Rapid Cerebral Metabolic Shift during Neonatal Sepsis Is Attenuated by Enteral Colostrum Supplementation in Preterm Pigs
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Experimental Procedures
2.2. NMR Sample Preparation
2.2.1. Liquid 1H NMR Spectroscopy
2.2.2. HR-MAS 1H NMR Spectroscopy
2.3. NMR Data Acquisition and Preprocessing
2.4. Data Analysis
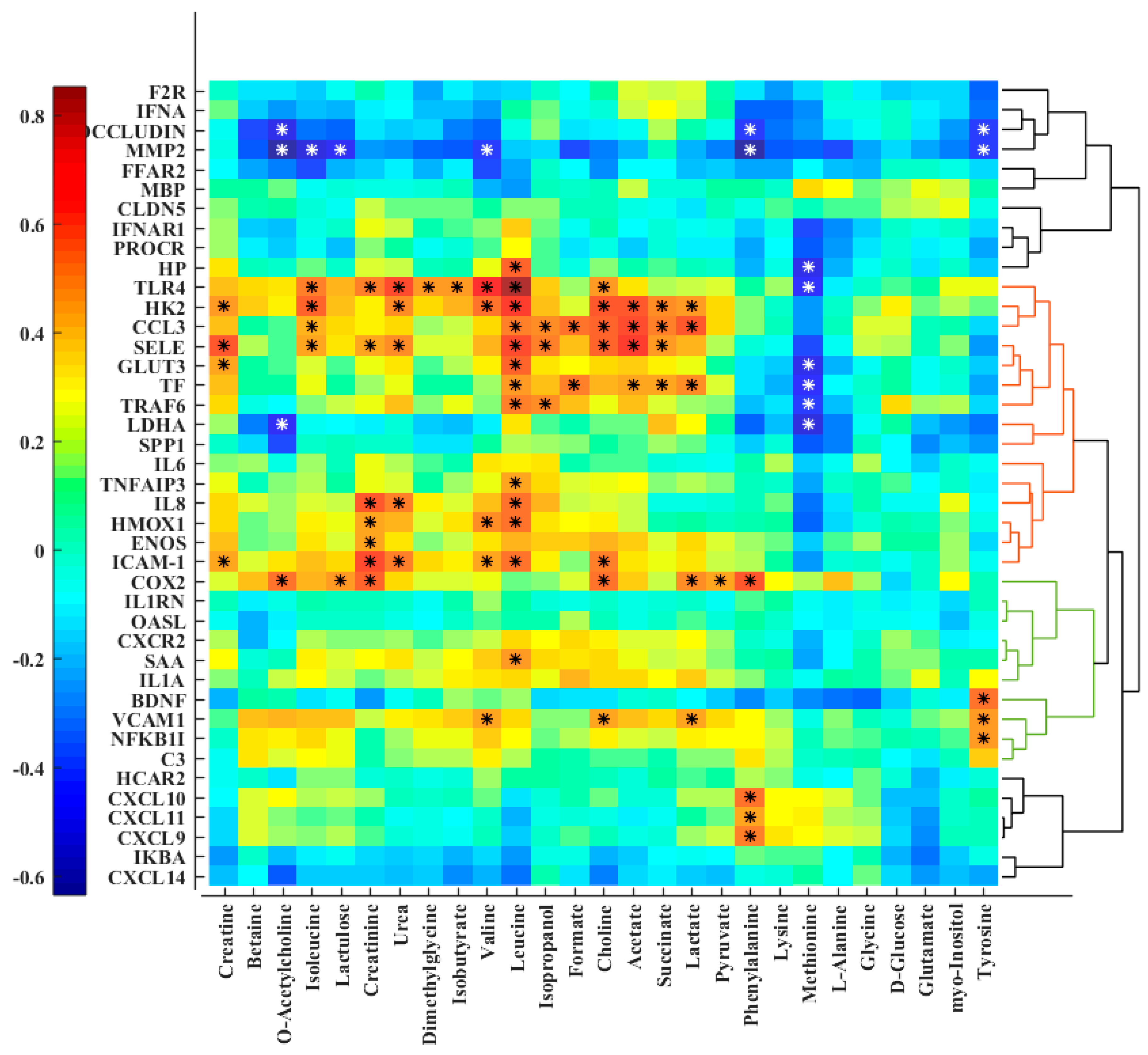
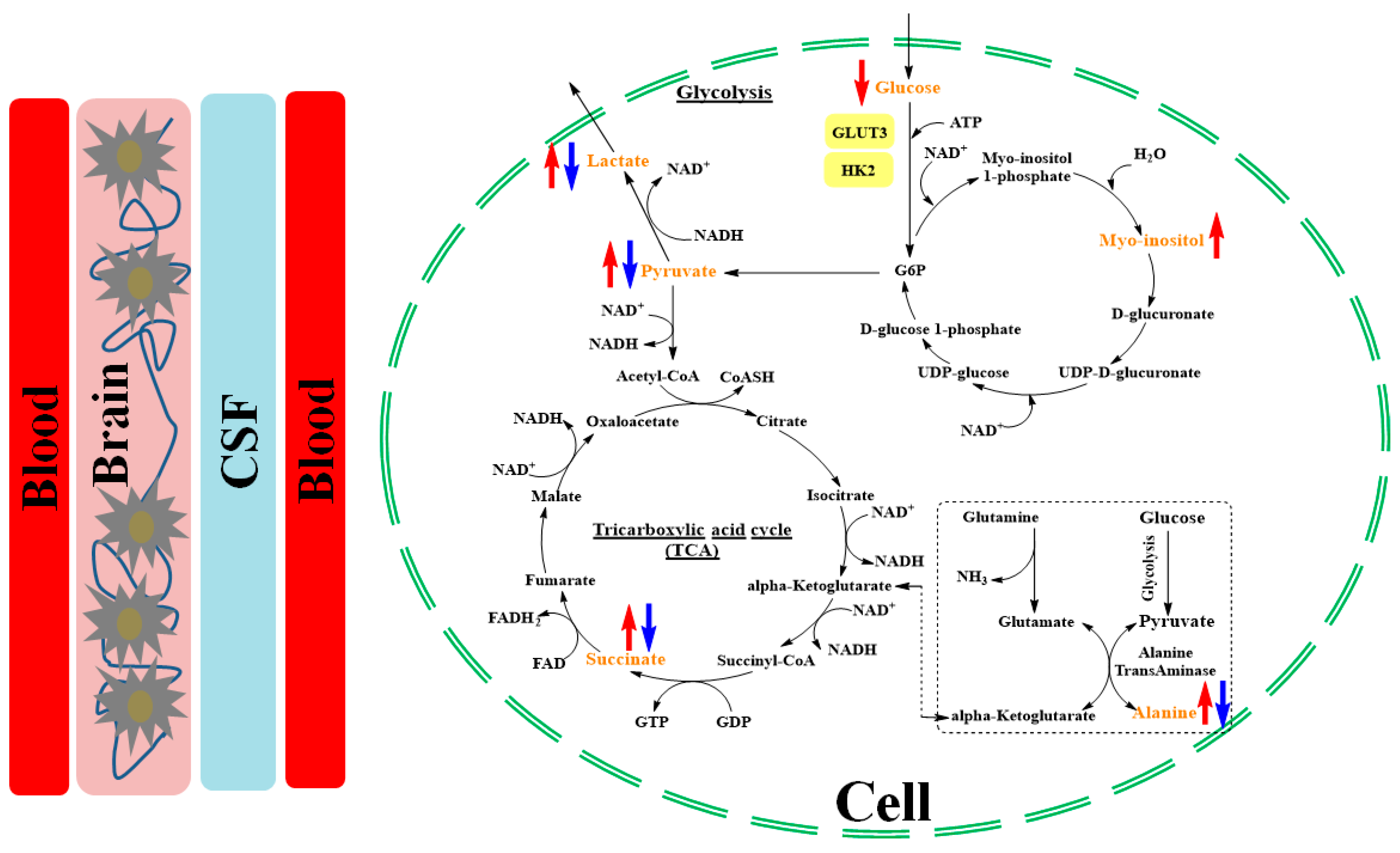
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stoll, B.J.; Hansen, N. Infections in VLBW infants: Studies from the NICHD Neonatal Research Network. Semin. Perinatol. 2003, 27, 293–301. [Google Scholar] [CrossRef]
- von Eiff, C.; Peters, G.; Heilmann, C. Pathogenesis of infections due to coagulasenegative staphylococci. Lancet Infect. Dis. 2002, 2, 677–685. [Google Scholar] [CrossRef]
- Levy, O. Innate immunity of the newborn: Basic mechanisms and clinical correlates. Nat. Rev. Immunol. 2007, 7, 379. [Google Scholar] [CrossRef] [PubMed]
- Bizzarro, M.J.; Raskind, C.; Baltimore, R.S.; Gallagher, P.G. Seventy-five years of neonatal sepsis at Yale: 1928–2003. Pediatrics 2005, 116, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Van Den Hoogen, A.; Gerards, L.J.; Verboon-Maciolek, M.A.; Fleer, A.; Krediet, T.G. Long-term trends in the epidemiology of neonatal sepsis and antibiotic susceptibility of causative agents. Neonatology 2010, 97, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Bassler, D.; Stoll, B.J.; Schmidt, B.; Asztalos, E.V.; Roberts, R.S.; Robertson, C.M.; Sauve, R.S. Using a count of neonatal morbidities to predict poor outcome in extremely low birth weight infants: Added role of neonatal infection. Pediatrics 2009, 123, 313–318. [Google Scholar] [CrossRef]
- Schlapbach, L.J.; Aebischer, M.; Adams, M.; Natalucci, G.; Bonhoeffer, J.; Latzin, P.; Nelle, M.; Bucher, H.U.; Latal, B. Impact of sepsis on neurodevelopmental outcome in a Swiss National Cohort of extremely premature infants. Pediatrics 2011, 128, e348–e357. [Google Scholar] [CrossRef]
- Stoll, B.J.; Hansen, N.I.; Adams-Chapman, I.; Fanaroff, A.A.; Hintz, S.R.; Vohr, B.; Higgins, R.D.; Health, N.I.o.C.; Network, H.D.N.R. Neurodevelopmental and growth impairment among extremely low-birth-weight infants with neonatal infection. JAMA 2004, 292, 2357–2365. [Google Scholar] [CrossRef]
