SGLT2 Inhibitors: Multifaceted Therapeutic Agents in Cardiometabolic and Renal Diseases
Abstract
1. Introduction
2. SGLT2 Inhibitors as a Paradigm Shift in Metabolic Therapy
3. Mechanisms of Action: From Glycosuria to Systemic Metabolic Effects
3.1. Renal Glucose Handling and Glycemic Control
3.2. Beyond Glycemia: Hemodynamic and Neurohormonal Effects
3.2.1. Natriuresis and Osmotic Diuresis
3.2.2. Tubuloglomerular Feedback (TGF) Modulation
3.2.3. Metabolic Shift to Ketone Utilization
3.2.4. Additional Systemic Effects
4. Cardiorenoprotective Effects of SGLT2 Inhibitors: Clinical Evidence and Mechanistic Insights
Atherosclerotic Cardiovascular Disease
5. SGLT2 Inhibitors in Obesity and Adipose Tissue Remodeling
5.1. Weight Loss: Mechanisms and Limitations
5.2. Adipose Tissue Inflammation and Browning
5.3. Adverse Effects and Recommendations in Special Populations
6. How Do SGLT2 Inhibitors Influence Specific Metabolic Markers of Adipose Tissue, Such as Lipolysis, Adipokine Secretion, and Mitochondrial Function?
6.1. Mitochondrial Biogenesis and Functional Remodeling
- -
- PGC-1α (peroxisome proliferator-activated receptor gamma coactivator 1-alpha), a master regulator of mitochondrial biogenesis, which showed increased expression in response to canagliflozin.
- -
- NRF1 (nuclear respiratory factor 1) and Tfam (mitochondrial transcription factor A), critical for mitochondrial DNA replication and respiratory chain assembly.
- -
- CPT1b (carnitine palmitoyltransferase 1b), which facilitates fatty acid β-oxidation, corroborated by elevated acylcarnitine levels.
6.2. Lipolytic Activation and Lipidomic Remodeling
- Upregulation of lipolytic enzymes: Hormone-sensitive lipase (HSL) and adipose triglyceride lipase (ATGL) expression were elevated in visceral and subcutaneous adipose depots, concomitant with reduced adipocyte size [66].
- Transient increases in free fatty acids (FFAs): Reflecting acute lipid mobilization, as seen in Yoshida et al. [67], though this was followed by improved adipose tissue insulin resistance (Adipo-IR), a paradoxical yet clinically beneficial outcome.
- FGF21-dependent pathways: Osataphan et al. [63] identified FGF21 as indispensable for SGLT2 inhibitor-induced lipolysis, with FGF21-null mice failing to exhibit adiposity reductions despite preserved ketogenesis.
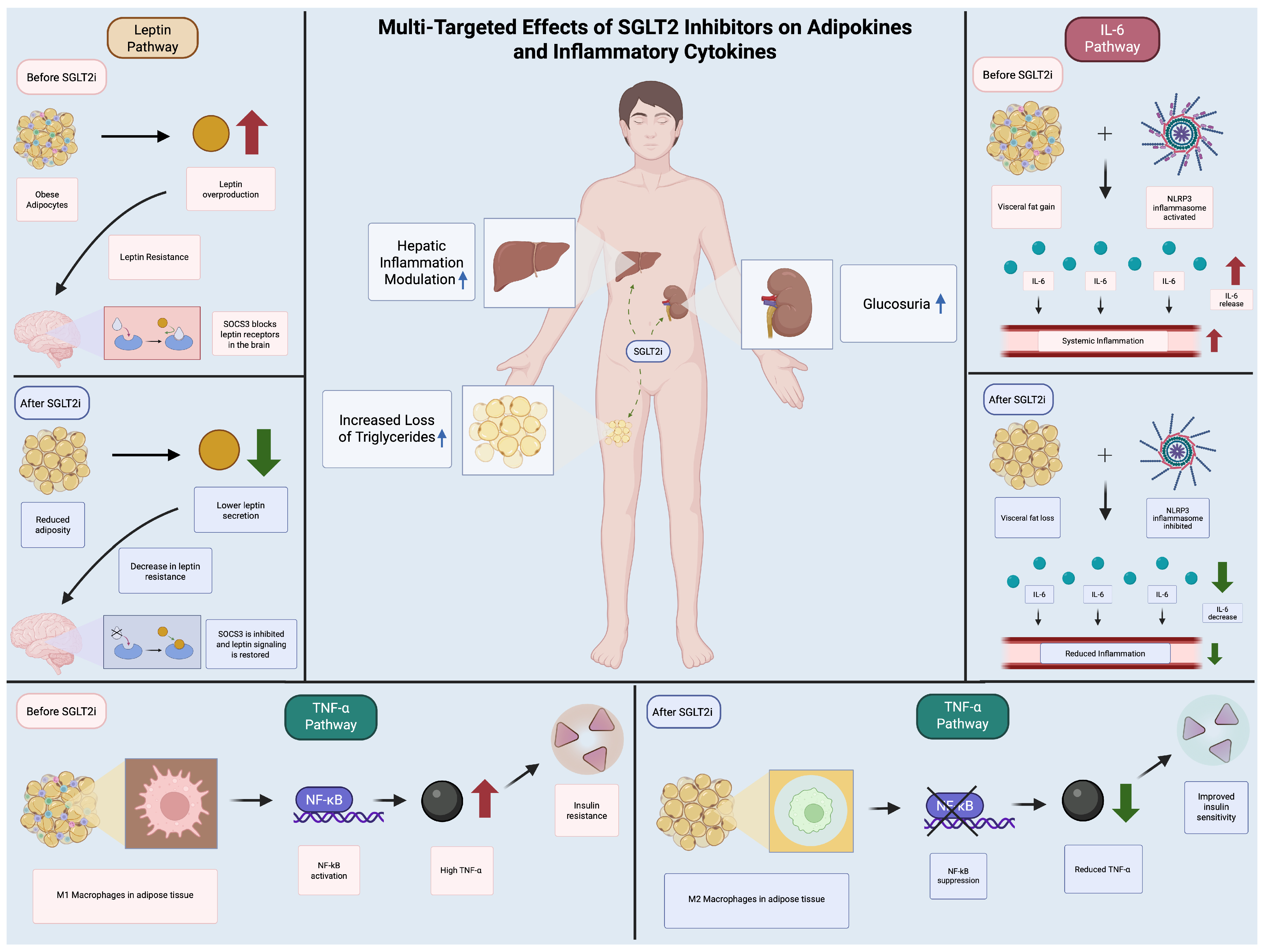
6.3. Adipokine Secretion and Anti-Inflammatory Effects
- Díaz-Rodríguez et al. (2018) [68]: Dapagliflozin suppressed chemokine secretion in epicardial adipose explants, alongside increased GLUT4 translocation, indicative of improved insulin sensitivity.
- Takano et al. (2023) [69]: Empagliflozin inhibited IL-6 and MCP-1 in human epicardial preadipocytes, attenuating pro-inflammatory differentiation.
