Metabolomic Signatures and Predictive Utility of LOXL1-Associated Genetic Risk Scores for Exfoliation Syndrome/Glaucoma in US Cohorts
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Blood Collection
2.2. GRS and LOXL1 SNPs
2.3. XFG Suspect (XFGS)/XFG Ascertainment
2.4. Metabolomic Profiling
2.5. Covariates
2.6. Statistical Analysis
2.6.1. Clinical Utility of XFS GRS
2.6.2. Metabolomic Analysis of High XFS Genetic Susceptibility
3. Results
3.1. Clinical Characteristics of XFGS/XFG Cases
3.2. Association Between XFS GRS8, GRS6, GRS2, LOXL1 SNPs and Incident XFGS/XFG
3.3. Model Prediction Performance for XFGS/XFG
3.4. Associations of Individual Metabolites and GRS and SNPs
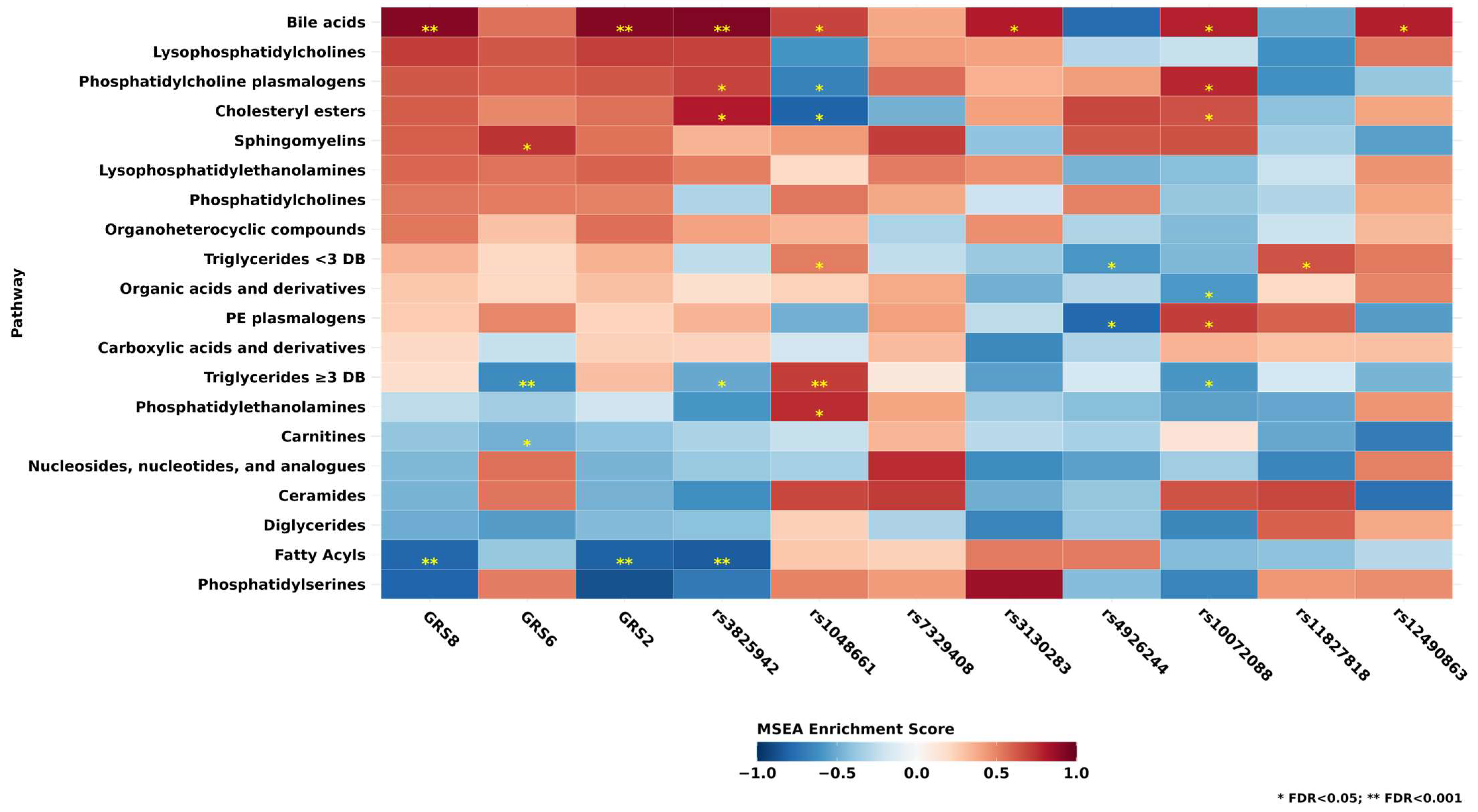
3.5. Associations of Metabolite Classes and GRS and SNPs
3.6. Subgroup Analyses
4. Discussion
4.1. LOXL1 and XFS GRS
4.2. Metabolomic Profiles of XFS GRS and SNPs Status
4.3. Strengths and Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ritch, R.; Schlötzer-Schrehardt, U. Exfoliation Syndrome. Surv. Ophthalmol. 2001, 45, 265–315. [Google Scholar] [CrossRef]
- Ritch, R. Why Is Glaucoma Associated with Exfoliation Syndrome? Prog. Retin. Eye Res. 2003, 22, 253–275. [Google Scholar] [CrossRef]
- Anastasopoulos, E.; Founti, P.; Topouzis, F. Update on Pseudoexfoliation Syndrome Pathogenesis and Associations with Intraocular Pressure, Glaucoma and Systemic Diseases. Curr. Opin. Ophthalmol. 2015, 26, 82–89. [Google Scholar] [CrossRef] [PubMed]
- De Vries, V.A.; Hanyuda, A.; Vergroesen, J.E.; Do, R.; Friedman, D.S.; Kraft, P.; Turman, C.; Luo, Y.; Tran, J.H.; Liefers, B.; et al. The Clinical Usefulness of a Glaucoma Polygenic Risk Score in 4 Population-Based European Ancestry Cohorts. Ophthalmology 2025, 132, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Schlotzer-Schrehardt, U. Genetics and Genomics of Pseudoexfoliation Syndrome/Glaucoma. Middle East Afr. J. Ophthalmol. 2011, 18, 30. [Google Scholar] [CrossRef] [PubMed]
- Greene, A.G.; Eivers, S.B.; Dervan, E.W.J.; O’Brien, C.J.; Wallace, D.M. Lysyl Oxidase Like 1: Biological Roles and Regulation. Exp. Eye Res. 2020, 193, 107975. [Google Scholar] [CrossRef]
- Challa, P. Genetics of Pseudoexfoliation Syndrome. Curr. Opin. Ophthalmol. 2009, 20, 88–91. [Google Scholar] [CrossRef]
- Kang, J.H.; Zeleznik, O.; Frueh, L.; Lasky-Su, J.; Eliassen, A.H.; Clish, C.; Rosner, B.A.; Pasquale, L.R.; Wiggs, J.L. Prediagnostic Plasma Metabolomics and the Risk of Exfoliation Glaucoma. Investig. Ophthalmol. Vis. Sci. 2022, 63, 15. [Google Scholar] [CrossRef]
