Extracellular Vesicle Metabolomics Holds Promise for Adult Axon Regeneration
Abstract
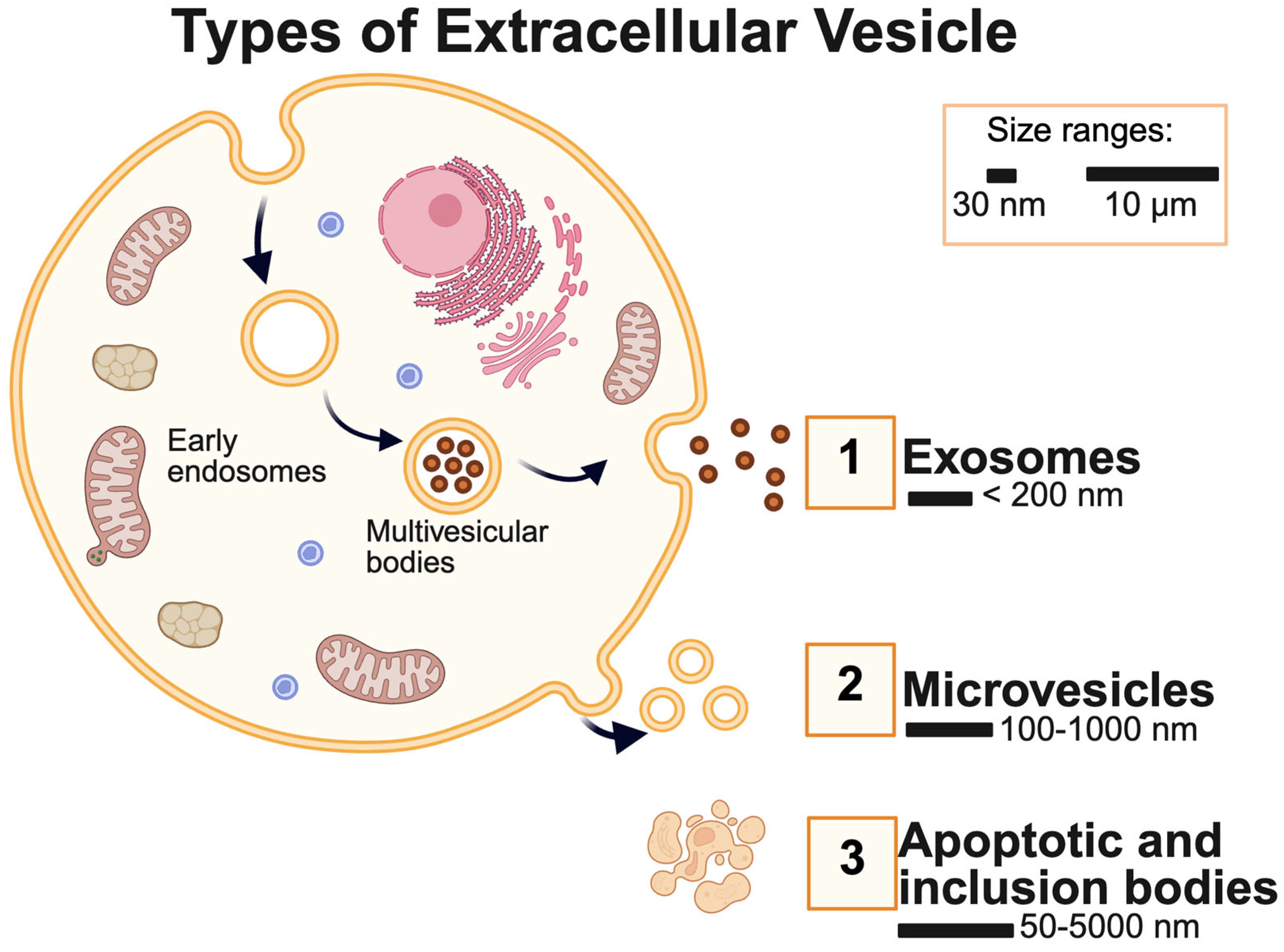
1. Introduction
2. Axon Regeneration
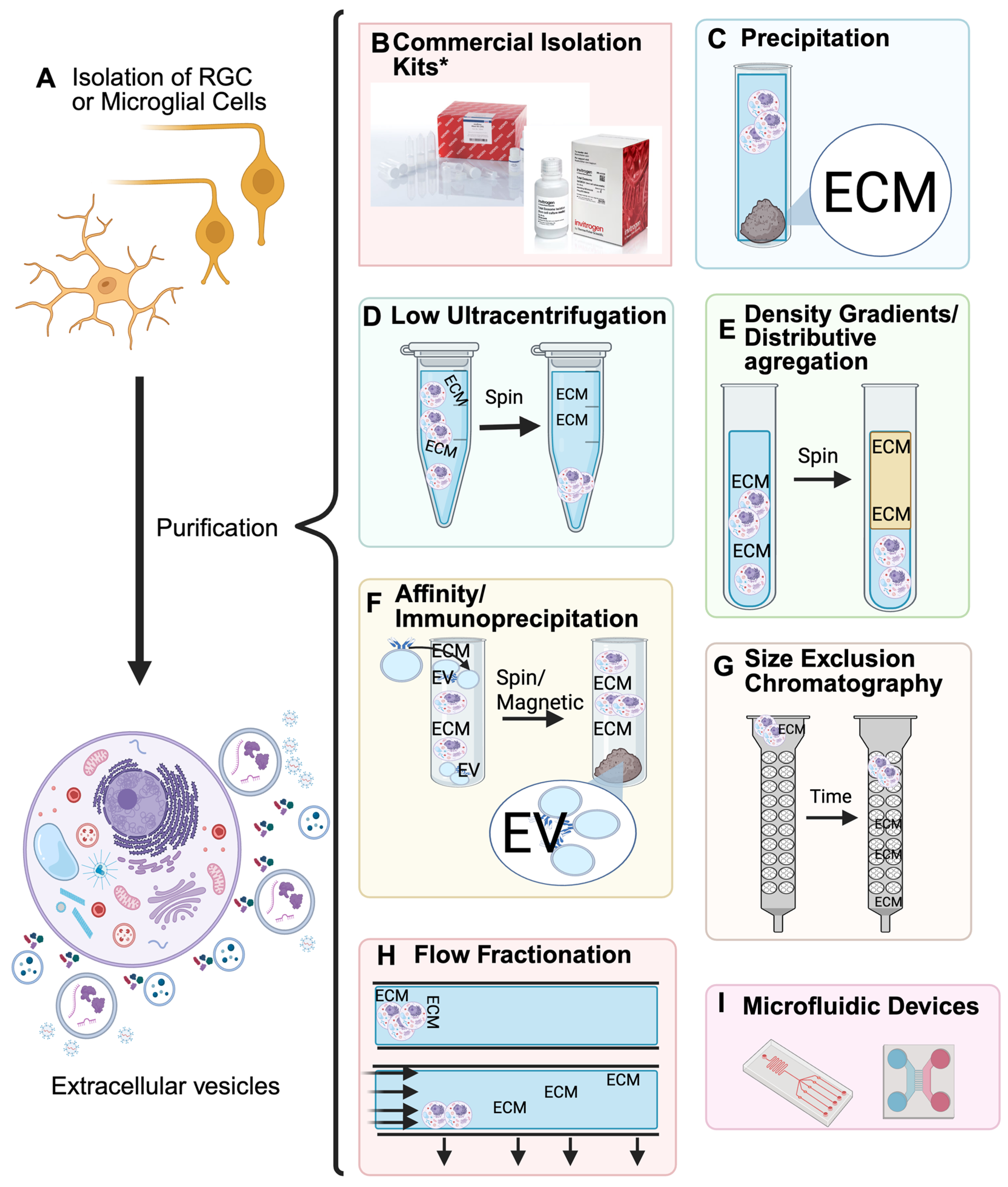
3. Isolation
| Isolation Kits | |||
|---|---|---|---|
| Company | Type of Isolation | Catalog # | Reference |
| ThermoFisher (Waltham, MA, USA) | Total Isolation from cell culture | 4478359 | [39] |