- Aragón-Herrera et al. (2023) [66]: Empagliflozin restored adiponectin levels in diabetic rats, paralleling reduced systemic inflammation.
6.4. Molecular Mechanisms: PPARα, FGF21, and AMPK/mTOR Crosstalk
- PPARα: Directly mediates mitochondrial biogenesis and fatty acid oxidation, with genetic/pharmacologic inhibition ablating these effects [62].
- FGF21: Acts as an endocrine mediator of lipolysis and energy expenditure, though its role is context-dependent [63].
- AMPK/mTOR: SGLT2 inhibitors activate AMPK while inhibiting mTOR, mimicking caloric restriction and promoting catabolic processes [65].
6.5. Upregulation of Mitochondrial Markers in Multiple Studies
6.6. Promotion of Browning and Energy Expenditure
6.7. Lack of Significant Improvement in Some Cases
6.8. Absence of Negative Effects on Mitochondrial Markers
6.9. Mechanistic Insights into Mitochondrial Enhancement
| Lipolysis and Lipid Metabolism | |||
|---|---|---|---|
| Lipid Parameter | Effect Direction | Tissue Location | Key Pathways |
| Fatty acid uptake (free fatty acids) | Increased (↑) | Visceral adipose tissue | Enhanced lipolysis |
| Hormone-sensitive lipase (HSL), adipose triglyceride lipase (ATGL) | Upregulated (↑) | Visceral and subcutaneous adipose tissue | Increased lipolysis |
| Adipose tissue insulin resistance (Adipo-IR), fasting free fatty acids | Upregulated (↑) | Subcutaneous adipose tissue | FGF21-dependent lipolysis |
| Beta-3 adrenergic receptor (3 AR), ATGL adipocyte size | Decreased (↓) | Subcutaneous adipose tissue | Increased lipolysis |
| Diglycerides, oxidized fatty acids | Increased (↑) | Visceral adipose tissue | Lipidomic remodeling |
7. Emerging Applications and Future Directions
- Non-Alcoholic Fatty Liver Disease (NAFLD): Preliminary data suggest improvements in hepatic steatosis and fibrosis. The REALM trial (dapagliflozin 10 mg/day) demonstrated that liver fat reduction was 47%, there was a ≥30% relative reduction in MRI-PDFF at 6 months (vs. 12% placebo), and fibrosis biomarkers, through FAST score, indicated an improvement of 52% vs. 29% (Δ23%, p = 0.008) [70]. Moreover, the synergistic potential is proven in the ongoing ENLIGHTENED trial (NCT05877547), which combines empagliflozin with semaglutide. This study shows a preliminary 62% NASH resolution rate (vs. 28% monotherapy) with a 5.2% absolute reduction in liver stiffness (FibroScan) [71].
- Polycystic Ovary Syndrome (PCOS): A 2024 meta-analysis by Javed et al. (Hum Reprod Update) found hyperandrogenism improvement, and free testosterone reduction (−1.8 pg/mL, 95% CI −2.4 to −1.2) was achieved with these drugs. Additionally, ovarian SGLT2 expression correlates with androgen production (r = 0.72, p < 0.001) [72].
