Effects of 12 Weeks of Calanus Oil Supplementation on Cardiac Diastolic Function in Obese and Prediabetic Women—A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Study Participants
2.2. Composition of Supplement
2.3. Anthropometry
2.4. Medication and Physical Activity
2.5. Blood Markers, Blood Pressure, and Aggregated Scores
2.6. Echocardiography
2.7. Statistical Analyses
3. Results
3.1. Baseline Characteristics
3.2. Body Composition, Blood Markers, Blood Pressure, Physical Activity
3.3. Cardiac Function
3.4. Relationships Between Cardiac Function and Other Variables
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Alx | Augmentation index |
| AFI | Automated function imaging |
| BMI | Body mass index |
| DBP | Central diastolic blood pressure |
| BP | Central blood pressure |
| DT | Deceleration time |
| DHA | Docosahexaenoic acid |
| E/A | Ratio of the early (E) to late (A) ventricular filling velocities |
| EPA | Eicosapentaenoic acid |
| ECW | Extracellular water |
| HOMA | The Homeostasis Model Assessment |
| HR | Heart rate |
| HDL-C | High-density lipoprotein cholesterol |
| HPLC | High-pressure liquid chromatography |
| IR | Insulin resistance |
| IVRT | Isovolumic relaxation time |
| LDL-C | Low-density lipoprotein cholesterol |
| LV | Left ventricle |
| LVEF | Left ventricular ejection fraction |
| LVEDV | Left ventricular end-diastolic volume |
| LVESV | Left ventricular end-systolic volume |
| LVSV | Left ventricular stroke volume |
| LS | Longitudinal strain |
| Met-S score | Metabolic syndrome severity score |
| n3 PUFAs | Long-chain omega-3 polyunsaturated fatty acids |
| NO | Nitric oxide |
| O3I | Omega-3 index |
| PWV | Pulse wave velocity |
References
- IDF Diabetes Atlas (11th Ed.). Available online: https://diabetesatlas.org/ (accessed on 23 May 2025).
- Gruss, S.M.; Nhim, K.; Gregg, E.; Bell, M.; Luman, E.; Albright, A. Public Health Approaches to Type 2 Diabetes Prevention: The US National Diabetes Prevention Program and Beyond. Curr. Diabetes Rep. 2019, 9, 78. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Larsson, S.C. An Atlas on Risk Factors for Type 2 Diabetes: A Wide-Angled Mendelian Randomisation Study. Diabetologia 2020, 63, 2359–2371. [Google Scholar] [CrossRef]
- Fritsche, A.; Wagner, R.; Heni, M.; Kantartzis, K.; Machann, J.; Schick, F.; Lehmann, R.; Peter, A.; Dannecker, C.; Fritsche, L.; et al. Different Effects of Lifestyle Intervention in High-and Low-Risk Prediabetes: Results of the Randomized Controlled Prediabetes Lifestyle Intervention Study (PLIS). Diabetes 2021, 70, 2785–2795. [Google Scholar] [CrossRef] [PubMed]
