Methods for Untargeted Analysis of Milk Metabolites: Influence of Extraction Method and Optimization of Separation
Abstract
1. Introduction
2. Materials and Methods
2.1. LC Column Selection
2.2. Collection of Milk Samples
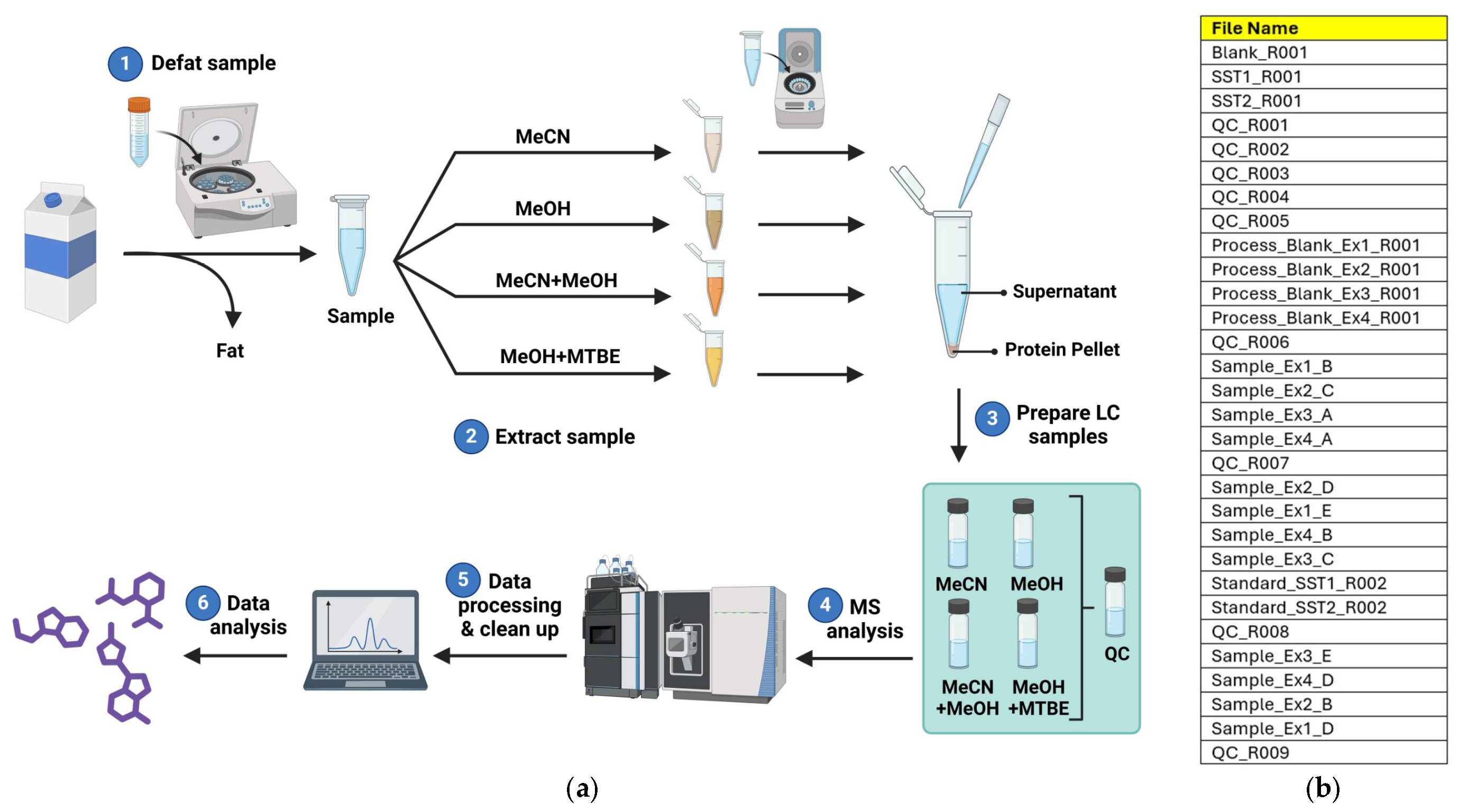
2.3. Extraction of Metabolites
2.4. System Suitability and Quality Controls
2.4.1. Data Quality
2.4.2. Quality Controls
2.5. Untargeted Metabolomics Analysis
2.6. Mass Spectrometry Method
2.7. Data Processing
2.8. Data Statistics and Visualization
3. Results
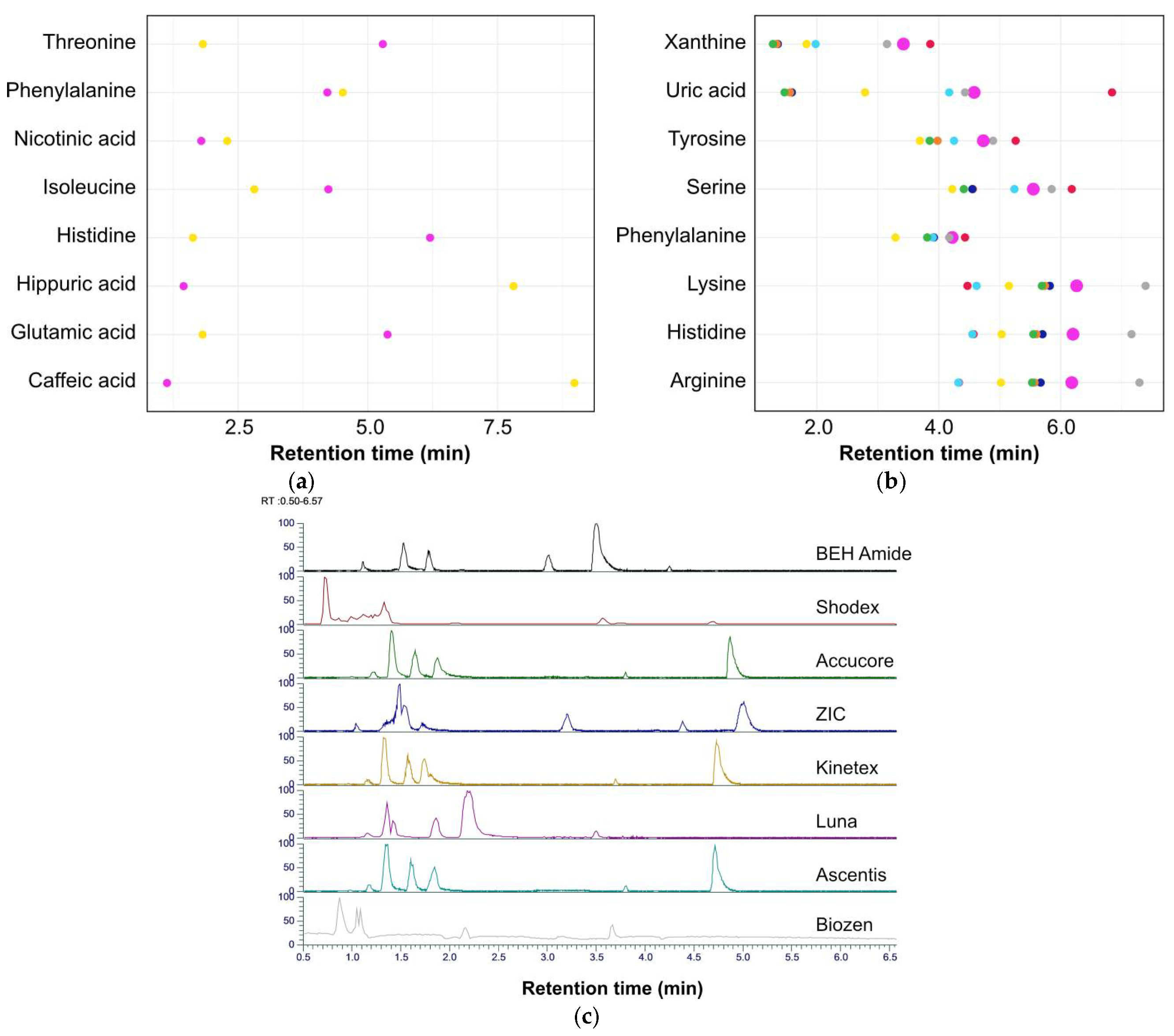
3.1. Column Selection
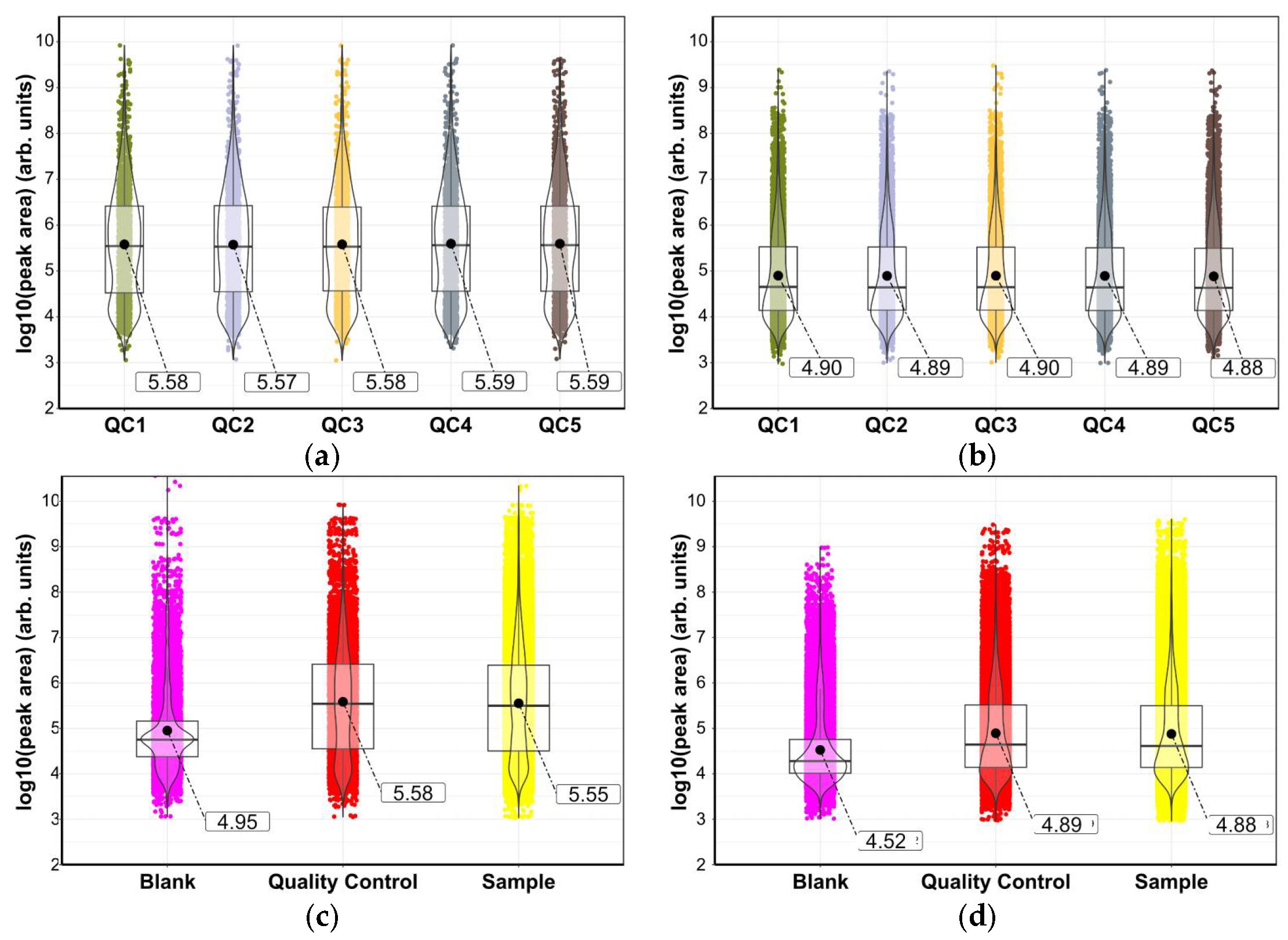
3.2. Quality Control
3.3. Data Processing and Clean-Up
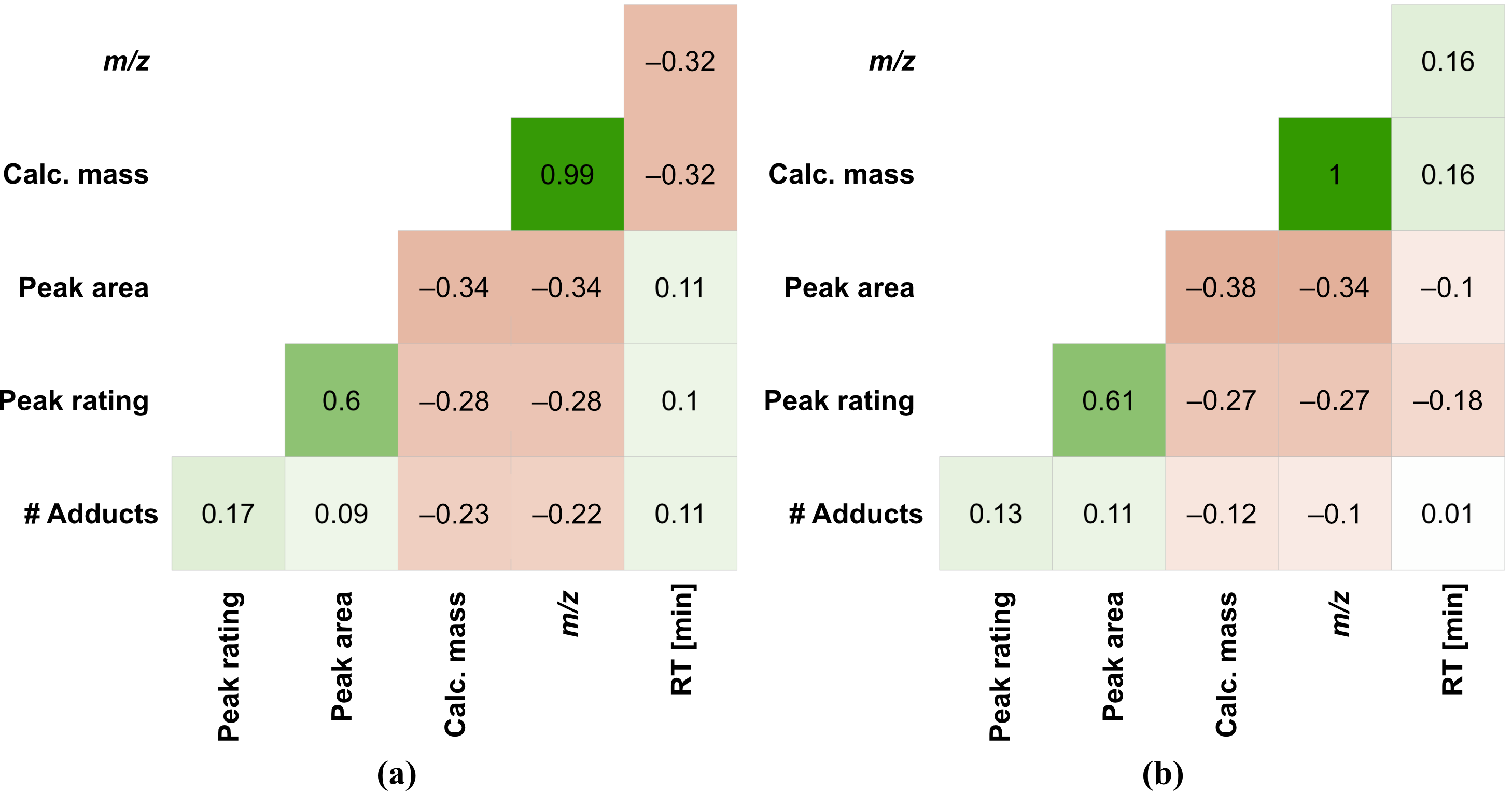
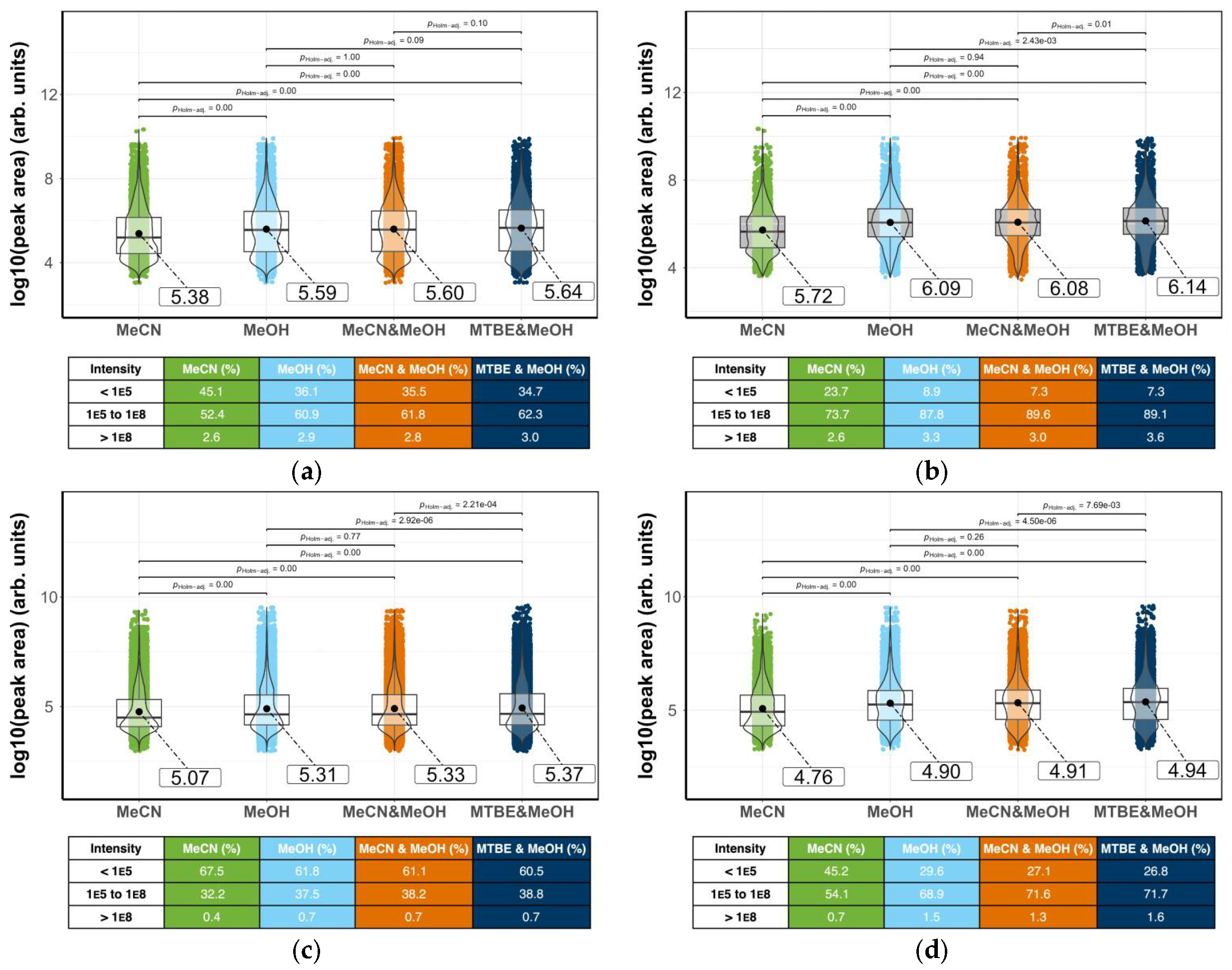
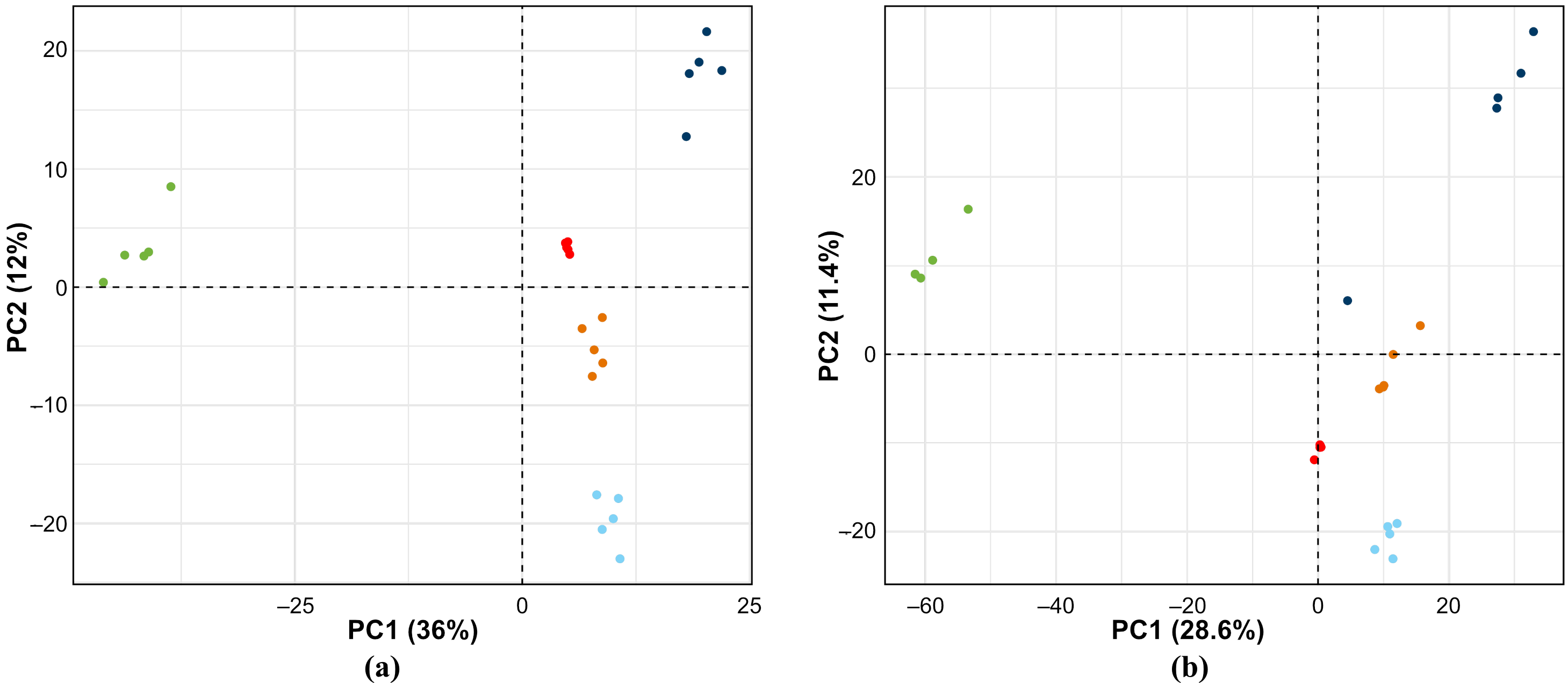
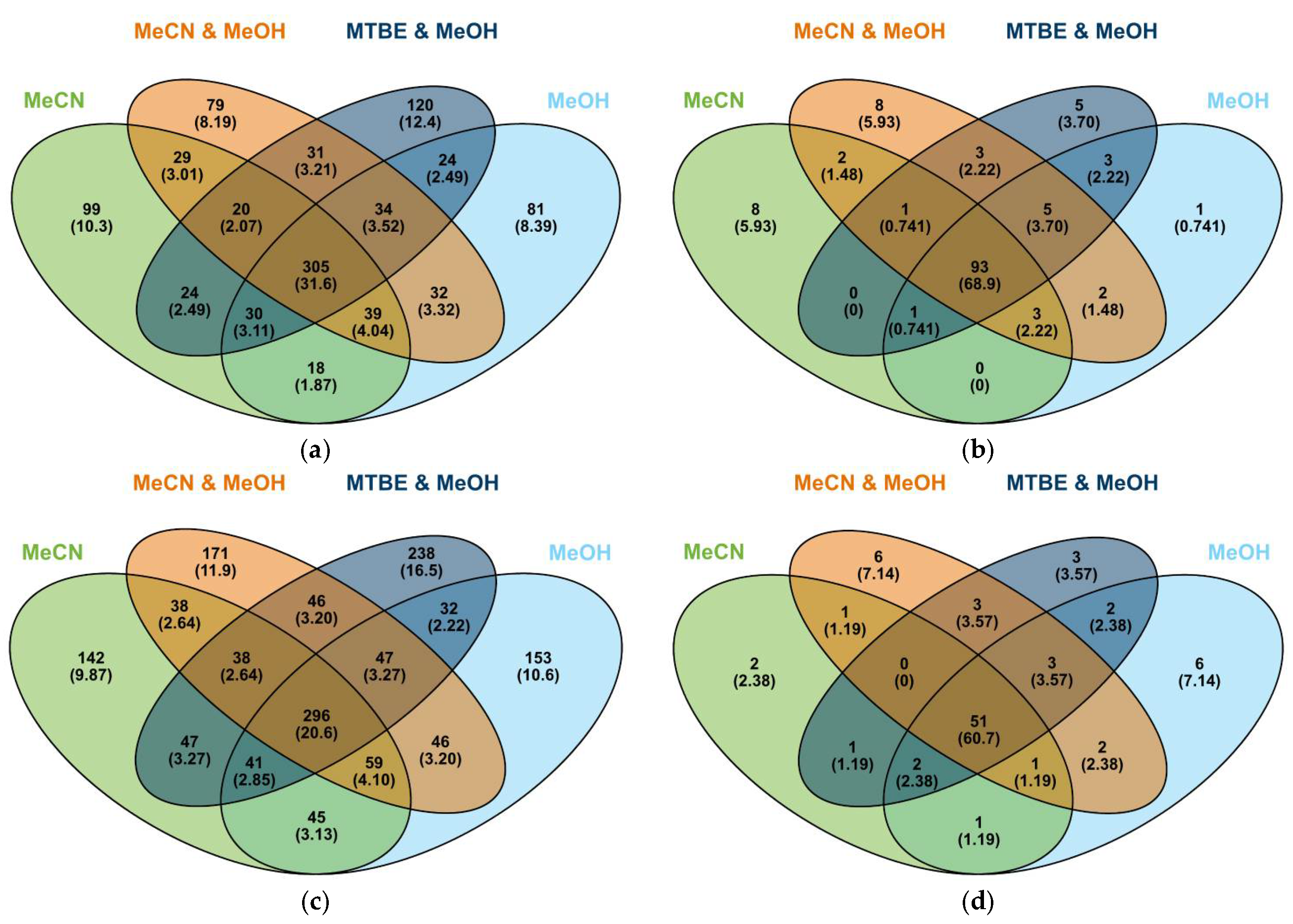
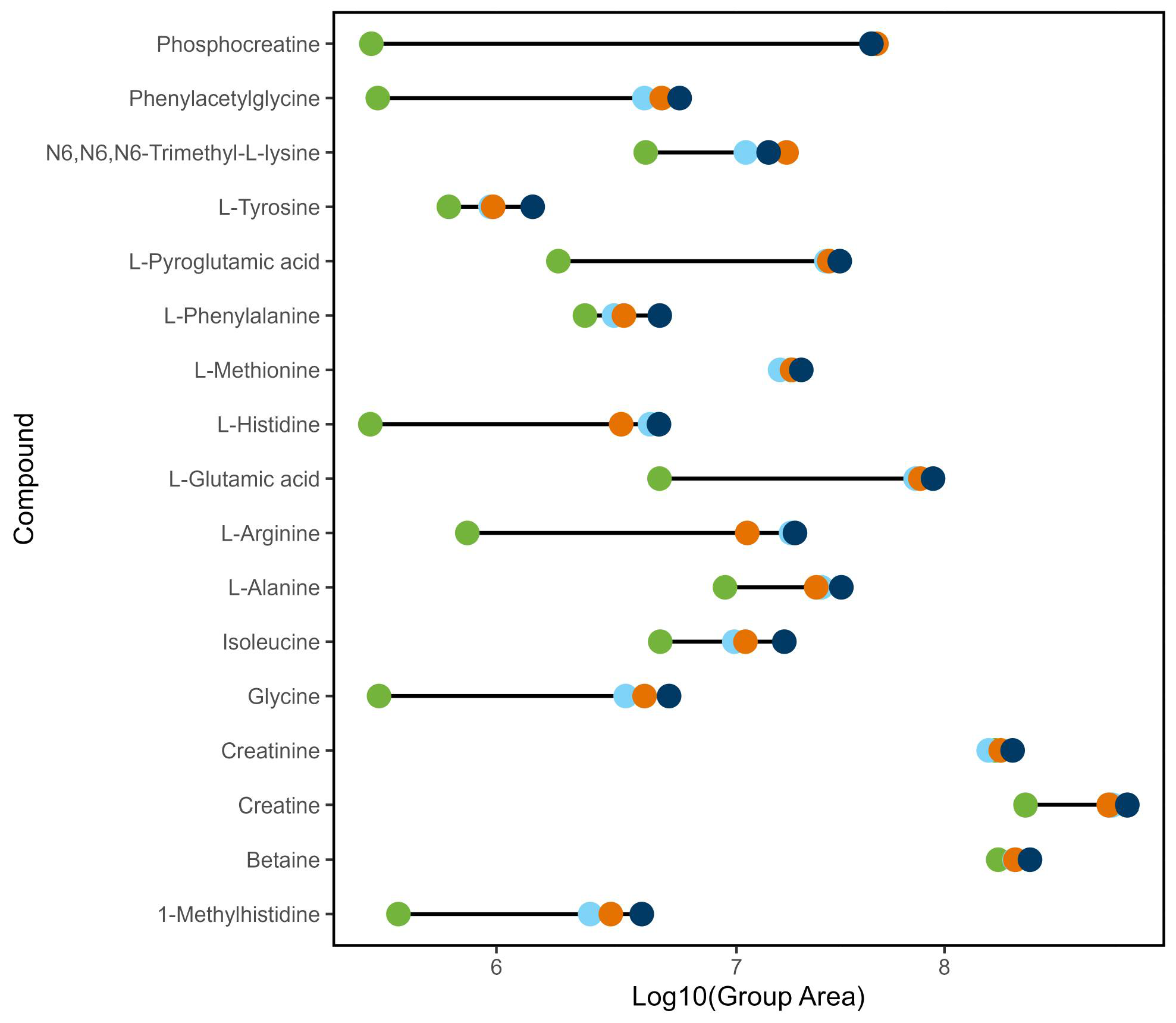
3.4. Statistical Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Foroutan, A.; Guo, A.C.; Vazquez-Fresno, R.; Lipfert, M.; Zhang, L.; Zheng, J.; Badran, H.; Budinski, Z.; Mandal, R.; Ametaj, B.N.; et al. Chemical Composition of Commercial Cow’s Milk. J. Agric. Food Chem. 2019, 67, 4897–4914. [Google Scholar] [CrossRef]
- Ametaj, B.N. Introducing Dairy: A Transdisciplinary Journal to Advance Understanding of Dairy Nutrition, Health and Productivity, Welfare and Well-Being as Well as Milk Synthesis-Composition and Health Effects of Its Products. Dairy 2020, 1, 1–5. [Google Scholar] [CrossRef]
- Visioli, F.; Strata, A. Milk, Dairy Products, and Their Functional Effects in Humans: A Narrative Review of Recent Evidence. Adv. Nutr. 2014, 5, 131–143. [Google Scholar] [CrossRef]