- Strunk, T.; Inder, T.; Wang, X.; Burgner, D.; Mallard, C.; Levy, O. Infection-induced inflammation and cerebral injury in preterm infants. Lancet Infect. Dis. 2014, 14, 751–762. [Google Scholar] [CrossRef]
- Vergnano, S.; Menson, E.; Kennea, N.; Embleton, N.; Russell, A.B.; Watts, T.; Robinson, M.J.; Collinson, A.; Heath, P.T. Neonatal infections in England: The NeonIN surveillance network. Arch. Dis. Child. Fetal Neonatal Ed. 2010, 96, F9–F14. [Google Scholar] [CrossRef]
- Lampropoulou, V.; Sergushichev, A.; Bambouskova, M.; Nair, S.; Vincent, E.E.; Loginicheva, E.; Cervantes-Barragan, L.; Ma, X.; Huang, S.C.-C.; Griss, T. Itaconate links inhibition of succinate dehydrogenase with macrophage metabolic remodeling and regulation of inflammation. Cell Metab. 2016, 24, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Olagnier, D.; Brandtoft, A.M.; Gunderstofte, C.; Villadsen, N.L.; Krapp, C.; Thielke, A.L.; Laustsen, A.; Peri, S.; Hansen, A.L.; Bonefeld, L. Nrf2 negatively regulates STING indicating a link between antiviral sensing and metabolic reprogramming. Nat. Commun. 2018, 9, 3506. [Google Scholar] [CrossRef] [PubMed]
- Vessichelli, M.; Mariggiò, S.; Varone, A.; Zizza, P.; Di Santo, A.; Amore, C.; Dell’Elba, G.; Cutignano, A.; Fontana, A.; Cacciapuoti, C. The Natural Phosphoinositide-Derivative Glycerophosphoinositol Inhibits the Lipopolysaccharide-Induced Inflammatory and Thrombotic Responses. J. Biol. Chem. 2017, 292, 12828–12841. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.L.; Dickinson, P.; Forster, T.; Craigon, M.; Ross, A.; Khondoker, M.R.; France, R.; Ivens, A.; Lynn, D.J.; Orme, J. Identification of a human neonatal immune-metabolic network associated with bacterial infection. Nat. Commun. 2014, 5, 4649. [Google Scholar] [CrossRef] [PubMed]
- Hylander, M.A.; Strobino, D.M.; Dhanireddy, R. Human milk feedings and infection among very low birth weight infants. Pediatrics 1998, 102, e38. [Google Scholar] [CrossRef] [PubMed]