- Myer, C.; Abdelrahman, L.; Banerjee, S.; Khattri, R.B.; Merritt, M.E.; Junk, A.K.; Lee, R.K.; Bhattacharya, S.K. Aqueous Humor Metabolite Profile of Pseudoexfoliation Glaucoma Is Distinctive. Mol. Omics 2020, 16, 425–435. [Google Scholar] [CrossRef]
- Leruez, S.; Bresson, T.; Chao De La Barca, J.M.; Marill, A.; De Saint Martin, G.; Buisset, A.; Muller, J.; Tessier, L.; Gadras, C.; Verny, C.; et al. A Plasma Metabolomic Signature of the Exfoliation Syndrome Involves Amino Acids, Acylcarnitines, and Polyamines. Investig. Ophthalmol. Vis. Sci. 2018, 59, 1025. [Google Scholar] [CrossRef]
- Thorleifsson, G.; Magnusson, K.P.; Sulem, P.; Walters, G.B.; Gudbjartsson, D.F.; Stefansson, H.; Jonsson, T.; Jonasdottir, A.; Jonasdottir, A.; Stefansdottir, G.; et al. Common Sequence Variants in the LOXL1 Gene Confer Susceptibility to Exfoliation Glaucoma. Science 2007, 317, 1397–1400. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, Y.; Gao, J.; Pawlyk, B.; Starcher, B.; Spencer, J.A.; Yanagisawa, H.; Zuo, J.; Li, T. Elastic Fiber Homeostasis Requires Lysyl Oxidase–like 1 Protein. Nat. Genet. 2004, 36, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Bertoia, M.L.; Lenart, E.B.; Stampfer, M.J.; Willett, W.C.; Speizer, F.E.; Chavarro, J.E. Origin, Methods, and Evolution of the Three Nurses’ Health Studies. Am. J. Public Health 2016, 106, 1573–1581. [Google Scholar] [CrossRef] [PubMed]
- Rimm, E.B.; Giovannucci, E.L.; Willett, W.C.; Colditz, G.A.; Ascherio, A.; Rosner, B.; Stampfer, M.J. Prospective Study of Alcohol Consumption and Risk of Coronary Disease in Men. Lancet 1991, 338, 464–468. [Google Scholar] [CrossRef] [PubMed]
- Aung, T.; Ozaki, M.; Lee, M.C.; Schlötzer-Schrehardt, U.; Thorleifsson, G.; Mizoguchi, T.; Igo, R.P.; Haripriya, A.; Williams, S.E.; Astakhov, Y.S.; et al. Genetic Association Study of Exfoliation Syndrome Identifies a Protective Rare Variant at LOXL1 and Five New Susceptibility Loci. Nat. Genet. 2017, 49, 993–1004. [Google Scholar] [CrossRef]
- Lindström, S.; Loomis, S.; Turman, C.; Huang, H.; Huang, J.; Aschard, H.; Chan, A.T.; Choi, H.; Cornelis, M.; Curhan, G.; et al. A Comprehensive Survey of Genetic Variation in 20,691 Subjects from Four Large Cohorts. PLoS ONE 2017, 12, e0173997. [Google Scholar] [CrossRef]
- Duffy, D.L.; Zhu, G.; Li, X.; Sanna, M.; Iles, M.M.; Jacobs, L.C.; Evans, D.M.; Yazar, S.; Beesley, J.; Law, M.H.; et al. Novel Pleiotropic Risk Loci for Melanoma and Nevus Density Implicate Multiple Biological Pathways. Nat. Commun. 2018, 9, 4774. [Google Scholar] [CrossRef]