- Alothman, M.; Hogan, S.A.; Hennessy, D.; Dillon, P.; Kilcawley, K.N.; O’Donovan, M.; Tobin, J.; Fenelon, M.A.; O’Callaghan, T.F. The “Grass-Fed” Milk Story: Understanding the Impact of Pasture Feeding on the Composition and Quality of Bovine Milk. Foods 2019, 8, 350. [Google Scholar] [CrossRef] [PubMed]
- Fiehn, O. Metabolomics—The link between genotypes and phenotypes. Plant Mol. Biol. 2002, 48, 155–171. [Google Scholar] [CrossRef] [PubMed]
- Guijas, C.; Montenegro-Burke, J.R.; Warth, B.; Spilker, M.E.; Siuzdak, G. Metabolomics activity screening for identifying metabolites that modulate phenotype. Nat. Biotechnol. 2018, 36, 316–320. [Google Scholar] [CrossRef]
- Johnson, C.H.; Ivanisevic, J.; Siuzdak, G. Metabolomics: Beyond biomarkers and towards mechanisms. Nat. Rev. Mol. Cell Biol. 2016, 17, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Yu, Z.; Zhu, D.; Meng, X.; Pang, X.; Liu, Y.; Frew, R.; Chen, H.; Chen, G. The application of NMR-based milk metabolite analysis in milk authenticity identification. J. Sci. Food Agric. 2017, 97, 2875–2882. [Google Scholar] [CrossRef]
- Loudiyi, M.; Temiz, H.T.; Sahar, A.; Haseeb Ahmad, M.; Boukria, O.; Hassoun, A.; Aït-Kaddour, A. Spectroscopic techniques for monitoring changes in the quality of milk and other dairy products during processing and storage. Crit. Rev. Food Sci. 2022, 62, 3063–3087. [Google Scholar] [CrossRef]
- Evans, A.M.; O’Donovan, C.; Playdon, M.; Beecher, C.; Beger, R.D.; Bowden, J.A.; Broadhurst, D.; Clish, C.B.; Dasari, S.; Dunn, W.B.; et al. Dissemination and analysis of the quality assurance (QA) and quality control (QC) practices of LC–MS based untargeted metabolomics practitioners. Metabolomics 2020, 16, 113. [Google Scholar] [CrossRef]
- Raftery, D. Mass Spectrometry in Metabolomics; Humana Press: Totowa, NJ, USA, 2014. [Google Scholar]