- Møller, H.K.; Thymann, T.; Fink, L.N.; Frokiaer, H.; Kvistgaard, A.S.; Sangild, P.T. Bovine colostrum is superior to enriched formulas in stimulating intestinal function and necrotising enterocolitis resistance in preterm pigs. Br. J. Nutr. 2011, 105, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, S.O.; Martin, L.; Østergaard, M.V.; Rudloff, S.; Li, Y.; Roggenbuck, M.; Bering, S.B.; Sangild, P.T. Bovine colostrum improves neonatal growth, digestive function, and gut immunity relative to donor human milk and infant formula in preterm pigs. Am. J. Physiol. Gastrointest. Liver Physiol. 2016, 311, G480–G491. [Google Scholar] [CrossRef]
- Lee, J.; Kim, H.-S.; Jung, Y.H.; Choi, K.Y.; Shin, S.H.; Kim, E.-K.; Choi, J.-H. Oropharyngeal colostrum administration in extremely premature infants: An RCT. Pediatrics 2015, 135, e357–e366. [Google Scholar] [CrossRef]
- Bardanzellu, F.; Fanos, V.; Reali, A. “Omics” in human colostrum and mature milk: Looking to old data with new eyes. Nutrients 2017, 9, 843. [Google Scholar] [CrossRef]
- Nasuf, A.W.A.; Ojha, S.; Dorling, J. Oropharyngeal colostrum in preventing mortality and morbidity in preterm infants. Cochrane Database Syst. Rev. 2018, 9, CD011921. [Google Scholar] [CrossRef]
- Trend, S.; Strunk, T.; Hibbert, J.; Kok, C.H.; Zhang, G.; Doherty, D.A.; Richmond, P.; Burgner, D.; Simmer, K.; Davidson, D.J. Antimicrobial protein and peptide concentrations and activity in human breast milk consumed by preterm infants at risk of late-onset neonatal sepsis. PLoS ONE 2015, 10, e0117038. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.-X.; Hu, G.-X.; Elsheikha, H.M.; He, S.; Chen, X.-Q.; Zhu, X.-Q. Profiling of the perturbed metabolomic state of mouse spleen during acute and chronic toxoplasmosis. Parasites Vectors 2017, 10, 339. [Google Scholar]
- Li, P.; Yin, Y.-L.; Li, D.; Kim, S.W.; Wu, G. Amino acids and immune function. Br. J. Nutr. 2007, 98, 237–252. [Google Scholar] [CrossRef] [PubMed]
- González-Domínguez, R.; García-Barrera, T.; Vitorica, J.; Gómez-Ariza, J.L. Metabolomics reveals significant impairments in the immune system of the APP/PS1 transgenic mice of Alzheimer’s disease. Electrophoresis 2015, 36, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Brunse, A.; Worsøe, P.; Pors, S.E.; Skovgaard, K.; Sangild, P.T. Oral Supplementation with Bovine Colostrum Prevents Septic Shock and Brain Barrier Disruption During Bloodstream Infection in Preterm Newborn Pigs. Shock 2018. [Google Scholar] [CrossRef] [PubMed]