- Neurodegenerative Diseases: In exploring links between ketone metabolism and neuroprotection, some ongoing trials or sub-studies, such as the Alzheimer’s EMPA-REG-NEURO trial (empagliflozin 25 mg), showed a 1.8-point ADAS-Cog14 improvement at 18 months (p = 0.03) and a cerebrospinal fluid β-amyloid 42/40 ratio of ↑15% (p = 0.02) following treatment [73].
- Availability and Access: SGLT-2 patents are set to expire in the not-so-far future, meaning that generic versions can be made by other manufacturers, not just by their original manufacturer. As a result, SGLT-2 inhibitors will become much cheaper and more widely available. Physicians will then be able to prescribe them more often and to a much larger number of patients, who may not have previously been able to access them due to their high costs.
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, Y.D.; Park, K.-G.; Lee, Y.-S.; Park, Y.-Y.; Kim, D.-K.; Nedumaran, B.; Jang, W.G.; Cho, W.-J.; Ha, J.; Lee, I.-K.; et al. Metformin Inhibits Hepatic Gluconeogenesis Through AMP-Activated Protein Kinase–Dependent Regulation of the Orphan Nuclear Receptor SHP. Diabetes 2008, 57, 306–314. [Google Scholar] [CrossRef]
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Bělohlávek, J.; et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef] [PubMed]
- Packer, M.; Butler, J.; Filippatos, G.; Zannad, F.; Ferreira, J.P.; Zeller, C.; Brueckmann, M.; Jamal, W.; Pocock, S.J.; Anker, S.D.; et al. Design of a prospective patient-level pooled analysis of two parallel trials of empagliflozin in patients with established heart failure. Eur. J. Heart Fail. 2020, 22, 2393–2398. [Google Scholar] [CrossRef] [PubMed]
- Ferrannini, G.; Savarese, G.; Rydén, L. Sodium-glucose transporter inhibition in heart failure: From an unexpected side effect to a novel treatment possibility. Diabetes Res. Clin. Pract. 2021, 175, 108796. [Google Scholar] [CrossRef]
- Mark, P.B.; Sattar, N. Implementation, not hesitation, for SGLT2 inhibition as foundational therapy for chronic kidney disease. Lancet 2022, 400, 1745–1747. [Google Scholar] [CrossRef]
- Davies, M.J.; D’Alessio, D.A.; Fradkin, J.; Kernan, W.N.; Mathieu, C.; Mingrone, G.; Rossing, P.; Tsapas, A.; Wexler, D.J.; Buse, J.B. Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2018, 41, 2669–2701. [Google Scholar] [CrossRef]
- Proks, P.; Reimann, F.; Green, N.; Gribble, F.; Ashcroft, F. Sulfonylurea Stimulation of Insulin Secretion. Diabetes 2002, 51, S368–S376. [Google Scholar] [CrossRef]
- Day, C. Thiazolidinediones: A new class of antidiabetic drugs. Diabet. Med. 1999, 16, 179–192. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Hompesch, M.; Kasichayanula, S.; Liu, X.; Hong, Y.; Pfister, M.; Morrow, L.A.; Leslie, B.R.; Boulton, D.W.; Ching, A.; et al. Characterization of Renal Glucose Reabsorption in Response to Dapagliflozin in Healthy Subjects and Subjects with Type 2 Diabetes. Diabetes Care 2013, 36, 3169–3176. [Google Scholar] [CrossRef]
- Komoroski, B.; Vachharajani, N.; Feng, Y.; Li, L.; Kornhauser, D.; Pfister, M. Dapagliflozin, a Novel, Selective SGLT2 Inhibitor, Improved Glycemic Control Over 2 Weeks in Patients with Type 2 Diabetes Mellitus. Clin. Pharmacol. Ther. 2009, 85, 513–519. [Google Scholar] [CrossRef]
- Lunati, M.E.; Cimino, V.; Gandolfi, A.; Trevisan, M.; Montefusco, L.; Pastore, I.; Pace, C.; Betella, N.; Favacchio, G.; Bulgheroni, M.; et al. SGLT2-inhibitors are effective and safe in the elderly: The SOLD study. Pharmacol. Res. 2022, 183, 106396. [Google Scholar] [CrossRef] [PubMed]
- Lyu, B.; Hwang, Y.J.; Selvin, E.; Jameson, B.C.; Chang, A.R.; Grams, M.E.; Shin, J.-I. Glucose-Lowering Agents and the Risk of Hypoglycemia: A Real-world Study. J. Gen. Intern. Med. 2023, 38, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Danpanichkul, P.; Manosroi, W.; Nilsirisuk, T.; Tosukhowong, T. Predictors of weight reduction effectiveness of SGLT2 inhibitors in diabetes mellitus type 2 patients. Front. Endocrinol. 2024, 14, 1251798. [Google Scholar] [CrossRef] [PubMed]
