Lung Protection Strategies during Cardiopulmonary Bypass Affect the Composition of Bronchoalveolar Fluid and Lung Tissue in Cardiac Surgery Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Bronchoalveolar Lavage Fluid
2.3. Lung Biopsy
2.4. Data Analysis
3. Results
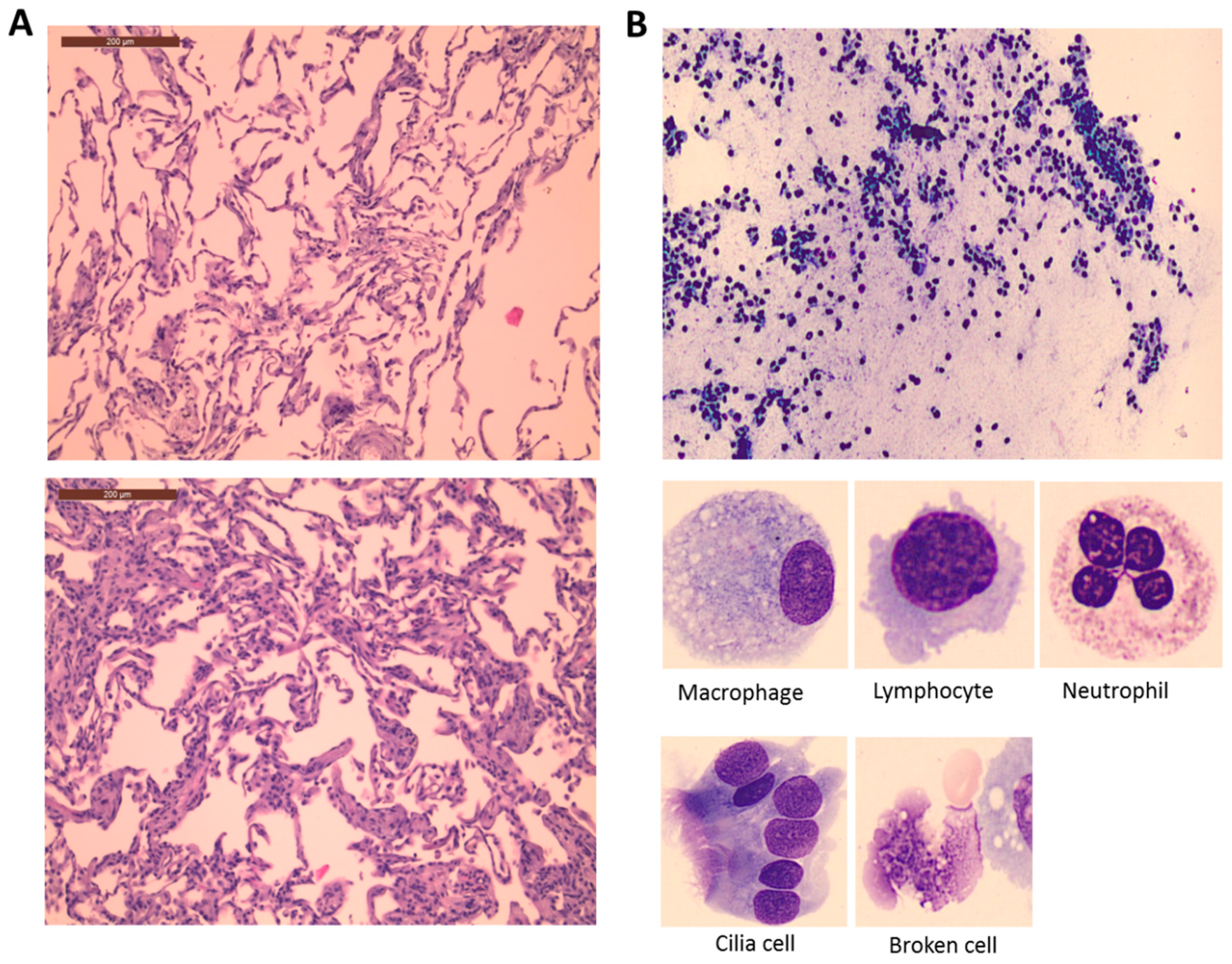
3.1. Lung Histological and Cellular Changes during Surgery
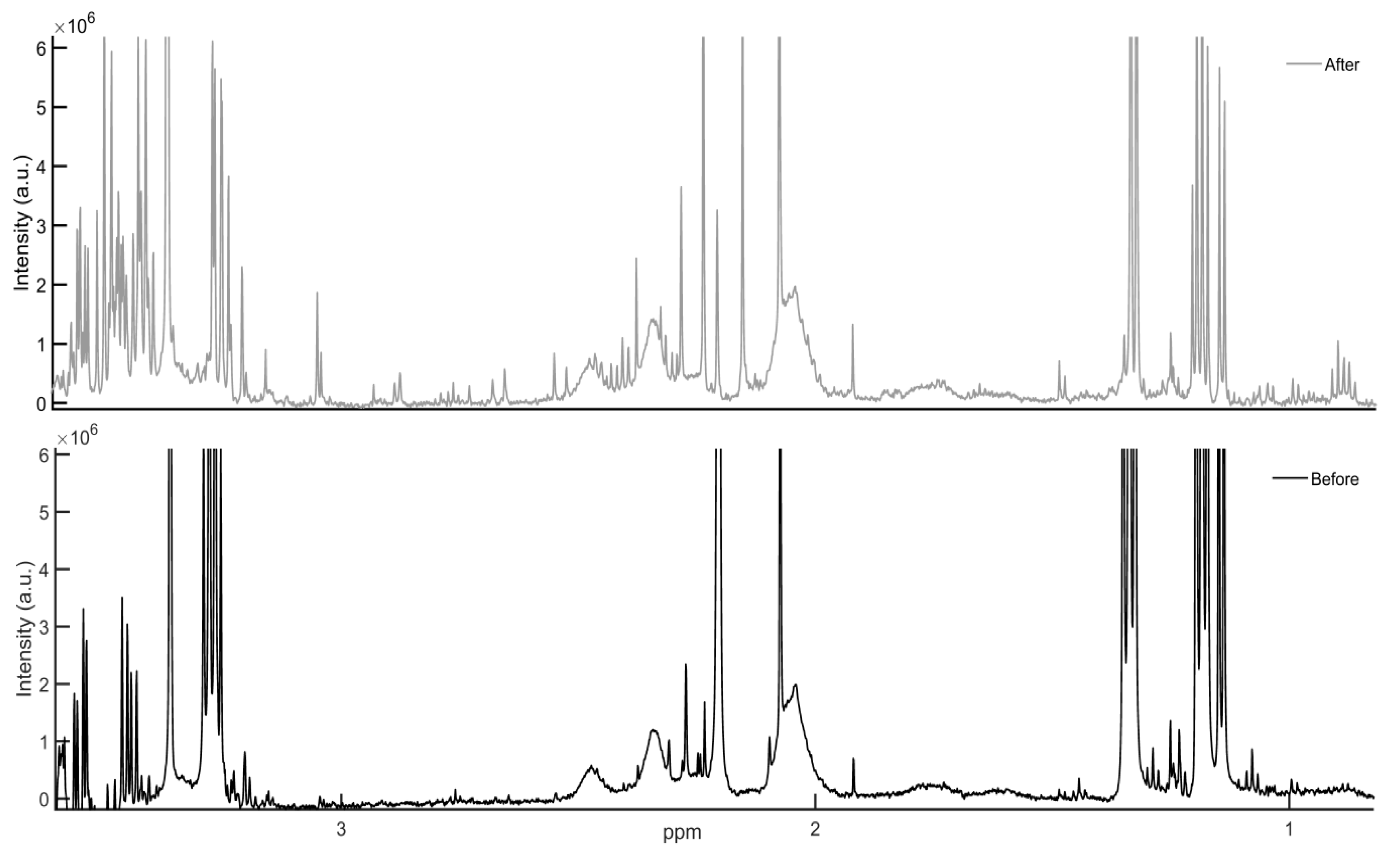
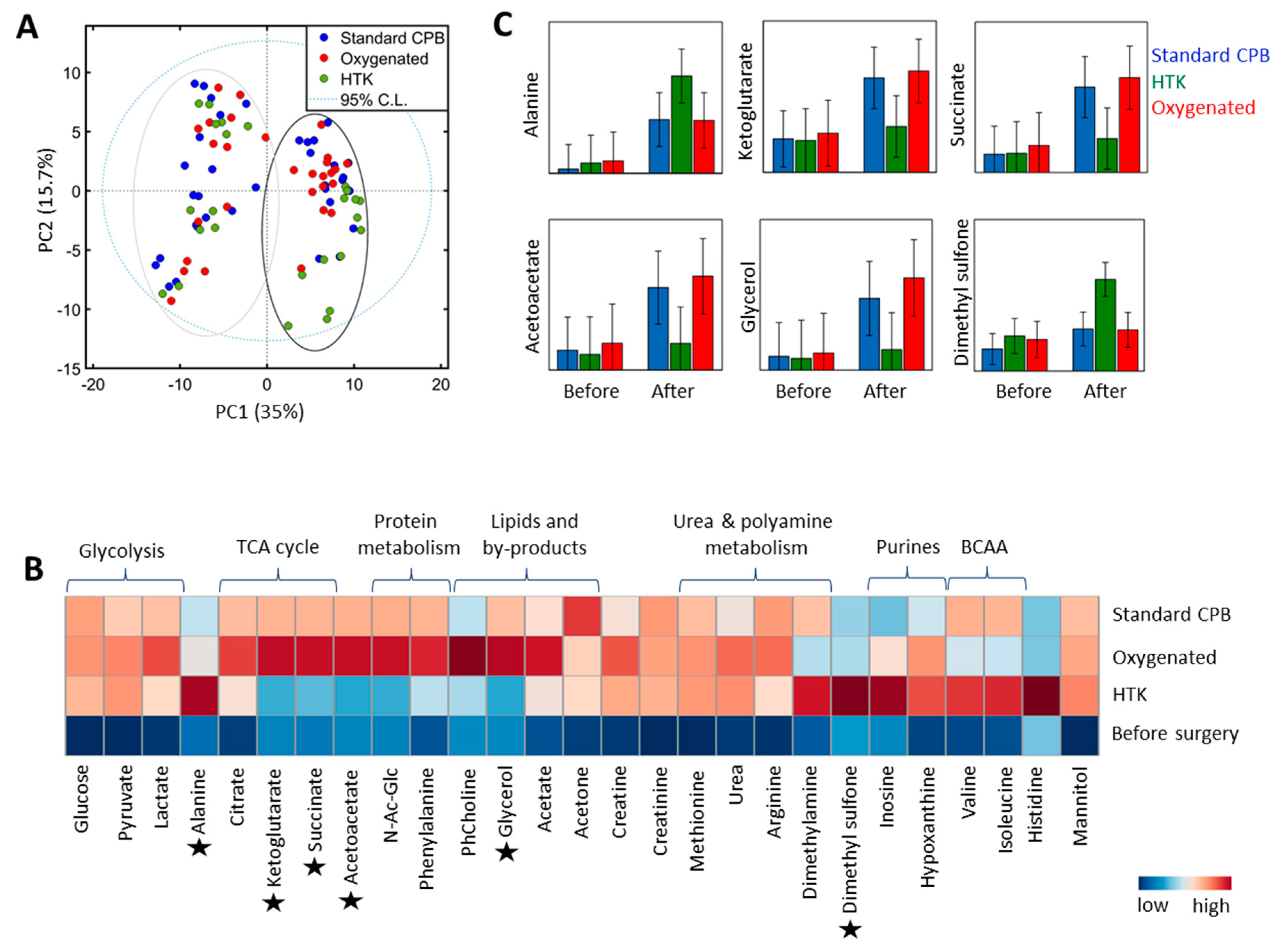
3.2. Lung Metabolite Profiles
4. Discussion
4.1. Lung Histological and Cellular Changes during Surgery
4.2. Lung Metabolite Profiles
4.3. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ng, C.S.; Wan, S.; Yim, A.P.; Arifi, A.A. Pulmonary dysfunction after cardiac surgery. Chest 2002, 121, 1269–1277. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.P.; Vargas, F.S.; Cukier, A.; Terra-Filho, M.; Teixeira, L.R.; Light, R.W. Arterial blood gases after coronary artery bypass surgery. Chest 1992, 102, 1337–1341. [Google Scholar] [CrossRef] [PubMed]
- Apostolakis, E.; Filos, K.S.; Koletsis, E.; Dougenis, D. Lung dysfunction following cardiopulmonary bypass. J. Card. Surg. 2009, 25, 47–55. [Google Scholar] [CrossRef] [PubMed]
- den Hengst, W.A.; Gielis, J.F.; Lin, J.Y.; Van Schil, P.E.; De Windt, L.J.; Moens, A.L. Lung ischemia-reperfusion injury: A molecular and clinical view on a complex pathophysiological process. Am. J. Physiol. Heart Circ. Physiol. 2010, 299, 1283–1299. [Google Scholar] [CrossRef] [PubMed]
- LaPar, D.J.; Gillen, J.R.; Crosby, I.K.; Sawyer, R.G.; Lau, C.L.; Kron, I.L.; Ailawadi, G. Predictors of operative mortality in cardiac surgical patients with prolonged intensive care unit duration. J. Am. Coll. Surg. 2013, 216, 1116–1123. [Google Scholar] [CrossRef] [PubMed]
- Kaul, T.K.; Fields, B.L.; Riggins, L.S.; Wyatt, D.A.; Jones, C.R.; Nagle, D. Adult respiratory distress syndrome following cardiopulmonary bypass: Incidence, prophylaxis and management. J. Cardiovasc. Surg. 1998, 39, 777–781. [Google Scholar]
- Milot, J.; Perron, J.; Lacasse, Y.; Létourneau, L.; Cartier, P.C.; Maltais, F. Incidence and predictors of ARDS after cardiac surgery. Chest 2001, 119, 884–888. [Google Scholar] [CrossRef] [PubMed]