- Shen, R.L.; Thymann, T.; Østergaard, M.V.; Støy, A.C.F.; Krych, Ł.; Nielsen, D.S.; Lauridsen, C.; Hartmann, B.; Holst, J.J.; Burrin, D.G. Early gradual feeding with bovine colostrum improves gut function and NEC resistance relative to infant formula in preterm pigs. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 309, G310–G323. [Google Scholar] [CrossRef] [PubMed]
- Beckonert, O.; Keun, H.C.; Ebbels, T.M.; Bundy, J.; Holmes, E.; Lindon, J.C.; Nicholson, J.K. Metabolic profiling, metabolomic and metabonomic procedures for NMR spectroscopy of urine, plasma, serum and tissue extracts. Nat. Protoc. 2007, 2, 2692–2703. [Google Scholar] [CrossRef]
- Wishart, D.S.; Jewison, T.; Guo, A.C.; Wilson, M.; Knox, C.; Liu, Y.; Djoumbou, Y.; Mandal, R.; Aziat, F.; Dong, E. HMDB 3.0—The human metabolome database in 2013. Nucleic Acids Res. 2012, 41, D801–D807. [Google Scholar] [CrossRef]
- Sweatman, B.C.; Farrant, R.D.; Holmes, E.; Ghauri, F.Y.; Nicholson, J.K.; Lindon, J.C. 600 MHz 1H-NMR spectroscopy of human cerebrospinal fluid: Effects of sample manipulation and assignment of resonances. J. Pharm. Biomed. Anal. 1993, 11, 651–664. [Google Scholar] [CrossRef]
- Wishart, D.S.; Lewis, M.J.; Morrissey, J.A.; Flegel, M.D.; Jeroncic, K.; Xiong, Y.; Cheng, D.; Eisner, R.; Gautam, B.; Tzur, D. The human cerebrospinal fluid metabolome. J. Chromatogr. B 2008, 871, 164–173. [Google Scholar] [CrossRef]
- Govindaraju, V.; Young, K.; Maudsley, A.A. Proton NMR chemical shifts and coupling constants for brain metabolites. NMR Biomed. 2000, 13, 129–153. [Google Scholar] [CrossRef]
- Nicholson, J.K.; Foxall, P.J.; Spraul, M.; Farrant, R.D.; Lindon, J.C. 750 MHz 1H and 1H-13C NMR spectroscopy of human blood plasma. Anal. Chem. 1995, 67, 793–811. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Wang, Y.; Nicholson, J.K.; Lindon, J.C. Use of relaxation-edited one-dimensional and two dimensional nuclear magnetic resonance spectroscopy to improve detection of small metabolites in blood plasma. Anal. Biochem. 2004, 325, 260–272. [Google Scholar] [CrossRef] [PubMed]
- Savorani, F.; Tomasi, G.; Engelsen, S.B. icoshift: A versatile tool for the rapid alignment of 1D NMR spectra. J. Magn. Reson. 2010, 202, 190–202. [Google Scholar] [CrossRef]