- Drucker, D.J. Mechanisms of Action and Therapeutic Application of Glucagon-like Peptide-1. Cell Metab. 2018, 27, 740–756. [Google Scholar] [CrossRef]
- Fonseca-Correa, J.I.; Correa-Rotter, R. Sodium-Glucose Cotransporter 2 Inhibitors Mechanisms of Action: A Review. Front. Med. 2021, 8, 777861. [Google Scholar] [CrossRef]
- Wang, D.D.; Naumova, A.V.; Isquith, D.; Sapp, J.; Huynh, K.A.; Tucker, I.; Balu, N.; Voronyuk, A.; Chu, B.; Ordovas, K.; et al. Dapagliflozin reduces systemic inflammation in patients with type 2 diabetes without known heart failure. Cardiovasc. Diabetol. 2024, 23, 197. [Google Scholar] [CrossRef]
- Cinti, F.; Leccisotti, L.; Sorice, G.P.; Capece, U.; D’aMario, D.; Lorusso, M.; Gugliandolo, S.; Morciano, C.; Guarneri, A.; Guzzardi, M.A.; et al. Dapagliflozin treatment is associated with a reduction of epicardial adipose tissue thickness and epicardial glucose uptake in human type 2 diabetes. Cardiovasc. Diabetol. 2023, 22, 349. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, N.M.; Fu, P.M.; An, Y.M.; Sun, F.M.; Wang, C.M.; Han, X.; Zhang, Y.; Yu, X.; Liu, Y. Dapagliflozin Attenuates Heart Failure with Preserved Ejection Fraction Remodeling and Dysfunction by Elevating β-Hydroxybutyrate–activated Citrate Synthase. J. Cardiovasc. Pharmacol. 2023, 82, 375–388. [Google Scholar] [CrossRef]
- Chagnac, A.; Herman, M.; Zingerman, B.; Erman, A.; Rozen-Zvi, B.; Hirsh, J.; Gafter, U. Obesity-induced glomerular hyperfiltration: Its involvement in the pathogenesis of tubular sodium reabsorption. Nephrol. Dial. Transplant. 2008, 23, 3946–3952. [Google Scholar] [CrossRef]
- Vallon, V.; Komers, R. Pathophysiology of the Diabetic Kidney. In Comprehensive Physiology; Wiley: Hoboken, NJ, USA, 2011; pp. 1175–1232. [Google Scholar] [CrossRef]
- Zhao, J.Z.; Weinhandl, E.D.; Carlson, A.M.; Peter, W.L.S. Hypoglycemia Risk With SGLT2 Inhibitors or Glucagon-like Peptide 1 Receptor Agonists Versus Sulfonylureas Among Medicare Insured Adults with CKD in the United States. Kidney Med. 2022, 4, 100510. [Google Scholar] [CrossRef]
- Vallon, V. State-of-the-Art-Review: Mechanisms of Action of SGLT2 Inhibitors and Clinical Implications. Am. J. Hypertens. 2024, 37, 841–852. [Google Scholar] [CrossRef] [PubMed]
- Masuda, T.; Watanabe, Y.; Fukuda, K.; Watanabe, M.; Onishi, A.; Ohara, K.; Imai, T.; Koepsell, H.; Muto, S.; Vallon, V.; et al. Unmasking a sustained negative effect of SGLT2 inhibition on body fluid volume in the rat. Am. J. Physiol. Physiol. 2018, 315, F653–F664. [Google Scholar] [CrossRef] [PubMed]
- Packer, M.; Anker, S.D.; Butler, J.; Filippatos, G.; Pocock, S.J.; Carson, P.; Januzzi, J.; Verma, S.; Tsutsui, H.; Brueckmann, M.; et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N. Engl. J. Med. 2020, 383, 1413–1424. [Google Scholar] [CrossRef] [PubMed]
- Vallon, V.; Gerasimova, M.; Rose, M.A.; Masuda, T.; Satriano, J.; Mayoux, E.; Koepsell, H.; Thomson, S.C.; Rieg, T. SGLT2 inhibitor empagliflozin reduces renal growth and albuminuria in proportion to hyperglycemia and prevents glomerular hyperfiltration in diabetic Akita mice. Am. J. Physiol. Physiol. 2014, 306, F194–F204. [Google Scholar] [CrossRef]
- Layton, A.T.; Vallon, V.; Edwards, A. Predicted consequences of diabetes and SGLT inhibition on transport and oxygen consumption along a rat nephron. Am. J. Physiol. Physiol. 2016, 310, F1269–F1283. [Google Scholar] [CrossRef]
- Škrtić, M.; Yang, G.K.; Perkins, B.A.; Soleymanlou, N.; Lytvyn, Y.; von Eynatten, M.; Woerle, H.J.; Johansen, O.E.; Broedl, U.C.; Hach, T.; et al. Characterisation of glomerular haemodynamic responses to SGLT2 inhibition in patients with type 1 diabetes and renal hyperfiltration. Diabetologia 2014, 57, 2599–2602. [Google Scholar] [CrossRef]
- Perkovic, V.; Jardine, M.J.; Neal, B.; Bompoint, S.; Heerspink, H.J.L.; Charytan, D.M.; Edwards, R.; Agarwal, R.; Bakris, G.; Bull, S.; et al. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N. Engl. J. Med. 2019, 380, 2295–2306. [Google Scholar] [CrossRef]