- Christenson, J.T.; Aeberhard, J.M.; Badel, P.; Pepcak, F.; Maurice, J.; Simonet, F.; Velebit, V.; Schmuziger, M. Adult respiratory distress syndrome after cardiac surgery. Cardiovasc. Surg. 1996, 4, 15–21. [Google Scholar] [CrossRef]
- Griese, M.; Wilnhammer, C.; Jansen, S.; Rinker, C. Cardiopulmonary bypass reduces pulmonary surfactant activity in infants. J. Thorac. Cardiovasc. Surg. 1999, 118, 237–244. [Google Scholar] [CrossRef]
- Haslam, P.L.; Baker, C.S.; Hughes, D.A.; Macnaughton, P.D.; Moat, N.E.; Dewar, A.; Aggarwal, A.; Evans, T.W. Pulmonary surfactant composition early in development of acute lung injury after cardiopulmonary bypass: Prophylactic use of surfactant therapy. Int. J. Exp. Pathol. 1997, 78, 277–289. [Google Scholar] [CrossRef] [PubMed]
- Wasowicz, M.; Sobczynski, P.F.; Biczysko, W.F.; Szulc, R. Ultrastructural changes in the lung alveoli after cardiac surgical operations with the use of cardiopulmonary bypass (CPB). Pol. J. Pathol. 1999, 50, 189–196. [Google Scholar] [PubMed]
- Schlensak, C.; Doenst, T.; Preusser, S.; Wunderlich, M.; Kleinschmidt, M.; Beyersdorf, F. Bronchial artery perfusion during cardiopulmonary bypass does not prevent ischemia of the lung in piglets: Assessment of bronchial artery blood flow with fluorescent microspheres. Eur. J. Cardiothorac. Surg. 2001, 19, 326–332. [Google Scholar] [CrossRef]
- Maltesen, R.G.; Rasmussen, B.S.; Pedersen, S.; Hanifa, M.A.; Kucheryavskiy, S.; Kristensen, S.R.; Wimmer, R. Metabotyping Patients’ Journeys Reveals Early Predisposition to Lung Injury after Cardiac Surgery. Sci. Rep. 2017, 7, 40275. [Google Scholar] [CrossRef] [PubMed]
- Maltesen, R.G.; Hanifa, M.A.; Kucheryavskiy, S.; Pedersen, S.; Kristensen, S.R.; Rasmussen, B.S.; Wimmer, R. Predictive biomarkers and metabolic hallmark of postoperative hypoxaemia. Metabolomics 2016, 12, 87–102. [Google Scholar] [CrossRef]
- Sievers, H.H.; Freund-Kaas, C.; Eleftheriadis, S.; Fischer, T.; Kuppe, H.; Kraatz, E.G.; Bechtel, J.F. Lung protection during total cardiopulmonary bypass by isolated lung perfusion: Preliminary results of a novel perfusion strategy. Ann. Thorac. Surg. 2002, 74, 1167–1172. [Google Scholar] [CrossRef]
- Suzuki, T.; Fukuda, T.; Ito, T.; Inoue, Y.; Cho, Y.; Kashima, I. Continuous pulmonary perfusion during cardiopulmonary bypass prevents lung injury in infants. Ann. Thorac. Surg. 2000, 69, 602–606. [Google Scholar] [CrossRef]