- Payton, L.; Noirot, C.; Last, K.S.; Grigor, J.; Hüppe, L.; Conway, D.V.P.; Dannemeyer, M.; Suin, A.; Meyer, B. Annual Transcriptome of a Key Zooplankton Species, the Copepod Calanus Finmarchicus. Ecol. Evol. 2022, 12, e8605. [Google Scholar] [CrossRef]
- Lee, R.F.; Hagen, W.; Kattner, G. Lipid Storage in Marine Zooplankton. Mar. Ecol. Prog. Ser. 2006, 307, 273–306. [Google Scholar] [CrossRef]
- Vosskötter, F.; Burhop, M.; Hahn, A.; Schuchardt, J.P. Equal Bioavailability of Omega-3 PUFA from Calanus Oil, Fish Oil and Krill Oil: A 12-Week Randomized Parallel Study. Lipids 2023, 58, 129–138. [Google Scholar] [CrossRef]
- Salma, W.; Franekova, V.; Lund, T.; Höper, A.; Ludvigsen, S.; Lund, J.; Aasum, E.; Ytrehus, K.; Belke, D.D.; Larsen, T.S. Dietary Calanus Oil Antagonizes Angiotensin II-Induced Hypertension and Tissue Wasting in Diet-Induced Obese Mice. Prostaglandins Leukot. Essent. Fat. Acids 2016, 108, 13–21. [Google Scholar] [CrossRef]
- Štěpán, M.; Dad’ová, K.; Matouš, M.; Krauzová, E.; Sontáková, L.; Koc, M.; Larsen, T.; Kuda, O.; Štich, V.; Rossmeislová, L.; et al. Exercise Training Combined with Calanus Oil Supplementation Improves the Central Cardiodynamic Function in Older Women. Nutrients 2021, 14, 149. [Google Scholar] [CrossRef]
- Bernasconi, A.A.; Wiest, M.M.; Lavie, C.J.; Milani, R.V.; Laukkanen, J.A. Effect of Omega-3 Dosage on Cardiovascular Outcomes: An Updated Meta-Analysis and Meta-Regression of Interventional Trials. Mayo Clin. Proc. 2021, 96, 304–313. [Google Scholar] [CrossRef]
- Harris, W.S.; Miller, M.; Tighe, A.P.; Davidson, M.H.; Schaefer, E.J. Omega-3 Fatty Acids and Coronary Heart Disease Risk: Clinical and Mechanistic Perspectives. Atherosclerosis 2008, 197, 12–24. [Google Scholar] [CrossRef]
- Marcus, M.D.; Mark, S. Link Omega-3 Fatty Acids and Arrhythmias. Circulation 2024, 150, 488–503. [Google Scholar] [CrossRef]
- Calder, P.C. Marine Omega-3 Fatty Acids and Inflammatory Processes: Effects, Mechanisms and Clinical Relevance. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2015, 1851, 469–484. [Google Scholar] [CrossRef]
- Zehr, K.R.; Walker, M.K. Omega-3 Polyunsaturated Fatty Acids Improve Endothelial Function in Humans at Risk for Atherosclerosis: A Review. Prostaglandins Other Lipid Mediat. 2018, 134, 131–140. [Google Scholar] [CrossRef]
