Untargeted NMR Spectroscopic Analysis of the Metabolic Variety of New Apple Cultivars
Abstract
:1. Introduction
2. Results
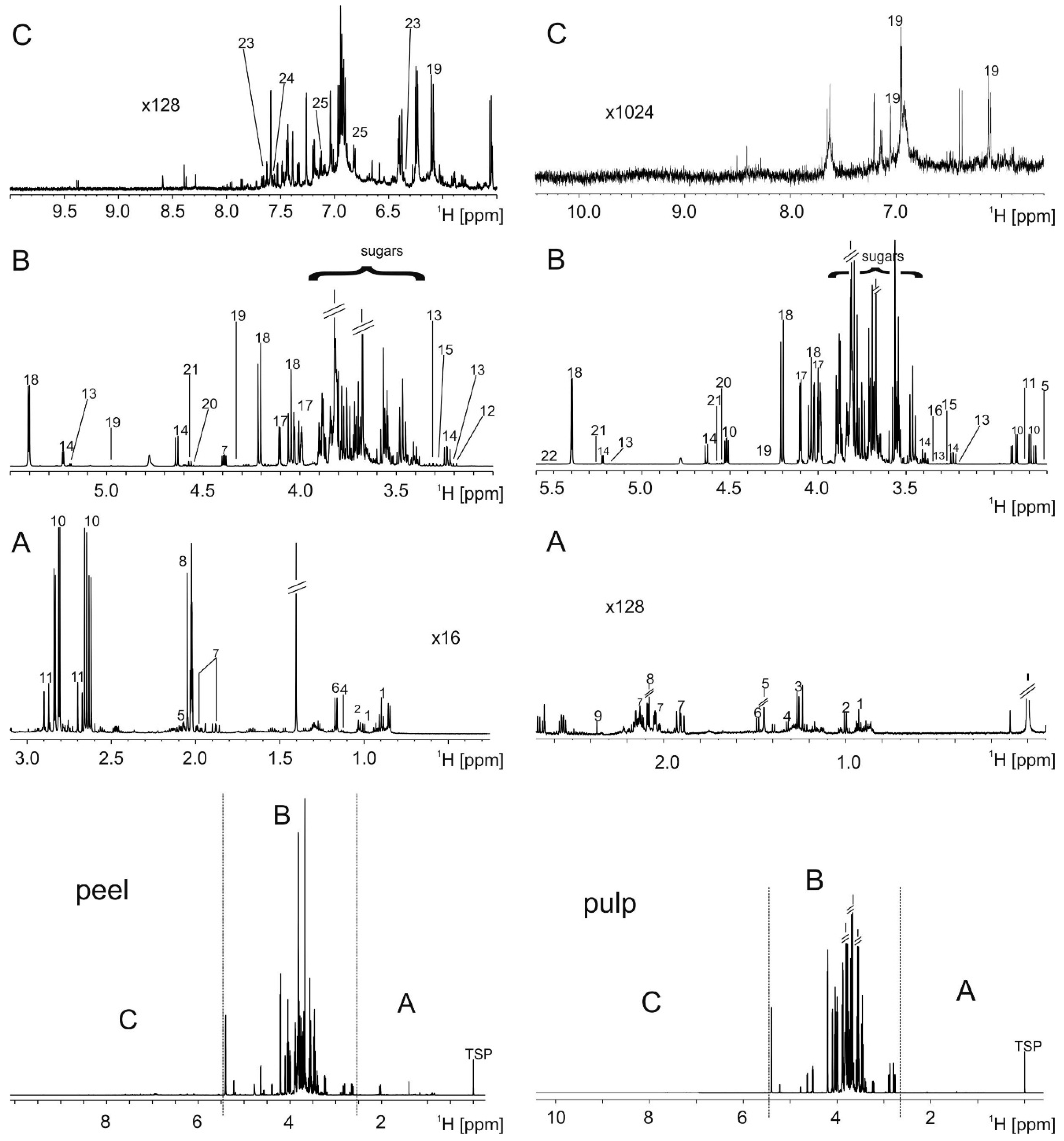
2.1. Metabolite Extraction and Identification
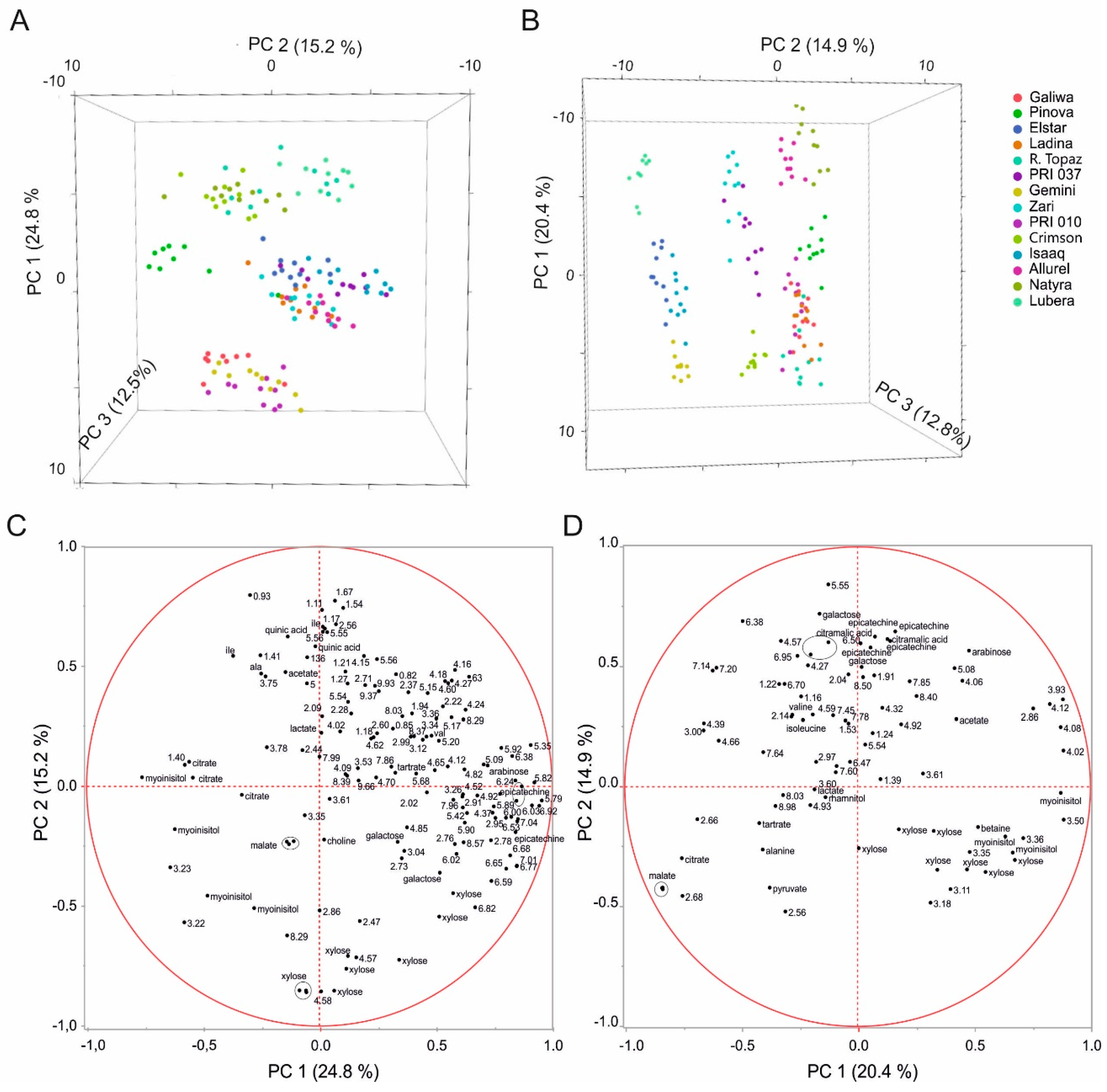
2.2. Multivariate Analysis to Differentiate Cultivars
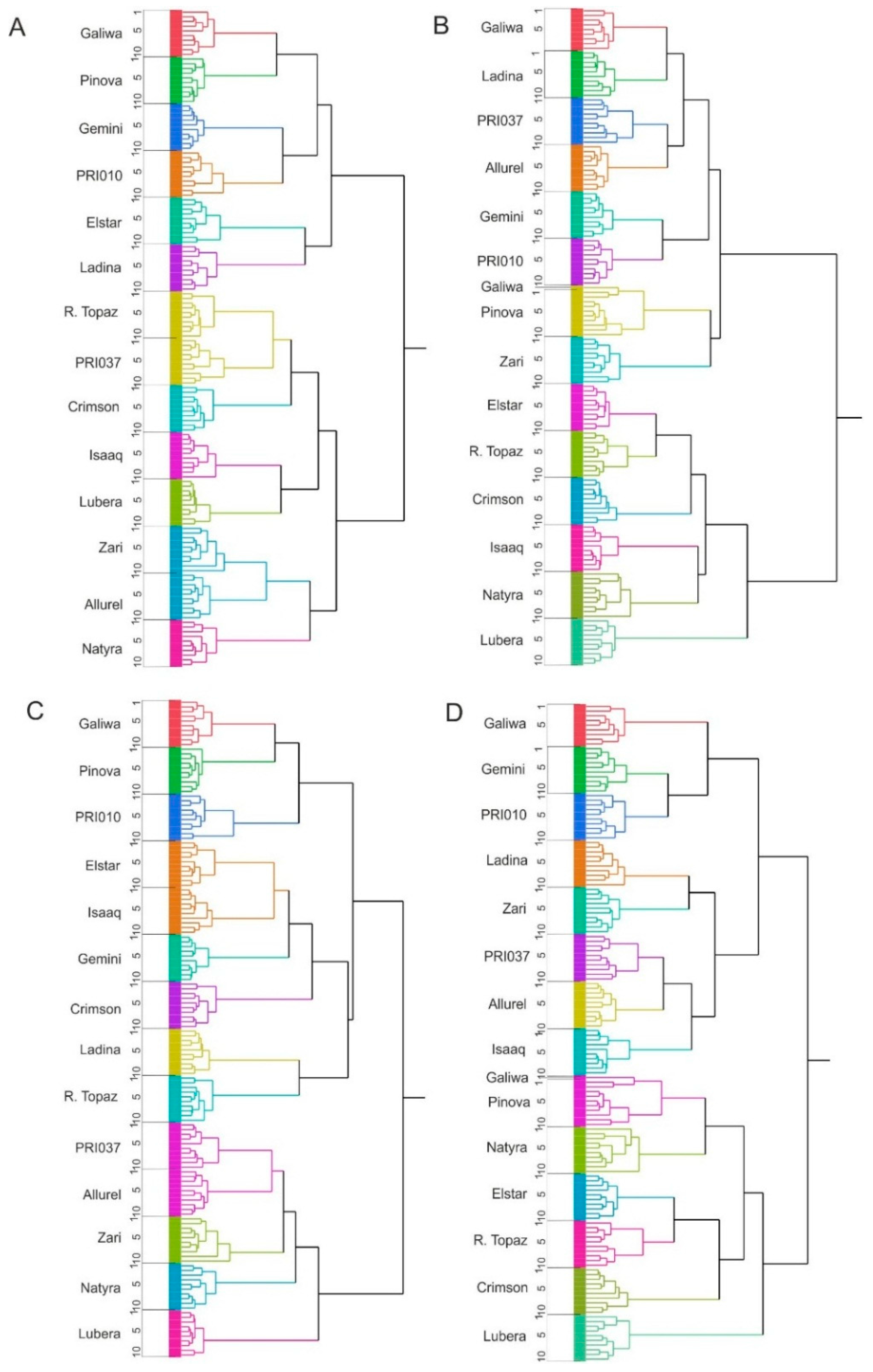
2.3. Cluster Analysis
3. Discussion
4. Materials and Methods
4.1. Plant Material and Sampling
4.2. Sample Preparation
4.3. 1H-NMR Spectroscopy
4.4. Data Processing of the NMR Spectra and Multivariate Pattern Recognition
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Monakhova, Y.B.; Godelmann, R.; Hermann, A.; Kuballa, T.; Cannet, C.; Schafer, H.; Spraul, M.; Rutledge, D.N. Synergistic effect of the simultaneous chemometric analysis of 1H-NMR spectroscopic and stable isotope (SNIF-NMR, 18O, 13C) data: Application to wine analysis. Anal. Chim. Acta 2014, 833, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Vanzo, A.; Jenko, M.; Vrhovsek, U.; Stopar, M. Metabolomic profiling and sensorial quality of ‘Golden Delicious’, ‘Liberty’, ‘Santana’, and ‘Topaz’ apples grown using organic and integrated production systems. J. Agric. Food Chem. 2013, 61, 6580–6587. [Google Scholar] [CrossRef] [PubMed]
- Troggio, M.; Gleave, A.; Salvi, S.; Chagné, D.; Cestaro, A.; Kumar, S.; Crowhurst, R.N.; Gardiner, S.E. Apple, from genome to breeding. Tree Genet. Genomes 2012, 8, 509–529. [Google Scholar] [CrossRef]
