | 0.082 | * |
| Albumen_Glycine | HMDB0000123 | 0.44 | ± | 1.28 | −0.70 | ± | 0.73 | 0.83 | ± | 0.42 | −0.57 | ± | 0.25 | 0.004 | 0.066 | * |
| Albumen_N-Butyrylglycine | HMDB0000808 | 0.12 | ± | 0.78 | −0.34 | ± | 0.72 | −0.04 | ± | 1.02 | 0.26 | ± | 1.24 | 0.000 | 0.007 | * |
| Albumen_Pyruvic acid | HMDB0000243 | −0.06 | ± | 1.36 | 0.19 | ± | 0.99 | −0.17 | ± | 1.07 | 0.04 | ± | 0.39 | 0.005 | 0.083 | * |
| Albumen_Ribitol | HMDB0000508 | −0.17 | ± | 0.65 | 0.59 | ± | 0.49 | −0.84 | ± | 1.27 | 0.42 | ± | 0.59 | 0.007 | 0.083 | * |
| Albumen_Threitol | HMDB0004136 | −0.70 | ± | 0.17 | 1.11 | ± | 0.74 | −1.01 | ± | 0.14 | 0.59 | ± | 0.34 | 0.001 | 0.020 | * |
| Albumen_Valine | HMDB0000883 | 0.54 | ± | 1.27 | −0.77 | ± | 0.36 | 0.89 | ± | 0.49 | −0.67 | ± | 0.10 | 0.006 | 0.088 | * |
| Metabolite | HMDB 1 | Relative Area (Mean ± SD) | Mixed Design ANOVA | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RIR | RIR | AUS | AUS | Breed × Feed | ||||||||||||
| Mixed | Fermented | Mixed | Fermented | P-Value | Q-Value | |||||||||||
| Albumen_N-Butyrylglycine | HMDB0000808 | 0.12 | ± | 0.78 | −0.34 | ± | 0.72 | −0.04 | ± | 1.02 | 0.26 | ± | 1.24 | 0.0000 | 0.0048 | * |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goto, T.; Mori, H.; Shiota, S.; Tomonaga, S. Metabolomics Approach Reveals the Effects of Breed and Feed on the Composition of Chicken Eggs. Metabolites 2019, 9, 224. https://doi.org/10.3390/metabo9100224
Goto T, Mori H, Shiota S, Tomonaga S. Metabolomics Approach Reveals the Effects of Breed and Feed on the Composition of Chicken Eggs. Metabolites. 2019; 9(10):224. https://doi.org/10.3390/metabo9100224
Chicago/Turabian StyleGoto, Tatsuhiko, Hiroki Mori, Shunsuke Shiota, and Shozo Tomonaga. 2019. "Metabolomics Approach Reveals the Effects of Breed and Feed on the Composition of Chicken Eggs" Metabolites 9, no. 10: 224. https://doi.org/10.3390/metabo9100224
APA StyleGoto, T., Mori, H., Shiota, S., & Tomonaga, S. (2019). Metabolomics Approach Reveals the Effects of Breed and Feed on the Composition of Chicken Eggs. Metabolites, 9(10), 224. https://doi.org/10.3390/metabo9100224