- van der Sar, S.A.; Zielman, R.; Terwindt, G.M.; van den Maagdenberg, A.M.; Deelder, A.M.; Mayboroda, O.A.; Meissner, A.; Ferrari, M.D. Ethanol contamination of cerebrospinal fluid during standardized sampling and its effect on 1H-NMR metabolomics. Anal. Bioanal. Chem. 2015, 407, 4835–4839. [Google Scholar] [CrossRef]
- Jansen, J.J.; Hoefsloot, H.C.; van der Greef, J.; Timmerman, M.E.; Westerhuis, J.A.; Smilde, A.K. ASCA: Analysis of multivariate data obtained from an experimental design. J. Chemometr. 2005, 19, 469–481. [Google Scholar] [CrossRef]
- Brealey, D.; Brand, M.; Hargreaves, I.; Heales, S.; Land, J.; Smolenski, R.; Davies, N.A.; Cooper, C.E.; Singer, M. Association between mitochondrial dysfunction and severity and outcome of septic shock. Lancet 2002, 360, 219–223. [Google Scholar] [CrossRef]
- Lin, Z.Y.; Xu, P.B.; Yan, S.K.; Meng, H.B.; Yang, G.J.; Dai, W.X.; Liu, X.R.; Li, J.B.; Deng, X.M.; Zhang, W.D. A metabonomic approach to early prognostic evaluation of experimental sepsis by 1H NMR and pattern recognition. NMR Biomed. 2009, 22, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Denko, N.C. Hypoxia, HIF1 and glucose metabolism in the solid tumour. Nat. Rev. Cancer 2008, 8, 705. [Google Scholar] [CrossRef] [PubMed]
- Schmerler, D.; Neugebauer, S.; Ludewig, K.; Bremer-Streck, S.; Brunkhorst, F.M.; Kiehntopf, M. Targeted metabolomics for discrimination of systemic inflammatory disorders in critically ill patients. J. Lipid Res. 2012, 53, 1369–1375. [Google Scholar] [CrossRef] [PubMed]
- Reinke, S.N.; Walsh, B.H.; Boylan, G.B.; Sykes, B.D.; Kenny, L.C.; Murray, D.M.; Broadhurst, D.I. 1H NMR derived metabolomic profile of neonatal asphyxia in umbilical cord serum: Implications for hypoxic ischemic encephalopathy. J. Proteome Res. 2013, 12, 4230–4239. [Google Scholar] [CrossRef] [PubMed]
- Khovidhunkit, W.; Kim, M.-S.; Memon, R.A.; Shigenaga, J.K.; Moser, A.H.; Feingold, K.R.; Grunfeld, C. Effects of infection and inflammation on lipid and lipoprotein metabolism: Mechanisms and consequences to the host. J. Lipid Res. 2004, 45, 1169–1196. [Google Scholar] [CrossRef] [PubMed]
- Drobnik, W.; Liebisch, G.; Audebert, F.-X.; Fröhlich, D.; Glück, T.; Vogel, P.; Rothe, G.; Schmitz, G. Plasma ceramide and lysophosphatidylcholine inversely correlate with mortality in sepsis patients. J. Lipid Res. 2003, 44, 754–761. [Google Scholar] [CrossRef]