- Suzuki, T.; Ito, T.; Kashima, I.; Teruya, K.; Fukuda, T. Continuous perfusion of pulmonary arteries during total cardiopulmonary bypass favorably affects levels of circulating adhesion molecules and lung function. J. Thorac. Cardiovasc. Surg. 2001, 122, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Kiessling, A.H.; Guo, F.W.; Gokdemir, Y.; Thudt, M.; Reyher, C.; Scherer, M.; Beiras-Fernandez, A.; Moritz, A. The influence of selective pulmonary perfusion on the inflammatory response and clinical outcome of patients with chronic obstructive pulmonary disease undergoing cardiopulmonary bypass. Interact Cardiovasc. Thorac. Surg. 2014, 18, 732–739. [Google Scholar] [CrossRef] [PubMed]
- Santini, F.; Onorati, F.; Telesca, M.; Menon, T.; Mazzi, P.; Berton, G.; Faggain, G.; Mazzucco, A. Selective pulmonary pulsatile perfusion with oxygenated blood during cardiopulmonary bypass attenuates lung tissue inflammation but does not affect circulating cytokine levels. Eur. J. Cardiothorac. Surg. 2012, 42, 942–950. [Google Scholar] [CrossRef] [PubMed]
- Santini, F.; Onorati, F.; Telesca, M.; Patelli, F.; Berton, G.; Franchi, G.; Faggain, G.; Mazzucco, A. Pulsatile pulmonary perfusion with oxygenated blood ameliorates pulmonary hemodynamic and respiratory indices in low-risk coronary artery bypass patients. Eur. J. Cardiothorac. Surg. 2011, 40, 794–803. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.M.; Hu, J.G.; Yin, B.L.; Yang, Y.F.; Zhang, W.; Yi, D.W.; Liu, S.Q. Lung protection of continuous pulmonary artery perfusion with oxygenated blood during cardiopulmonary bypass. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2005, 30, 413–416. (In Chinese) [Google Scholar] [PubMed]
- De Santo, L.S.; Romano, G.; Amarelli, C.; Della Corte, A.; Onorati, F.; Torella, M.; De Feo, M.; Nappi, G.A.; Cotrufo, M. Pilot study on prevention of lung injury during surgery for type A acute aortic dissection: No evident improvements with celsior flushing through the pulmonary artery. Int. J. Artif. Organs 2003, 26, 1032–1038. [Google Scholar] [CrossRef] [PubMed]
