Changes in the Elemental and Metabolite Profile of Wheat Phloem Sap during Grain Filling Indicate a Dynamic between Plant Maturity and Time of Day
Abstract
1. Introduction
2. Results
2.1. Variability: Elemental
2.2. Within-Day Variability: Metabolite Profile
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Aphid Stylectomy
4.3. Microscope Measurement of Nanoliter Phloem Exudate Volumes
4.4. Sample Analysis
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Atwell, B.B.J.; Kriedmann, P.E.; Turnbull, C.G.N. Plants in Action: Adaptation in Nature, Performance in Cultivation; MacMillan Education Australia: Melbourne, Australia, 1999. [Google Scholar]
- Brinch-Pedersen, H.; Borg, S.; Tauris, B.; Holm, P.B. Molecular genetic approaches to increasing mineral availability and vitamin content of cereals. J. Cereal Sci. 2007, 46, 308–326. [Google Scholar] [CrossRef]
- Zee, S.Y.; O’Brien, T.P. A special type of tracheary element associated with “xylem discontinuity” in the floral axis of wheat. Aust. J. Biol. Sci. 1970, 23, 783–791. [Google Scholar] [CrossRef]
- Blindauer, C.A.; Schmid, R. Cytosolic metal handling in plants: Determinants for zinc specificity in metal transporters and metallothioneins. Metallomics 2010, 2, 510–529. [Google Scholar] [CrossRef] [PubMed]
- Harris, W.R.; Sammons, R.D.; Grabiak, R.C. A speciation model of essential trace metal ions in phloem. J. Inorg. Biochem. 2012, 116, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, R.; Kato, M.; Nagata, S.; Yanagisawa, S.; Yoneyama, T. Identification of zn–nicotianamine and fe–2′-deoxymugineic acid in the phloem sap from rice plants (Oryza sativa L.). Plant Cell Physiol. 2012, 53, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Rengel, Z.; Römheld, V. Root exudation and Fe uptake and transport in wheat genotypes differing in tolerance to Zn deficiency. Plant Soil 2000, 222, 25–34. [Google Scholar] [CrossRef]