- O’Connell, J.; Gurdasani, D.; Delaneau, O.; Pirastu, N.; Ulivi, S.; Cocca, M.; Traglia, M.; Huang, J.; Huffman, J.E.; Rudan, I.; et al. A General Approach for Haplotype Phasing across the Full Spectrum of Relatedness. PLoS Genet. 2014, 10, e1004234. [Google Scholar] [CrossRef]
- Das, S.; Forer, L.; Schönherr, S.; Sidore, C.; Locke, A.E.; Kwong, A.; Vrieze, S.I.; Chew, E.Y.; Levy, S.; McGue, M.; et al. Next-Generation Genotype Imputation Service and Methods. Nat. Genet. 2016, 48, 1284–1287. [Google Scholar] [CrossRef]
- Pasquale, L.R.; Aschard, H.; Kang, J.H.; Bailey, J.N.C.; Lindström, S.; Chasman, D.I.; Christen, W.G.; Allingham, R.R.; Ashley-Koch, A.; Lee, R.K.; et al. Age at Natural Menopause Genetic Risk Score in Relation to Age at Natural Menopause and Primary Open-Angle Glaucoma in a US-Based Sample. Menopause 2017, 24, 150–156. [Google Scholar] [CrossRef]
- Townsend, M.K.; Clish, C.B.; Kraft, P.; Wu, C.; Souza, A.L.; Deik, A.A.; Tworoger, S.S.; Wolpin, B.M. Reproducibility of Metabolomic Profiles among Men and Women in 2 Large Cohort Studies. Clin. Chem. 2013, 59, 1657–1667. [Google Scholar] [CrossRef] [PubMed]
- Zeleznik, O.A.; Eliassen, A.H.; Kraft, P.; Poole, E.M.; Rosner, B.A.; Jeanfavre, S.; Deik, A.A.; Bullock, K.; Hitchcock, D.S.; Avila-Pacheco, J.; et al. A Prospective Analysis of Circulating Plasma Metabolites Associated with Ovarian Cancer Risk. Cancer Res. 2020, 80, 1357–1367. [Google Scholar] [CrossRef]
- Wei, R.; Wang, J.; Su, M.; Jia, E.; Chen, S.; Chen, T.; Ni, Y. Missing Value Imputation Approach for Mass Spectrometry-Based Metabolomics Data. Sci. Rep. 2018, 8, 663. [Google Scholar] [CrossRef]
- Price, A.L.; Patterson, N.J.; Plenge, R.M.; Weinblatt, M.E.; Shadick, N.A.; Reich, D. Principal Components Analysis Corrects for Stratification in Genome-Wide Association Studies. Nat. Genet. 2006, 38, 904–909. [Google Scholar] [CrossRef]
- Cole, S.R.; Hernan, M.A. Constructing Inverse Probability Weights for Marginal Structural Models. Am. J. Epidemiol. 2008, 168, 656–664. [Google Scholar] [CrossRef]
- Gao, X.; Starmer, J.; Martin, E.R. A Multiple Testing Correction Method for Genetic Association Studies Using Correlated Single Nucleotide Polymorphisms. Genet. Epidemiol. 2008, 32, 361–369. [Google Scholar] [CrossRef]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene Set Enrichment Analysis: A Knowledge-Based Approach for Interpreting Genome-Wide Expression Profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Challa, P.; Schmidt, S.; Liu, Y.; Qin, X.; Vann, R.R.; Gonzalez, P.; Allingham, R.R.; Hauser, M.A. Analysis of LOXL1 Polymorphisms in a United States Population with Pseudoexfoliation Glaucoma. Mol. Vis. 2008, 14, 146–149. [Google Scholar]