- De Santo, L.S.; Romano, G.; Amarelli, C.; Onorati, F.; Torella, M.; Renzulli, A.; Galdieri, N.; Cotrufo, M. Surgical repair of acute type an aortic dissection: Continuous pulmonary perfusion during retrograde cerebral perfusion prevents lung injury in a pilot study. J. Thorac. Cardiovasc. Surg. 2003, 126, 826–831. [Google Scholar] [CrossRef]
- Wei, B.; Liu, Y.; Wang, Q.; Yu, C.; Long, C.; Chang, Y.; Ruan, Y.M. Lung perfusion with protective solution relieves lung injury in corrections of Tetralogy of Fallot. Ann. Thorac. Surg. 2004, 77, 918–924. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, Y.; Liu, J.P.; Li, X. Pulmonary artery perfusion with HTK solution prevents lung injury in infants after cardiopulmonary bypass. Chin. Med. J. 2010, 123, 2645–2650. [Google Scholar] [PubMed]
- Buggeskov, K.B.; Sundskard, M.M.; Jonassen, T.; Andersen, L.W.; Secher, N.H.; Ravn, H.B.; Steinbrüchel, D.A.; Jakobsen, J.C.; Wetterslev, J. Pulmonary artery perfusion versus no pulmonary perfusion during cardiopulmonary bypass in patients with COPD: A randomised clinical trial. BMJ Open Respir. Res. 2016, 3, e000146. [Google Scholar] [CrossRef] [PubMed]
- Schlensak, C.; Doenst, T.; Preusser, S.; Wunderlich, M.; Kleinschmidt, M.; Beyersdorf, F. Cardiopulmonary bypass reduction of bronchial blood flow: A potential mechanism for lung injury in a neonatal pig model. J. Thorac. Cardiovasc. Surg. 2002, 123, 1199–1205. [Google Scholar] [CrossRef] [PubMed]
- Griffith, B.P.; Zenati, M. The pulmonary donor. Clin. Chest Med. 1990, 11, 217–226. [Google Scholar] [PubMed]
- Warnecke, G.; Struber, M.; Hohlfeld, J.M.; Niedermeyer, J.; Sommer, S.P.; Haverich, A. Pulmonary preservation with Bretscheider’s HTK and Celsior solution in minipigs. Eur. J. Cardiothorac. Surg. 2002, 21, 1073–1079. [Google Scholar] [CrossRef]
- Li, J.; Wu, Y.; Tian, X.; Wang, J.; Dong, M.; Wang, A.; Ma, S.J. Protective effect of HTK solution on postoperative pulmonary function in infants with CHD and PAH. Biosci. Rep. 2017, 37, BSR20170984. [Google Scholar] [CrossRef] [PubMed]
- GmbH, F.K.C. 510 (k) Summary. Custodiol HTK Solution. Available online: https://www.accessdata.fda.gov/cdrh_docs/pdf3/K032794.pdf?utm_campaign=Google2&utm_source=fdaSearch&utm_medium=website&utm_term=custodiol&utm_content=1 (accessed on 18 August 2018).