- Zhang, F.-S.; Römheld, V.; Marschner, H. Diurnal rhythm of release of phytosiderophores and uptake rate of Zinc in iron-deficient wheat. Soil Sci. Plant Nutr. 1991, 37, 671–678. [Google Scholar] [CrossRef]
- Reichman, S.M.; Parker, D.R. Probing the effects of light and temperature on diurnal rhythms of phytosiderophore release in wheat. New Phytol. 2007, 174, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Nozoye, T.; Itai, R.N.; Nagasaka, S.; Takahashi, M.; Nakanishi, H.; Mori, S.; Nishizawa, N.K. Diurnal changes in the expression of genes that participate in phytosiderophore synthesis in rice. Soil Sci. Plant Nutr. 2004, 50, 1125–1131. [Google Scholar] [CrossRef]
- Selby-Pham, J.; Lutz, A.; Moreno-Moyano, L.T.; Boughton, B.A.; Roessner, U.; Johnson, A.A.T. Diurnal changes in transcript and metabolite levels during the iron deficiency response of rice. Rice 2017, 10, 14. [Google Scholar] [CrossRef] [PubMed]
- Negishi, T.; Nakanishi, H.; Yazaki, J.; Kishimoto, N.; Fujii, F.; Shimbo, K.; Yamamoto, K.; Sakata, K.; Sasaki, T.; Kikuchi, S.; et al. Cdna microarray analysis of gene expression during fe-deficiency stress in barley suggests that polar transport of vesicles is implicated in phytosiderophore secretion in Fe-deficient barley roots. Plant J. 2002, 30, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Andrés-Colás, N.; Perea-García, A.; Puig, S.; Peñarrubia, L. Deregulated copper transport affects arabidopsis development especially in the absence of environmental cycles. Plant Physiol. 2010, 153, 170–184. [Google Scholar] [CrossRef] [PubMed]
- Duc, C.; Cellier, F.; Lobréaux, S.; Briat, J.-F.; Gaymard, F. Regulation of iron homeostasis in Arabidopsis thaliana by the clock regulator time for coffee. J. Biol. Chem. 2009, 284, 36271–36281. [Google Scholar] [CrossRef] [PubMed]
- Ravet, K.; Touraine, B.; Boucherez, J.; Briat, J.-F.; Gaymard, F.; Cellier, F. Ferritins control interaction between iron homeostasis and oxidative stress in Arabidopsis. Plant J. 2009, 57, 400–412. [Google Scholar] [CrossRef] [PubMed]