- Wolf, C.; Gramer, E.; Müller-Myhsok, B.; Pasutto, F.; Gramer, G.; Wissinger, B.; Weisschuh, N. Lysyl Oxidase-like 1 Gene Polymorphisms in German Patients With Normal Tension Glaucoma, Pigmentary Glaucoma and Exfoliation Glaucoma. J. Glaucoma 2010, 19, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Jia, L.; Wang, N.; Tang, G.; Zhang, C.; Fan, S.; Liu, W.; Meng, H.; Zeng, W.; Liu, N.; et al. Evaluation of LOXL1 Polymorphisms in Exfoliation Syndrome in a Chinese Population. Mol. Vis. 2009, 15, 2349–2357. [Google Scholar]
- Csiszar, K. Lysyl Oxidases: A Novel Multifunctional Amine Oxidase Family. In Progress in Nucleic Acid Research and Molecular Biology; Elsevier: Amsterdam, The Netherlands, 2001; Volume 70, pp. 1–32. [Google Scholar] [CrossRef]
- Whigham, B.T.; Allingham, R.R. Review: The Role of LOXL1 in Exfoliation Syndrome/Glaucoma. Saudi J. Ophthalmol. 2011, 25, 347–352. [Google Scholar] [CrossRef]
- Lee, K.Y.C.; Ho, S.L.; Thalamuthu, A.; Venkatraman, A.; Venkataraman, D.; Pek, D.C.K.; Aung, T.; Vithana, E.N. Association of LOXL1 Polymorphisms with Pseudoexfoliation in the Chinese. Mol. Vis. 2009, 15, 1120–1126. [Google Scholar]
- Fuse, N.; Miyazawa, A.; Nakazawa, T.; Mengkegale, M.; Otomo, T.; Nishida, K. Evaluation of LOXL1 Polymorphisms in Eyes with Exfoliation Glaucoma in Japanese. Mol. Vis. 2008, 14, 1338–1343. [Google Scholar]
- Kolovos, A.; Qassim, A.; Hassall, M.M.; Marshall, H.N.; Schmidt, J.; Nguyen, T.T.; He, W.; Mullany, S.; Hollitt, G.L.; Berry, E.C.; et al. A Multitrait Open-Angle Glaucoma Polygenic Risk Score Stratifies Risk of Glaucoma Diagnosis and Severity in Eyes with Pseudoexfoliation. Ophthalmology 2025, 132, 878–887. [Google Scholar] [CrossRef]
- Küchle, M.; Nguyen, N.X.; Hannappel, E.; Naumann, G.O.H. The Blood-Aqueous Barrier in Eyes with Pseudoexf Oliation Syndrome. Ophthalmic Res. 1995, 27, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.M.; Khalsa, S.B.S.; Scheer, F.A.J.L.; Cajochen, C.; Lockley, S.W.; Czeisler, C.A.; Wright, K.P. Acute Effects of Bright Light Exposure on Cortisol Levels. J. Biol. Rhythm. 2010, 25, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Pasquale, L.R.; Borrás, T.; Fingert, J.H.; Wiggs, J.L.; Ritch, R. Exfoliation Syndrome: Assembling the Puzzle Pieces. Acta Ophthalmol. 2016, 94, e505–e512. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.P.; Bali, A.; Singh, N.; Jaggi, A.S. Advanced Glycation End Products and Diabetic Complications. Korean J. Physiol. Pharmacol. 2014, 18, 1–14. [Google Scholar] [CrossRef]