- Simões, E.A.; Cardoso, P.F.; Pêgo-Fernandes, P.M.; Canzian, M.; Pazetti, R.; Braga, K.A.; Nepomuceno, N.A.; Jatene, F.A. An experimental rat model of ex vivo lung perfusion for the assessment of lungs regarding histopathological findings and apoptosis: Low-potassium dextran vs. histidine-tryptophan-ketoglutarate. J. Bras. Pneumol. 2012, 38, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Buggeskov, K.B.; Wetterslev, J.; Secher, N.H.; Andersen, L.W.; Jonassen, T.; Steinbrüchel, D.A. Pulmonary perfusion with oxygenated blood or custodiol HTK solution during cardiac surgery for postoperative pulmonary function in COPD patients: A trial protocol for the randomized, clinical, parallel group, assessor and data analyst blinded Pulmonary Protection Trial. Trials 2013, 14, 30. [Google Scholar] [PubMed]
- Meyer, K.C.; Raghu, G.; Baughman, R.P.; Brown, K.K.; Costabel, U.; du Bois, R.M.; Haslam, P.L.; Kim, D.S.; Nagai, S.; Rottoli, P.; et al. An Official American Thoracic Society Clinical Practice Guideline: The Clinical Utility of Bronchoalveolar Lavage Cellular Analysis in Interstitial Lung Disease. Am. J. Respir. Crit. Care Med. 2012, 185, 1004–1014. [Google Scholar] [CrossRef] [PubMed]
- Meiboom, S.; Gill, D. Modified Spin-Echo Method for Measuring Nuclear Relaxation Times. Rev. Sci. Instrum. 1958, 29, 688–691. [Google Scholar] [CrossRef]
- Mikawa, K.; Nishina, K.; Takao, Y.; Obara, H. ONO-1714, a Nitric Oxide Synthase Inhibitor, Attenuates Endotoxin-Induced Acute Lung Injury in Rabbits. Anesth. Analg. 2003, 97, 1751–1755. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yao, J.H.; Hu, X.W.; Fan, Z.; Huang, L.; Jing, H.R.; Liu, K.X.; Tian, X.F. Inhibition of Rho kinase by fasudil hydrochloride attenuates lung injury induced by intestinal ischemia and reperfusion. Life Sci. 2011, 88, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Yao, J.; Li, Y.; Hu, X.; Shao, H.; Tian, X. Anti-inflammatory and antioxidant effects of curcumin on acute lung injury in a rodent model of intestinal ischemia reperfusion by inhibiting the pathway of NF-Kb. Int. J. Clin. Exp. Pathol. 2015, 8, 3451–3459. [Google Scholar] [PubMed]
- Wishart, D.S.; Jewison, T.F.; Fau, G.A.; Wilson, M.F.; Knox, C.F.; Liu, Y.F.; Djoumbou, Y.; Mandal, R.; Aziat, F.; Dong, E.; et al. HMDB 3.0-The Human Metabolome Database in 2013. Nucleic Acids Res. 2013, 41, D801–D807. [Google Scholar] [CrossRef] [PubMed]
- Freitas, C.R.; Malbouisson, L.M.; Benicio, A.; Negri, E.M.; Bini, F.M.; Massoco, C.O.; Otsuki, D.A.; Melo, M.F.; Carmona, M.J. Lung Perfusion and Ventilation During Cardiopulmonary Bypass Reduces Early Structural Damage to Pulmonary Parenchyma. Anesth. Analg. 2016, 122, 943–952. [Google Scholar] [CrossRef] [PubMed]
- Tilney, N.L.; Hester, W.J. Physiologic and histologic changes in the lungs of patients dying after prolonged cardiopulmonary bypass: An inquiry into the nature of post-perfusion lung. Ann. Surg. 1967, 166, 759–766. [Google Scholar] [CrossRef] [PubMed]
- Ratliff, N.B.; Young, W.G.; Hackel, D.B.; Mikat, E.; Wilson, J.W.; Durham, N.C. Pulmonary injury secondary to extracorporeal circulation. J. Thorac. Cardiovasc. Surg. 1972, 65, 425–432. [Google Scholar]
- Frass, O.M.; Bühling, F.; Täger, M.; Frass, H.; Ansorge, S.; Huth, C.; Welte, T. Antioxidant and antiprotease status in peripheral blood and BAL fluid after cardiopulmonary bypass. Chest 2001, 120, 1599–1608. [Google Scholar] [CrossRef] [PubMed]
- Marsh, M.J.; Metters, E.E.; Hellewell, S.A.; George, A.; Henderson, N.G.; Postle, A.D. Cardiopulmonary bypass and inflammatory markers in bronchoalveolar lavage fluid(BALF). Crit. Care Med. 2005, 33, A121. [Google Scholar] [CrossRef]
