Essences in Metabolic Engineering of Lignan Biosynthesis
Abstract
:1. Introduction
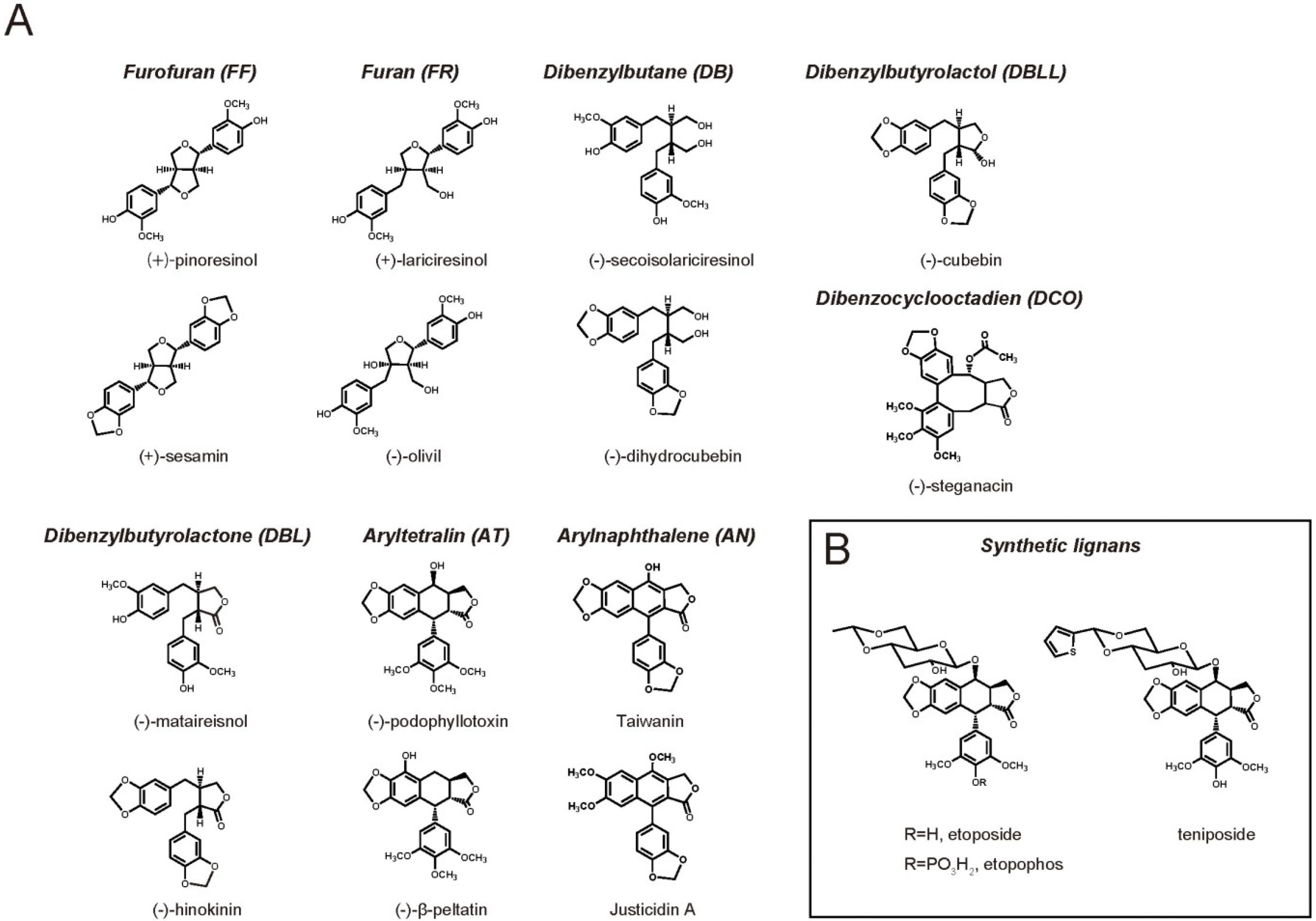
2. Lignan Biological Activity on Mammals
3. Lignan Biosynthesis Pathways
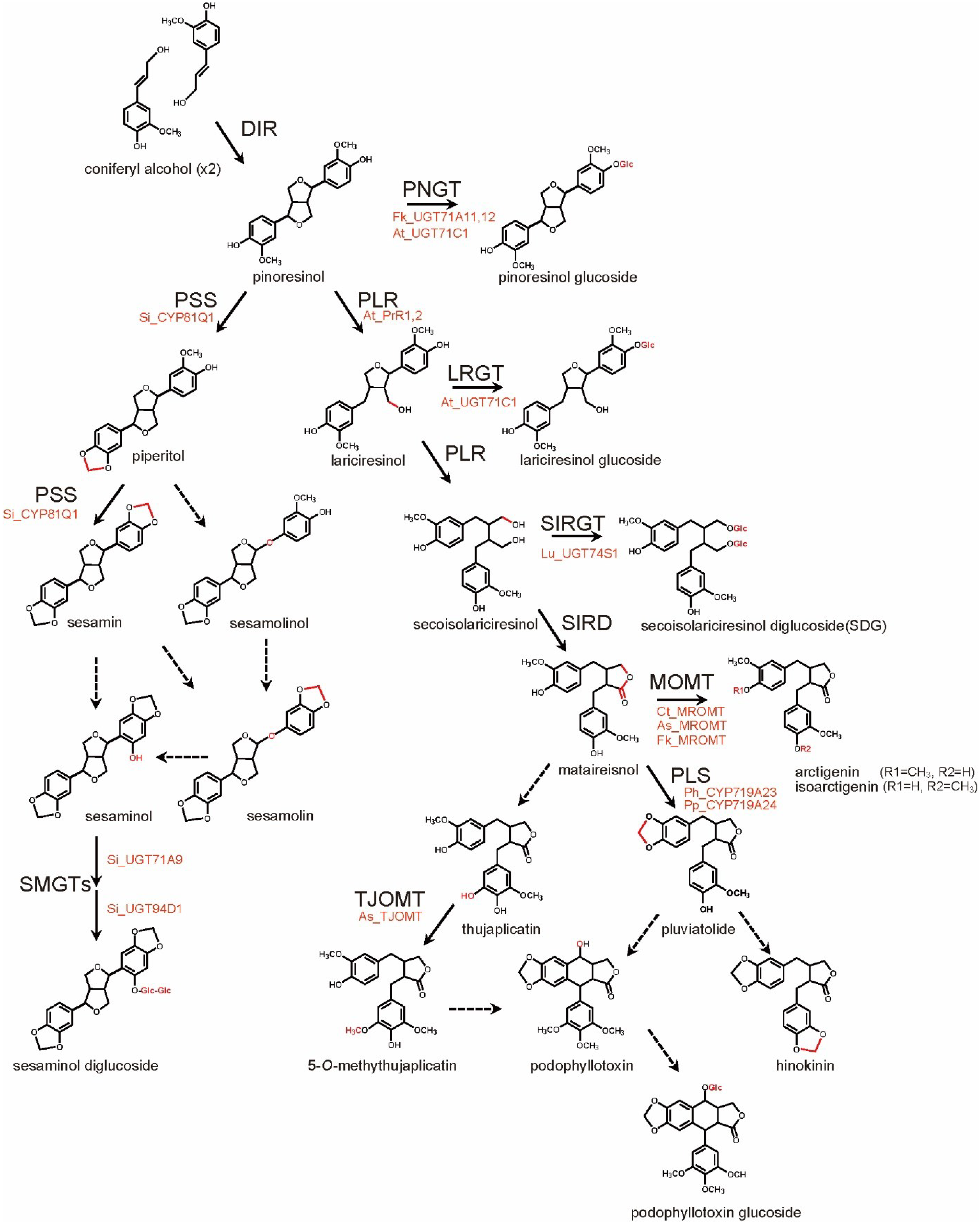
4. Metabolic Engineering of Lignan Biosynthesis
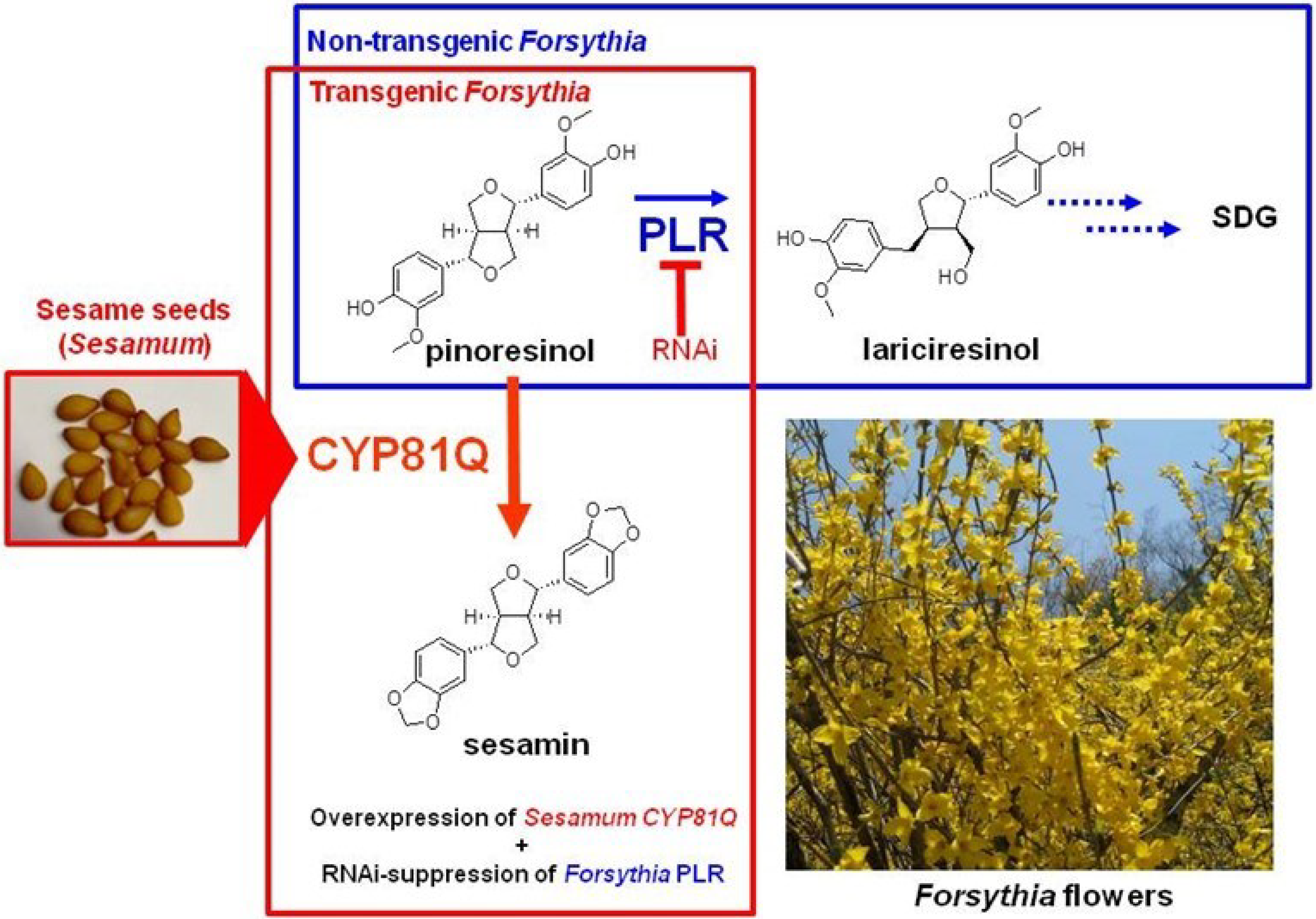
4.1. Gene Transfection or Silencing
4.2. Light Irradiation
4.3. Elicitation
| Elicitor | Target | Effect | References |
|---|---|---|---|
| Chito-oligosaccharides (1 mg) | Juniperus chinensis callus culture | Increased PTOX production | [104] |
| Methyl jasmonate (MeJA) (100 μM) | Forsythia intermedia cell suspension culture | Increased pinoresinol and matairesinol production | [103] |
| Mannan (0.1 mg mL-1) β-1,3-glucan (0.1 mg mL-1) Ancymidol (10-7 M) | L. austriacum callus culture | Enhanced activity of tyrosine ammonia-lyase (TAL), coumarate 3-hydroxylase (C3H), polyphenoloxidase (PPO) and PAL | [101] |
| Increased PTOX, 6-MPTOX, dPTOX, α- and β-peltatins production | |||
| Increaded PTOX and α-peltatins production Increaded PTOX, 6-MPTOX, dPTOX and α- peltatins production | |||
| Indanoyl-isoleucine (5-100 µM) Coronalon, (10-50 µM) MeJA(100 μM) | L. nodiflorum cell suspension culture | Increased deoxypodophyllotoxin production | [97] |
| Enhanced activity of 6-hydroxylase and β -peltatin 6-O-methyltransferas, | |||
| Increased 6-MPTOX and 5’-d-6-MPTOX production | |||
| MeJA (100 μM) | L. album cell suspension culture | Increased PTOX production | [98] |
| Botrytis cinerea extract (3 % v/v) | L. usitatissimum cell suspension culture | Rapid stimulation of the monolignol pathway, enhanced PAL activity and expression of genes encoding PAL, CCR and CAD | [108] |
| Phoma exigua extract (3 % v/v) | |||
| Fusarium oxysporum extract (3 % v/v) | |||
| MeJA (50–200 μM) | L. tauricum hairy root culture | Increased 6MPTOX and 4’-DM6MPTOX production | [102] |
| Salicylic acid (SA) (10 μM ) | L. album cell suspension culture | Enhanced PAL, CCR and CAD gene expression and PTOX production | [99] |
| Chitin (100 mg l-1) | L. album cell suspension culture | Increased lariciresinol and/or PTOX production | [105] |
| Chitosan (100–200 mg L-1) | |||
| MeJA (100–200 μM) | |||
| Fusarium graminearum extract (1 % v/v) | L. album cell suspension culture | Enhanced PAL, CCR, CAD, and PLR gene expression Increased PTOX and lariciresinol production | [105,109] |
| Sclerotinia sclerotiorum extract (1 % v/v) | |||
| Rhizopus stolonifer extract (1 % v/v) | |||
| Rhizoctonia solani extract (1 % v/v) | |||
| MeJA (10–100 μM) | Podophyllum hexandrum cell suspension cultute | Changes in cell proteome, Increased PTOX production | [109] |
| Fusarium graminearum extract (1 %v/v) | L. album hairy root culture | Enhanced PAL, CCR, CAD and PLR gene expression, Increased PTOX, 6MPTOX, and lariciresinol production | [106] |
| Sclerotinia sclerotiorum extract (1 %v/v) | |||
| Trichoderma viride extract (1 %v/v) | |||
| Chitosan (100 mg l-1) | |||
| Chitosan and chitin oligomers (100 mg L-1) | L. album cell suspension culture | Enhanced PAL, CCR, CAD and PLR gene expression, | [107] |
| Increased PTOX, 6MPTOX . and lariciresinol production | |||
| Fusarium graminearum culture filtrate (1 % v/v) | L. album cell suspension culture | Increased phenolic compound, PTOX and lariciresinol production | [110] |
| Enhanced PAL activity, |
5. Conclusions
Acknowledgments
Author contributions
Conflicts of Interest
References
- Umezawa, T. Diversity in lignan biosynthesis. Phytochem. Rev. 2003, 2, 371–390. [Google Scholar] [CrossRef]
- Suzuki, S.; Umezawa, T. Biosynthesis of lignans and norlignans. J. Wood. Sci. 2007, 53, 273–284. [Google Scholar] [CrossRef]
- Macías, F.A.; López, A.; Varela, R.M.; Torres, A.; Molinillo, J.M.G. Bioactive lignans from a cultivar of Helianthus annuus. J. Agric. Food Chem. 2004, 52, 6443–6447. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Choe, E. Extraction of lignan compounds from roasted sesame oil and their effects on the autoxidation of methyl linoleate. J. Food Sci. 2006, 71, C430–C436. [Google Scholar] [CrossRef]