- Schrimpe-Rutledge, A.C.; Codreanu, S.G.; Sherrod, S.D.; McLean, J.A. Untargeted Metabolomics Strategies—Challenges and Emerging Directions. J. Am. Soc. Mass Spectrom. 2016, 27, 1897–1905. [Google Scholar] [CrossRef]
- Imperiale, S.; Morozova, K.; Ferrentino, G.; Scampicchio, M. Analysis of milk with liquid chromatography–mass spectrometry: A review. Eur. Food Res. Technol. 2023, 249, 861–902. [Google Scholar] [CrossRef]
- Yuan, X.; Shi, W.; Jiang, J.; Li, Z.; Fu, P.; Yang, C.; Rehman, S.u.; Pauciullo, A.; Liu, Q.; Shi, D. Comparative metabolomics analysis of milk components between Italian Mediterranean buffaloes and Chinese Holstein cows based on LC-MS/MS technology. PLoS ONE 2022, 17, e0262878. [Google Scholar] [CrossRef]
- Suh, J.H. Critical review: Metabolomics in dairy science—Evaluation of milk and milk product quality. Food Res. Int. 2022, 154, 110984. [Google Scholar] [CrossRef]
- Rocchetti, G.; Gallo, A.; Nocetti, M.; Lucini, L.; Masoero, F. Milk metabolomics based on ultra-high-performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry to discriminate different cows feeding regimens. Food Res. Int. 2020, 134, 109279. [Google Scholar] [CrossRef] [PubMed]
- Barnes, S.; Benton, H.P.; Casazza, K.; Cooper, S.J.; Cui, X.; Du, X.; Engler, J.; Kabarowski, J.H.; Li, S.; Pathmasiri, W.; et al. Training in metabolomics research. I. Designing the experiment, collecting and extracting samples and generating metabolomics data. J. Mass Spectrom. 2016, 51, 461–475. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Locasale, J.W. Metabolomics: A Primer. Trends Biochem. Sci. 2017, 42, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Naz, S.; Vallejo, M.; García, A.; Barbas, C. Method validation strategies involved in non-targeted metabolomics. J. Chromatogr. A 2014, 1353, 99–105. [Google Scholar] [CrossRef]