- Kan, B.; Michalski, C.; Fu, H.; Au, H.H.; Lee, K.; Marchant, E.A.; Cheng, M.F.; Anderson-Baucum, E.; Aharoni-Simon, M.; Tilley, P. Cellular metabolism constrains innate immune responses in early human ontogeny. Nat. Commun. 2018, 9, 4822. [Google Scholar] [CrossRef] [PubMed]
- Turrin, N.P.; Gayle, D.; Ilyin, S.E.; Flynn, M.C.; Langhans, W.; Schwartz, G.J.; Plata-Salamán, C.R. Pro-inflammatory and anti-inflammatory cytokine mRNA induction in the periphery and brain following intraperitoneal administration of bacterial lipopolysaccharide. Brain Res. Bull. 2001, 54, 443–453. [Google Scholar] [CrossRef]
- DeRisi, J.L.; Iyer, V.R.; Brown, P.O. Exploring the metabolic and genetic control of gene expression on a genomic scale. Science 1997, 278, 680–686. [Google Scholar] [CrossRef] [PubMed]
- Kelly, B.; O’neill, L.A. Metabolic reprogramming in macrophages and dendritic cells in innate immunity. Cell Res. 2015, 25, 771. [Google Scholar] [CrossRef]
- Krawczyk, C.M.; Holowka, T.; Sun, J.; Blagih, J.; Amiel, E.; DeBerardinis, R.J.; Cross, J.R.; Jung, E.; Thompson, C.B.; Jones, R.G. Toll-like receptor induced changes in glycolytic metabolism regulate dendritic cell activation. Blood 2010, 115, 4742–4749. [Google Scholar] [CrossRef]
- Rodríguez-Prados, J.-C.; Través, P.G.; Cuenca, J.; Rico, D.; Aragonés, J.; Martín-Sanz, P.; Cascante, M.; Boscá, L. Substrate fate in activated macrophages: A comparison between innate, classic, and alternative activation. J. Immunol. 2010, 185, 605–614. [Google Scholar] [CrossRef]
- Tannahill, G.; Curtis, A.; Adamik, J.; Palsson-McDermott, E.; McGettrick, A.; Goel, G.; Frezza, C.; Bernard, N.; Kelly, B.; Foley, N. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature 2013, 496, 238. [Google Scholar] [CrossRef] [PubMed]
- Shing, C.M.; Peake, J.M.; Suzuki, K.; Jenkins, D.G.; Coombes, J.S. Bovine colostrum modulates cytokine production in human peripheral blood mononuclear cells stimulated with lipopolysaccharide and phytohemagglutinin. J. Interferon Cytokine Res. 2009, 29, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Balachandran, B.; Dutta, S.; Singh, R.; Prasad, R.; Kumar, P. Bovine colostrum in prevention of necrotizing enterocolitis and sepsis in very low birth weight neonates: A randomized, double-blind, placebo-controlled pilot trial. J. Trop. Pediatr. 2017, 63, 10–17. [Google Scholar] [CrossRef]