- Miller, P.E.; Van Elswyk, M.; Alexander, D.D. Long-Chain Omega-3 Fatty Acids Eicosapentaenoic Acid and Docosahexaenoic Acid and Blood Pressure: A Meta-Analysis of Randomized Controlled Trials. Am. J. Hypertens. 2014, 27, 885–896. [Google Scholar] [CrossRef]
- Barbarawi, M.; Lakshman, H.; Barbarawi, O.; Alabdouh, A.; Al Kasasbeh, M.; Djousse, L.; Manson, J.A.E. Omega-3 Supplementation and Heart Failure: A Meta-Analysis of 12 Trials Including 81,364 Participants. Contemp. Clin. Trials 2021, 107, 106458. [Google Scholar] [CrossRef] [PubMed]
- Kerlikowsky, F.; Bartsch, M.; Jonas, W.; Hahn, A.; Schuchardt, J.P. Calanus Oil and Lifestyle Interventions Improve Glucose Homeostasis in Obese Subjects with Insulin Resistance. Mar. Drugs 2025, 23, 139. [Google Scholar] [CrossRef] [PubMed]
- Frey, I.; Berg, A.; Grathwohl, D.; Keul, J. Freiburg Questionnaire of Physical Activity--Development, Evaluation and Application. Soz. Praventivmed. 1999, 44, 55–64. [Google Scholar] [CrossRef]
- DeBoer, M.D.; Filipp, S.L.; Gurka, M.J. Use of a Metabolic Syndrome Severity z Score to Track Risk during Treatment of Prediabetes: An Analysis of the Diabetes Prevention Program. Diabetes Care 2018, 41, 2421–2430. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.R.; Hosker, J.R.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis Model Assessment: Insulin Resistance and Fl-Cell Function from Fasting Plasma Glucose and Insulin Concentrations in Man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef]
- Dang, K.; Wang, X.; Hu, J.; Zhang, Y.; Cheng, L.; Qi, X.; Liu, L.; Ming, Z.; Tao, X.; Li, Y. The Association between Triglyceride-Glucose Index and Its Combination with Obesity Indicators and Cardiovascular Disease: NHANES 2003–2018. Cardiovasc. Diabetol. 2024, 23. [Google Scholar] [CrossRef]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F.; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2016, 29, 277–314. [Google Scholar] [CrossRef]
- Aneja, A.; Tang, W.H.W.; Bansilal, S.; Garcia, M.J.; Farkouh, M.E. Diabetic Cardiomyopathy: Insights into Pathogenesis, Diagnostic Challenges, and Therapeutic Options. Am. J. Med. 2008, 121, 748–757. [Google Scholar] [CrossRef] [PubMed]
- Bertero, E.; Maack, C. Metabolic Remodelling in Heart Failure. Nat. Rev. Cardiol. 2018, 15, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Stahrenberg, R.; Edelmann, F.; Mende, M.; Kockskämper, A.; Düngen, H.D.; Scherer, M.; Kochen, M.M.; Binder, L.; Herrmann-Lingen, C.; Gelbrich, G.; et al. Association of Glucose Metabolism with Diastolic Function along the Diabetic Continuum. Diabetologia 2010, 53, 1331–1340. [Google Scholar] [CrossRef] [PubMed]