- Heerspink, H.J.L.; Stefánsson, B.V.; Correa-Rotter, R.; Chertow, G.M.; Greene, T.; Hou, F.-F.; Mann, J.F.E.; McMurray, J.J.V.; Lindberg, M.; Rossing, P.; et al. Dapagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2020, 383, 1436–1446. [Google Scholar] [CrossRef]
- Ferrannini, E.; Baldi, S.; Frascerra, S.; Astiarraga, B.; Heise, T.; Bizzotto, R.; Mari, A.; Pieber, T.R.; Muscelli, E. Shift to Fatty Substrate Utilization in Response to Sodium–Glucose Cotransporter 2 Inhibition in Subjects Without Diabetes and Patients with Type 2 Diabetes. Diabetes 2016, 65, 1190–1195. [Google Scholar] [CrossRef]
- Merovci, A.; Solis-Herrera, C.; Daniele, G.; Eldor, R.; Fiorentino, T.V.; Tripathy, D.; Xiong, J.; Perez, Z.; Norton, L.; Abdul-Ghani, M.A.; et al. Dapagliflozin improves muscle insulin sensitivity but enhances endogenous glucose production. J. Clin. Investig. 2014, 124, 509–514. [Google Scholar] [CrossRef]
- Goedeke, L.; Ma, Y.; Gaspar, R.C.; Nasiri, A.; Lee, J.; Zhang, D.; Galsgaard, K.D.; Hu, X.; Zhang, J.; Guerrera, N.; et al. SGLT2 inhibition alters substrate utilization and mitochondrial redox in healthy and failing rat hearts. J. Clin. Investig. 2024, 134, e176708. [Google Scholar] [CrossRef] [PubMed]
- Tsampasian, V.; Elghazaly, H.; Chattopadhyay, R.; Ali, O.; Corballis, N.; Chousou, P.; Clark, A.; Garg, P.; Vassiliou, V. SGLT2 inhibitors in heart failure with preserved and reduced ejection fraction: A systematic review and meta-analysis. Eur. J. Prev. Cardiol. 2022, 29, zwac056-020. [Google Scholar] [CrossRef]
- Svanström, H.; Mkoma, G.F.; Hviid, A.; Pasternak, B. SGLT-2 inhibitors and mortality among patients with heart failure with reduced ejection fraction: Linked database study. BMJ 2024, 387, e080925. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.Y.; Ephraums, L.A.; Inglis, J.M.; Nguyen, H.T.T.; Umapathysivam, M.M.; Simpson, N.J.; Harris, J.H.; Burdeniuk, C.M.; De Pasquale, C.G.; Thynne, T.R.J. A Cross-Sectional Study of Capillary Blood Ketone Concentrations in Heart Failure Based on Sodium-Glucose Co-Transporter-2 Inhibitor Use and Heart Failure Type. Heart Lung Circ. 2025, 34, 34–39. [Google Scholar] [CrossRef]
- Gangat, N.; Szuber, N.; Alkhateeb, H.B.; Al-Kali, A.; Pardanani, A.D.; Tefferi, A. JAK2 wild-type erythrocytosis associated with sodium-glucose cotransporter 2 inhibitor therapy. Blood 2021, 138, 2886–2889. [Google Scholar] [CrossRef]
- Herat, L.Y.; Magno, A.L.; Rudnicka, C.; Hricova, J.; Carnagarin, R.; Ward, N.C.; Arcambal, A.; Kiuchi, M.G.; Head, G.A.; Schlaich, M.P.; et al. SGLT2 Inhibitor–Induced Sympathoinhibition. JACC Basic Transl. Sci. 2020, 5, 169–179. [Google Scholar] [CrossRef]
- Byrne, N.J.; Matsumura, N.; Maayah, Z.H.; Ferdaoussi, M.; Takahara, S.; Darwesh, A.M.; Levasseur, J.L.; Jahng, J.W.S.; Vos, D.; Parajuli, N.; et al. Empagliflozin Blunts Worsening Cardiac Dysfunction Associated With Reduced NLRP3 (Nucleotide-Binding Domain-Like Receptor Protein 3) Inflammasome Activation in Heart Failure. Circ. Heart Fail. 2020, 13, e006277. [Google Scholar] [CrossRef]
- Kim, S.R.; Lee, S.-G.; Kim, S.H.; Kim, J.H.; Choi, E.; Cho, W.; Rim, J.H.; Hwang, I.; Lee, C.J.; Lee, M.; et al. SGLT2 inhibition modulates NLRP3 inflammasome activity via ketones and insulin in diabetes with cardiovascular disease. Nat. Commun. 2020, 11, 2127. [Google Scholar] [CrossRef]
- Solomon, S.D.; McMurray, J.J.; Claggett, B.; de Boer, R.A.; DeMets, D.; Hernandez, A.F.; Inzucchi, S.E.; Kosiborod, M.N.; Lam, C.S.; Martinez, F.; et al. Dapagliflozin in Heart Failure with Mildly Reduced or Preserved Ejection Fraction. N. Engl. J. Med. 2022, 387, 1089–1098. [Google Scholar] [CrossRef]
