Triglyceride–Glucose Index and New-Onset Type 2 Diabetes Mellitus in Middle-Aged Men
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Examination Procedures
2.3. Statistical Analysis
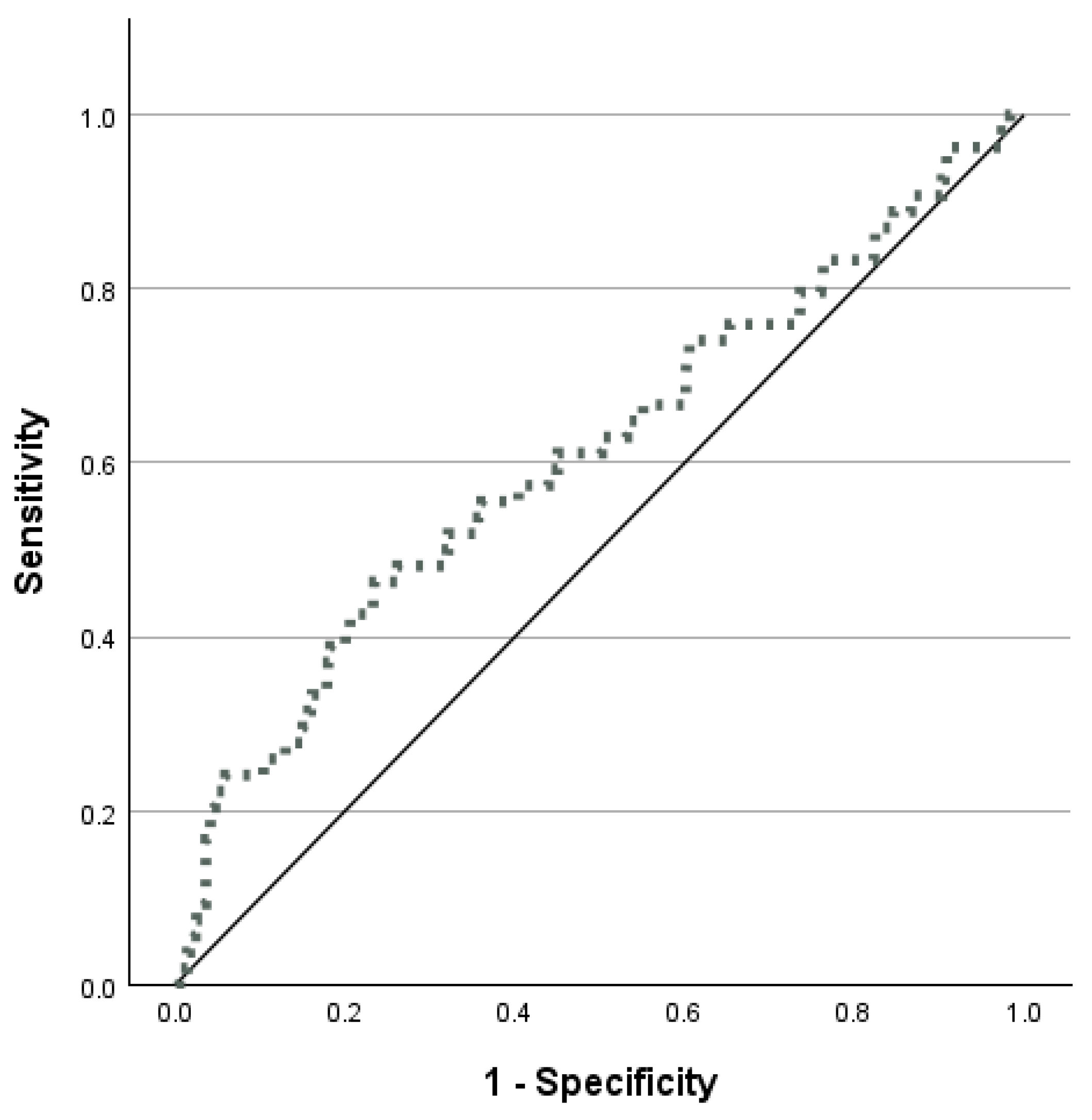
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AUC | Area under the curve |
| BMI | Body mass index |
| eGFR | estimated glomerular filtration rate |
| HOMA | Homeostatic Model Assessment |
| IR | Insulin resistance |
| OHS | Olivetti Heart Study |
| RCS | Restricted cubic splines |
| ROC | Receiver-operating characteristic |
| STROBE | STrengthening the Reporting of OBservational studies in Epidemiology |
| T2DM | Type 2 Diabetes Mellitus |
| TyG | Triglyceride–glucose index |
| WC | Waist circumference |
References
- International Diabetes Federation. IDF Diabetes Atlas, 9th ed.; International Diabetes Federation: Brussels, Belgium, 2019. [Google Scholar]
- Mancia, G.; Kreutz, R.; Brunström, M.; Burnier, M.; Grassi, G.; Januszewicz, A.; Muiesan, M.L.; Tsioufis, K.; Agabiti-Rosei, E.; Algharably, E.A.E.; et al. 2023 ESH Guidelines for the management of arterial hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension. J. Hypertens. 2023, 41, 1874–2071. [Google Scholar] [CrossRef]
- D’Elia, L.; Strazzullo, P. Excess body weight, insulin resistance and isolated systolic hypertension: Potential pathophysiological links. High Blood Press. Cardiovasc. Prev. 2018, 25, 17–23. [Google Scholar] [CrossRef]
- Cersosimo, E.; Solis Herrera, C.; Trautmann, M.E.; Malloy, J.; Triplitt, C.L. Assessment of pancreatic β cell function: Review of methods and clinical applications. Curr. Diabetes Rev. 2014, 10, 2–42. [Google Scholar] [CrossRef] [PubMed]
- Simental Mendía, L.E.; Rodríguez Morán, M.; Guerrero Romero, F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab. Syndr. Relat. Disord. 2008, 6, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Guerrero Romero, F.; Simental Mendía, L.E.; González Ortiz, M.; Martínez Abundis, E.; Ramos Zavala, M.G.; Hernández González, S.O.; Jacques Camarena, O.; Rodríguez Morán, M. The product of triglycerides and glucose, a simple measure of insulin sensitivity: Comparison with the euglycemic–hyperinsulinemic clamp. J. Clin. Endocrinol. Metab. 2010, 95, 3347–3351. [Google Scholar] [CrossRef]
- Sánchez García, A.; Rodríguez Gutiérrez, R.; Mancillas Adame, L.; González Nava, V.; Díaz González Colmenero, A.; Solis, R.C.; Álvarez-Villalobos, N.A.; González-González, J.G. Diagnostic accuracy of the triglyceride and glucose index for insulin resistance: A systematic review. Int. J. Endocrinol. 2020, 2020, 4678526. [Google Scholar] [CrossRef]
- D’Elia, L. Is the triglyceride–glucose index ready for cardiovascular risk assessment? Nutr. Metab. Cardiovasc. Dis. 2025, 35, 103834. [Google Scholar] [CrossRef]