- Manzoni, P.; Rinaldi, M.; Cattani, S.; Pugni, L.; Romeo, M.G.; Messner, H.; Stolfi, I.; Decembrino, L.; Laforgia, N.; Vagnarelli, F. Bovine lactoferrin supplementation for prevention of late-onset sepsis in very low-birth-weight neonates: A randomized trial. JAMA 2009, 302, 1421–1428. [Google Scholar] [CrossRef] [PubMed]
- Hay, W.W., Jr.; Hendrickson, K.C. Preterm formula use in the preterm very low birth weight infant. Semin. Fetal Neonatal Med. 2017, 22, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Hauser, G.; Finelli, V.N. The biosynthesis of free and phosphatide myo-inositol from glucose by mammalian tissue slices. J. Biol. Chem. 1963, 238, 64. [Google Scholar]
- Fisher, S.K.; Novak, J.E.; Agranoff, B.W. Inositol and higher inositol phosphates in neural tissues: Homeostasis, metabolism and functional significance. J. Neurochem. 2002, 82, 736–754. [Google Scholar] [CrossRef]
- Battaglia, F.C.; Meschia, G.; Blechner, J.N.; Barron, D.H. The free myo-inositol concentration of adult and fetal tissues of several species. Exp. Physiol. 1961, 46, 188–193. [Google Scholar] [CrossRef]
- Nissen, P.M.; Nebel, C.; Oksbjerg, N.; Bertram, H.C. Metabolomics reveals relationship between plasma inositols and birth weight: Possible markers for fetal programming of type 2 diabetes. BioMed Res. Int. 2011, 2011, 378268. [Google Scholar] [CrossRef]
- Hallman, M.; Bry, K.; Hoppu, K.; Lappi, M.; Pohjavuori, M. Inositol supplementation in premature infants with respiratory distress syndrome. N. Engl. J. Med. 1992, 326, 1233–1239. [Google Scholar] [CrossRef]
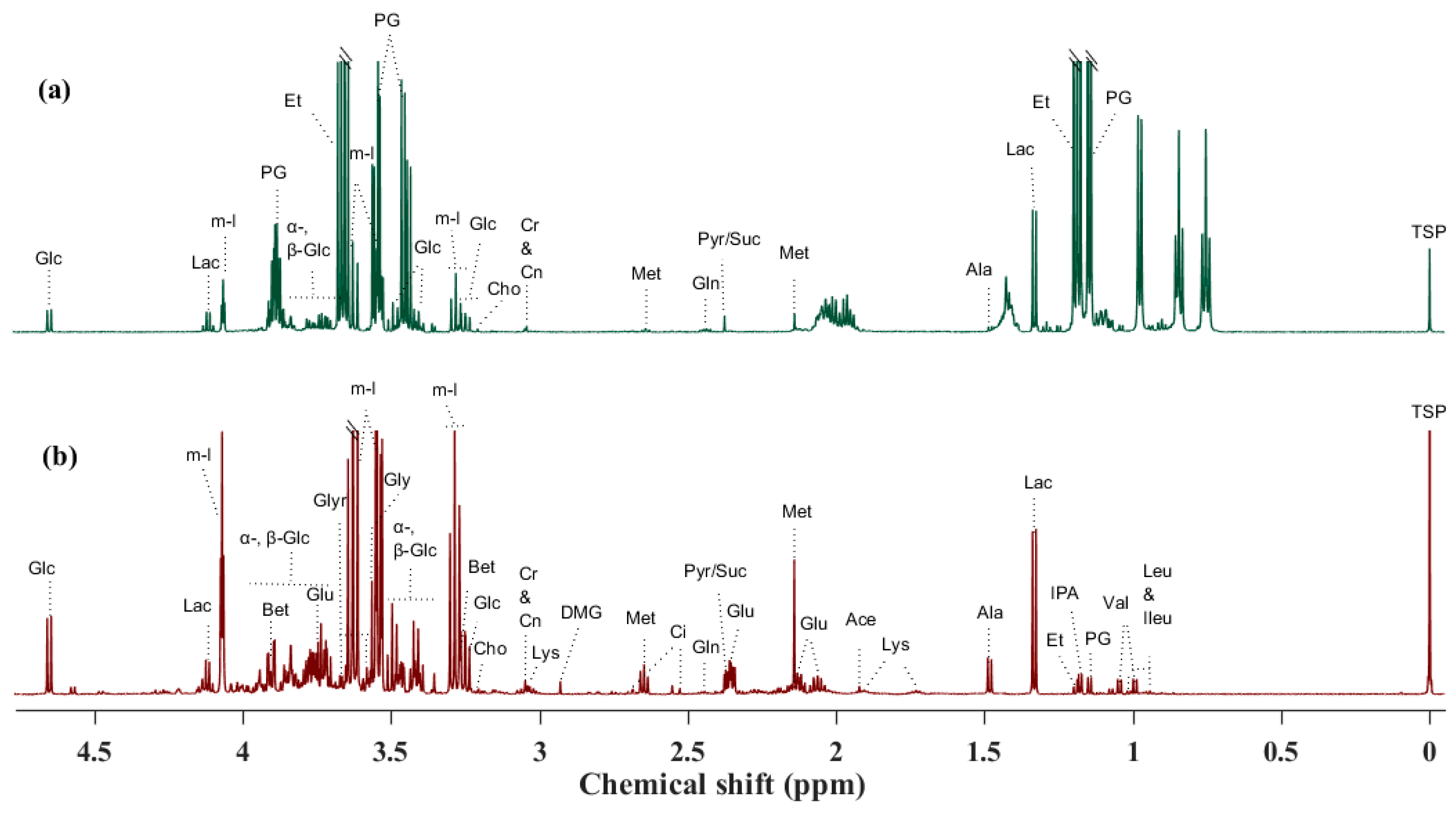
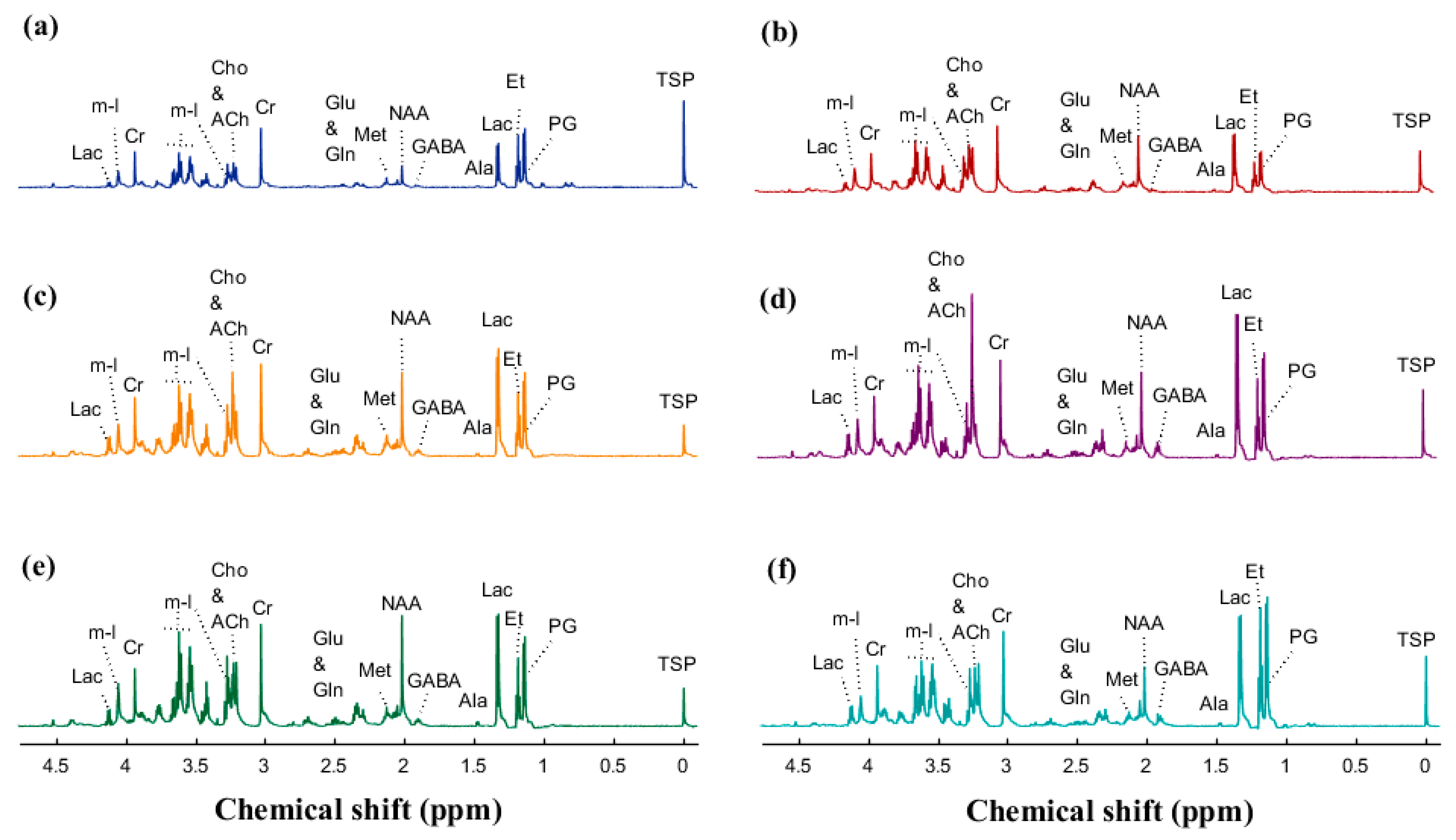
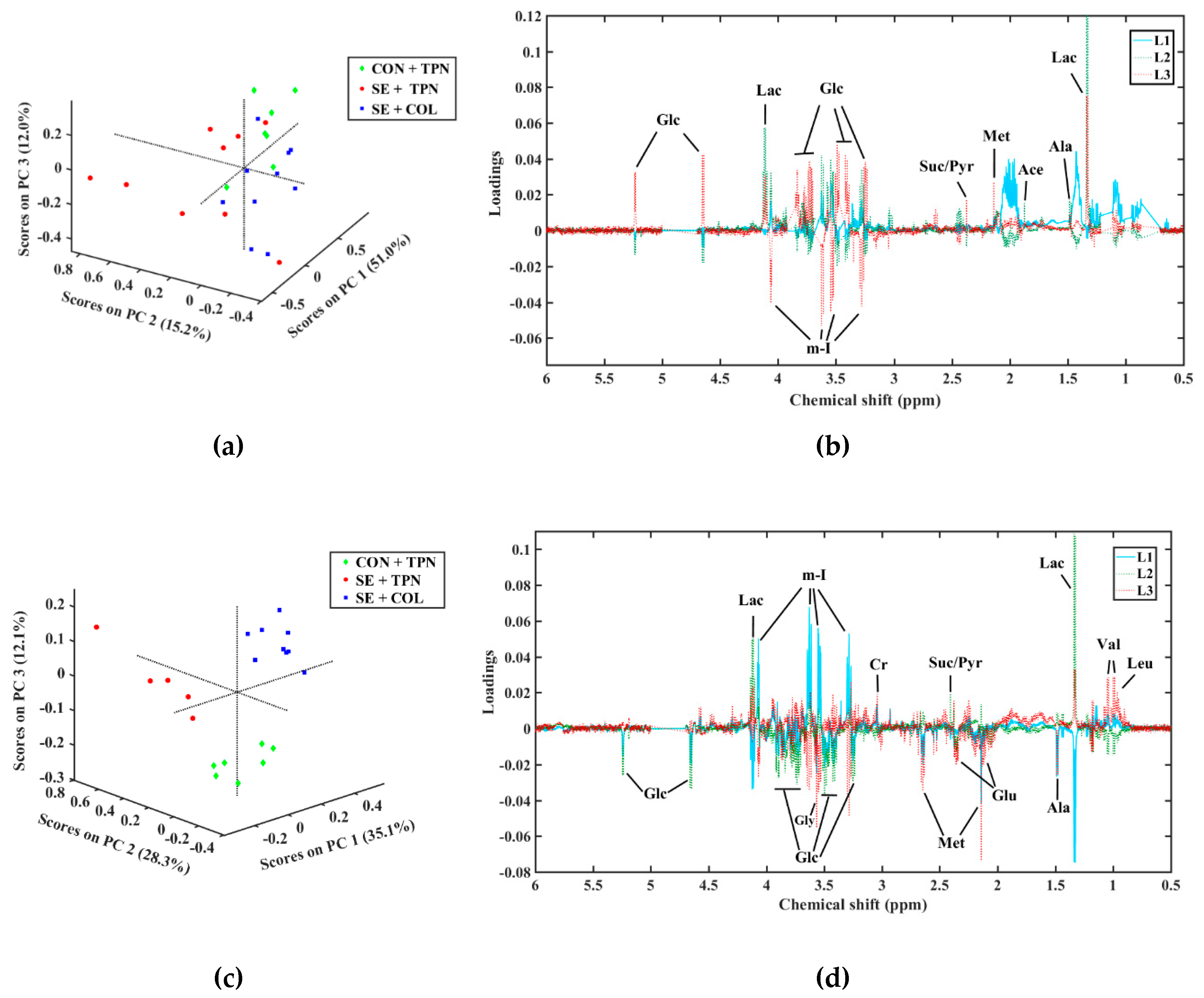
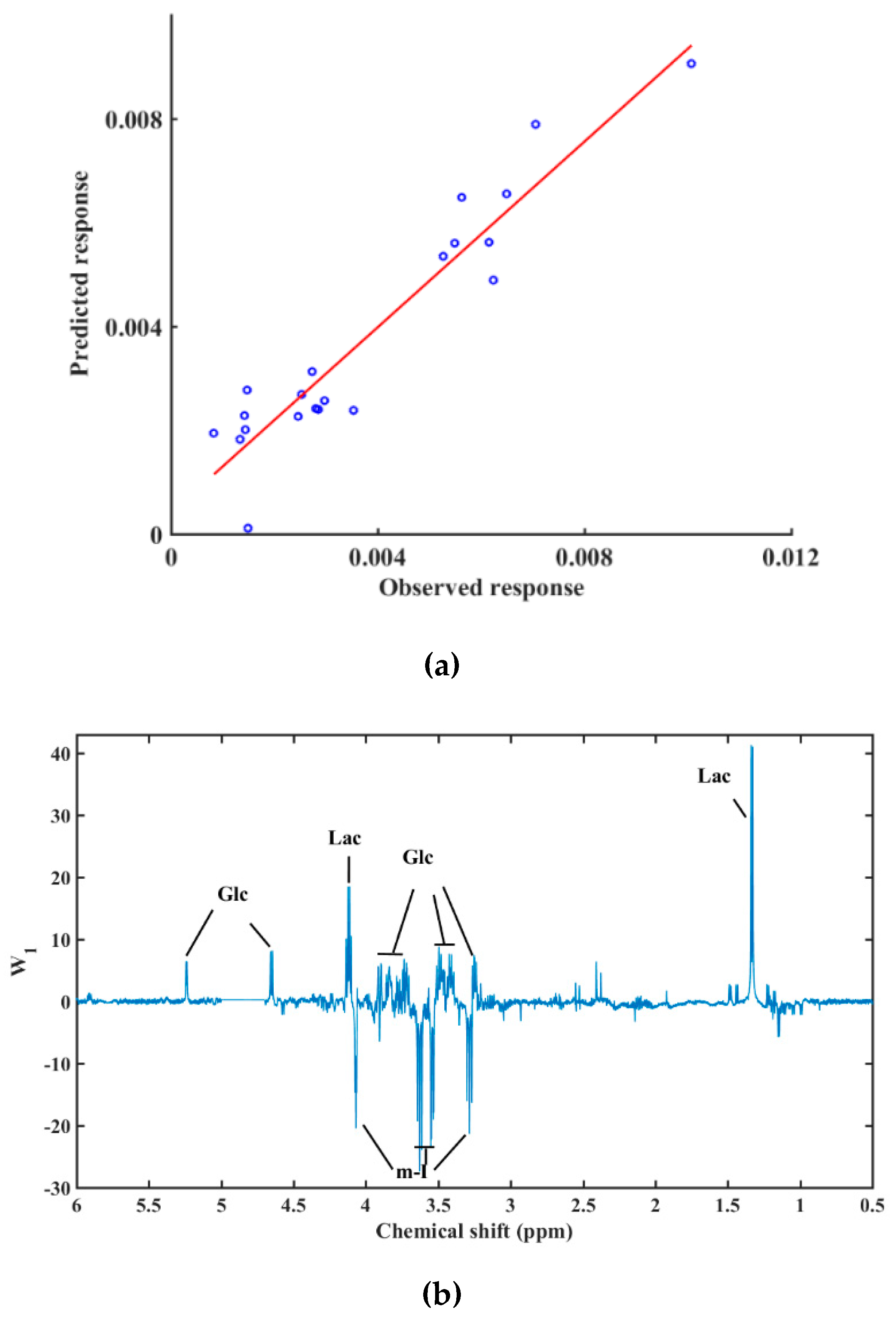
| Metabolite | Mean ± SD | Treatment p-Value | |||
|---|---|---|---|---|---|
| CON + TPN | SE + TPN | SE + COL | |||
| Plasma | Lactate | 1.46 ± 0.52 a | 6.00 ± 0.63 b | 1.32 ± 0.46 a | <0.001 |
| Alanine | 0.33 ± 0.05 ab | 0.43 ± 0.06 b | 0.22 ± 0.04 a | 0.046 | |
| Succinate/pyruvate | 0.02 ± 0.01 a | 0.08 ± 0.01 b | 0.01 ± 0.01 a | 0.001 | |
| Choline | 0.03 ± 0.01 a | 0.06 ± 0.01 b | 0.04 ± 0.00 ab | 0.021 | |