- Anker, S.D.; Butler, J.; Filippatos, G.; Ferreira, J.P.; Bocchi, E.; Böhm, M.; Brunner–La Rocca, H.-P.; Choi, D.-J.; Chopra, V.; Chuquiure-Valenzuela, E.; et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N. Engl. J. Med. 2021, 385, 1451–1461. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2023 Focused Update of the 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2023, 44, 3627–3639. [Google Scholar] [CrossRef]
- Htoo, P.T.; Tesfaye, H.; Schneeweiss, S.; Wexler, D.J.; Everett, B.M.; Glynn, R.J.; Schmedt, N.; Koeneman, L.; Déruaz-Luyet, A.; Paik, J.M.; et al. Cardiorenal effectiveness of empagliflozin vs. glucagon-like peptide-1 receptor agonists: Final-year results from the EMPRISE study. Cardiovasc. Diabetol. 2024, 23, 57. [Google Scholar] [CrossRef]
- Quagliariello, V.; Canale, M.L.; Bisceglia, I.; Iovine, M.; Paccone, A.; Maurea, C.; Scherillo, M.; Merola, A.; Giordano, V.; Palma, G.; et al. Sodium-glucose cotransporter 2 inhibitor dapagliflozin prevents ejection fraction reduction, reduces myocardial and renal NF-κB expression and systemic pro-inflammatory biomarkers in models of short-term doxorubicin cardiotoxicity. Front. Cardiovasc. Med. 2024, 11, 1289663. [Google Scholar] [CrossRef]
- Sato, S.; Takayanagi, K.; Shimizu, T.; Kanozawa, K.; Iwashita, T.; Hasegawa, H. Correlation between albuminuria and interstitial injury marker reductions associated with SGLT2 inhibitor treatment in diabetic patients with renal dysfunction. Eur. J. Med. Res. 2022, 27, 140. [Google Scholar] [CrossRef] [PubMed]
- Devenny, J.J.; Godonis, H.E.; Harvey, S.J.; Rooney, S.; Cullen, M.J.; Pelleymounter, M.A. Weight Loss Induced by Chronic Dapagliflozin Treatment Is Attenuated by Compensatory Hyperphagia in Diet-Induced Obese (DIO) Rats. Obesity 2012, 20, 1645–1652. [Google Scholar] [CrossRef] [PubMed]
- Polidori, D.; Sanghvi, A.; Seeley, R.J.; Hall, K.D. How Strongly Does Appetite Counter Weight Loss? Quantification of the Feedback Control of Human Energy Intake. Obesity 2016, 24, 2289–2295. [Google Scholar] [CrossRef] [PubMed]
- Ferrannini, G.; Hach, T.; Crowe, S.; Sanghvi, A.; Hall, K.D.; Ferrannini, E. Energy Balance After Sodium–Glucose Cotransporter 2 Inhibition. Diabetes Care 2015, 38, 1730–1735. [Google Scholar] [CrossRef]
- Horie, I.; Abiru, N.; Hongo, R.; Nakamura, T.; Ito, A.; Haraguchi, A.; Natsuda, S.; Sagara, I.; Ando, T.; Kawakami, A. Increased sugar intake as a form of compensatory hyperphagia in patients with type 2 diabetes under dapagliflozin treatment. Diabetes Res. Clin. Pract. 2018, 135, 178–184. [Google Scholar] [CrossRef]
- Lin, X.-F.; Cui, X.-N.; Yang, J.; Jiang, Y.-F.; Wei, T.-J.; Xia, L.; Liao, X.-Y.; Li, F.; Wang, D.-D.; Li, J.; et al. SGLT2 inhibitors ameliorate NAFLD in mice via downregulating PFKFB3, suppressing glycolysis and modulating macrophage polarization. Acta Pharmacol. Sin. 2024, 45, 2579–2597. [Google Scholar] [CrossRef]
- Xu, L.; Xu, C.; Liu, X.; Li, X.; Li, T.; Yu, X.; Xue, M.; Yang, J.; Kosmas, C.E.; Moris, D.; et al. Empagliflozin Induces White Adipocyte Browning and Modulates Mitochondrial Dynamics in KK Cg-Ay/J Mice and Mouse Adipocytes. Front. Physiol. 2021, 12, 745058. [Google Scholar] [CrossRef]
- Qu, J.; Tian, L.; Zhang, M.; Sun, B.; Chen, L. SGLT2 inhibitor canagliflozin reduces visceral adipose tissue in db/db mice by modulating AMPK/KLF4 signaling and regulating mitochondrial dynamics to induce browning. Mol. Cell Endocrinol. 2024, 592, 112320. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Liu, Q.; Li, Y.; Ding, Y.; Zhao, Y.; Tang, Q.; Wu, T.; Chen, L.; Pu, S.; Cheng, S.; et al. Inhibition of the sodium–glucose co-transporter SGLT2 by canagliflozin ameliorates diet-induced obesity by increasing intra-adipose sympathetic innervation. Br. J. Pharmacol. 2021, 178, 1756–1771. [Google Scholar] [CrossRef] [PubMed]
- Chow, E.; Clement, S.; Garg, R. Euglycemic diabetic ketoacidosis in the era of SGLT-2 inhibitors. BMJ Open Diabetes Res. Care 2023, 11, e003666. [Google Scholar] [CrossRef] [PubMed]