- Garwolińska, D.; Namieśnik, J.; Kot-Wasik, A.; Hewelt-Belka, W. State of the art in sample preparation for human breast milk metabolomics—Merits and limitations. TrAC Trends Anal. Chem. 2019, 114, 1–10. [Google Scholar] [CrossRef]
- Li, M.; Kang, S.; Zheng, Y.; Shao, J.; Zhao, H.; An, Y.; Cao, G.; Li, Q.; Yue, X.; Yang, M. Comparative metabolomics analysis of donkey colostrum and mature milk using ultra-high-performance liquid tandem chromatography quadrupole time-of-flight mass spectrometry. J. Dairy Sci. 2020, 103, 992–1001. [Google Scholar] [CrossRef]
- Zhan, X. (Ed.) Metabolomics—Methodology and Applications in Medical Sciences and Life Sciences. IntechOpen: Rijeka, Croatia, 2021. [Google Scholar]
- Canelas, A.B.; ten Pierick, A.; Ras, C.; Seifar, R.M.; van Dam, J.C.; van Gulik, W.M.; Heijnen, J.J. Quantitative Evaluation of Intracellular Metabolite Extraction Techniques for Yeast Metabolomics. Anal. Chem. 2009, 81, 7379–7389. [Google Scholar] [CrossRef]
- Lin, Y.; Caldwell, G.W.; Li, Y.; Lang, W.; Masucci, J. Inter-laboratory reproducibility of an untargeted metabolomics GC–MS assay for analysis of human plasma. Sci. Rep. 2020, 10, 10918. [Google Scholar] [CrossRef]
- Chiavelli, L.U.R.; Godoy, A.C.; Silveira, R.d.; Santos, P.D.S.; Lopes, T.A.M.; Santos, O.O.; Visentainer, J.V. Optimization of Milk Sample Cleanup Using Response Surface Methodology. Food Anal. Methods 2020, 13, 166–175. [Google Scholar] [CrossRef]
- Hu, C.; Duan, Q.; Han, X. Strategies to Improve/Eliminate the Limitations in Shotgun Lipidomics. Proteomics 2020, 20, 1900070. [Google Scholar] [CrossRef] [PubMed]
- Koivusalo, M.; Haimi, P.; Heikinheimo, L.; Kostiainen, R.; Somerharju, P. Quantitative determination of phospholipid compositions by ESI-MS: Effects of acyl chain length, unsaturation, and lipid concentration on instrument response. J. Lipid Res. 2001, 42, 663–672. [Google Scholar] [CrossRef]