- Hanyuda, A.; Zeleznik, O.A.; Raita, Y.; Negishi, K.; Pasquale, L.R.; Lasky-Su, J.; Wiggs, J.L.; Kang, J.H. Machine Learning on Prediagnostic Metabolite Data Identifies Etiologic Endotypes of Exfoliation Glaucoma in United States Health Professionals. Ophthalmol. Sci. 2025, 5, 100678. [Google Scholar] [CrossRef]
- Jacob, M.M.; Pai, H.V.; Gnanaharan, J.; Kamath, S. Serum Bile Acids in Patients with Primary Open-Angle Glaucoma. J. Glaucoma 2018, 27, 687–690. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Cai, H.; Xue, S.; You, W.; Liu, B.; Jiang, H. Bile Acids Induce Activation of Alveolar Epithelial Cells and Lung Fibroblasts through Farnesoid X Receptor-dependent and Independent Pathways. Respirology 2016, 21, 1075–1080. [Google Scholar] [CrossRef] [PubMed]
- Qin, P.; Tang, X.; Elloso, M.M.; Harnish, D.C. Bile Acids Induce Adhesion Molecule Expression in Endothelial Cells through Activation of Reactive Oxygen Species, NF-κB, and P38. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, H741–H747. [Google Scholar] [CrossRef]
- Türkyılmaz, K.; Öner, V.; Kırbas, A.; Sevim, M.S.; Sekeryapan, B.; Özgür, G.; Durmus, M. Serum YKL-40 Levels as a Novel Marker of Inflammation and Endothelial Dysfunction in Patients with Pseudoexfoliation Syndrome. Eye 2013, 27, 854–859. [Google Scholar] [CrossRef]
- Wang, Y.E.; Tseng, V.L.; Yu, F.; Caprioli, J.; Coleman, A.L. Association of Dietary Fatty Acid Intake With Glaucoma in the United States. JAMA Ophthalmol. 2018, 136, 141–147. [Google Scholar] [CrossRef]
- Romeo Villadóniga, S.; Rodríguez García, E.; Sagastagoia Epelde, O.; Álvarez Díaz, M.D.; Domingo Pedrol, J.C. Effects of Oral Supplementation with Docosahexaenoic Acid (DHA) plus Antioxidants in Pseudoexfoliative Glaucoma: A 6-Month Open-Label Randomized Trial. J. Ophthalmol. 2018, 2018, 8259371. [Google Scholar] [CrossRef]
- Callaghan, B.; Vallabh, N.A.; Willoughby, C.E. Deuterated Polyunsaturated Fatty Acids Provided Protection against Oxidative Stress in Ocular Fibroblasts Derived from Glaucoma Patients. Mech. Ageing Dev. 2023, 211, 111778. [Google Scholar] [CrossRef] [PubMed]
- Mayers, J.R.; Wu, C.; Clish, C.B.; Kraft, P.; Torrence, M.E.; Fiske, B.P.; Yuan, C.; Bao, Y.; Townsend, M.K.; Tworoger, S.S.; et al. Elevation of Circulating Branched-Chain Amino Acids Is an Early Event in Human Pancreatic Adenocarcinoma Development. Nat. Med. 2014, 20, 1193–1198. [Google Scholar] [CrossRef]
- Zeleznik, O.A.; Balasubramanian, R.; Zhao, Y.; Frueh, L.; Jeanfavre, S.; Avila-Pacheco, J.; Clish, C.B.; Tworoger, S.S.; Eliassen, A.H. Circulating Amino Acids and Amino Acid-Related Metabolites and Risk of Breast Cancer among Predominantly Premenopausal Women. Npj Breast Cancer 2021, 7, 54. [Google Scholar] [CrossRef]