- Di Pino, A.; Mangiafico, S.; Urbano, F.; Scicali, R.; Scandura, S.; D’Agate, V.; Piro, S.; Tamburino, C.; Purrello, F.; Rabuazzo, A.M. HbA1c Identifies Subjects with Prediabetes and Subclinical Left Ventricular Diastolic Dysfunction. J. Clin. Endocrinol. Metab. 2017, 102, 3756–3764. [Google Scholar] [CrossRef]
- Gui, T.; Li, Y.; Zhang, S.; Zhang, N.; Sun, Y.; Liu, F.; Chen, Q.; Gai, Z. Docosahexaenoic Acid Protects against Palmitate-Induced Mitochondrial Dysfunction in Diabetic Cardiomyopathy. Biomed. Pharmacother. 2020, 128, 110306. [Google Scholar] [CrossRef]
- Eraky, S.M.; Ramadan, N.M. Effects of Omega-3 Fatty Acids and Metformin Combination on Diabetic Cardiomyopathy in Rats through Autophagic Pathway. J. Nutr. Biochem. 2021, 97, 108798. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Gao, M.; Xu, H. Ginger Extract and Omega-3 Fatty Acids Supplementation: A Promising Strategy to Improve Diabetic Cardiomyopathy. Physiol. Res. 2024, 73, 351–367. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Wu, J.H.Y. Omega-3 Fatty Acids and Cardiovascular Disease: Effects on Risk Factors, Molecular Pathways, and Clinical Events. J. Am. Coll. Cardiol. 2011, 58, 2047–2067. [Google Scholar] [CrossRef]
- Wang, J.; Chen, S.; Chen, X.; Qin, C.; Hu, J.; Zeng, X.; Luo, H.; Yang, P.; Luo, H.; Yuan, C.; et al. Higher Triglyceride Glucose-Waist Height Ratio Index Is Associated with Higher Prevalence of Gallstone: A Population-Based Study. Front. Med. 2024, 11, 1481620. [Google Scholar] [CrossRef]
- Lopaschuk, G.D.; Karwi, Q.G.; Tian, R.; Wende, A.R.; Abel, E.D. Cardiac Energy Metabolism in Heart Failure. Circ. Res. 2021, 128, 1487–1513. [Google Scholar] [CrossRef]
- Kenny, H.C.; Abel, E.D. Heart Failure in Type 2 Diabetes Mellitus: Impact of Glucose-Lowering Agents, Heart Failure Therapies, and Novel Therapeutic Strategies. Circ. Res. 2019, 124, 121–141. [Google Scholar] [CrossRef] [PubMed]
- Peterson, L.R.; Herrero, P.; Schechtman, K.B.; Racette, S.B.; Waggoner, A.D.; Kisrieva-Ware, Z.; Dence, C.; Klein, S.; Marsala, J.A.; Meyer, T.; et al. Effect of Obesity and Insulin Resistance on Myocardial Substrate Metabolism and Efficiency in Young Women. Circulation 2004, 109, 2191–2196. [Google Scholar] [CrossRef] [PubMed]