- Ashokan, M.; Ramesha, K.P.; Hallur, S.; Karthikkeyan, G.; Rana, E.; Azharuddin, N.; Raj, S.R.; Jeyakumar, S.; Kumaresan, A.; Kataktalware, M.A.; et al. Differences in milk metabolites in Malnad Gidda (Bos indicus) cows reared under pasture-based feeding system. Sci. Rep. 2021, 11, 2831. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Xiao, J.F.; Tuli, L.; Ressom, H.W. LC-MS-based metabolomics. Mol. BioSyst. 2012, 8, 470–481. [Google Scholar] [CrossRef] [PubMed]
- Wernisch, S.; Pennathur, S. Evaluation of coverage, retention patterns, and selectivity of seven liquid chromatographic methods for metabolomics. Anal. Bioanal. Chem. 2016, 408, 6079–6091. [Google Scholar] [CrossRef]
- Manis, C.; Scano, P.; Nudda, A.; Carta, S.; Pulina, G.; Caboni, P. LC-QTOF/MS Untargeted Metabolomics of Sheep Milk under Cocoa Husks Enriched Diet. Dairy 2021, 2, 112–121. [Google Scholar] [CrossRef]
- Nobile, M.; Chiesa, L.M.; Danesi, L.; Fontana, M.; Ghidini, S.; Villa, R.E.; Panseri, S. Metabolomic and volatilome profiling of milk to assess the application of infrared radiation processing. Food Control 2025, 171, 111131. [Google Scholar] [CrossRef]
- Yan, R.; Ji, Z.; Fan, J.; Li, J.; Ren, Y. Evaluation of the Efficacy of a Lactobacilli-Based Teat Detergents for the Microbiota of Cows Teats Using an Untargeted Metabolomics Approach. J. Microbiol. Biotechnol. 2024, 34, 103–115. [Google Scholar] [CrossRef]
- Zhang, Y.D.; Li, P.; Zheng, N.; Jia, Z.W.; Meruva, N.; Ladak, A.; Cleland, G.; Wen, F.; Li, S.L.; Zhao, S.G.; et al. A metabolomics approach to characterize raw, pasteurized, and ultra-high temperature milk using ultra-performance liquid chromatography—Quadrupole time-of-flight mass spectrometry and multivariate data analysis. J. Dairy Sci. 2018, 101, 9630–9636. [Google Scholar] [CrossRef]