- Aprea, E.; Gika, H.; Carlin, S.; Theodoridis, G.; Vrhovsek, U.; Mattivi, F. Metabolite profiling on apple volatile content based on solid phase microextraction and gas-chromatography time of flight mass spectrometry. J. Chromatogr. A 2011, 1218, 4517–4524. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, J.S.; Lauridsen, M.B.; Dragsted, L.O.; Nielsen, J.; Staerk, D. Development of a bioassay-coupled HPLC-SPE-ttNMR platform for identification of alpha-glucosidase inhibitors in apple peel (Malus xdomestica Borkh.). Food Chem. 2012, 135, 1692–1699. [Google Scholar] [CrossRef] [PubMed]
- Tomita, S.; Nemoto, T.; Matsuo, Y.; Shoji, T.; Tanaka, F.; Nakagawa, H.; Ono, H.; Kikuchi, J.; Ohnishi-Kameyama, M.; Sekiyama, Y. A NMR-based, non-targeted multistep metabolic profiling revealed l-rhamnitol as a metabolite that characterised apples from different geographic origins. Food Chem. 2015, 174, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Vermathen, M.; Marzorati, M.; Baumgartner, D.; Good, C.; Vermathen, P. Investigation of different apple cultivars by high resolution magic angle spinning NMR. A feasibility study. J. Agric. Food Chem. 2011, 59, 12784–12793. [Google Scholar] [CrossRef] [PubMed]
- Vermathen, M.; Marzorati, M.; Vermathen, P. Exploring high-resolution magic angle spinning (HR-MAS) NMR spectroscopy for metabonomic analysis of apples. Chimia 2012, 66, 747–751. [Google Scholar] [CrossRef] [PubMed]
