Traditionally, dietary TA has been viewed as an antinutritional factor that negatively impacts animal feed intake and growth performance [
18]. A study by Schiavone et al. showed that growth performance was significantly improved in broilers fed 0.2% natural extract of chestnut wood, with no adverse effects on organ health being detected [
19]. In line with these reports, we observed that the dietary inclusion of 0.18% TA (TA3 group) significantly improved the ADG, while simultaneously decreasing diarrhea index scores in weaned piglets. However, some negative effects were noted with the addition of 0.24% TA (TA4 group) to the diet. This may be due to the ability of tannic acid to bind starch, proteins and digestive enzymes to form insoluble complexes, resulting in higher levels of tannic acid in diets that reduce palatability, nutrient digestibility and growth.
Villus height, crypt depth and their ratio are commonly used parameters for evaluating gut function and absorption status [
20,
21]. Zhao et al. found that the addition of 0.1% tannins to the diet of Hu sheep increased the VH in the jejunum and ileum and had a tendency to decrease CD in the ileum [
22]. A separate study revealed that adding 0.2% TA to the diet decreased the CD and increased the V/C ratio in the duodenum, while 1.0% TA tended to reduce CD and VH in the ileum [
12]. Our findings showed that supplementing the diet of weaned piglets with an appropriate amount of TA improved the VH and the V/C ratio and exerted a positive effect on the morphology of the gut mucosa. In turn, this enhanced the growth and intestinal health of the weaned piglets, with the best effect being achieved with the addition of 0.18% TA. Meanwhile, there was a dose-dependent effect of TA, with a reduced efficacy of TA4 (0.24%) indicating a dose-dependent threshold.
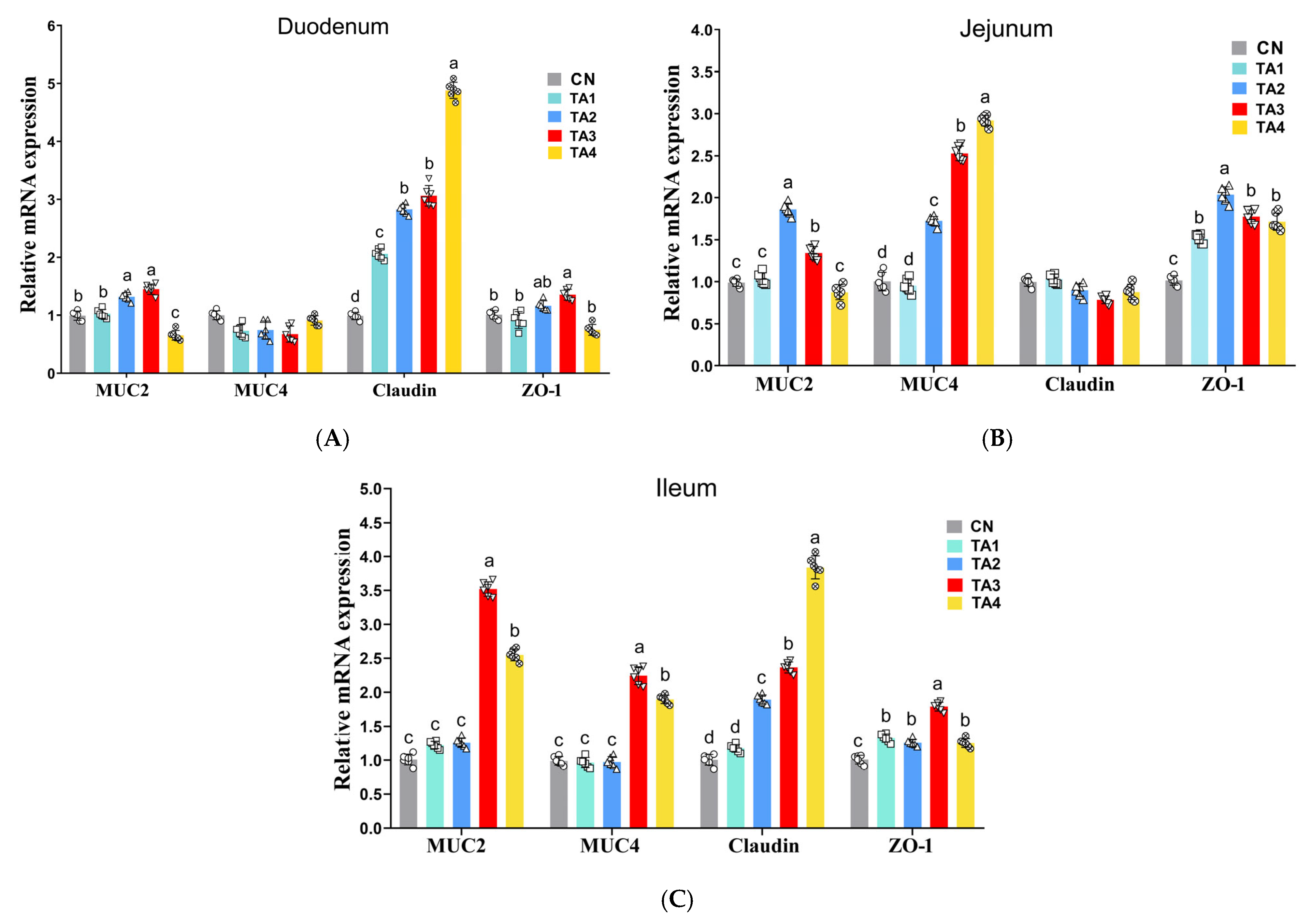
Tight junction proteins and mucins are essential components of the intestinal barrier, and a decrease in their expression can lead to increased intestinal permeability and inflammation [
23]. Research has shown that early weaning in piglets is often accompanied by a reduction in tight junction protein levels, an increase in intestinal permeability and the occurrence of diarrhea [
1]. Our findings suggested that TA supplementation in piglet diets upregulated the expression of
ZO-1,
claudin,
MUC2 and
MUC4 in the intestine of weaned piglets, implying that TA can alleviate weaning stress-induced damage in the gut barrier.