- Liu, Z.; Jiang, A.; Lv, X.; Zhou, C.; Tan, Z. Metabolic Changes in Serum and Milk of Holstein Cows in Their First to Fourth Parity Revealed by Biochemical Analysis and Untargeted Metabolomics. Animals 2024, 14, 407. [Google Scholar] [CrossRef]
- Riboni, N.; Piergiovanni, M.; Mattarozzi, M.; Robotti, E.; Stocco, G.; Ablondi, M.; Cipolat-Gotet, C.; Summer, A.; Bianchi, F.; Careri, M. Ultra-high performance liquid chromatography ion mobility-high-resolution mass spectrometry for the assessment of raw milk traceability. Food Chem. 2025, 471, 142796. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, M.C.; Soria, E.; Llama-Palacios, A.; Almirón, F.; Valdés, A.; Cifuentes, A.; Hernández, M.; Ciudad, M.J.; Collado, L. Lactic Microbiota and Metabolites in Raw Cow’s Milk: Implications for Consumer Health. Dairy 2025, 6, 24. [Google Scholar] [CrossRef]
- Ma, S.; Wang, D.; Zhang, M.; Xu, L.; Fu, X.; Zhang, T.; Yan, M.; Huang, X. Untargeted metabonomic analysis reveals the composition and changes of milk metabolites in dual-purpose cattle (Bos taurus) population. J. Agric. Food Res. 2025, 21, 101922. [Google Scholar] [CrossRef]
- Zhuang, J.; Hou, Y.; Wang, Y.; Gao, Y.; Chen, Y.; Qi, J.; Li, P.; Bian, Y.; Ju, N. Relationship between microorganisms and milk metabolites during quality changes in refrigerated raw milk: A metagenomic and metabolomic exploration. Int. J. Food Microbiol. 2024, 425, 110891. [Google Scholar] [CrossRef]