- Liu, X.; Tan, Z.; Huang, Y.; Zhao, H.; Liu, M.; Yu, P.; Ma, J.; Zhao, Y.; Zhu, W.; Wang, J. Relationship between the triglyceride–glucose index and risk of cardiovascular diseases and mortality in the general population: A systematic review and meta-analysis. Cardiovasc. Diabetol. 2022, 21, 124. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, I.F.; Bao, X.; Nilsson, P.M.; Zaigham, S. Triglyceride–glucose (TyG) index is a predictor of arterial stiffness, incidence of diabetes, cardiovascular disease, and all-cause and cardiovascular mortality: A longitudinal two-cohort analysis. Front. Cardiovasc. Med. 2023, 9, 1035105. [Google Scholar] [CrossRef]
- Lopez Jaramillo, P.; Gomez Arbelaez, D.; Martinez Bello, D.; Abat, M.E.M.; Alhabib, K.F.; Avezum, Á.; Barbarash, O.; Chifamba, J.; Diaz, M.L.; Gulec, S. Association of the triglyceride–glucose index as a measure of insulin resistance with mortality and cardiovascular disease in populations from five continents (PURE study): A prospective cohort study. Lancet Healthy Longev. 2023, 4, e23–e33. [Google Scholar] [CrossRef]
- D’Elia, L.; Masulli, M.; Virdis, A.; Casiglia, E.; Tikhonoff, V.; Angeli, F.; Barbagallo, C.M.; Bombelli, M.; Cappelli, F.; Cianci, R.; et al. Triglyceride–glucose index and mortality in a large regional based Italian database (Urrah Project). J. Clin. Endocrinol. Metab. 2025, 110, e470–e477. [Google Scholar] [CrossRef]
- He, G.; Zhang, Z.; Wang, C.; Wang, W.; Bai, X.; He, L.; Chen, S.; Li, G.; Yang, Y.; Zhang, X.; et al. Association of the triglyceride–glucose index with all-cause and cause specific mortality: A population-based cohort study of 3.5 million adults in China. Lancet Reg. Health West. Pac. 2024, 49, 101135. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.; Qian, L.; Che, X.; Lv, P.; Xu, Q. TyG index is a predictor of all-cause mortality during long-term follow-up in middle-aged and elderly individuals with hypertension. Clin. Exp. Hypertens. 2023, 45, 2272581. [Google Scholar] [CrossRef]
- Li, X.; Sun, M.; Yang, Y.; Yao, N.; Yan, S.; Wang, L.; Hu, W.; Guo, R.; Wang, Y.; Li, B. Predictive effect of triglyceride–glucose-related parameters, obesity indices, and lipid ratios for diabetes in a Chinese population: A prospective cohort study. Front. Endocrinol. 2022, 13, 862919. [Google Scholar] [CrossRef]
- Li, X.; Li, G.; Cheng, T.; Liu, J.; Song, G.; Ma, H. Association between triglyceride–glucose index and risk of incident diabetes: A secondary analysis based on a Chinese cohort study. Lipids Health Dis. 2020, 19, 236. [Google Scholar] [CrossRef]
- Shan, Y.; Liu, Q.; Gao, T. Triglyceride–glucose index in predicting the risk of new onset diabetes in the general population aged ≥45 years: A national prospective cohort study. BMC Endocr. Disord. 2025, 25, 25. [Google Scholar] [CrossRef]