- Hall, A.; Bastow, R.M.; Davis, S.J.; Hanano, S.; McWatters, H.G.; Hibberd, V.; Doyle, M.R.; Sung, S.; Halliday, K.J.; Amasino, R.M.; et al. The time for coffee gene maintains the amplitude and timing of Arabidopsis circadian clocks. Plant Cell Online 2003, 15, 2719–2729. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Millar, A.J.; Davis, A.M.; Davis, S.J. Time for coffee encodes a nuclear regulator in the Arabidopsis thaliana circadian clock. Plant Cell Online 2007, 19, 1522–1536. [Google Scholar] [CrossRef] [PubMed]
- Tissot, N.; Przybyla-Toscano, J.; Reyt, G.; Castel, B.; Duc, C.; Boucherez, J.; Gaymard, F.; Briat, J.-F.; Dubos, C. Iron around the clock. Plant Sci. 2014, 224, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, B.L.; Oh, S.; Karakkat, B. Molecular basis and fitness implications of the interplay between light and the regulation of iron homeostasis in photosynthetic organisms. Environ. Exp. Bot. 2015, 114, 48–56. [Google Scholar] [CrossRef]
- Stein, R.J.; Ricachenevsky, F.K.; Fett, J.P. Differential regulation of the two rice ferritin genes (osfer1 and osfer2). Plant Sci. 2009, 177, 563–569. [Google Scholar] [CrossRef]
- Borg, S.; Brinch-Pedersen, H.; Tauris, B.; Madsen, L.H.; Darbani, B.; Noeparvar, S.; Holm, P.B. Wheat ferritins: Improving the iron content of the wheat grain. J. Cereal Sci. 2012, 56, 204–213. [Google Scholar] [CrossRef]
- Stomph, T.J.; Choi, E.Y.; Stangoulis, J.C.R. Temporal dynamics in wheat grain zinc distribution: Is sink limitation the key? Ann. Bot. 2011, 107, 927–937. [Google Scholar] [CrossRef] [PubMed]
- Pearson, J.N.; Rengel, Z. Distribution and remobilization of Zn and Mn during grain development in wheat. J. Exp. Bot. 1994, 45, 1829–1835. [Google Scholar] [CrossRef]
- Dinant, S.; Kehr, J. Sampling and analysis of phloem sap. In Plant Mineral Nutrients; Maathuis, F.J.M., Ed.; Humana Press: New York, NY, USA, 2013; Volume 953, pp. 185–194. [Google Scholar]