- Kenney, D.H.; Paffenroth, R.C.; Timko, M.T.; Teixeira, A.R. Dimensionally reduced machine learning model for predicting single component octanol–water partition coefficients. J. Cheminform. 2023, 15, 9. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.; Zhang, X.; Su, M.; Jia, M.; Zhu, D.; Kebede, B.; Wu, H.; Chen, G. Establishing an untargeted-to-MRM liquid chromatography–mass spectrometry method for discriminating reconstituted milk from ultra-high temperature milk. Food Chem. 2021, 337, 127946. [Google Scholar] [CrossRef] [PubMed]
- Broadhurst, D.; Goodacre, R.; Reinke, S.N.; Kuligowski, J.; Wilson, I.D.; Lewis, M.R.; Dunn, W.B. Guidelines and considerations for the use of system suitability and quality control samples in mass spectrometry assays applied in untargeted clinical metabolomic studies. Metabolomics 2018, 14, 72. [Google Scholar] [CrossRef]
- Patil, I. Visualizations with statistical details: The ’ggstatsplot’ approach. J. Open Source Softw. 2021, 6, 3167. [Google Scholar] [CrossRef]
- Chen, H.; Boutros, P.C. VennDiagram: A package for the generation of highly-customizable Venn and Euler diagrams in R. BMC Bioinform. 2011, 12, 35. [Google Scholar] [CrossRef]
- Lewis, M.R.; Pearce, J.T.M.; Spagou, K.; Green, M.; Dona, A.C.; Yuen, A.H.Y.; David, M.; Berry, D.J.; Chappell, K.; Horneffer-van der Sluis, V.; et al. Development and Application of Ultra-Performance Liquid Chromatography-TOF MS for Precision Large Scale Urinary Metabolic Phenotyping. Anal. Chem. 2016, 88, 9004–9013. [Google Scholar] [CrossRef]
- Want, E.J.; Wilson, I.D.; Gika, H.; Theodoridis, G.; Plumb, R.S.; Shockcor, J.; Holmes, E.; Nicholson, J.K. Global metabolic profiling procedures for urine using UPLC-MS. Nat. Protoc. 2010, 5, 1005–1018. [Google Scholar] [CrossRef]
- Broeckling, C.D.; Beger, R.D.; Cheng, L.L.; Cumeras, R.; Cuthbertson, D.J.; Dasari, S.; Davis, W.C.; Dunn, W.B.; Evans, A.M.; Fernández-Ochoa, A.; et al. Current Practices in LC-MS Untargeted Metabolomics: A Scoping Review on the Use of Pooled Quality Control Samples. Anal. Chem. 2023, 95, 18645–18654. [Google Scholar] [CrossRef] [PubMed]