- Katagiri, R.; Goto, A.; Nakagawa, T.; Nishiumi, S.; Kobayashi, T.; Hidaka, A.; Budhathoki, S.; Yamaji, T.; Sawada, N.; Shimazu, T.; et al. Increased Levels of Branched-Chain Amino Acid Associated with Increased Risk of Pancreatic Cancer in a Prospective Case–Control Study of a Large Cohort. Gastroenterology 2018, 155, 1474–1482.e1. [Google Scholar] [CrossRef]
- Tobias, D.K.; Hazra, A.; Lawler, P.R.; Chandler, P.D.; Chasman, D.I.; Buring, J.E.; Lee, I.-M.; Cheng, S.; Manson, J.E.; Mora, S. Circulating Branched-Chain Amino Acids and Long-Term Risk of Obesity-Related Cancers in Women. Sci. Rep. 2020, 10, 16534. [Google Scholar] [CrossRef] [PubMed]
- Hur, B.; Gupta, V.K.; Huang, H.; Wright, K.A.; Warrington, K.J.; Taneja, V.; Davis, J.M.; Sung, J. Plasma Metabolomic Profiling in Patients with Rheumatoid Arthritis Identifies Biochemical Features Predictive of Quantitative Disease Activity. Arthritis Res. Ther. 2021, 23, 164. [Google Scholar] [CrossRef] [PubMed]

| Characteristics a | Incident XFGS/XFG Cases (n = 118) | Non-Cases (n = 39,354) | p-Values |
|---|---|---|---|
| Genetic risk score-8 | 0.841 (0.461) | −0.002 (0.999) | <0.001 |
| Female, % | 79.6 | 74.1 | <0.001 |
| Age, years b | 54.3 (8.4) | 49.3 (8.9) | 0.001 |
| Scandinavian ancestry, % | 11.9 | 9.3 | 0.80 |
| Family history of glaucoma, %,b | 26.2 | 16.3 | 0.01 |
| Self-reported diabetes diagnosis, % | 0 | 2.8 | 0.02 |
| Self-reported hypertension diagnosis, % | 16.3 | 17.7 | 0.43 |
| Self-reported high cholesterol diagnosis, % | 5.4 | 17.4 | <0.001 |
| Self-reported history of myocardial infarction, % | 0 | 1.5 | 0.04 |
| Total calories, kcal/day | 1631 (580) | 1779 (521) | 0.01 |
| Total vitamin A intake, IU/day | 13,501 (6011) | 13,519 (8113) | 0.33 |
| Total caffeine intake, mg/day | 384 (265) | 291 (237) | <0.001 |
| Folate intake, μg/day | 434 (221) | 44 (243) | 0.26 |
| Weighted lifetime average latitude of residence, °N | 40.6 (3.6) | 39.5 (3.9) | 0.01 |
| Annual UV flux, ×10−4 mW/m2 | 181 (18) | 186 (27) | 0.03 |
| Total alcoholic intake, g/day | 9.0 (16.3) | 6.8 (11.2) | 0.27 |
| Cigarette smoking, pack-years | 7.0 (12.5) | 9.2 (15.1) | 0.04 |
| Body mass index, kg/m2 | 23.4 (3.1) | 24.8 (4.1) | <0.001 |
| In top 25th percentile for physical activity, % | 14.5 | 23.9 | 0.53 |
| Alternate healthy eating index 2010 | 44.6 (9.8) | 45.3 (9.7) | 0.48 |
| Variables | T1 (n = 38) | T2 (n = 38) | T3 (n = 42) |
|---|---|---|---|
| XFS GRS8, median (min, max) | 0.408 (−1.020, 0.793) | 0.946 (0.808, 1.075) | 1.183 (1.076, 1.731) |
| Age at diagnosis, mean year | 70.5 | 70.3 | 70.3 |