- Kuang, M.; Yang, R.; Huang, X.; Wang, C.; Sheng, G.; Xie, G.; Zou, Y. Assessing temporal differences in the predictive power of baseline TyG related parameters for future diabetes: An analysis using time dependent receiver operating characteristics. J. Transl. Med. 2023, 21, 299. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Lim, N.K.; Park, H.Y. The product of fasting plasma glucose and triglycerides improves risk prediction of type 2 diabetes in middle aged Koreans. BMC Endocr. Disord. 2018, 18, 33. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.L.; Liu, L.; Lo, K.; Huang, J.Y.; Yu, Y.L.; Huang, Y.Q.; Feng, Y.Q. Association between triglyceride–glucose index and risk of new onset diabetes among Chinese adults: Findings from the China Health and Retirement Longitudinal Study. Front. Cardiovasc. Med. 2020, 7, 610322. [Google Scholar] [CrossRef]
- da Silva, A.; Caldas, A.P.S.; Rocha, D.M.U.P.; Bressan, J. Triglyceride–glucose index predicts type 2 diabetes mellitus risk: A systematic review and meta-analysis of cohort studies. Prim. Care Diabetes 2020, 14, 584–593. [Google Scholar] [CrossRef]
- Pranata, R.; Huang, I.; Irvan; Lim, M.A.; Vania, R. The association between triglyceride–glucose index and the incidence of type 2 diabetes mellitus: A systematic review and dose response meta-analysis. Endocrine 2021, 74, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Brahimaj, A.; Rivadeneira, F.; Muka, T.; Sijbrands, E.J.G.; Franco, O.H.; Dehghan, A.; Kavousi, M. Novel metabolic indices and incident type 2 diabetes among women and men: The Rotterdam Study. Diabetologia 2019, 62, 1581–1590. [Google Scholar] [CrossRef] [PubMed]
- Navarro González, D.; Sánchez Íñigo, L.; Pastrana Delgado, J.; Fernández Montero, A.; Martinez, J.A. Triglyceride–glucose index (TyG index) in comparison with fasting plasma glucose improves diabetes prediction in patients with normal fasting glucose: The Vascular Metabolic CUN cohort. Prev. Med. 2016, 86, 99–105. [Google Scholar] [CrossRef] [PubMed]
- D’Elia, L.; De Palma, D.; Rossi, G.; Strazzullo, V.; Russo, O.; Iacone, R.; Fazio, V.; Strazzullo, P.; Galletti, F. Not smoking is associated with lower risk of hypertension: Results of the Olivetti Heart Study. Eur. J. Public. Health 2014, 24, 226–230. [Google Scholar] [CrossRef] [PubMed]
- D’Elia, L.; Manfredi, M.; Sabino, P.; Strazzullo, P.; Galletti, F. The Olivetti Heart Study: Predictive value of a new adiposity index on risk of hypertension, blood pressure, and subclinical organ damage. Nutr. Metab. Cardiovasc. Dis. 2016, 26, 630–636. [Google Scholar] [CrossRef] [PubMed]
- EQUATOR Network. STROBE Guidelines. Available online: https://www.equator-network.org/reporting-guidelines/strobe/ (accessed on 10 June 2025).