- Cuthbertson, D.; Andrews, P.K.; Reganold, J.P.; Davies, N.M.; Lange, B.M. Utility of metabolomics toward assessing the metabolic basis of quality traits in apple fruit with an emphasis on antioxidants. J. Agric. Food Chem. 2012, 60, 8552–8560. [Google Scholar] [CrossRef] [PubMed]
- De Paepe, D.; Valkenborg, D.; Noten, B.; Servaes, K.; Diels, L.; de Loose, M.; Van Droogenbroeck, B.; Voorspoels, S. Variability of the phenolic profiles in the fruits from old, recent and new apple cultivars cultivated in Belgium. Metabolomics 2015, 11, 739–752. [Google Scholar] [CrossRef]
- Vaclavik, L.; Schreiber, A.; Lacina, O.; Cajka, T.; Hajslova, J. Liquid chromatography–mass spectrometry-based metabolomics for authenticity assessment of fruit juices. Metabolomics 2012, 8, 793–803. [Google Scholar] [CrossRef]
- Farneti, B.; Khomenko, I.; Cappellin, L.; Ting, V.; Romano, A.; Biasioli, F.; Costa, G.; Costa, F. Comprehensive VOC profiling of an apple germplasm collection by PTR-ToF-MS. Metabolomics 2015, 11, 838–850. [Google Scholar] [CrossRef]
- Sansavini, S.; Donati, F.; Costa, F.; Tartarini, S. Advances in apple breeding for enhanced fruit quality and resistance to biotic stresses: New varieties for the European market. J. Fruit Ornam. Plant Res. 2004, 12, 13–52. [Google Scholar]