- Zhang, J.; Jiang, Z.; Bao, C.; Mei, J.U.; Zhu, J. Cardiopulmonary bypass increases pulmonary microvascular permeability through the Src kinase pathway: Involvement of caveolin-1 and vascular endothelial cadherin. Mol. Med. Rep. 2016, 13, 2918–2924. [Google Scholar] [CrossRef] [PubMed]
- Wolak, J.E.; Esther, J.C.R.; O’Connell, T.M. Metabolomic analysis of bronchoalveolar lavage fluid from cystic fibrosis patients. Biomarkers 2009, 14, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Rai, R.K.; Azim, A.; Sinha, N.; Sahoo, J.N.; Singh, C.; Ahmed, A.; Saigal, S.; Baronia, A.K.; Gupta, D.; Gurjar, M.; et al. Metabolic profiling in human lung injuries by high-resolution nuclear magnetic resonance spectroscopy of bronchoalveolar lavage fluid (BALF). Metabolomics 2013, 9, 667–676. [Google Scholar] [CrossRef]
- Edelman, J.J.; Seco, M.; Dunne, B.; Matzelle, S.J.; Murphy, M.; Joshi, P.; Yan, T.D.; Wilson, M.K.; Bannon, P.G.; Vallely, M.P.; et al. Custodiol for myocardial protection and preservation: A systematic review. Ann. Cardiothorac. Surg. 2013, 2, 717–728. [Google Scholar] [PubMed]
- Dailey, L.A.; Hernández-Prieto, R.; Casas-Ferreira, A.; Jones, M.; Riffo-Vasquez, Y.; Rodríguez-Gonzalo, E.; Spina, D.; Jones, S.A.; Smith, N.W.; Forbes, B.; et al. Adenosine monophosphate is elevated in the bronchoalveolar lavage fluid of mice with acute respiratory toxicity induced by nanoparticles with high surface hydrophobicity. Nanotoxicology 2015, 9, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Esther, C.R.; Alexis, N.E.; Clas, M.L.; Lazarowski, E.R.; Donaldson, S.H.; Pedrosa-Ribeiro, C.M.; Moore, C.G.; Davis, S.D.; Boucher, R.C. Extracellular purines are biomarkers of neutrophilic airway inflammation. Eur. Respir. J. 2008, 31, 949–956. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, R.S.; Andrade, C.F. Oxidative Stress and Lung Ischemia-Reperfusion Injury. Oxid. Med. Cell. Longev. 2015, 2015, 590987. [Google Scholar] [CrossRef] [PubMed]
- Evans, C.R.; Karnovsky, A.; Kovach, M.A.; Standiford, T.J.; Burant, C.F.; Stringer, K.A. Untargeted LC-MS metabolomics of bronchoalveolar lavage fluid differentiates acute respiratory distress syndrome from health. J. Proteome Res. 2014, 13, 640–649. [Google Scholar] [CrossRef] [PubMed]
- Hoet, P.H.M.; Nemery, B. Polyamines in the lung: Polyamine uptake and polyamine-linked pathological or toxicological conditions. Am. J. Physiol. Lung Cell. Mol. Physiol. 2000, 278, 417–433. [Google Scholar] [CrossRef] [PubMed]
- Naz, S.; Garcia, A.; Rusak, M.; Barbas, C. Method development and validation for rat serum fingerprinting with CE–MS: Application to ventilator-induced-lung-injury study. Anal. Bioanal. Chem. 2013, 405, 4849–4858. [Google Scholar] [CrossRef] [PubMed]
- Lemire, J.; Milandu, Y.; Auger, C.; Bignucolo, A.; Appanna, V.P.; Appanna, V.D. Histidine is a source of the antioxidant, alpha-ketoglutarate, in Pseudomonas fluorescens challenged by oxidative stress. FEMS Microbiol. Lett. 2010, 309, 170–177. [Google Scholar] [PubMed]
| HTK/Oxygenated/Standard CPB | |
|---|---|
| Patient no. | BALF: 13/15/19 (Lung biopsy: 7/9/9) |
| Age-years (mean ± SD) | 72 ± 9; 71 ± 12; 70 ± 8 |
| Gender (male/female) | 10/3; 12/3; 17/2 |
| Cardiopulmonary bypass time, min. (mean ± SD) | 96 ± 22; 104 ± 63; 107 ± 52 |
| Aortic cross-clamp time, min. (mean ± SD) | 61 ± 13; 57 ± 44; 67 ± 42 |
| End-CPB PaO2/FiO2 (kPa) (mean ± SD) | 29 ± 10; 32 ± 10; 27 ± 11 |
| Lung Tissue | Groups | Before | After | Before vs. After | Time-Group | ||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | Min | Max | Mean | Min | Max | p-Value | p-Value | ||