- Guo, H.; Liu, A-H.; Ye, M.; Yang, M.; Guo, D.-A. Characterization of phenolic compounds in the fruits of Forsythia suspense by high-performance liquid chromatography coupled with electrospray ionization tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2007, 21, 715–729. [Google Scholar] [CrossRef] [PubMed]
- Peñalvo, J.L.; Adlercreutz, H.; Uehara, M.; Ristimaki, A.; Watanabe, S. Lignan content of selected foods from Japan. J. Agric. Food Chem. 2008, 56, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Piao, X.-L.; Jang, M.-H.; Cui, J.; Piao, X. Lignans from the fruits of Forsythia suspensa. Bioorg. Med. Chem. Lett. 2008, 18, 1980–1984. [Google Scholar] [CrossRef] [PubMed]
- Hata, N.; Hayashi, Y.; Okazawa, A.; Ono, E.; Satake, H.; Kobayashi, A. Comparison of sesamin contents and CYP81Q1 gene expressions in aboveground vegetative organs between two Japanese sesame (Sesamum indicum L.) varieties differing in seed sesamin contents. Plant Sci. 2010, 178, 510–516. [Google Scholar] [CrossRef]
- Hata, N.; Hayashi, Y.; Ono, E.; Satake, H.; Kobayashi, A.; Muranaka, T.; Okazawa, A. Differences in plant growth and leaf sesamin content of the lignan-rich sesame variety “Gomazou” under continuous light of different wavelengths. Plant Biotechnol. 2013, 30, 1–8. [Google Scholar] [CrossRef]
- Okazawa, A.; Hori, K.; Okumura, R.; Izumi, Y.; Hata, N.; Bamba, T.; Fukusaki, E.; Ono, E.; Satake, H.; Kobayashi, A. Simultaneous quantification of lignans in Arabidopsis thaliana by highly sensitive capillary liquid chromatography-electrospray ionization-ion trap mass spectrometry. Plant Biotechnol. 2011, 28, 287–293. [Google Scholar] [CrossRef]
- Schmidt, T.J.; Klaes, M.; Sendker, J. Lignans in seeds of Linum species. Phytochemistry 2012, 82, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Satake, H.; Ono, E.; Murata, J. Recent advances in metabolic engineering of lignan biosynthesis pathways for the production of transgenic plant-based foods and supplements. J. Agric. Food Chem. 2013, 61, 11721–11729. [Google Scholar] [CrossRef] [PubMed]
- Chaurasia, O.P.; Ballabh, B.; Tayade, A.; Kumar, R.; Kumar, G.P.; Singh, S.B. Podophyllum L.: An endangered and anticancerous medicinal plant–An overview. Indian J. Tradit. Know. 2012, 11, 234–241. [Google Scholar]
- Ionkova, I. Biotechnological approaches for the production of lignans. Phcog. Rev. 2007, 1, 57–68. [Google Scholar]
- Ionkova, I.; Antonova, I.; Momekov, G.; Fuss, E. Production of podophyllotoxin in Linum linearifolium in vitro cultures. Pharmacogn. Mag. 2010, 6, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Ionkova, I. Anticancer lignans–from discovery to biotechnology. Mini. Rev. Med. Chem. 2011, 11, 843–856. [Google Scholar] [CrossRef]
- Lata, H.; Mizuno, C.S.; Moraes, R.M. The role of biotechnology in the production of the anticancer compound podophyllotoxin. Methods. Mol. Biol. 2009, 547, 387–402. [Google Scholar] [PubMed]
- Malik, S.; Biba, O.; Grúz, J.; Arroo, R.R.J.; Strnad, M. Biotechnological approaches for producing aryltetralin lignans from Linum species. Phytochem. Rev. 2014, 13, 893–913. [Google Scholar] [CrossRef]
- Oliva, A.; Moraes, R.A.; Watson, S.B.; Duke, S.O.; Dayan, F.E. Aryltertralin lignans inhibit plant growth by affecting formation of mitotic microtubular organizing centers. Pestic. Biochem. Phys. 2002, 72, 45–54. [Google Scholar] [CrossRef]
- Harmatha, J.; Dinan, L. Biological activities of lignans and stilbenoids associated with plant-insect chemical interaction. Phytochem. Rev. 2003, 2, 321–330. [Google Scholar] [CrossRef]
- Schroeder, F.C.; del Campo, M.L.; Grant, J.B.; Weibel, D.B.; Smedley, S.R.; Bolton, K.L.; Meinwald, J.; Eisner, T. Pinoresinol: A lignol of plant origin serving for defense in a caterpillar. Proc. Natl. Acad. Sci. USA 2006, 103, 15497–15501. [Google Scholar] [CrossRef] [PubMed]
- Cutillo, F.; D'Abrosca, B.; DellaGreca, M.; Fiorentino, A.; Zarrelli, A. Lignans and neolignans from Brassica fruticulosa: Effects on seed germination and plant growth. J. Agric. Food Chem. 2003, 51, 6165–6172. [Google Scholar] [CrossRef] [PubMed]