- Tanrıverdi, M.; Baştemir, M.; Demirbakan, H.; Ünalan, A.; Türkmen, M.; Tanrıverdi, G.Ö. Association of SGLT-2 inhibitors with bacterial urinary tract infection in type 2 diabetes. BMC Endocr. Disord. 2023, 23, 211. [Google Scholar] [CrossRef]
- Papadokostaki, E.; Rizos, E.; Tigas, S.; Liberopoulos, E.N. Canagliflozin and Amputation Risk: Evidence So Far. Int. J. Low. Extrem. Wounds 2020, 19, 21–26. [Google Scholar] [CrossRef]
- Xiang, L.; Liu, M.; Xiang, G.; Yue, L.; Zhang, J.; Xu, X.; Dong, J. Dapagliflozin promotes white adipose tissue browning though regulating angiogenesis in high fat induced obese mice. BMC Pharmacol. Toxicol. 2024, 25, 26. [Google Scholar] [CrossRef]
- Selvaraj, S.; Patel, S.; Sauer, A.J.; McGarrah, R.W.; Jones, P.; Kwee, L.C.; Windsor, S.L.; Ilkayeva, O.; Muehlbauer, M.J.; Newgard, C.B.; et al. Metabolic Effects of the SGLT2 Inhibitor Dapagliflozin in Heart Failure Across the Spectrum of Ejection Fraction. Circ. Heart Fail. 2024, 17, e011980. [Google Scholar] [CrossRef]
- Pruett, J.E.; Lirette, S.T.; Romero, D.G.; Cardozo, L.L.Y. Sodium-Glucose Cotransporter-2 Inhibition Benefits in Cardiorenal Risk in Men and Women. J. Endocr. Soc. 2022, 7, bvac191. [Google Scholar] [CrossRef]
- Yang, C.-C.; Chen, K.-H.; Yue, Y.; Cheng, B.-C.; Hsu, T.-W.; Chiang, J.Y.; Chen, C.-H.; Liu, F.; Xiao, J.; Yip, H.-K. SGLT2 inhibitor downregulated oxidative stress via activating AMPK pathway for cardiorenal (CR) protection in CR syndrome rodent fed with high protein diet. J. Mol. Histol. 2024, 55, 803–823. [Google Scholar] [CrossRef]
- Scisciola, L.; Cataldo, V.; Taktaz, F.; Fontanella, R.A.; Pesapane, A.; Ghosh, P.; Franzese, M.; Puocci, A.; De Angelis, A.; Sportiello, L.; et al. Anti-inflammatory role of SGLT2 inhibitors as part of their anti-atherosclerotic activity: Data from basic science and clinical trials. Front. Cardiovasc. Med. 2022, 9, 1008922. [Google Scholar] [CrossRef]
- Wei, D.; Liao, L.; Wang, H.; Zhang, W.; Wang, T.; Xu, Z. Canagliflozin ameliorates obesity by improving mitochondrial function and fatty acid oxidation via PPARα in vivo and in vitro. Life Sci. 2020, 247, 117414. [Google Scholar] [CrossRef]
- Osataphan, S.; Macchi, C.; Singhal, G.; Chimene-Weiss, J.; Sales, V.; Kozuka, C.; Dreyfuss, J.M.; Pan, H.; Tangcharoenpaisan, Y.; Morningstar, J.; et al. SGLT2 inhibition reprograms systemic metabolism via FGF21-dependent and -independent mechanisms. J. Clin. Investig. 2019, 4, e123130. [Google Scholar] [CrossRef]
- Nishitani, S.; Fukuhara, A.; Shin, J.; Okuno, Y.; Otsuki, M.; Shimomura, I. Metabolomic and microarray analyses of adipose tissue of dapagliflozin-treated mice, and effects of 3-hydroxybutyrate on induction of adiponectin in adipocytes. Sci. Rep. 2018, 8, 8805. [Google Scholar] [CrossRef]
- Xu, L.; Nagata, N.; Nagashimada, M.; Zhuge, F.; Ni, Y.; Chen, G.; Mayoux, E.; Kaneko, S.; Ota, T. SGLT2 Inhibition by Empagliflozin Promotes Fat Utilization and Browning and Attenuates Inflammation and Insulin Resistance by Polarizing M2 Macrophages in Diet-induced Obese Mice. EBioMedicine 2017, 20, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Aragón-Herrera, A.; Moraña-Fernández, S.; Otero-Santiago, M.; Anido-Varela, L.; Campos-Toimil, M.; García-Seara, J.; Román, A.; Seijas, J.; García-Caballero, L.; Rodríguez, J.; et al. The lipidomic and inflammatory profiles of visceral and subcutaneous adipose tissues are distinctly regulated by the SGLT2 inhibitor empagliflozin in Zucker diabetic fatty rats. Biomed. Pharmacother. 2023, 161, 114535. [Google Scholar] [CrossRef]
- Yoshida, Y.; Cheng, X.; Shao, H.; Fonseca, V.A.; Shi, L. A Systematic Review of Cost-Effectiveness of Sodium-Glucose Cotransporter Inhibitors for Type 2 Diabetes. Curr. Diabetes Rep. 2020, 20, 12. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Rodríguez, E.; Agra, R.M.; Fernández, Á.L.; Adrio, B.; García-Caballero, T.; González-Juanatey, J.R.; Eiras, S. Effects of dapagliflozin on human epicardial adipose tissue: Modulation of insulin resistance, inflammatory chemokine production, and differentiation ability. Cardiovasc. Res. 2018, 114, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Takano, M.; Kondo, H.; Harada, T.; Takahashi, M.; Ishii, Y.; Yamasaki, H.; Shan, T.; Akiyoshi, K.; Shuto, T.; Teshima, Y.; et al. Empagliflozin Suppresses the Differentiation/Maturation of Human Epicardial Preadipocytes and Improves Paracrine Secretome Profile. JACC Basic Transl. Sci. 2023, 8, 1081–1097. [Google Scholar] [CrossRef]