- Wolfe, K.; Wu, X.; Liu, R.H. Antioxidant activity of apple peels. J. Agric. Food Chem. 2003, 51, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Sciubba, F.; di Cocco, M.E.; Gianferri, R.; Capuani, G.; de Salvador, F.R.; Fontanari, M.; Gorietti, D.; Delfini, M. Nuclear Magnetic Resonance-Based Metabolic Comparative Analysis of Two Apple Varieties with Different Resistances to Apple Scab Attacks. J. Agric. Food Chem. 2015, 63, 8339–8347. [Google Scholar] [CrossRef] [PubMed]
- Noiton, D.A.M.; Alspach, P.A. Founding Clones, Inbreeding, Coancestry, and Status Number of Modern Apple Cultivars. J. Am. Soc. Hortic. Sci. 1996, 121, 773–782. [Google Scholar]
- Patzak, J.; Paprstein, F.; Henychova, A.; Sedlak, J. Genetic diversity of Czech apple cultivars inferred from microsatellite markers analysis. Hortic. Sci. 2012, 39, 149–157. [Google Scholar]
- Wishart, D.S.; Tzur, D.; Knox, C.; Eisner, R.; Guo, A.C.; Young, N.; Cheng, D.; Jewell, K.; Arndt, D.; Sawhney, S.; et al. HMDB: The Human Metabolome Database. Nucleic Acids Res. 2007, 35, D521–D526. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S. Metabolomics: Applications to food science and nutrition research. Trends Food Sci. Technol. 2008, 19, 482–493. [Google Scholar] [CrossRef]


| Cultivar | Characteristics |
|---|---|
| Galiwa | Scab mildew resistant (SMR) |
| Pinova Evelina | Established cultivar, colour mutant |
| Elstar v. d. Zalm | Established cultivar |
| Ladina | SMR |
| Red Topaz | SMR |