- Jia, G.; DeMarco, V.G.; Sowers, J.R. Insulin Resistance and Hyperinsulinaemia in Diabetic Cardiomyopathy. Nat. Rev. Endocrinol. 2016, 12, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Burhop, M.; Schuchardt, J.P.; Nebl, J.; Müller, M.; Lichtinghagen, R.; Hahn, A. Marine Oil from C. Finmarchicus Enhances Glucose Homeostasis and Liver Insulin Resistance in Obese Prediabetic Individuals. Nutrients 2022, 14, 396. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Geelen, A.; Brouwer, I.A.; Geleijnse, J.M.; Zock, P.L.; Katan, M.B. Effect of Fish Oil on Heart Rate in Humans: A Meta-Analysis of Randomized Controlled Trials. Circulation 2005, 112, 1945–1952. [Google Scholar] [CrossRef]
- Geleijnse, J.M.; Giltay, E.J.; Grobbee, E.; Donders, A.R.T.; Kok, F.J. Blood Pressure Response to Fish Oil Supplementation: Metaregression Analysis of Randomized Trials. J. Hypertens. 2002, 20, 1493–1499. [Google Scholar] [CrossRef]
- Zhang, X.; Ritonja, J.A.; Zhou, N.; Chen, B.E.; Li, X. Omega-3 Polyunsaturated Fatty Acids Intake and Blood Pressure: A Dose-Response Meta-Analysis of Randomized Controlled Trials. J. Am. Heart Assoc. 2022, 11, e025071. [Google Scholar] [CrossRef]
- Jia, G.; Whaley-Connell, A.; Sowers, J.R. Diabetic Cardiomyopathy: A Hyperglycaemia- and Insulin-Resistance-Induced Heart Disease. Diabetologia 2018, 61, 21–28. [Google Scholar] [CrossRef]
- Bender, A.; Lange, S. Adjusting for multiple testing--when and how? J. Clin. Epidemiol. 2001, 54, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Cuming, G. The new statistics: Why and how. Psychol. Sci. 2014, 25, 7–29. [Google Scholar] [CrossRef] [PubMed]
- Wasserstein, R.; Lazar, N. The ASA Statement on p-Values: Context, Process, and Purpose. Am. Stat. 2016, 70, 129–133. [Google Scholar] [CrossRef]
| Components | g/ 100 g Calanus Oil | mg/ 4 g Calanus Oil |
|---|---|---|
| MUFA | 9.7 | 388 |
| PUFA | 26.2 | 1048 |
| n3 PUFAs | 25.0 | 1000 |
| ALA | 1.4 | 56 |
| SDA | 8.4 | 336 |
| EPA | 6.9 | 276 |
| DHA | 6.4 | 256 |
| n6 PUFAs | 1.1 | 44 |
| LA | 0.7 | 28 |
| ARA | 0.2 | 8 |
| Fatty alcohols | 28.8 | 1152 |
| Sterols | 0.35 | 14 |
| Astaxanthin | 0.1 | 4 |
| Parameters | Mean ± SD |
|---|---|
| Age [year] | 59.25 ± 9.60 |
| BMI [kg/m2] | 34.24 ± 3.70 |
| Smoking status Current smoker, n (%] Previous smoker, n %] Never smoke, n [%] | 1 [5] 0 [0] 19 [95] |
| Medical drug intake No intake, n [%] Antihypertensive drug, n [%] other, n [%] | 4 [20] 11 [55] 5 [25] |