- Nishiwaki, H.; Kumamoto, M.; Shuto, Y.; Yamauchi, S. Stereoselective syntheses of all stereoisomers of lariciresinol and their plant growth inhibitory activities. J. Agric. Food. Chem. 2011, 59, 13089–13095. [Google Scholar] [CrossRef] [PubMed]
- Carillo, P.; Cozzolino, C.; D’Abrosca, B.; Nacca, F.; DellaGreca, M.; Fiorentio, A.; Fuggi, A. Effects of the allelochemicals dihydrodiconiferylalcohol and lariciresinol on metabolism of Lactuca sativa. Open Bioact. Compd .J. 2010, 3, 18–24. [Google Scholar] [CrossRef]
- Heinonen, S.; Nurmi, T.; Liukkonen, K.; Poutanen, K.; Wähälä, K.; Deyama, T.; Nishibe, S.; Adlercreutz, H. In vitro metabolism of plant lignans: New precursors of mammalian lignans enterolactone and enterodiol. J. Agric. Food Chem. 2001, 49, 3178–3186. [Google Scholar] [CrossRef] [PubMed]
- Lampe, J.W.; Atkinson, C.; Hullar, M.A. Assessing exposure to lignans and their metabolites in humans. J. AOAC Int. 2006, 89, 1174–1181. [Google Scholar] [PubMed]
- Liu, Z.; Saarinen, N.M.; Thompson, L.U. Sesamin is one of the major precursors of mammalian lignans in sesame seed (Sesamum indicum) as observed in vitro and in rats. J. Nutr. 2006, 136, 906–912. [Google Scholar] [PubMed]
- Mueller, S.O.; Simon, S.; Chae, K.; Metzler, M.; Korach, K.S. Phytoestrogens and their human metabolites show distinct agonistic and antagonistic properties on estrogen receptor alpha (ERα) and ERβ in human cells. Toxicol. Sci. 2004, 80, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Penttinen, P.; Jaehrling, J.; Damdimopoulos, A.E.; Inzunza, J.; Lemmen, J.G.; van der Saag, P.; Pettersson, K.; Gauglitz, G.; Mäkelä, S.; Pongratz, I. Diet-derived polyphenol metabolite enterolactone is a tissue-specific estrogen receptor activator. Endocrinology 2007, 148, 4875–4886. [Google Scholar] [CrossRef] [PubMed]
- During, A.; Debouche, C.; Raas, T.; Larondelle, Y. Among plant lignans, pinoresinol has the strongest antiinflammatory properties in human intestinal Caco-2 cells. J. Nutr. 2012, 142, 1798–1805. [Google Scholar] [CrossRef] [PubMed]
- Adlercreutz, H. Lignans and human health. Crit. Rev. Cl. Lab. Sci. 2007, 44, 483–525. [Google Scholar] [CrossRef]
- Bergman Jungeström, M.; Thompson, L.U.; Dabrosin, C. Flaxseed and its lignans inhibit estradiol-induced growth, angiogenesis, and secretion of vascular endothelial growth factor in human breast cancer xenografts in vivo. Clin. Cancer. Res. 2007, 13, 1061–1067. [Google Scholar]
- Power, K.A.; Saarinen, N.M.; Chen, J.-M.; Thompson, L.U. Mammalian lignans enterolactone and enterodiol, alone and in combination with the isoflavone genistein, do not promote the growth of MCF-7 xenografts in ovariectomized athymic nude mice. Int. J. Cancer 2006, 118, 1316–1320. [Google Scholar] [CrossRef]
- Mense, S.M.; Hei, T.K.; Ganju, R.K.; Bhat, H.K. Phytoestrogens and breast cancer prevention: Possible mechanisms of action. Environ. Health Persp. 2008, 116, 426–433. [Google Scholar] [CrossRef]
- Saarinen, N.M.; Wärri, A.; Dings, R.P.M.; Airio, M.; Smeds, A.I.; Mäkelä, S. Dietary lariciresinol attenuates mammary tumor growth and reduces blood vessel density in human MCF-7 breast cancer xenografts and carcinogen-induced mammary tumors in rats. Int. J. Cancer 2008, 123, 1196–1204. [Google Scholar] [CrossRef]
- Adolphe, J.L.; Whiting, S.J.; Juurlink, B.H.J.; Thorpe, L.U.; Alcorn, J. Health effects with consumption of the flax lignan secoisolariciresinol diglucoside. Br. J. Nutr. 2010, 103, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Barre, D.E.; Mizier-Barre, K.A.; Stelmach, E.; Hobson, J.; Griscti, O.; Rudiuk, A.; Muthuthevar, D. Flaxseed lignan complex administration in older human type 2 diabetics manages central obesity and prothrombosis-an invitation to further investigation into polypharmacy reduction. J. Nutr. Metab. 2012. 585170. [Google Scholar]
- Hano, C.; Renouard, S.; Molinié, R.; Corbin, C.; Barakzoy, E.; Doussot, J.; Lamblin, F.; Lainé, E. Flaxseed (Linum usitatissimum L.) extract as well as (+)-secoisolariciresinol diglucoside and its mammalian derivatives are potent inhibitors of α-amylase activity. Bioorg. Med. Chem. Lett. 2013, 23, 3007–3012. [Google Scholar] [CrossRef] [PubMed]