- Palmer, L.; Dias, D.; Boughton, B.; Roessner, U.; Graham, R.; Stangoulis, J. Metabolite profiling of wheat (Triticum aestivum L.) phloem exudate. Plant Methods 2014, 10, 27. [Google Scholar] [CrossRef] [PubMed]
- Palmer, L.J.; Palmer, L.T.; Rutzke, M.A.; Graham, R.D.; Stangoulis, J.C.R. Nutrient variability in phloem: Examining changes in K, Mg, Zn and Fe concentration during grain loading in common wheat (Triticum aestivum L.). Physiol. Plant 2014, 152, 729–737. [Google Scholar] [CrossRef] [PubMed]
- Gattolin, S.; Newbury, H.J.; Bale, J.S.; Tseng, H.-M.; Barrett, D.A.; Pritchard, J. A diurnal component to the variation in sieve tube amino acid content in wheat. Plant Physiol. 2008, 147, 912–921. [Google Scholar] [CrossRef] [PubMed]
- Thompson, M.V.; Zwieniecki, M.A. The role of potassium in long distance transport. In Vascular Transport in Plants; Holbrook, N.M., Zwieniecki, M.A., Eds.; Elsevier Science: Burlington, MA, USA, 2011; pp. 221–240. [Google Scholar]
- Marschner, H. Mineral Nutrition of Higher Plants; Academic Press: Cambridge, MA, USA, 1995. [Google Scholar]
- Servaites, J.C.; Schrader, L.E.; Jung, D.M. Energy-dependent loading of amino acids and sucrose into the phloem of soybean. Plant Physiol. 1979, 64, 546–550. [Google Scholar] [CrossRef] [PubMed]
- Philippar, K.; Büchsenschütz, K.; Abshagen, M.; Fuchs, I.; Geiger, D.; Lacombe, B.; Hedrich, R. The k+ channel kzm1 mediates potassium uptake into the phloem and guard cells of the c4 grass Zea mays. J. Biol. Chem. 2003, 278, 16973–16981. [Google Scholar] [CrossRef] [PubMed]
- Gajdanowicz, P.; Michard, E.; Sandmann, M.; Rocha, M.; Corrêa, L.G.G.; Ramírez-Aguilar, S.J.; Gomez-Porras, J.L.; González, W.; Thibaud, J.-B.; van Dongen, J.T.; et al. Potassium (k+) gradients serve as a mobile energy source in plant vascular tissues. Proc. Natl. Acad. Sci. USA 2011, 108, 864–869. [Google Scholar] [CrossRef] [PubMed]