| Alveolar congestion | Control | 1.1 | 1 | 2 | 1.2 | 1 | 2 | 0.06 * | 0.62 |
| HTK | 1.0 | 1 | 1 | 1.3 | 1 | 2 | |||
| O2 | 1.1 | 1 | 2 | 1.3 | 1 | 2 | |||
| Heamorhage | Control | 0.4 | 0 | 2 | 0.4 | 0 | 2 | 0.72 | 0.64 |
| HTK | 0.1 | 0 | 1 | 0.3 | 0 | 1 | |||
| O2 | 0.2 | 0 | 1 | 0.2 | 0 | 1 | |||
| Neutrophilic infiltration | Control | 1.1 | 1 | 2 | 1.6 | 1 | 2 | <0.001 | 0.32 |
| HTK | 1.1 | 1 | 2 | 1.5 | 1 | 2 | |||
| O2 | 1.2 | 1 | 2 | 1.3 | 1 | 2 | |||
| Thickness of alveolar wall hyaline membranes | Control | 1.4 | 1 | 3 | 1.6 | 1 | 3 | 0.01 * | 0.58 |
| HTK | 1.6 | 1 | 3 | 2.1 | 1 | 3 | |||
| O2 | 1.5 | 1 | 3 | 1.6 | 1 | 3 | |||
| Total score | Control | 4.1 | 3 | 6 | 4.6 | 3 | 7 | 0.001 | 0.36 |
| HTK | 3.7 | 3 | 5 | 5.1 | 3 | 7 | |||
| O2 | 3.9 | 3 | 7 | 4.3 | 3 | 7 | |||
| BALF Cells | Group | Before | After | Before vs. After | Time-Group | ||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | Min | Max | Mean | Min | Max | p-Value | p-Value | ||
| Total white blood cells | Control | 157,435 | 22,000 | 990,000 | 239,777 | 52,000 | 1,020,000 | 0.015 * | 0.59 |
| HTK | 163,846 | 9000 | 401,000 | 173,615 | 48,000 | 415,000 | |||
| O2 | 205,071 | 43,000 | 504,000 | 328,333 | 74,000 | 1,185,000 | |||
| Neutrophils | Control | 18.5 | 0 | 89 | 23.9 | 1 | 145 | 0.44 | 0.79 |
| HTK | 20.1 | 2 | 79 | 19.5 | 2 | 110 | |||
| O2 | 21.0 | 1 | 164 | 27.0 | 0 | 81 | |||
| Lymphocytes | Control | 7.9 | 1 | 25 | 4.1 | 0 | 18 | 0.007 * | 0.78 |
| HTK | 17.5 | 0 | 86 | 13.8 | 0 | 46 | |||
| O2 | 10.9 | 0 | 54 | 10.1 | 0 | 50 | |||
| Eosinocytes | Control | 0.9 | 0 | 3 | 0.8 | 0 | 5 | 0.84 | 0.96 |
| HTK | 0.9 | 0 | 3 | 0.9 | 0 | 6 | |||
| O2 | 2.4 | 0 | 21 | 2.2 | 0 | 17 | |||
| Macrophages | Control | 161.2 | 3 | 274 | 119.7 | 12 | 225 | 0.02 * | 0.26 |
| HTK | 147.9 | 42 | 265 | 158.2 | 37 | 227 | |||
| O2 | 170.4 | 12 | 216 | 135.5 | 10 | 217 | |||
| Other cells including cilia | Control | 12.4 | 0 | 89 | 54.1 | 0 | 272 | 0003 * | 0.19 |
| HTK | 22.0 | 0 | 230 | 24.3 | 0 | 122 | |||
| O2 | 5.1 | 0 | 41 | 30.9 | 0 | 176 | |||
| Non-nucleated cells including broken cells and smudge | Control | 58.4 | 3 | 471 | 98.1 | 2 | 293 | 0.05 * | 0.63 |
| HTK | 51.8 | 3 | 184 | 48.2 | 6 | 221 | |||
| O2 | 36.9 | 1 | 278 | 65.7 | 2 | 200 | |||
| BALF Metabolites | Before CPB | After CPB | CPB Effect | Treatment Effect | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | HTK | O2 | Control | HTK | O2 | Time (After vs. Before) | Time * Group | HTK/Control | O2/Control | ||||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | FC | p-Value | p-Value | FC | p-Value | FC | p-Value | |
| Glycerol | 31.1 | 34 | 33.4 | 43 | 36.8 | 40 | 143.3 | 37 | 46.6 | 41 | 185.4 | 38 | - | - | 0.11 | 0.3 | 0.029 | 1.3 | 0.7 |
| Phosphocholine | 7.7 | 7 | 5.1 | 9 | 8.0 | 8 | 18.6 | 7 | 14.5 | 8 | 37.0 | 8 | 2 | <0.0001 | 0.7 | 0.9 | 0.6 | 2.2 | 0.3 |
| N-Acetyl-Glucosamine | 1.7 | 2 | 1.8 | 2 | 2.9 | 2 | 9.6 | 2 | 3.3 | 2 | 11.2 | 2 | - | - | 0.05 | 0.3 | 0.013 | 1.1 | 0.8 |
| Acetate | 9.0 | 1 | 9.6 | 2 | 7.6 | 1 | 13.7 | 1 | 11.4 | 2 | 13.9 | 1 | 1.6 | <0.0001 | 0.9 | 0.9 | 0.3 | 1 | 0.9 |
| Acetoacetic acid | 7.5 | 6 | 7.2 | 8 | 9.8 | 7 | 29.3 | 7 | 10.6 | 7 | 34.2 | 7 | - | - | 0.14 | 0.3 | 0.027 | 1.1 | 0.8 |
| Acetone | 5.6 | 2 | 4.6 | 3 | 6.2 | 2 | 15.8 | 2 | 10.5 | 2 | 10.7 | 2 | 2 | <0.0001 | 0.9 | 0.6 | 0.3 | 0.7 | 0.3 |
| Glucose | 8.8 | 9 | 12.1 | 12 | 11.1 | 11 | 83.4 | 10 | 66.3 | 11 | 71.9 | 11 | 10 | <0.0001 | 1.0 | 0.9 | 0.7 | 0.9 | 0.7 |
| Lactate | 70.6 | 19 | 73.8 | 24 | 83.2 | 22 | 224.0 | 21 | 178.0 | 23 | 228.1 | 21 | 2.9 | <0.0001 | 0.4 | 0.8 | 0.2 | 1 | 0.9 |