- Sirato-Yasumoto, S.; Katsuta, M.; Okuyama, Y.; Takahashi, Y.; Ide, T. Effect of sesame seeds rich in sesamin and sesamolin on fatty acid oxidation in rat liver. J. Agric. Food Chem. 2001, 49, 2647–2651. [Google Scholar] [CrossRef] [PubMed]
- Nakano, D.; Itoh, C.; Takaoka, M.; Kiso, Y.; Tanaka, T.; Matsumura, Y. Antihypertensive effect of sesamin. IV. Inhibition of vascular superoxide production by sesamin. Biol. Pharm. Bull. 2002, 25, 1247–1249. [Google Scholar] [CrossRef] [PubMed]
- Nakai, M.; Harada, M.; Nakahara, K.; Akimoto, K.; Shibata, H.; Miki, W.; Kiso, Y. Novel antioxidative metabolites in rat liver with ingested sesamin. J. Agric. Food. Chem. 2003, 51, 1666–1670. [Google Scholar] [CrossRef] [PubMed]
- Akimoto, K.; Kitagawa, Y.; Akamatsu, T.; Hirose, N.; Sugano, M.; Shimizu, S.; Yamada, H. Protective effects of sesamin against liver damage caused by alcohol or carbon tetrachloride in rodents. Ann. Nutr. Metab. 1993, 37, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Tada, M.; Ono, Y.; Nakai, M.; Harada, M.; Shibata, H.; Kiso, Y.; Ogata, T. Evaluation of antioxidative effects of sesamin on the in vivo hepatic reducing abilities by a radiofrequency ESR method. Anal. Sci. 2013, 29, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-M.; Zheng, G.-H.; Ming, Q.-L.; Cheng, C.; Sun, J.M. Sesamin protects mouse liver against nickel-induced oxidative DNA damage and apoptosis by the PI3K/Akt pathway. J. Agri. Food Chem. 2013, 61, 1146–1154. [Google Scholar] [CrossRef]
- Saarinen, N.M.; Wärri, A.; Airio, M.; Smeds, A.; Mäkelä, S. Role of dietary lignans in the reduction of breast cancer risk. Mol. Nutr. Food Res. 2007, 51, 857–866. [Google Scholar] [CrossRef] [PubMed]
- Velentzis, L.S.; Cantwell, M.M.; Cardwell, C.; Keshtgar, M.R.; Leathem, A.J.; Woodside, J.V. Lignans and breast cancer risk in pre- and post-menopausal women: Meta-analyses of observational studies. Br. J. Cancer 2009, 100, 1492–1498. [Google Scholar] [CrossRef] [PubMed]
- Velentzis, L.S.; Keshtgar, M.R.; Woodside, J.V.; Leathem, A.J.; Titcomb, A.; Perkins, K.A.; Mazurowska, M.; Anderson, V.; Wardell, K.; Cantwell, M.M. Significant changes in dietary intake and supplement use after breast cancer diagnosis in a UK multicentre study. Breast Cancer Res. Treat. 2011, 128, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Buck, K.; Zaineddin, A.K.; Vrieling, A.; Linseisen, J.; Chang-Claude, J. Meta-analyses of lignans and enterolignans in relation to breast cancer risk. Am. J. Clin. Nutr. 2010, 92, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Buck, K.; Zaineddin, A.K.; Vrieling, A.; Heinz, J.; Linseisen, J.; Flesch-Janys, D.; Chang-Claude, J. Estimated enterolignans, lignan-rich foods, and fibre in relation to survival after postmenopausal breast cancer. Br. J. Cancer 2011, 105, 1151–1157. [Google Scholar] [CrossRef]
- Zaineddin, A.K.; Buck, K.; Vrieling, A.; Heinz, J.; Flesch-Janys, D.; Linseisen, J.; Chang-Claude, J. The association between dietary lignans, phytoestrogen-rich foods, and fiber intake and postmenopausal breast cancer risk: A German case-control study. Nutr. Cancer 2012, 64, 652–665. [Google Scholar] [CrossRef] [PubMed]
- Buck, K.; Vrieling, A.; Zaineddin, A.K.; Becker, S.; Hüsing, A.; Kaaks, R.; Linseisen, J.; Flesch-Janys, D.; Chang-Claude, J. Serum enterolactone and prognosis of postmenopausal breast cancer. J. Clin. Oncol. 2011, 29, 3730–3738. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.M.; Saggar, J.K.; Corey, P.; Thompson, L.U. Flaxseed and pure secoisolariciresinol diglucoside, but not flaxseed hull, reduce human breast tumor growth (MCF-7) in athymic mice. J. Nutr. 2009, 139, 2061–2066. [Google Scholar] [CrossRef] [PubMed]