| t0 (n = 20) | t12 (n = 20) | p-Value | Effect-Size, d (95%-CI) | |
|---|---|---|---|---|
| Variables | Mean ± SD | Mean ± SD | ||
| Body weight [kg] WC [cm] | 94.52 ± 8.98 110.2 ± 9.66 | 94.18 ± 8.58 105.9 ± 10.47 | 0.242 0.049 | 0.022 (−0.43; 0.47) 0.573 (0.09; 1.04) |
| Absolute fat mass [%] Visceral fat mass [L] TBW [kg] ECW [kg] | 44.37 ± 7.49 3.38 ± 1.21 38.01 ± 3.11 17.56 ± 1.38 | 42.75 ± 6.75 2.97 ± 1.21 38.86 ± 3.41 17.82 ± 1.45 | 0.003 0.023 0.001 0.040 | 0.874 (0.35; 1.33) 0.479 (0.01; 0.94) −0.578 (0.10; 1.05) −0.421 (0.01; 0.08) |
| Fasting glucose [mg/dL] Fasting insulin [µE/mL] HOMA index [AU] HbA1c [%] | 106.3 ± 7.77 14.29 ± 6.58 3.77 ± 1.75 5.65 ± 0.30 | 103.7 ± 8.11 14.02 ± 5.89 3.60 ± 1.61 5.66 ± 0.28 | 0.010 0.418 0.310 0.360 | 0.572 (0.09; 1.04) 0.047 (−0.39; 0.49) 0.113 (−0.32; 0.55) 0.081 (−0.52; 0.36) |
| TAG [mg/dL] | 151.9 ± 78.39 | 119.9 ± 45.37 | 0.001 | 0.782 (0.27; 1,28) |
| TC [mg/dL] | 233.2 ± 47.24 | 236.45 ± 36.10 | 0.337 | −0.294 (−0.75; 0.17) |
| HDL-C [mg/dL] | 62.4 ± 11.74 | 63.7 ± 12.63 | 0.199 | −0.328 (−0.79; 0.14) |
| LDL-C [mg/dL] | 141.4 ± 33.04 | 139.9 ± 24.81 | 0.395 | −0.080 (−0.53; 0.37) |
| CRP [mg/L] | 2.78 ± 2.86 | 2.93 ± 3.52 | 0.358 | −0.083 (−0.52; 0.36) |
| Central SBP * [mmHg] | 130.8 ± 11.64 | 130.1 ± 13.33 | 0.372 | 0.842 (0.31; 1.36) |
| Central DBP * [mmHg] | 81.08 ± 8.66 | 78.53 ± 9.22 | 0.048 | 0.814 (0.28; 1.32) |
| HR [bpm] | 77.5 ± 8.71 | 74.43 ± 7.19 | 0.047 | 0.263 (0.72; 0.19) |
| Alx [%] | 27.15 ± 10.69 | 29.15 ± 9.20 | 0.163 | −0.195 (−0.65; 0.26) |
| PWV [m/s] | 8.64 ± 1.35 | 8.6 ± 1.56 | 0.332 | 0.320 (−0.15; 0.78) |
| Met-S-score WC [AU] | 0.76 ± 0.58 | 0.41 ± 0.53 | 0.001 | 1.344 (0.71; 1.96) |
| TyG-WHtR [AU] | 6.34 ± 0.79 | 5.97 ± 0.76 | 0.001 | 0.930 (0.68; 1.50) |
| Variables | t0 (n = 20) | t12 (n = 20) | p-Value | Effect-Size, d (95%-CI) |
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | |||
| LV EDV * [mL] | 100.72 ± 19.65 | 106.5 ± 17.32 | 0.166 | −0.330 (−0.78; 0.18) |
| LV ESV * [mL] | 46.03 ± 7.37 | 46.38 ± 9.03 | 0.908 | −0.004 (−0.52; 0.44) |
| LV SV * [mL] | 56.9 ± 13.56 | 60.72 ± 11.95 | 0.206 | −0.280 (−0.76; 0.20) |
| LV EF * [%] | 55.22 ± 5.39 | 56.31 ± 5.75 | 0.299 | −0.21 (−0.68; 0.28) |
| LV CO * [L/min] | 4.11 ± 0.96 | 4.12 ± 0.83 | 0.489 | −0.010 (−0.47; 0.45) |
| LS [%] | −16.35 ± 2.88 | −15.15 ± 2.98 | 0.116 | −0.412 (−0.89; 0.07) |
| DecT [ms] | 222.38 ± 30.29 | 218.28 ± 27.7 | 0.333 | 0.098 (−0.34; 0.54) |