- Welch, R.M. Micronutrient nutrition of plants. Crit. Rev. Plant Sci. 1995, 14, 49–82. [Google Scholar] [CrossRef]
- Takagi, S.I.; Nomoto, K.; Takemoto, T. Physiological aspect of mugineic acid, a possible phytosiderophore of graminaceous plants. J. Plant Nutr. 1984, 7, 469–477. [Google Scholar] [CrossRef]
- Nozoye, T.; Nagasaka, S.; Bashir, K.; Takahashi, M.; Kobayashi, T.; Nakanishi, H.; Nishizawa, N.K. Nicotianamine synthase 2 localizes to the vesicles of iron-deficient rice roots, and its mutation in the YXXφ or LL motif causes the disruption of vesicle formation or movement in rice. Plant J. 2014, 77, 246–260. [Google Scholar] [CrossRef] [PubMed]
- Reid, R.; Brookes, J.; Tester, M.; Smith, F.A. The mechanism of zinc uptake in plants. Planta 1996, 198, 39–45. [Google Scholar] [CrossRef]
- Grotz, N.; Guerinot, M.L. Molecular aspects of Cu, Fe and Zn homeostasis in plants. Biochim. Biophys. Acta Mol. Cell Res. 2006, 1763, 595–608. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.K. Heavy metals toxicity in plants: An overview on the role of glutathione and phytochelatins in heavy metal stress tolerance of plants. S. Afr. J. Bot. 2010, 76, 167–179. [Google Scholar] [CrossRef]
- Mendoza-Cózatl, D.G.; Butko, E.; Springer, F.; Torpey, J.W.; Komives, E.A.; Kehr, J.; Schroeder, J.I. Identification of high levels of phytochelatins, glutathione and cadmium in the phloem sap of Brassica napus. A role for thiol-peptides in the long-distance transport of cadmium and the effect of cadmium on iron translocation. Plant J. 2008, 54, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Ye, W.-L.; Wood, B.A.; Stroud, J.L.; Andralojc, P.J.; Raab, A.; McGrath, S.P.; Feldmann, J.; Zhao, F.-J. Arsenic speciation in phloem and xylem exudates of castor bean. Plant Physiol. 2010, 154, 1505–1513. [Google Scholar] [CrossRef] [PubMed]