- Truan, J.S.; Chen, J.M.; Thompson, L.U. Comparative effects of sesame seed lignan and flaxseed lignan in reducing the growth of human breast tumors (MCF-7) at high levels of circulating estrogen in athymic mice. Nutr. Cancer. 2012, 64, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Yousefzadi, M.; Sharifi, M.; Behmanesh, M.; Moyano, E.; Bonfill, M.; Cusido, R.M.; Palazon, J. Podophyllotoxin: Current approaches to its biotechnological production and future challenges. Eng. Life Sci. 2010, 10, 281–292. [Google Scholar] [CrossRef]
- Davin, L.B.; Lewis, N.G. A historical perspective on lignan biosynthesis: Monolignol, allylphenol and hydroxycinnamic acid coupling and downstream metabolism. Phytochem. Rev. 2003, 2, 257–288. [Google Scholar] [CrossRef]
- Dinkova-Kostova, A.T.; Gang, D.R.; Davin, L.B.; Bedgar, D.L.; Chu, A.; Lewis, N.G. (+)-pinoresinol/(+)-lariciresinol reductase from Forsythia intermedia. J. Biol. Chem. 1996, 271, 29473–29482. [Google Scholar] [CrossRef] [PubMed]
- Gang, D.R.; Kasahara, H.; Xia, Z.-Q.; Vander-Mijnsbrugge, K.; Bauw, G.; Boerjan, W.; van Montagu, M.; Davin, L.B.; Lewis, N.G. Evolution of plant defense mechanisms. Relationships of phenylcoumaran benzylic ether reductases to pinoresinol-lariciresinol and isoflavone reductases. J. Biol. Chem. 1999, 274, 7516–7527. [Google Scholar] [CrossRef] [PubMed]
- Hemmati, S.; Schmidt, T.J.; Fuss, E. (+)-pinoresinol/(-)-lariciresinol reductase from Linum perenne Himmelszelt involved in the biosynthesis of justicidin B. FEBS Lett. 2007, 581, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Bayindir, Ü.; Alfermann, A.W.; Fuss, E. Hinokinin Biosynthesis in Linum corymbulosum Reichenb. Plant J. 2008, 55, 810–820. [Google Scholar] [CrossRef] [PubMed]
- Nakatsubo, T.; Mizutani, M.; Suzuki, S.; Hattori, T.; Umezawa, T. Characterization of Arabidopsis thaliana pinoresinol reductase, a new type of enzyme involved in lignan biosynthesis. J. Biol. Chem. 2008, 283, 15550–15557. [Google Scholar] [CrossRef] [PubMed]
- Wankhede, D.P.; Biswas, D.K.; Rajkumar, S.; Sinha, A.K. Expressed sequence tags and molecular cloning and characterization of gene encoding pinoresinol/lariciresinol reductase from Podophyllum hexandrum. Protoplasma 2013, 250, 1239–1249. [Google Scholar] [CrossRef] [PubMed]
- Ono, E.; Kim, H.-J.; Murata, J.; Morimoto, K.; Okazawa, A.; Kobayashi, A.; Umezawa, T.; Satake, H. Molecular and functional characterization of novel furofuran-class lignan glucosyltransferases from Forsythia. Plant Biotechnol. 2010, 27, 317–324. [Google Scholar] [CrossRef]
- Kim, H.-J.; Ono, E.; Morimoto, K.; Yamagaki, T.; Okazawa, A.; Kobayashi, A.; Satake, H. Metabolic engineering of lignan biosynthesis in Forsythia cell culture. Plant Cell Physiol. 2009, 50, 2200–2209. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, K.; Satake, H. Seasonal alteration in amounts of lignans and their glucosides and gene expression of the relevant biosynthetic enzymes in the Forsythia suspense leaf. Biol. Pharm. Bull. 2013, 36, 1519–1523. [Google Scholar] [CrossRef]
- Okazawa, A.; Kusunose, T.; Ono, E.; Kim, H.-J.; Satake, H.; Shimizu, B.; Mizutani, M.; Seki, H.; Muranaka, T. Glucosyltransferase activity of Arabidopsis UGT71C1 towards pinoresinol and lariciresinol. Plant Biotechnol. 2014. [CrossRef]
- Xia, Z-Q.; Costa, M. A.; Pélissier, H.C.; Davin, L.B.; Lewis, N.G. Secoisolariciresinol dehydrogenase purification, cloning, and functional expression. J. Biol. Chem. 2001, 276, 12614–12623. [Google Scholar] [CrossRef] [PubMed]
- Ghose, K.; Selvaraj, K.; McCallum, J.; Kirby, C.W.; Sweeney-Nixon, M.; Cloutier, S.J.; Deyholos, M.; Datla, R.; Fofana, B. Identification and functional characterization of a flax UDP-glycosyltransferase glucosylating secoisolariciresinol (SECO) into secoisolariciresinol monoglucoside (SMG) and diglucoside (SDG). BMC Plant. Biol. 2014. [CrossRef]
- Umezawa, T.; Ragamustari, S.K.; Nakatsubo, T.; Wada, S.; Li, L.; Yamamura, M.; Sakakibara, N.; Hattori, T.; Suzuki, S.; Chiang, V.L. A lignan O-methyltransferase catalyzing the regioselective methylation of matairesinol in Carthamus tinctorius. Plant Biotechnol. 2013, 30, 97–109. [Google Scholar] [CrossRef]
- Ragamustari, S.K.; Yamamura, M.; Ono, E.; Hattori, T.; Suzuki, S.; Suzuki, H.; Shibata, D.; Umezawa, T. Substrate-enantiomer selectivity of matairesinol O-methyltransferases. Plant Biotechnol. 2014, 31, 257–267. [Google Scholar] [CrossRef]