- American Diabetes Association Professional Practice Committee. 2. Diagnosis and Classification of Diabetes: Standards of Care in Diabetes—2024. Diabetes Care 2024, 47 (Suppl. 1), S20–S42. [Google Scholar] [CrossRef]
- Levey, A.S.; Inker, L.A.; Coresh, J. GFR estimation: From physiology to public health. Am. J. Kidney Dis. 2014, 63, 820–834. [Google Scholar] [CrossRef]
- World Health Organization. Obesity and Overweight. World Health Organization, 2025. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 12 June 2025).
- National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Final Report. Circulation 2002, 106, 3143–3421. [Google Scholar] [CrossRef]
- Harrell, F.E. Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis; Springer: New York, NY, USA, 2001. [Google Scholar]
- D’Elia, L.; Masulli, M.; Rendina, D.; Iacone, R.; Russo, O.; Zarrella, A.F.; Abate, V.; Strazzullo, P.; Galletti, F. Predictive role of triglyceride–glucose index and HOMA index on development of arterial stiffening in non diabetic men. Nutr. Metab. Cardiovasc. Dis. 2024, 34, 2464–2471. [Google Scholar] [CrossRef]
- D’Elia, L.; Masulli, M.; Barbato, A.; Rendina, D.; Iacone, R.; Russo, O.; Strazzullo, P.; Galletti, F. Triglyceride–glucose index, HOMA index and metabolic syndrome in a sample of adult men. Minerva Med. 2024, 115, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Wells, J.C.K. Sexual dimorphism of body composition. Best. Pract. Res. Clin. Endocrinol. Metab. 2007, 21, 415–430. [Google Scholar] [CrossRef] [PubMed]
- Mauvais Jarvis, F.; Clegg, D.J.; Hevener, A.L. The role of estrogens in control of energy balance and glucose homeostasis. Endocr. Rev. 2013, 34, 309–338. [Google Scholar] [CrossRef]
- Nedungadi, T.P.; Clegg, D.J. Sexual dimorphism in body fat distribution and risk for cardiovascular diseases. J. Cardiovasc. Transl. Res. 2009, 2, 321–327. [Google Scholar] [CrossRef]
- D’Elia, L. Potassium intake and human health. Nutrients 2024, 16, 833. [Google Scholar] [CrossRef]
- Chatterjee, R.; Yeh, H.C.; Shafi, T.; Selvin, E.; Anderson, C.; Pankow, J.S.; Miller, E.; Brancati, F. Serum and dietary potassium and risk of incident type 2 diabetes mellitus: The Atherosclerosis Risk in Communities (ARIC) Study. Arch. Intern. Med. 2010, 170, 1745–1751. [Google Scholar] [CrossRef]
- Conen, K.; Scanni, R.; Gombert, M.T.; Hulter, H.N.; Krapf, R. Effects of potassium citrate or potassium chloride in patients with combined glucose intolerance: A placebo controlled pilot study. J. Diabetes Complicat. 2016, 30, 1158–1161. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, R.; Slentz, C.; Davenport, C.A.; Johnson, J.; Lin, P.H.; Muehlbauer, M.; D’Alessio, D.; Svetkey, L.P.; Edelman, D. Effects of potassium supplementation on glucose metabolism in African Americans with prediabetes: A pilot trial. Am. J. Clin. Nutr. 2017, 106, 1431–1438. [Google Scholar] [CrossRef] [PubMed]