- Noctor, G.; Mhamdi, A.; Chaouch, S.; Han, Y.I.; Neukermans, J.; Marquez-Garcia, B.; Queval, G.; Foyer, C.H. Glutathione in plants: An integrated overview. Plant Cell Environ. 2012, 35, 454–484. [Google Scholar] [CrossRef] [PubMed]
- Andreini, C.; Banci, L.; Bertini, I.; Rosato, A. Zinc through the three domains of life. J. Proteome Res. 2006, 5, 3173–3178. [Google Scholar] [CrossRef] [PubMed]
- Downing, N.; Unwin, D.M. A new method for cutting the mouth-parts of feeding aphids, and for collecting plant sap. Physiol. Entomol. 1977, 2, 275–277. [Google Scholar] [CrossRef]
- Palmer, L.J.; Palmer, L.T.; Pritchard, J.; Graham, R.D.; Stangoulis, J.C.R. Improved techniques for measurement of nanolitre volumes of phloem exudate from aphid stylectomy. Plant Methods 2013, 9, 18. [Google Scholar] [CrossRef] [PubMed]
- Temmerman, L.; Livera, A.M.D.; Bowne, J.B.; Sheedy, J.R.; Callahan, D.L.; Nahid, A.; Souza, D.P.D.; Schoofs, L.; Tull, D.L.; McConville, M.J.; et al. Cross-platform urine metabolomics of experimental hyperglycemia in type 2 diabetes. J. Diabetes Metab. 2012, 6, 2–7. [Google Scholar] [CrossRef]
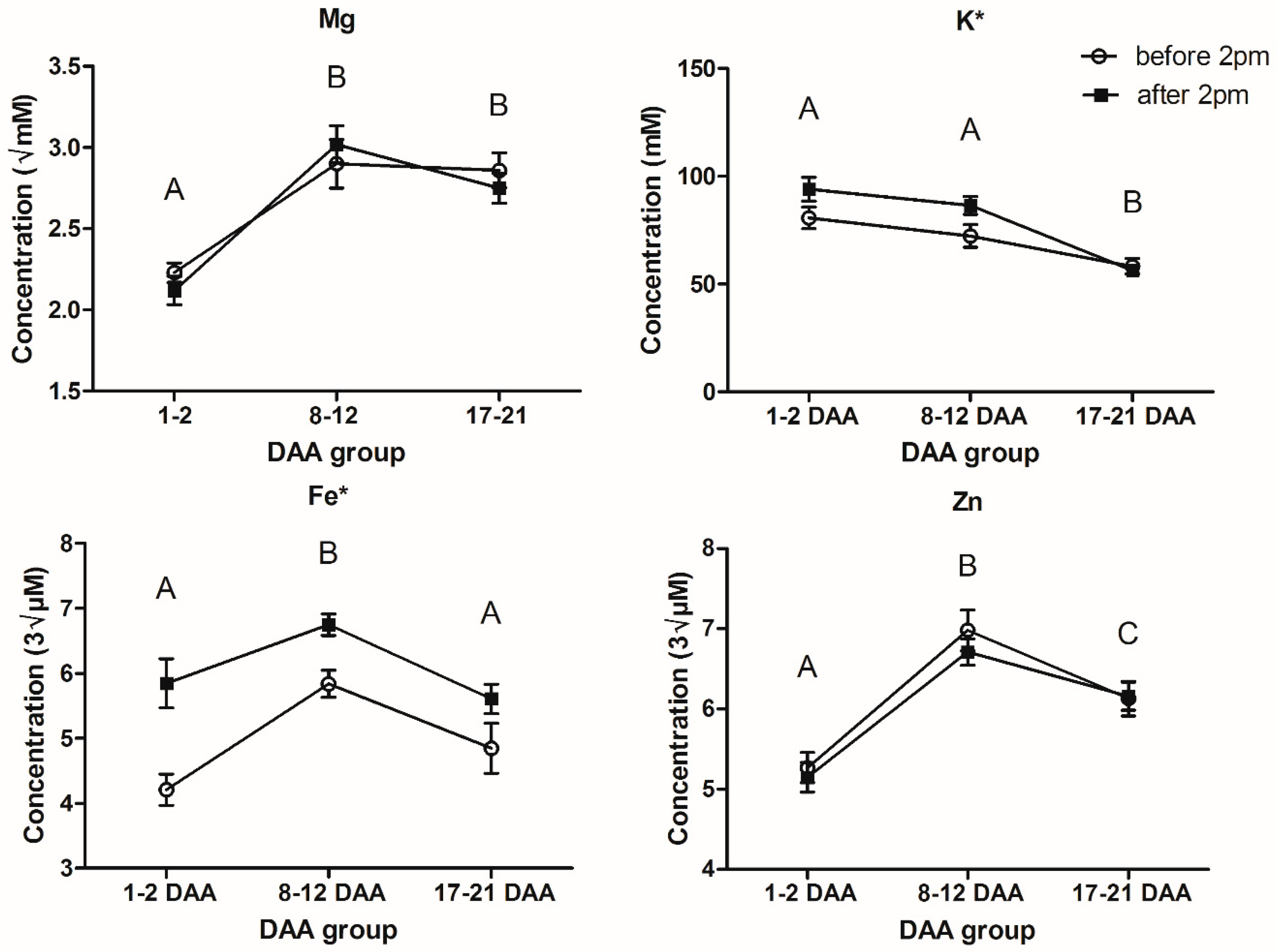
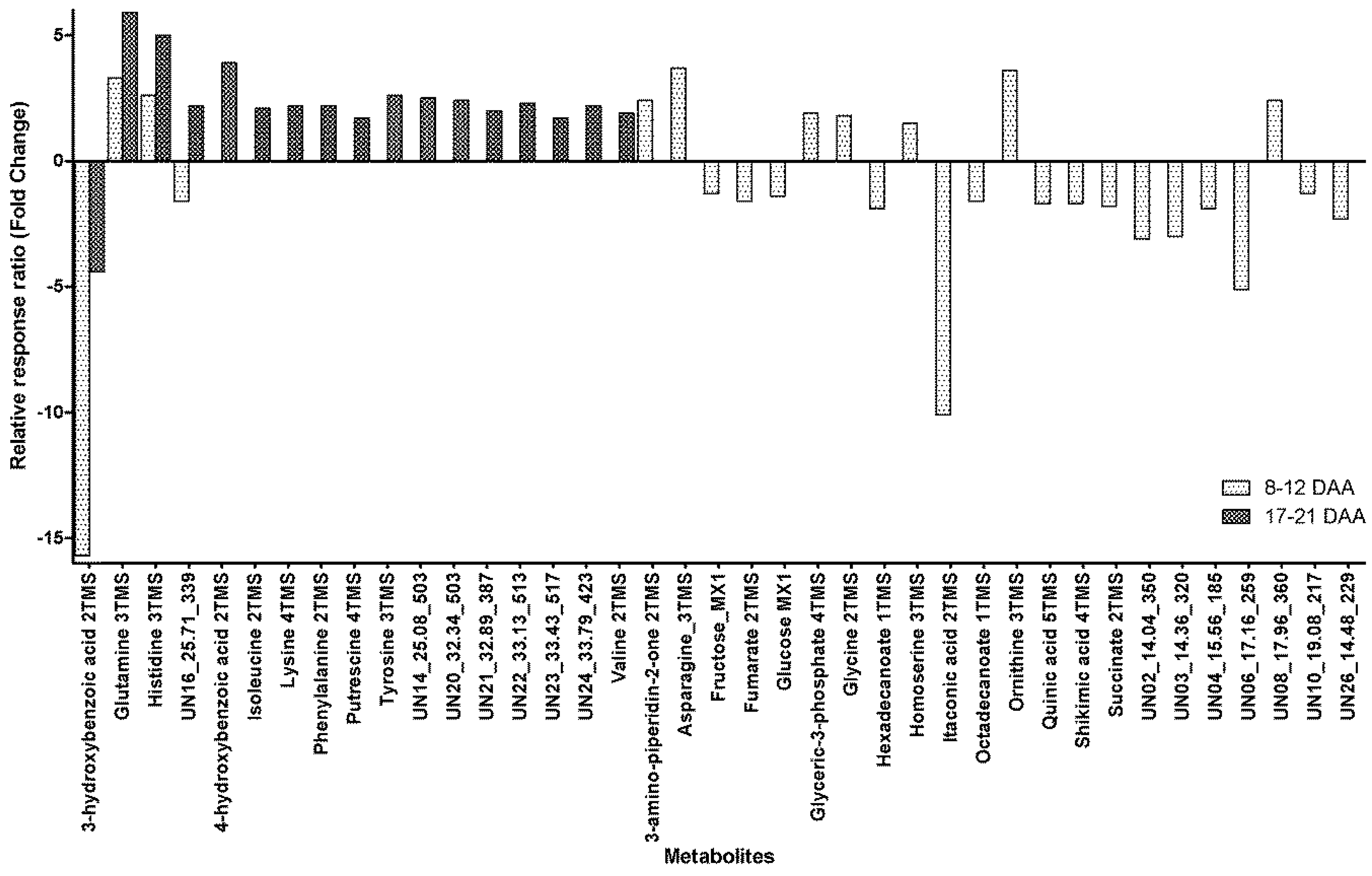
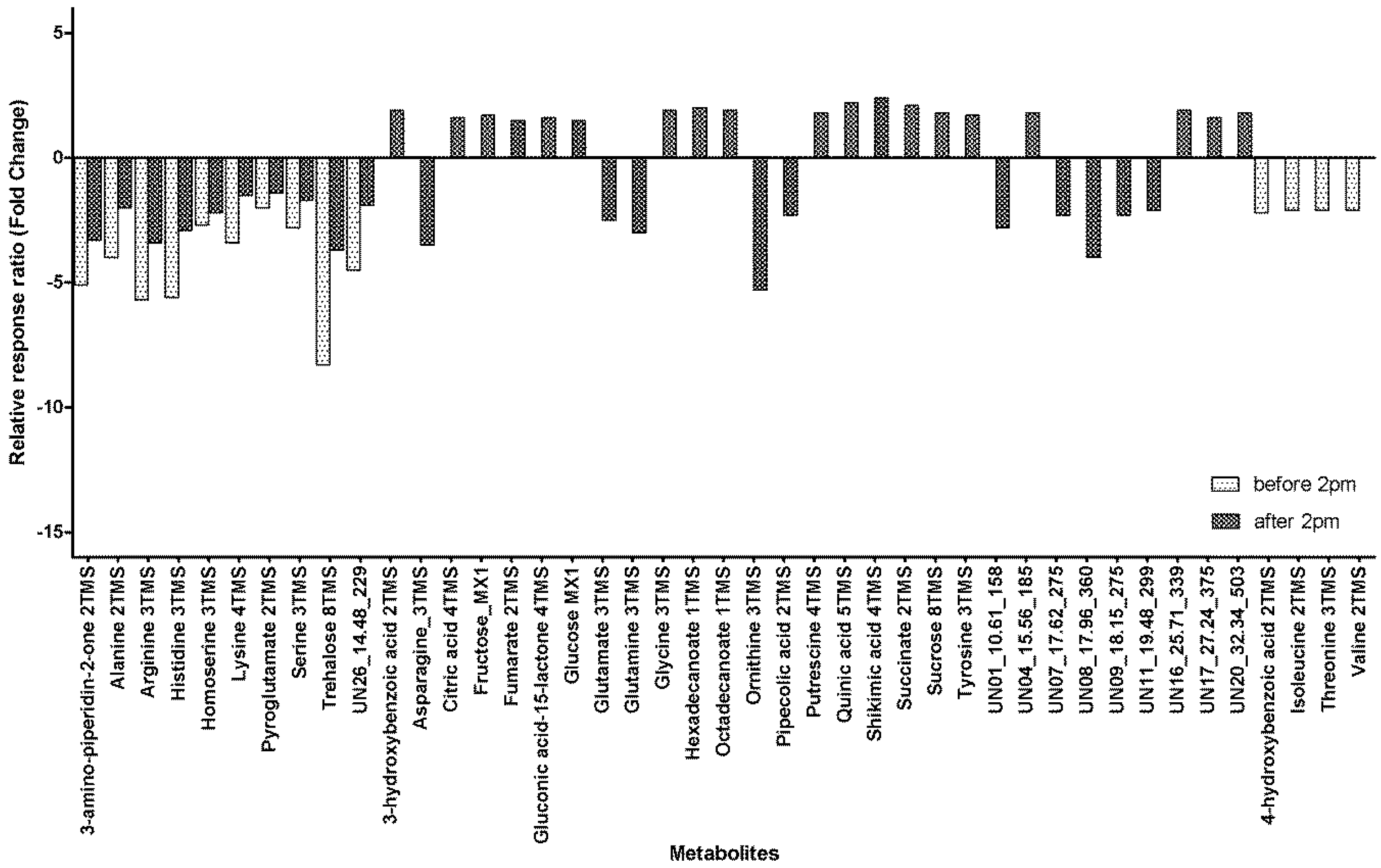
| Element | Within-Day Group | Mean Difference (I-J) | Std. Error | Sig. | Fold Change (I → J) | 95% Confidence Interval for Difference | ||
|---|---|---|---|---|---|---|---|---|
| I | J | Lower Bound | Upper Bound | |||||
| Mg () # | before 14:00 | after 14:00 | 0.032 | 0.119 | 0.790 | −1.01 | −0.203 | 0.266 |
| K (mM) | before 14:00 | after 14:00 | −8.52 * | 4.184 | 0.043 | 1.12 | −16.783 | −0.255 |
| Fe () | before 14:00 | after 14:00 | −1.10 * | 0.252 | 0.000 | 1.22 | −1.60 | −0.61 |
| Zn () | before 14:00 | after 14:00 | 0.12 | 0.193 | 0.536 | −1.02 | −0.26 | 0.50 |
| Element | DAA Group | Mean Difference (I-J) | Std. Error | Sig. | Fold Change (I → J) | 95% Confidence Interval | ||
|---|---|---|---|---|---|---|---|---|
| I | J | Lower Bound | Upper Bound | |||||