- Ragamustari, S.K.; Nakatsubo, T.; Hattori, T.; Ono, E.; Kitamura, Y.; Suzuki, S.; Yamamura, M.; Umezawa, T. A novel O-methyltransferase involved in the first methylation step of yatein biosynthesis from matairesinol in Anthriscus sylvestris. Plant Biotechnol. 2013, 30, 375–384. [Google Scholar] [CrossRef]
- Marques, J.V.; Kim, K.-W.; Lee, C.; Costa, M.A.; May, G.D.; Crow, J.A.; Davin, L.B.; Lewis, N.G. Next generation sequencing in predicting gene function in podophyllotoxin biosynthesis. J. Biol. Chem. 2013, 288, 466–479. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yu, S.; Tong, C.; Zhao, Y.; Liu, Y.; Song, C.; Zhang, Y.; Zhang, X.; Wang, Y.; Hua, W.; et al. Genome sequencing of the high oil crop sesame provides insight into oil biosynthesis. Genome Biol. 2014. [Google Scholar] [CrossRef]
- Wang, L.; Han, X.; Zhang, Y.; Li, D.; Wei, X.; Ding, X.; Zhang, X. Deep resequencing reveals allelic variation in Sesamum indicum. BMC Plant Biol. 2014. [Google Scholar] [CrossRef]
- Wu, K.; Yang, M.; Liu, H.; Tao, Y.; Mei, J.; Zhao, Y. Genetic analysis and molecular characterization of Chinese sesame (Sesamum indicum L.) cultivars using insertion-deletion (InDel) and simple sequence repeat (SSR) markers. BMC Genet. 2014. [Google Scholar] [CrossRef]
- Ono, E.; Nakai, M.; Fukui, Y.; Tomimori, N.; Fukuchi-Mizutani, M.; Saito, M.; Satake, H.; Tanaka, T.; Katsuta, M.; Umezawa, T.; Tanaka, Y. Formation of two methylenedioxy bridges by a Sesamum CYP81Q protein yielding a furofuran lignan, (+)-sesamin. Proc. Natl. Acad. Sci. USA 2006, 103, 10116–10121. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, A.; Fukui, Y.; Iuchi-Okada, A.; Kakutani, S.; Satake, H.; Iwashita, T.; Nakao, M.; Umezawa, T.; Ono, E. Sequential glucosylation of a furofuran lignan, (+)-sesaminol, by Sesamum indicum UGT71A9 and UGT94D1. Plant J. 2008, 54, 415–427. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Hobson, N.; Galindo, L.; Zhu, S.; Shi, D.; McDill, J.; Yang, L.; Hawkins, S.; Neutelings, G.; Datla, R.; et al. The genome of flax (Linum usitatissimum) assembled de novo from short shotgun sequence reads. Plant J. 2012, 72, 461–473. [Google Scholar] [CrossRef] [PubMed]
- Barvkar, V.T.; Pardeshi, V.C.; Kale, S.M.; Kadoo, N.Y.; Gupta, V.S. Phylogenomic analysis of UDP glycosyltransferase 1 multigene family in Linum usitatissimum identified genes with varied expression patterns. BMC Genomics 2012. [Google Scholar] [CrossRef]
- Babu, P.R.; Rao, K.V.; Reddy, V.D. Structural organization and classification of cytochrome P450 genes in flax (Linum usitatissimum L.). Gene 2013, 513, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, D.; Sinha, R.; Hazra, S.; Datta, R.; Chattopadhyay, S. De novo transcriptome analysis using 454 pyrosequencing of the Himalayan Mayapple, Podophyllum hexandrum. BMC Genomics 2013. [Google Scholar] [CrossRef]
- Marques, J.V.; Dalisay, D.S.; Yang, H.; Lee, C.; Davin, L.B.; Lewis, N.G. A multi-omics strategy resolves the elusive nature of alkaloids in Podophyllum species. Mol. Biosyst. 2014, 10, 2838–2849. [Google Scholar] [CrossRef] [PubMed]
- Eyberger, A.L.; Dondapati, R.; Porter, J.R. Endophyte fungal isolates from Podophyllum peltatum produce podophyllotoxin. J. Nat. Prod. 2006, 69, 1121–1124. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, J.; Petersen, M. Pinoresinol and matairesinol accumulation in a Forsythia × intermedia cell suspension culture. Plant Cell Tiss. Org. 2002, 68, 91–98. [Google Scholar] [CrossRef]
- Renouard, S.; Tribalatc, M.-A.; Lamblin, F.; Mongelard, G.; Fliniaux, O.; Corbin, C.; Marosevic, D.; Pilard, S.; Demailly, H.; Gutierrez, L.; et al. RNAi-mediated pinoresinol lariciresinol reductase gene silencing in flax (Linum usitatissimum L.) seed coat: Consequences on lignans and neolignans accumulation. J. Plant Physiol. 2014, 171, 1372–1377. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, K.; Ono, E.; Kim, H.-J.; Okazawa, A.; Kobayashi, A.; Satake, H. The construction of transgenic Forsythia plants: Comparative study of three Forsythia species. Plant Biotechnol. 2011, 28, 273–280. [Google Scholar] [CrossRef]