| Bilateral, % | 35.7 | 44.1 | 32.6 |
| Highest untreated intraocular pressure, mean mmHg | 27.8 | 27.7 | 28.2 |
| Cup to disc ratio, mean | 0.56 | 0.55 | 0.59 |
| Presence of glaucomatous visual field loss, % | 46.6 | 61.5 | 40.3 |
| Family history of glaucoma, % | 22.4 | 35.8 | 9.2 |
| Q1 + Q2 | Q3 | Q4 | Q5 | |
|---|---|---|---|---|
| Median GRS8 (min, max) | −0.91 (−3.25, 0.02) | 0.24 (0.03, 0.40) | 0.63 (0.40, 0.95) | 1.13 (0.95, 1.89) |
| XFGS/XFG cases (n = 118) | 5 | 13 | 41 | 59 |
| Person-years (%) | 292,345 (40.0) | 146,095 (20.0) | 146,257 (20.0) | 146,158 (20.0) |
| Model 1 | 0.13 (0.04, 0.48) | REF = 1.0 | 1.78 (0.78, 4.06) | 3.82 (1.76, 8.29) |
| Model 2 | 0.13 (0.04, 0.48) | REF = 1.0 | 2.36 (1.15, 4.85) | 4.30 (2.15, 8.59) |
| Model 3 | 0.18 (0.05, 0.62) | REF = 1.0 | 4.29 (1.78, 10.36) | 7.03 (3.07, 16.09) |
| Model 4 | 0.13 (0.04, 0.51) | REF = 1.0 | 2.29 (1.11, 4.74) | 4.31 (2.12, 8.76) |
| (a) | ||||
| rs1048661 allele | TT | TG | GG | |
| XFGS/XFG cases (n = 118) | 4 | 29 | 85 | |
| Person-years (%) | 75,918 (10.4) | 320,648 (43.9) | 334,289 (45.7) | |
| Model 1 | REF = 1.0 | 1.51 (0.39, 5.77) | 3.82 (1.09, 13.45) | |
| Model 2 | REF = 1.0 | 1.65 (0.42, 6.52) | 4.47 (1.22, 16.31) | |
| Model 3 | REF = 1.0 | 2.17 (0.71, 6.63) | 8.53 (3.02, 24.15) | |
| Model 4 | REF = 1.0 | 1.68 (0.41, 6.81) | 4.62 (1.25, 17.06) | |
| rs3825942 allele | AA + AG | GG | ||
| XFGS/XFG cases (n = 118) | 2 | 116 | ||
| Person-years (%) | 216,204 (29.6) | 514,651 (70.4) | ||
| Model 1 | REF = 1.0 | 70.65 (17.18, 290.52) | ||
| Model 2 | REF = 1.0 | 99.72 (19.22, 517.26) | ||
| Model 3 | REF = 1.0 | 75.90 (17.63, 326.87) | ||
| Model 4 | REF = 1.0 | 106.08 (15.77, 713.75) | ||
| (b) | ||||
| Q1 + Q2 | Q3 | Q4 | Q5 | |
| Median GRS6 (min, max) | −0.85 (−2.09, −0.21) | −0.13 (−0.21, 0.15) | 0.48 (0.15, 0.88) | 1.33 (0.89, 5.12) |
| XFGS/XFG cases (n = 118) | 42 | 27 | 26 | 23 |
| Person-years (%) | 292,672 (40.0) | 145,382 (20.0) | 147,016 (20.0) | 145,785 (20.0) |
| Model 1 | 0.82 (0.45, 1.52) | REF = 1.0 | 0.97 (0.45, 2.10) | 0.71 (0.37, 1.38) |
| Model 2 | 0.94 (0.51, 1.72) | REF = 1.0 | 0.99 (0.47, 2.10) | 0.82 (0.41, 1.63) |
| Model 3 | 0.94 (0.50, 1.79) | REF = 1.0 | 0.92 (0.39, 2.14) | 0.79 (0.39, 1.62) |
| Model 4 | 0.93 (0.50, 1.72) | REF = 1.0 | 1.00 (0.49, 2.04) | 0.83 (0.42, 1.65) |
| (c) | ||||
| Q1 + Q2 | Q3 | Q4 | Q5 | |
| Median GRS2 (min, max) | −0.88 (−2.96, −0.32) | 0.36 (0.34, 0.39) | 0.40 (0.40, 1.09) | 1.12 (1.10, 1.15) |
| XFGS/XFG cases (n = 118) | 6 | 24 | 25 | 63 |
| Person-years (%) | 292,121 (40.0) | 154,440 (21.1) | 139,422 (19.1) | 144,872 (19.8) |