| Pyruvate | 0.9 | 1 | 1.4 | 1 | 1.9 | 1 | 7.6 | 1 | 7.3 | 1 | 7.5 | 1 | 6.2 | <0.0001 | 1.0 | 1 | 1 | 1 | 0.9 |
| Alanine | 1.1 | 2 | 2.3 | 3 | 2.8 | 3 | 11.0 | 3 | 22.1 | 3 | 10.9 | 3 | 7.9 | <0.0001 | 0.05 | 1.8 | 0.13 | 1 | 1 |
| Citrate | 1.9 | 1 | 3.8 | 1 | 4.6 | 1 | 9.6 | 1 | 7.3 | 1 | 9.7 | 1 | 2.4 | <0.0001 | 0.7 | 0.8 | 0.3 | 1.1 | 0.8 |
| 2-Ketoglutarate | 12.3 | 5 | 14.0 | 6 | 13.9 | 6 | 33.9 | 6 | 16.0 | 6 | 38.1 | 6 | - | - | 0.02 | 0.5 | 0.005 | 1.1 | 0.8 |
| Succinate | 4.0 | 3 | 5.1 | 4 | 5.5 | 3 | 16.9 | 3 | 7.5 | 3 | 19.5 | 3 | - | - | 0.08 | 0.4 | 0.025 | 1.1 | 0.8 |
| Creatine | 0.4 | 1 | 0.7 | 1 | 0.6 | 1 | 3.0 | 1 | 3.0 | 1 | 3.6 | 1 | 5.8 | <0.0001 | 0.7 | 1 | 1 | 1.2 | 0.7 |
| Creatinine | 2.3 | 2 | 1.8 | 2 | 3.5 | 2 | 18.8 | 2 | 14.9 | 2 | 15.5 | 2 | 6.5 | <0.0001 | 0.8 | 0.8 | 0.5 | 0.8 | 0.5 |
| Isoleucine | 1.1 | 1 | 1.4 | 1 | 0.8 | 1 | 3.9 | 1 | 3.3 | 1 | 2.3 | 1 | 2.3 | 0.0004 | 0.3 | 0.9 | 0.4 | 0.7 | 0.2 |
| Valine | 1.6 | 1 | 2.1 | 1 | 1.9 | 1 | 5.1 | 1 | 5.1 | 1 | 3.5 | 1 | 2.2 | 0.0001 | 0.07 | 0.9 | 0.7 | 0.7 | 0.2 |
| Urea | 8.8 | 6 | 4.3 | 8 | 7.2 | 7 | 39.5 | 7 | 44.2 | 7 | 47.2 | 7 | 19.9 | <0.0001 | 0.6 | 1.4 | 0.3 | 1.2 | 0.4 |
| Arginine | 2.7 | 11 | 2.2 | 14 | 2.8 | 13 | 74.6 | 12 | 50.2 | 14 | 69.3 | 13 | 28.1 | <0.0001 | 0.5 | 0.7 | 0.5 | 1 | 0.9 |
| Methionine | 0.2 | 3 | 0.0 | 3 | 0.0 | 3 | 13.2 | 3 | 12.5 | 3 | 12.7 | 3 | 88.6 | <0.0001 | 1.0 | 1.1 | 0.8 | 1 | 1 |
| Dimethylamine | 0.4 | 0 | 0.6 | 0 | 0.3 | 0 | 1.7 | 0 | 2.0 | 0 | 1.1 | 0 | 3.3 | <0.0001 | 0.14 | 1.2 | 0.5 | 0.6 | 0.2 |
| Dimethyl sulfone | 3.3 | 1 | 3.9 | 1 | 4.1 | 1 | 6.0 | 1 | 11.7 | 1 | 5.4 | 1 | - | - | 0.004 | 2.1 | 0.004 | 0.9 | 0.8 |
| Histidine | 0.1 | 29 | 0.0 | 36 | 0.5 | 33 | 3.0 | 31 | 250.8 | 35 | 3.2 | 32 | - | - | <0.0001 | 67.8 | 0.0002 | 1.1 | 0.9 |
| Phenylalanine | 22.8 | 4 | 18.2 | 6 | 23.7 | 5 | 36.0 | 5 | 26.8 | 5 | 34.1 | 5 | 2 | 0.01 | 0.3 | 0.7 | 0.14 | 0.9 | 0.5 |
| Inosine | 4.5 | 1 | 4.2 | 1 | 3.4 | 1 | 5.1 | 1 | 6.2 | 1 | 4.9 | 1 | 1.3 | 0.008 | 0.5 | 1.2 | 0.4 | 0.9 | 0.5 |
| Hypoxanthine | 4.6 | 1 | 4.4 | 2 | 4.4 | 2 | 9.0 | 1 | 10.4 | 2 | 9.6 | 1 | 3.3 | 0.0002 | 0.9 | 1.1 | 0.7 | 1 | 1 |
| Mannitol | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 687.7 | 69 | 675.4 | 76 | 624.8 | 71 | 90 | <0.0001 | 0.9 | 1 | 1 | 0.9 | 0.6 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maltesen, R.G.; Buggeskov, K.B.; Andersen, C.B.; Plovsing, R.; Wimmer, R.; Ravn, H.B.; Rasmussen, B.S. Lung Protection Strategies during Cardiopulmonary Bypass Affect the Composition of Bronchoalveolar Fluid and Lung Tissue in Cardiac Surgery Patients. Metabolites 2018, 8, 54. https://doi.org/10.3390/metabo8040054
Maltesen RG, Buggeskov KB, Andersen CB, Plovsing R, Wimmer R, Ravn HB, Rasmussen BS. Lung Protection Strategies during Cardiopulmonary Bypass Affect the Composition of Bronchoalveolar Fluid and Lung Tissue in Cardiac Surgery Patients. Metabolites. 2018; 8(4):54. https://doi.org/10.3390/metabo8040054
Chicago/Turabian StyleMaltesen, Raluca G., Katrine B. Buggeskov, Claus B. Andersen, Ronni Plovsing, Reinhard Wimmer, Hanne B. Ravn, and Bodil S. Rasmussen. 2018. "Lung Protection Strategies during Cardiopulmonary Bypass Affect the Composition of Bronchoalveolar Fluid and Lung Tissue in Cardiac Surgery Patients" Metabolites 8, no. 4: 54. https://doi.org/10.3390/metabo8040054
APA StyleMaltesen, R. G., Buggeskov, K. B., Andersen, C. B., Plovsing, R., Wimmer, R., Ravn, H. B., & Rasmussen, B. S. (2018). Lung Protection Strategies during Cardiopulmonary Bypass Affect the Composition of Bronchoalveolar Fluid and Lung Tissue in Cardiac Surgery Patients. Metabolites, 8(4), 54. https://doi.org/10.3390/metabo8040054