- Rosati, C.; Cadic, A.; Renou, J.-P.; Duron, M. Regeneration and Agrobacterium-mediated transformation of Forsythia x intermedia “Spring Glowly”. Plant Cell Rep. 1996, 16, 114–117. [Google Scholar] [PubMed]
- Rosati, C.; Simoneau, P.; Treutter, D.; Poupard, P.; Cadot, Y.; Cadic, A.; Duron, M. Engineering of flower color in forsythia by expression of two independently-transformed dihydroflavonol 4-reductase and anthocyanidin synthase genes of flavonoid pathway. Mol. Breeding 2003, 12, 197–208. [Google Scholar] [CrossRef]
- Chen, Q.; Lai, H.; Hurtado, J.; Stahnke, J.; Leuzinger, K.; Dent, M. Agroinfiltration as an Effective and scalable strategy of gene delivery for production of pharmaceutical proteins. Adv. Tech. Biol. Med. 2013. [Google Scholar] [CrossRef]
- Jackson, J.A.; Fuglevand, G.; Brown, B.A.; Shaw, M.J.; Jenkins, G.I. Isolation of Arabidopsis mutants altered in the light-regulation of chalcone synthase gene expression using a transgenic screening approach. Plant J. 1995, 8, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Bharti, A.K.; Khurana, J.P. Mutants of Arabidopsis as tools to understand the regulation of phenylpropanoid pathway and UVB protection mechanisms. Photochem. Photobiol. 1997, 65, 765–776. [Google Scholar] [CrossRef] [PubMed]
- Von Lintig, J.; Welsch, R.; Bonk, M.; Giuliano, G.; Batschauer, A.; Kleinig, H. Light-dependent regulation of carotenoid biosynthesis occurs at the level of phytoene synthase expression and is mediated by phytochrome in Sinapis alba and Arabidopsis thaliana seedlings. Plant J. 1997, 12, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Yazaki, K.; Matsuoka, H.; Ujihara, T.; Sato, F. Shikonin biosynthesis in Lithospermum erythrorhizon: Light-induced negative regulation of secondary metabolism. Plant Biotechnol. 1999, 16, 335–342. [Google Scholar] [CrossRef]
- Morimoto, K.; Kim, H.-J.; Ono, E.; Kobayashi, A.; Okazawa, A.; Satake, H. Effects of light on production of endogenous and exogenous lignans by Forsythia koreana wildtype and transgenic cells. Plant Biotechnol. 2011, 28, 331–337. [Google Scholar] [CrossRef]
- Yousefzadi, M.; Sharifi, M.; Behmanesh, M.; Ghasempour, A.; Moyano, E.; Palazon, J. The effect of light on gene expression and podophyllotoxin biosynthesis in Linum album cell culture. Plant Physiol. Biochem. 2012, 56, 41–56. [Google Scholar] [CrossRef] [PubMed]
- Hata, N.; Hayashi, Y.; Okazawa, A.; Ono, E.; Satake, H.; Kobayashi, A. Effect of photoperiod on growth of the plants, and sesamin content and CYP81Q1 gene expression in the leaves of sesame (Sesamum indicum L.). Environ. Exp. Bot. 2012, 75, 212–219. [Google Scholar] [CrossRef]
- Zhao, J.; Davis, L.C.; Verpoorte, R. Elicitor signal transduction leading to production of plant secondary metabolites. Biotechnol. Adv. 2005, 23, 283–333. [Google Scholar] [CrossRef]
- Berim, A.; Spring, O.; Conrad, J.; Maitrejean, M.; Boland, W.; Petersen, M. Enhancement of lignan biosynthesis in suspension cultures of Linum nodiflorum by coronalon, indanoyl-isoleucine and methyl jasmonate. Planta 2005, 222, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Van Fürden, B.; Humburg, A.; Fuss, E. Influence of methyl jasmonate on podophyllotoxin and 6-methoxypodophyllotoxin accumulation in Linum album cell suspension cultures. Plant Cell Rep. 2005, 24, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Yousefzadi, M.; Sharifi, M.; Behmanesh, M.; Ghasempour, A.; Moyano, E.; Palazon, J. Salicylic acid improves podophyllotoxin production in cell cultures of Linum album by increasing the expression of genes related with its biosynthesis. Biotechnol. Lett. 2010, 32, 1739–1743. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, D.; Sinha, R.; Ghanta, S.; Chakraborty, A.; Hazra, S.; Chattopadhyay, S. Proteins differentially expressed in elicited cell suspension culture of Podophyllum hexandrum with enhanced podophyllotoxin content. Proteome Sci. 2012. [Google Scholar] [CrossRef]