| Model 1 | 0.14 (0.04, 0.45) | REF = 1.0 | 2.06 (1.03, 4.11) | 3.47 (1.82, 6.61) |
| Model 2 | 0.12 (0.04, 0.43) | REF = 1.0 | 2.24 (1.00, 4.98) | 3.70 (1.93, 7.12) |
| Model 3 | 0.13 (0.05, 0.37) | REF = 1.0 | 2.69 (1.14, 6.34) | 4.99 (2.62, 9.53) |
| Model 4 | 0.13 (0.04, 0.47) | REF = 1.0 | 2.44 (1.12, 5.31) | 3.67 (1.90, 7.08) |
| Univariate Models | C-Index (95% CI) |
| Model 1a: GRS8 | 0.76 (0.72, 0.79) |
| Model 1b: GRS2 | 0.73 (0.70, 0.77) |
| Model 1c: rs3825942 | 0.64 (0.62, 0.65) |
| Model 1d: rs1048661 | 0.63 (0.59, 0.68) |
| Model 1e: rs3825942 + rs1048661 | 0.76 (0.72, 0.79) |
| Model 1f: GRS6 | 0.51 (0.46, 0.57) |
| Model 1g: GRS6 + rs3825942 + rs1048661 | 0.76 (0.73, 0.80) |
| Multivariable-Adjusted Models | C-Index (95% CI) |
| Model 2: Age + sex + period at risk + age × sex | 0.81 (0.77, 0.85) |
| Model 3: Age + sex + period at risk + age × sex + IOP > 25 mmHg | 0.88 (0.84, 0.92) |
| Model 4: Age + sex + period at risk + age × sex + IOP > 25 mmHg + family history of glaucoma | 0.88 (0.84, 0.92) |
| Model 5a: Model 4 + GRS8 | 0.93 (0.91, 0.95) 1 |
| Model 5b: Model 4 + rs3825942 | 0.91 (0.89, 0.93) |
| Model 5c: Model 4 + rs1048661 | 0.90 (0.88, 0.92) |
| Model 5d: Model 4 + rs3825942 + rs1048661 | 0.93 (0.91, 0.95) |
| Model 5e: Model 4 + GRS2 | 0.93 (0.90, 0.95) 2 |
| Model 5f: Model 4 + GRS6 | 0.88 (0.84, 0.92) 3 |
| Model 5g: Model 4 + GRS6 + rs3825942 + rs1048661 | 0.93 (0.91, 0.95) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juramt, N.; Zeleznik, O.A.; Pasquale, L.R.; Wiggs, J.L.; Kang, J.H. Metabolomic Signatures and Predictive Utility of LOXL1-Associated Genetic Risk Scores for Exfoliation Syndrome/Glaucoma in US Cohorts. Metabolites 2025, 15, 582. https://doi.org/10.3390/metabo15090582
Juramt N, Zeleznik OA, Pasquale LR, Wiggs JL, Kang JH. Metabolomic Signatures and Predictive Utility of LOXL1-Associated Genetic Risk Scores for Exfoliation Syndrome/Glaucoma in US Cohorts. Metabolites. 2025; 15(9):582. https://doi.org/10.3390/metabo15090582
Chicago/Turabian StyleJuramt, Namuunaa, Oana A. Zeleznik, Louis R. Pasquale, Janey L. Wiggs, and Jae H. Kang. 2025. "Metabolomic Signatures and Predictive Utility of LOXL1-Associated Genetic Risk Scores for Exfoliation Syndrome/Glaucoma in US Cohorts" Metabolites 15, no. 9: 582. https://doi.org/10.3390/metabo15090582
APA StyleJuramt, N., Zeleznik, O. A., Pasquale, L. R., Wiggs, J. L., & Kang, J. H. (2025). Metabolomic Signatures and Predictive Utility of LOXL1-Associated Genetic Risk Scores for Exfoliation Syndrome/Glaucoma in US Cohorts. Metabolites, 15(9), 582. https://doi.org/10.3390/metabo15090582