| PRI 037 | SMR, bred in Wageningen |
| Gemini | Scab resistant, from Italy |
| Zari | from Belgium |
| PRI 010 | SMR |
| Crimson Crisp | SMR, established cultivar from USA |
| Isaaq | Scab resistant, from Italy |
| Allurel | SMR |
| Natyra | SMR |
| Lubera | red-fleshed |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eisenmann, P.; Ehlers, M.; Weinert, C.H.; Tzvetkova, P.; Silber, M.; Rist, M.J.; Luy, B.; Muhle-Goll, C. Untargeted NMR Spectroscopic Analysis of the Metabolic Variety of New Apple Cultivars. Metabolites 2016, 6, 29. https://doi.org/10.3390/metabo6030029
Eisenmann P, Ehlers M, Weinert CH, Tzvetkova P, Silber M, Rist MJ, Luy B, Muhle-Goll C. Untargeted NMR Spectroscopic Analysis of the Metabolic Variety of New Apple Cultivars. Metabolites. 2016; 6(3):29. https://doi.org/10.3390/metabo6030029
Chicago/Turabian StyleEisenmann, Philipp, Mona Ehlers, Christoph H. Weinert, Pavleta Tzvetkova, Mara Silber, Manuela J. Rist, Burkhard Luy, and Claudia Muhle-Goll. 2016. "Untargeted NMR Spectroscopic Analysis of the Metabolic Variety of New Apple Cultivars" Metabolites 6, no. 3: 29. https://doi.org/10.3390/metabo6030029
APA StyleEisenmann, P., Ehlers, M., Weinert, C. H., Tzvetkova, P., Silber, M., Rist, M. J., Luy, B., & Muhle-Goll, C. (2016). Untargeted NMR Spectroscopic Analysis of the Metabolic Variety of New Apple Cultivars. Metabolites, 6(3), 29. https://doi.org/10.3390/metabo6030029