- Vardapetyan, H.R.; Kirakosyan, A.B.; Oganesyan, A.A.; Penesyan, A.R.; Alfermann, W.A. Effect of various elicitors on lignan biosynthesis in callus cultures of Linum austriacum. Russ. J. Plant Physl. 2003, 50, 297–300. [Google Scholar] [CrossRef]
- Ionkova, I. Effect of methyl jasmonate on production of ariltetralin lignans in hairy root cultures of Linum tauricum. Pharmacogn. Res. 2009, 1, 102–105. [Google Scholar]
- Schmitt, J.; Petersen, M. Influence of methyl jasmonate and coniferyl alcohol on pinoresinol and matairesinol accumulation in a Forsythia × intermedia suspension culture. Plant Cell Rep. 2002, 20, 885–890. [Google Scholar] [CrossRef]
- Muranaka, T.; Miyata, M.; Ito, K.; Tachibana, S. Production of podophyllotoxin in Juniperus chinensis callus cultures treated with oligosaccharides and a biogenetic precursor. Phytochemistry 1998, 49, 491–496. [Google Scholar] [CrossRef]
- Bahabadi, S.E.; Sharifi, M.; Safaie, N.; Murata, J.; Yamagaki, T.; Satake, H. Increased lignan biosynthesis in the suspension cultures of Linum album by fungal extracts. Plant Biotechnol. Rep. 2011, 5, 367–373. [Google Scholar] [CrossRef]
- Bahabadi, S.E.; Sharifi, M.; Chashmi, N.A.; Murata, J.; Satake, H. Significant enhancement of lignans accumulation in hairy root cultures of Linum album using biotic elicitors. Acta. Physiol. Plant 2014, 36, 3325–3331. [Google Scholar] [CrossRef]
- Bahabadi, S.E.; Sharifi, M.; Murata, J.; Satake, H. The effect of chitosan and chitin oligomers on gene expression and lignan production in Linum album cell cultures. J. Med. Plant 2014, 13, 46–53. [Google Scholar]
- Hano, C.; Addi, M.; Bensaddek, L.; Crônier, D.; Baltora-Rosset, S.; Doussot, J.; Maury, S.; Mesnard, B.; Chabbert, B.; Hawkins, S.; Lainé, E.; Lamblin, F. Differential accumulation of monolignol-drived compounds in elicited flax (Linum usitatissimum) cell suspension cultures. Planta 2006, 223, 975–989. [Google Scholar] [CrossRef] [PubMed]
- Bahabadi, S.E.; Sharifi, M.; Behmanesh, M.; Safaie, N.; Murata, J.; Araki, R.; Yamagaki, T.; Satake, H. Time-course changes in fungal elicitor-induced lignan synthesis and expression of the relevant genes in cell cultures of Linum album. J. Plant Physiol. 2012, 169, 487–491. [Google Scholar] [CrossRef]
- Tahsili, J.; Sharifi, M.; Safaie, N.; Bahabadi, S.E.; Behmanesh, M. Induction of lignans and phenolic compounds in cell culture of Linum album by culture filtrate of Fusarium graminearum. J. Plant Interact. 2014, 9, 412–417. [Google Scholar] [CrossRef]
- Chun, C.; Kozai, T. A closed transplant production system, a hybrid of scaled-up micropropagation system and plant factory. J Plant Biotechnol 2001, 3, 59–66. [Google Scholar]
- Hirai, T.; Fukukawa, G.; Kakuta, H.; Fukuda, N.; Ezura, H. Production of recombinant miraculin using transgenic tomatoes in a closed cultivation system. J. Agric. Food Chem. 2010, 58, 6096–6101. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Yoshida, R.; Kikuzaki, A.; Hirai, T.; Kuroda, H.; Hiwasa-Tanase, K.; Takane, K.; Ezura, H.; Mizoguchi, T. Molecular breeding of tomato lines for mass production of miraculin in a plant factory. J. Agric. Food Chem. 2010, 58, 9505–9510. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Satake, H.; Koyama, T.; Bahabadi, S.E.; Matsumoto, E.; Ono, E.; Murata, J. Essences in Metabolic Engineering of Lignan Biosynthesis. Metabolites 2015, 5, 270-290. https://doi.org/10.3390/metabo5020270
Satake H, Koyama T, Bahabadi SE, Matsumoto E, Ono E, Murata J. Essences in Metabolic Engineering of Lignan Biosynthesis. Metabolites. 2015; 5(2):270-290. https://doi.org/10.3390/metabo5020270
Chicago/Turabian StyleSatake, Honoo, Tomotsugu Koyama, Sedigheh Esmaeilzadeh Bahabadi, Erika Matsumoto, Eiichiro Ono, and Jun Murata. 2015. "Essences in Metabolic Engineering of Lignan Biosynthesis" Metabolites 5, no. 2: 270-290. https://doi.org/10.3390/metabo5020270
APA StyleSatake, H., Koyama, T., Bahabadi, S. E., Matsumoto, E., Ono, E., & Murata, J. (2015). Essences in Metabolic Engineering of Lignan Biosynthesis. Metabolites, 5(2), 270-290. https://doi.org/10.3390/metabo5020270