- Shi, M.; Zhang, H.; Wang, W.; Zhang, X.; Liu, J.; Wang, Q.; Wang, Y.; Zhang, C.; Guo, X.; Qiao, Q.; et al. Effect of dapagliflozin on liver and pancreatic fat in patients with type 2 diabetes and non-alcoholic fatty liver disease. J. Diabetes Its Complicat. 2023, 37, 108610. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. A Clinical Study of Efinopegdutide in Participants with Precirrhotic Nonalcoholic Steatohepatitis (NASH) (MK-6024-013). Available online: https://clinicaltrials.gov/study/NCT05877547 (accessed on 5 June 2025).
- Zhang, L.; Wang, Z.; Kong, L.; Liu, H.; Ma, Z.; Xu, M.; Yushanjiang, S.; Yuan, D.; Yu, L. Effect of SGLT2 Inhibitors on Improving Glucolipid Metabolism and Reproductive Hormone Status in Overweight/Obese Women with PCOS: A Systematic Review and Meta-Analysis. Reprod. Sci. 2024, 31, 1190–1203. [Google Scholar] [CrossRef]
- Hierro-Bujalance, C.; Infante-Garcia, C.; del Marco, A.; Herrera, M.; Carranza-Naval, M.J.; Suarez, J.; Alves-Martinez, P.; Lubian-Lopez, S.; Garcia-Alloza, M. Empagliflozin reduces vascular damage and cognitive impairment in a mixed murine model of Alzheimer’s disease and type 2 diabetes. Alzheimer’s Res. Ther. 2020, 12, 40. [Google Scholar] [CrossRef]
| Adverse Effect | Description |
|---|---|
| Euglycemic ketoacidosis | This is when there is metabolic acidosis in the blood with normal-to-low glucose levels as opposed to classic ketoacidosis, which has hyperglycemia. The use of SGLT-2 inhibitors reduces glucose levels, which may cause insulinopenia, and increases glucagon, leading to ketogenesis and an increase in ketone bodies in the blood. This is a possible risk with SGLT-2 inhibitor use in general, and it should thus be closely monitored in subsequent follow-ups [54]. |
| Urinary tract infections | Because SGLT2 inhibitors prevent excess glucose from being reabsorbed in the kidneys, glucose is excreted in the urine. This glycosuria creates a favorable environment for bacteria and fungi in the genitourinary tract, increasing the risk of infections in patients using these medications. This should be closely monitored in subsequent follow-ups [55]. |
| Increased risk of amputations (specifically canagliflozin) | Preliminary data from the CANVAS program has raised concerns about a potentially increased risk of lower limb amputations concurrent with the use of SGLT2 inhibitors, especially canagliflozin. This remains a serious consideration in patients with peripheral artery diseases, prior amputations, or other high-risk features. Therefore, careful patient selection and monitoring are advised when prescribing these medications [56]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Checa-Ros, A.; Okojie, O.-J.; D’Marco, L. SGLT2 Inhibitors: Multifaceted Therapeutic Agents in Cardiometabolic and Renal Diseases. Metabolites 2025, 15, 536. https://doi.org/10.3390/metabo15080536
Checa-Ros A, Okojie O-J, D’Marco L. SGLT2 Inhibitors: Multifaceted Therapeutic Agents in Cardiometabolic and Renal Diseases. Metabolites. 2025; 15(8):536. https://doi.org/10.3390/metabo15080536
Chicago/Turabian StyleCheca-Ros, Ana, Owahabanun-Joshua Okojie, and Luis D’Marco. 2025. "SGLT2 Inhibitors: Multifaceted Therapeutic Agents in Cardiometabolic and Renal Diseases" Metabolites 15, no. 8: 536. https://doi.org/10.3390/metabo15080536
APA StyleCheca-Ros, A., Okojie, O.-J., & D’Marco, L. (2025). SGLT2 Inhibitors: Multifaceted Therapeutic Agents in Cardiometabolic and Renal Diseases. Metabolites, 15(8), 536. https://doi.org/10.3390/metabo15080536