Weaning readily leads to excessive free radical production and oxidative stress in piglets [
24]. The activities of T-SOD and GSH-Px, along with T-AOC and MDA levels, serve as important indicators for assessing antioxidant capacity [
25]. Our findings showed that adding TA to the diet of weaned piglets improved GSH-Px activity and T-AOC in the duodenum and jejunum, increased T-SOD activity in the intestine and tended to lower MDA generation. Again, 0.18% was found to be the optimal TA supplementation level. These findings suggest that TA improves intestinal morphology and barrier integrity, at least in part by enhancing intestinal antioxidant capacity and reducing intestinal damage.
The gut microbiota and their metabolites are closely related to the physiological activities of animals, such as nutrition, metabolism and stress responses [
26]. Accordingly, gut dysbiosis is considered to be a key factor responsible for post-weaning diarrhea and intestinal infections. In this study, alpha diversity analysis showed that dietary TA increased the Chao1 and Faith’s PD indexes, which implied that TA increased the diversity of the gut flora in the weaned piglets. In this study, Good’s coverage in each group exceeded 99%, thereby accurately reflecting the community composition of the fecal flora of piglets.
In this work, at the phylum level, we observed that the dominant cecal microbial communities in the piglets were Bacteroidetes and Firmicutes. Firmicutes can break down cellulose and degrade complex macromolecules, thereby improving the body’s immunity, digestion rate and resistance to pathogens. These effects are primarily mediated through the production of SCFAs, which modulate inflammation and serve as an energy source for gut epithelial cells. Bacteroidetes have high polysaccharide-degrading capacity, which contributes to enhanced host immunity and improved intestinal mucosal barrier function [
27]. Here, we found that TA supplementation elevated the relative abundance of Firmicutes and the Firmicutes/Bacteroidetes ratio while concurrently reducing the relative abundance of Bacteroidetes. At the family level, the most abundant cecal microorganisms in the weaned piglets were
Lactobacillaceae, followed by
Prevotellaceae and
Ruminococcaceae. Notably, TA addition increased the abundances of
Lactobacillaceae and
Rumatobacteriaceae and decreased that of
Prevotellaceae in the piglet cecum.
Lactobacillaceae are the prominent probiotic in the gut and can ameliorate intestinal permeability and barrier function by increasing occludin expression and suppressing the proliferation of harmful bacteria [
28].
Prevotellaceae are involved in the regulation of glucose metabolism and were reported to be positively associated with gestational diabetes [
29].
Rumatococcaceae are the main microorganisms responsible for transforming primary bile acids into secondary ones, thereby stabilizing the intestinal barrier and reducing gut inflammation [
30]. At the genus level, we found that TA elevated the relative abundances of
Lactobacillus and
SMB53 and reduced that of
Prevotella in the cecal flora of the piglets. In pigs,
Lactobacillus plays an important part in regulating immunity, sustaining homeostasis and health, aiding digestion and improving growth [
25].
SMB53, a member of the
Clostridiaceae family, can consume intestinal mucus and plant-derived sugars, suggesting that it is involved in sugar metabolism [
31].
Prevotella is an opportunistic pathogen that not only ferments non-starch polysaccharides, yielding SCFAs, but also increases intestinal permeability, which leads to inflammation [
32]. LefSe analyses showed that the cecal flora of the weaned piglets in the Control group was rich in
Slackia and
Collinsella, potentially explaining the occurrence of diarrhea in the piglets.
Slackia abundance is notably elevated in chickens with low body weight, and these bacteria promote the deposition of body fat in animals [
33]. The influence of
Collinsella in glucose-lipid metabolism may be related to its involvement in the inflammatory response and the disruption of the gut mucosal barrier [
34]. Piglets in the TA2 group had the highest abundances of
Lactobacillaceae,
Lactobacillus,
Lactobacillales and
Bacilli, while the greatest relative abundances of
Clostridiaceae,
SMB53,
Clostridium,
Desulfovibrio,
Deliaproterobacteria,
Desulfovibrionales,
Desulfovibrionaceae and
Peptostreptococaceae were recorded in the TA3 group. These results suggest that dietary TA supplementation improves gut health and growth in weaned piglets by increasing the abundance of probiotics and lowering that of pathogenic bacteria in the intestinal microbiota.
Short-chain fatty acids, the main metabolites generated by intestinal flora through the fermentation of dietary fiber and starch, are saturated fatty acids with a carbon chain of six or fewer carbon atoms. These include formic, acetic, propionic, butyric, valeric and hexanoic acids, along with their isomers. A previous has demonstrated that SCFAs can promote the growth and maturation of intestinal tissue, maintain the integrity of the gut barrier and mitigate gut inflammation [
35]. In this work, we found that dietary TA supplementation led to an increase in the contents of acetic, propionic, isobutyric, hexanoic, valeric and isovaleric acids, as well as in the total SCFA content in the cecum of piglets, indicating that adding TA to the diet exerts a positive effect on the gut flora of weaned piglets. The best effect was seen with the 0.18% TA inclusion level. However, there was no significant change in the content of butyric acid, which was inconsistent with the previous report [
8], probably due to the difference in the type and added amount of TA, and the specific reason needs to be further investigated. We speculate that dietary TA improves the intestinal environment in weaned piglets by elevating the production of SCFAs, thereby contributing to improved growth performance.