| E [cm/s] | 67.13 ± 13.72 | 69.67 ± 9.78 | 0.159 | −0.212 (−0.069; 0.27) |
| A [cm/s] | 71.28 ± 15.42 | 72.22 ± 16.51 | 0.338 | −0.061 (−0.54; 0.42) |
| E/A Ratio [AU] | 0.97 ± 0.30 | 1.03 ± 0.30 | 0.023 | −0.479 (−0.94; −0.10) |
| E’septal [cm/s] | 8.62 ± 2.04 | 8.82 ± 1.58 | 0.307 | −0.046 (−0.48; 0.39) |
| E/e’ septal [AU] | 8.00 ± 1.51 | 8.06 ± 1.38 | 0.419 | −0.004 (−0.52; 0.44) |
| IVRT septal [ms] | 81.12 ± 19.79 | 84.98 ± 21.38 | 0.240 | −0.190 (−0.64; 0.29) |
| n (%) | n (%) | p-value | - | |
| Normal diastolic function | 18 (90) | 18 (90) | 0.500 | |
| Indeterminate diastolic function | 2 (10) | 2 (10) | 0.500 |
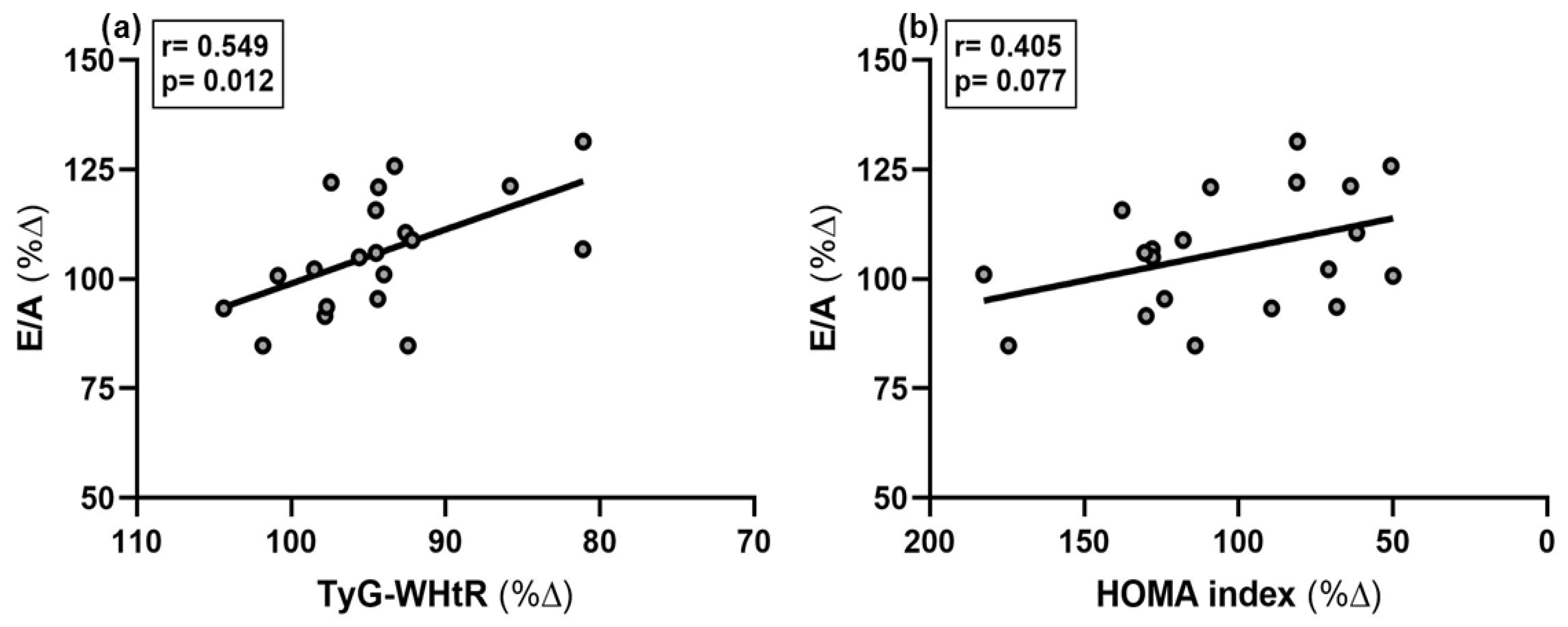
| % Δ Variables | r-Value | Beta-Coeff. | p-Value |
|---|---|---|---|
| % Δ TyG-WHtR % Δ HOMA index | 0.549 0.405 | −0.549 −0.405 | 0.012 0.077 |
| % Δ Fasting glucose | 0.189 | −0.203 | 0.426 |
| % Δ HbA1c | 0.037 | 0.059 | 0.877 |
| % Δ Met-S-score WC | 0.127 | −0.127 | 0.605 |
| % Δ Central DBP * | 0.080 | 0.080 | 0.665 |
| % Δ Absolute fat mass | 0.249 | −0.249 | 0.290 |
| % Δ Visceral fat mass % Δ Visceral fat mass | 0.388 0.283 | −0.235 −0.283 | 0.091 0.241 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kerlikowsky, F.; Spahiu, F.; Stöhr, E.J.; Junge, S.; Jonas, W.; Flierdt, E.v.d.; Schuchardt, J.P.; Hahn, A. Effects of 12 Weeks of Calanus Oil Supplementation on Cardiac Diastolic Function in Obese and Prediabetic Women—A Pilot Study. Metabolites 2025, 15, 596. https://doi.org/10.3390/metabo15090596
Kerlikowsky F, Spahiu F, Stöhr EJ, Junge S, Jonas W, Flierdt Evd, Schuchardt JP, Hahn A. Effects of 12 Weeks of Calanus Oil Supplementation on Cardiac Diastolic Function in Obese and Prediabetic Women—A Pilot Study. Metabolites. 2025; 15(9):596. https://doi.org/10.3390/metabo15090596
Chicago/Turabian StyleKerlikowsky, Felix, Fabian Spahiu, Eric J. Stöhr, Sina Junge, Wiebke Jonas, Edda van de Flierdt, Jan Philipp Schuchardt, and Andreas Hahn. 2025. "Effects of 12 Weeks of Calanus Oil Supplementation on Cardiac Diastolic Function in Obese and Prediabetic Women—A Pilot Study" Metabolites 15, no. 9: 596. https://doi.org/10.3390/metabo15090596
APA StyleKerlikowsky, F., Spahiu, F., Stöhr, E. J., Junge, S., Jonas, W., Flierdt, E. v. d., Schuchardt, J. P., & Hahn, A. (2025). Effects of 12 Weeks of Calanus Oil Supplementation on Cardiac Diastolic Function in Obese and Prediabetic Women—A Pilot Study. Metabolites, 15(9), 596. https://doi.org/10.3390/metabo15090596