| Mg () # | 1–2 DAA | 8–12 DAA | −0.84 * | 0.141 | 0.000 | 1.39 | −1.18 | −0.51 |
| 17–21 DAA | −0.63 * | 0.136 | 0.000 | 1.29 | −0.96 | −0.30 | ||
| 8–12 DAA | 17–21 DAA | 0.22 | 0.120 | 0.148 | −1.08 | −0.05 | 0.48 | |
| K (mM) | 1–2 DAA | 8–12 DAA | 4.97 | 4.994 | 0.685 | −1.06 | −7.08 | 17.02 |
| 17–21 DAA | 31.94 * | 4.763 | 0.000 | −1.56 | 20.45 | 43.43 | ||
| 8–12 DAA | 17–21 DAA | 26.97 * | 3.940 | 0.000 | −1.47 | 17.46 | 36.48 | |
| Fe () | 1–2 DAA | 8–12 DAA | −1.39 * | 0.287 | 0.000 | 1.27 | −2.08 | −0.69 |
| 17–21 DAA | −0.14 | 0.293 | 0.947 | 1.03 | −0.85 | 0.57 | ||
| 8–12 DAA | 17–21 DAA | 1.24 * | 0.246 | 0.000 | −1.23 | 0.65 | 1.84 | |
| Zn () | 1–2 DAA | 8–12 DAA | −1.57 * | 0.229 | 0.000 | 1.30 | −2.12 | −1.01 |
| 17–21 DAA | −0.95 * | 0.222 | 0.000 | 1.18 | −1.49 | −0.41 | ||
| 8–12 DAA | 17–21 DAA | 061 * | 0.188 | 0.004 | −1.10 | 0.16 | 1.07 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palmer, L.J.; Stangoulis, J.C.R. Changes in the Elemental and Metabolite Profile of Wheat Phloem Sap during Grain Filling Indicate a Dynamic between Plant Maturity and Time of Day. Metabolites 2018, 8, 53. https://doi.org/10.3390/metabo8030053
Palmer LJ, Stangoulis JCR. Changes in the Elemental and Metabolite Profile of Wheat Phloem Sap during Grain Filling Indicate a Dynamic between Plant Maturity and Time of Day. Metabolites. 2018; 8(3):53. https://doi.org/10.3390/metabo8030053
Chicago/Turabian StylePalmer, Lachlan J., and James C. R. Stangoulis. 2018. "Changes in the Elemental and Metabolite Profile of Wheat Phloem Sap during Grain Filling Indicate a Dynamic between Plant Maturity and Time of Day" Metabolites 8, no. 3: 53. https://doi.org/10.3390/metabo8030053
APA StylePalmer, L. J., & Stangoulis, J. C. R. (2018). Changes in the Elemental and Metabolite Profile of Wheat Phloem Sap during Grain Filling Indicate a Dynamic between Plant Maturity and Time of Day. Metabolites, 8(3), 53. https://doi.org/10.3390/metabo8030053