- Dudzik, D.; Barbas-Bernardos, C.; García, A.; Barbas, C. Quality assurance procedures for mass spectrometry untargeted metabolomics. a review. J. Pharm. Biomed. Anal. 2018, 147, 149–173. [Google Scholar] [CrossRef]
- Schymanski, E.L.; Jeon, J.; Gulde, R.; Fenner, K.; Ruff, M.; Singer, H.P.; Hollender, J. Identifying Small Molecules via High Resolution Mass Spectrometry: Communicating Confidence. Environ. Sci. Technol. 2014, 48, 2097–2098. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilkie, D.; White, B.; Heidari, G.; Naffa, R.; Peddie, G.; Rowlands, G.J.; Plieger, P.G. Methods for Untargeted Analysis of Milk Metabolites: Influence of Extraction Method and Optimization of Separation. Metabolites 2025, 15, 597. https://doi.org/10.3390/metabo15090597
Wilkie D, White B, Heidari G, Naffa R, Peddie G, Rowlands GJ, Plieger PG. Methods for Untargeted Analysis of Milk Metabolites: Influence of Extraction Method and Optimization of Separation. Metabolites. 2025; 15(9):597. https://doi.org/10.3390/metabo15090597
Chicago/Turabian StyleWilkie, Daisy, Brad White, Golnaz Heidari, Rafea Naffa, Gaile Peddie, Gareth J. Rowlands, and Paul G. Plieger. 2025. "Methods for Untargeted Analysis of Milk Metabolites: Influence of Extraction Method and Optimization of Separation" Metabolites 15, no. 9: 597. https://doi.org/10.3390/metabo15090597
APA StyleWilkie, D., White, B., Heidari, G., Naffa, R., Peddie, G., Rowlands, G. J., & Plieger, P. G. (2025). Methods for Untargeted Analysis of Milk Metabolites: Influence of Extraction Method and Optimization of Separation. Metabolites, 15(9), 597. https://doi.org/10.3390/metabo15090597