| N. of Participants | 789 |
|---|---|
| Age (yrs) | 51.3 ± 7.2 |
| BMI (kg/m2) | 26.9 ± 3.0 |
| Excess body weight (%) | 73.7 |
| Waist Circumference (cm) | 94.4 ± 8.4 |
| Abdominal obesity (%) | 16.9 |
| eGFR (mL/min/1.73 m2) 1 | 97.7 ± 1.2 |
| Renal damage (eGFR < 60 mL/min/1.73 m2) (%) | 0.5 |
| Lipid-lowering therapy—yes (%) | 12.0 |
| Physical activity—yes (%) | 35.0 |
| Alcohol consumption—yes (%) | 79.7 |
| TyG (Units) | 4.72 ± 0.25 |
| Incident T2DM | YES | NO | p-Value |
|---|---|---|---|
| N. of participants | 54 | 735 | |
| Age (yrs) | 51.9 ± 5.6 | 51.2 ± 7.3 | 0.5 |
| BMI (kg/m2) | 28.4 ± 2.9 | 26.8 ± 3.0 | <0.001 |
| Excess body weight (%) | 90.7 | 9.3 | <0.001 |
| Waist Circumference (cm) | 98.0 ± 8.1 | 94.2 ± 8.3 | 0.001 |
| Abdominal obesity (%) | 28.3 | 16.1 | 0.02 |
| eGFR (mL/min/1.73 m2) 1 | 95.5 ± 1.1 | 97.7 ± 1.2 | 0.7 |
| Renal damage (eGFR < 60 mL/min/1.73 m2) (%) | 0 | 0.5 | 0.6 |
| Lipid-lowering therapy—yes (%) | 25.9 | 11.0 | 0.001 |
| Physical activity—yes (%) | 43.4 | 34.4 | 0.2 |
| Alcohol consumption—yes (%) | 73.6 | 80.1 | 0.2 |
| TyG (Unit) | 4.82 ± 0.29 | 4.71 ± 0.25 | 0.002 |
| High-TyG (>4.88) | Low-TyG (≤4.88) | |
|---|---|---|
| N. of participants | 202 | 587 |
| Age (yrs) | 51.6 ± 6.8 | 51.2 ± 7.3 |
| BMI (kg/m2) | 27.7 ± 2.8 * | 26.6 ± 3.0 |
| Excess body weight (%) | 83.2 * | 70.5 |
| Waist circumference (cm) | 97.1 ± 7.7 * | 93.5 ± 8.4 |
| Abdominal obesity (%) | 24.0 * | 14.5 |
| eGFR (mL/min/1.73 m2) 1 | 94.8 ± 1.1 * | 97.7 ± 1.2 |
| Renal damage (eGFR < 60 mL/min/1.73 m2) (%) | 0 | 0.5 |
| Lipid-lowering therapy—yes (%) | 19.8 * | 9.4 |
| Physical activity—yes (%) | 36.7 | 34.4 |
| Alcohol consumption—yes (%) | 80.6 | 79.4 |
| TyG (Unit) | 5.0 ± 0.1 * | 4.6 ± 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Elia, L.; Rendina, D.; Iacone, R.; Strazzullo, P.; Galletti, F. Triglyceride–Glucose Index and New-Onset Type 2 Diabetes Mellitus in Middle-Aged Men. Metabolites 2025, 15, 537. https://doi.org/10.3390/metabo15080537
D’Elia L, Rendina D, Iacone R, Strazzullo P, Galletti F. Triglyceride–Glucose Index and New-Onset Type 2 Diabetes Mellitus in Middle-Aged Men. Metabolites. 2025; 15(8):537. https://doi.org/10.3390/metabo15080537
Chicago/Turabian StyleD’Elia, Lanfranco, Domenico Rendina, Roberto Iacone, Pasquale Strazzullo, and Ferruccio Galletti. 2025. "Triglyceride–Glucose Index and New-Onset Type 2 Diabetes Mellitus in Middle-Aged Men" Metabolites 15, no. 8: 537. https://doi.org/10.3390/metabo15080537
APA StyleD’Elia, L., Rendina, D., Iacone, R., Strazzullo, P., & Galletti, F. (2025). Triglyceride–Glucose Index and New-Onset Type 2 Diabetes Mellitus in Middle-Aged Men. Metabolites, 15(8), 537. https://doi.org/10.3390/metabo15080537