| Glutamate | 1.15 ± 0.16 a | 1.05 ± 0.19 a | 1.00 ± 0.14 a | N.S. * | |
| myo-Inositol | 8.35 ± 1.26 a | 8.66 ± 1.51 a | 8.56 ± 1.11 a | N.S. | |
| Methionine | 0.57 ± 0.06 b | 0.58 ± 0.07 b | 0.24 ± 0.05 a | 0.001 | |
| Valine | 0.20 ± 0.03 a | 0.26 ± 0.03 a | 0.37 ± 0.03 b | 0.001 | |
| Leucine | 0.02 ± 0.01 a | 0.03 ± 0.01 a | 0.07 ± 0.00 b | <0.001 | |
| CSF | Lactate | 0.34 ± 0.09 a | 0.62 ± 0.08 b | 0.30 ± 0.08 a | 0.021 |
| Alanine | 0.01 ± 0.00 a | 0.02 ± 0.00 a | 0.01 ± 0.00 a | 0.066 | |
| Choline | 0.004 ± 0.001 a | 0.004 ± 0.001 a | 0.004 ± 0.001 a | N.S. | |
| Succinate/pyruvate | 0.03 ± 0.00 a | 0.02 ± 0.00 a | 0.02 ± 0.00 a | 0.064 | |
| Glutamate | 0.13 ± 0.01 a | 0.1 ± 0.01 a | 0.09 ± 0.01 a | N.S. | |
| myo-Inositol | 0.56 ± 0.06 a | 0.69 ± 0.05 a | 0.67 ± 0.05 a | N.S. | |
| Methionine | 0.004 ± 0.00 b | 0.003 ± 0.00 b | 0.001 ± 0.00 a | <0.001 | |
| PVMW | Choline | 4.83 ± 2.06 a | 0.85 ± 2.46 a | 8.54 ± 1.74 a | 0.060 |
| Lactate | 8.44 ± 3.09 a | 2.10 ± 3.70 a | 12.66 ± 2.62 a | 0.095 | |
| N-acetylaspartate | 8.09 ± 3.10 a | 1.84 ± 3.70 a | 12.84 ± 2.62 a | 0.079 | |
| Creatine | 7.45 ± 3.34 a | 1.67 ± 3.99 a | 13.79 ± 2.82 a | 0.066 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alinaghi, M.; Jiang, P.-P.; Brunse, A.; Sangild, P.T.; Bertram, H.C. Rapid Cerebral Metabolic Shift during Neonatal Sepsis Is Attenuated by Enteral Colostrum Supplementation in Preterm Pigs. Metabolites 2019, 9, 13. https://doi.org/10.3390/metabo9010013
Alinaghi M, Jiang P-P, Brunse A, Sangild PT, Bertram HC. Rapid Cerebral Metabolic Shift during Neonatal Sepsis Is Attenuated by Enteral Colostrum Supplementation in Preterm Pigs. Metabolites. 2019; 9(1):13. https://doi.org/10.3390/metabo9010013
Chicago/Turabian StyleAlinaghi, Masoumeh, Ping-Ping Jiang, Anders Brunse, Per Torp Sangild, and Hanne Christine Bertram. 2019. "Rapid Cerebral Metabolic Shift during Neonatal Sepsis Is Attenuated by Enteral Colostrum Supplementation in Preterm Pigs" Metabolites 9, no. 1: 13. https://doi.org/10.3390/metabo9010013
APA StyleAlinaghi, M., Jiang, P.-P., Brunse, A., Sangild, P. T., & Bertram, H. C. (2019). Rapid Cerebral Metabolic Shift during Neonatal Sepsis Is Attenuated by Enteral Colostrum Supplementation in Preterm Pigs. Metabolites, 9(1), 13. https://doi.org/10.3390/metabo9010013