| Total Isolation from plasma | 4484450 | [40,41] | |
| Total Isolation from serum | 4478360 | [39] | |
| Total Isolation from urine | 4484452 | [39] | |
| Total Exosome RNA and Protein Isolation | 4478545 | [39] | |
| QIAGEN (Hilden, Germany) | exoEasy Maxi Kit | 76064 | [42] |
| miRCURY Exosome Serum/Plasma Kit | 76603 | [42] | |
| System bioscience (Palo Alto, CA, USA) | ExoQuick-TC® ULTRA for Tissue Culture Media | EQULTRA-20TC-1 | [43,44] |
| The Original ExoQuick | EXOQ5A-1 | [44] | |
| ExoQuick® Exosome Isolation and RNA Purification Kit (for Tissue Culture Media) | EQ806TC-1 | [43] | |
| ExoQuick® Exosome Isolation and RNA Purification Kit (for Serum and Plasma) | EQ806A-1 | [41] | |
| IZON (Christchurch, New Zealand) | qEVoriginal Columns | ICO-35 | [42,44] |
| Fujifilm (Tokyo, Japan) | MagCapture ™ Exosome Isolation Kit PS Ver.2 | 294-84101 | [45,46] |
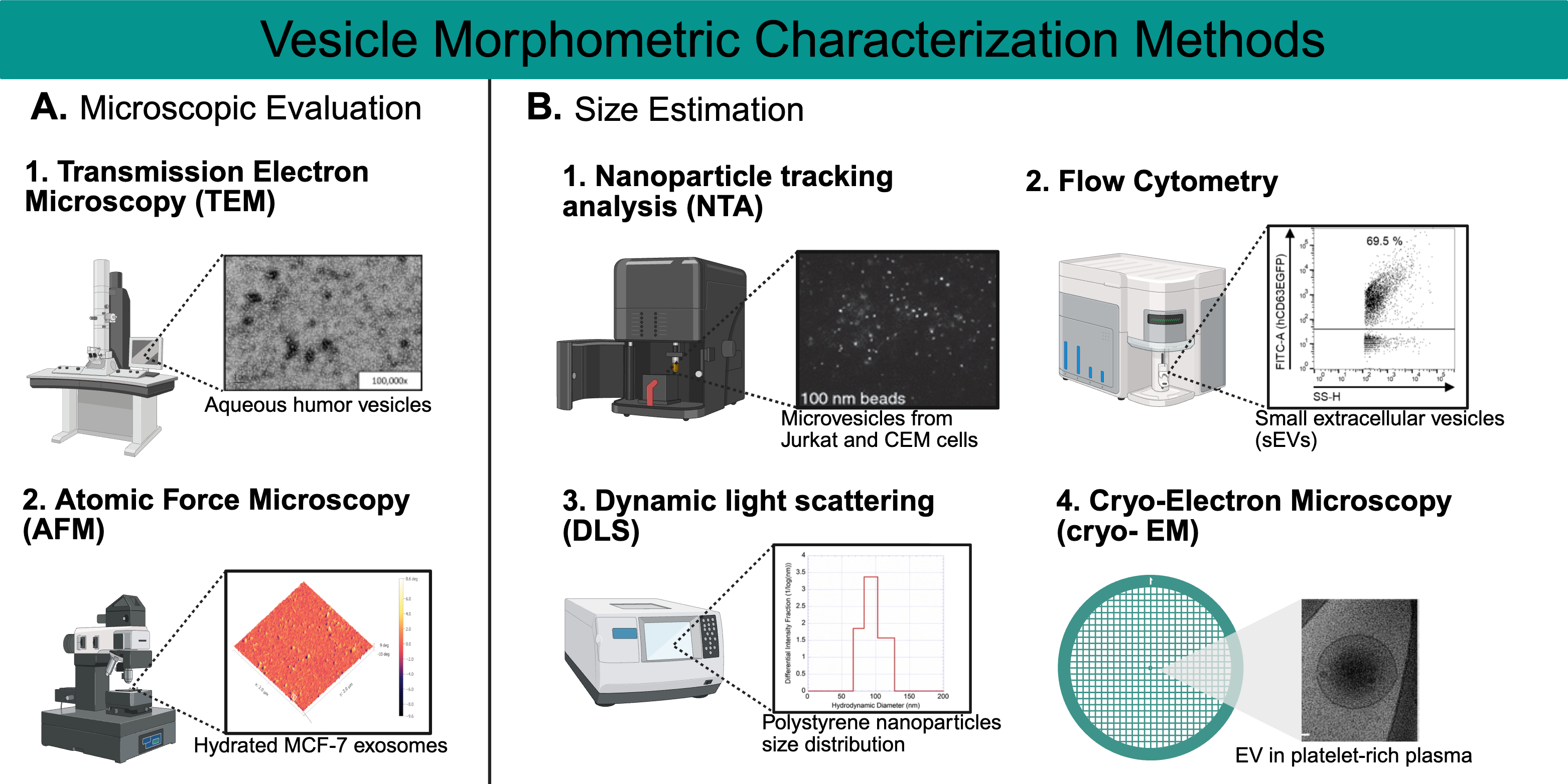
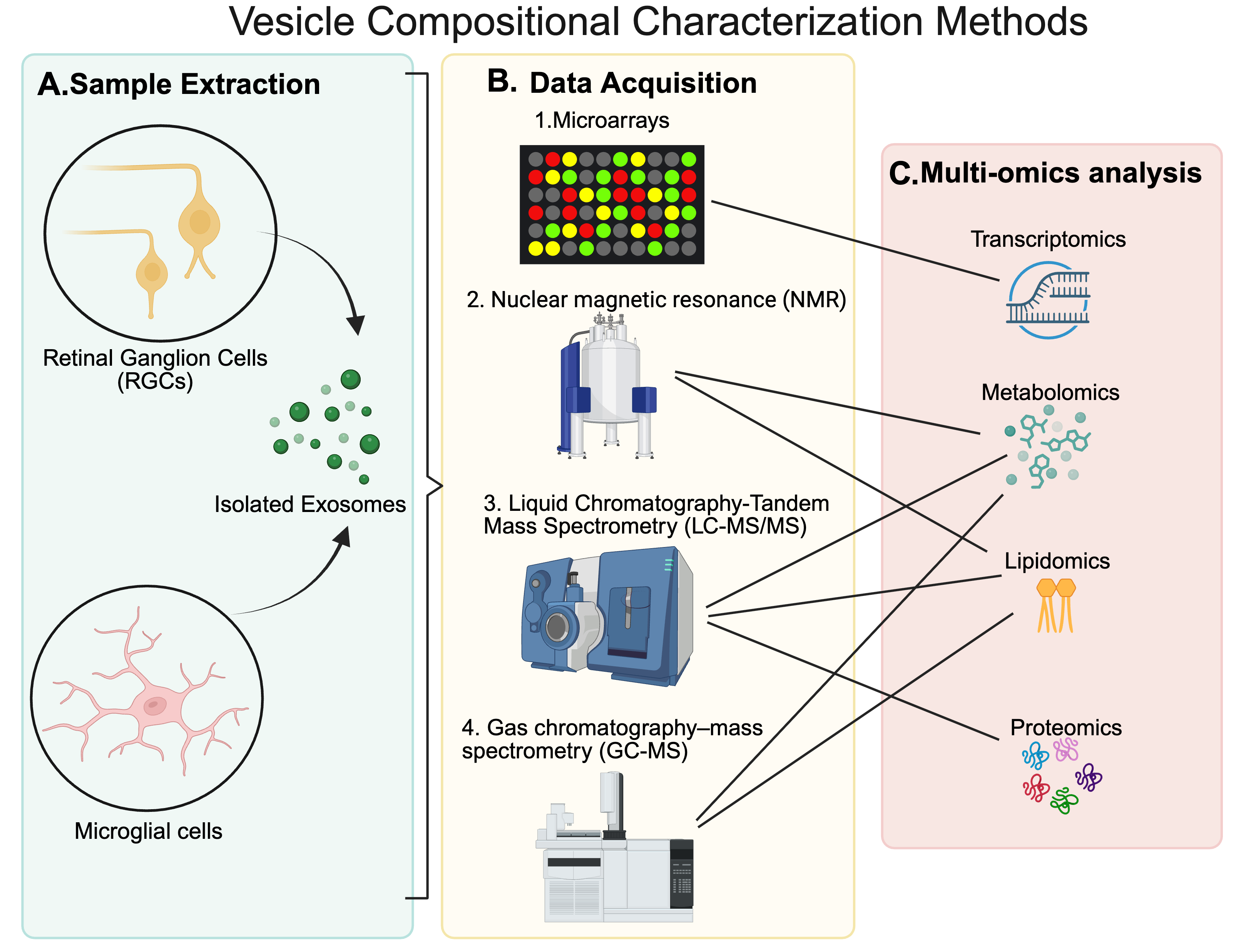
4. Characterization
4.1. Morphometric Characterization
4.2. Compositional Characterization
4.3. Data Processing and Analysis in MS-Based Exosome Research
| Data Analysis and Processing Resources | |||
|---|---|---|---|
| Omics Type | Data Analysis and Processing | Use(s) | |
| Proteomics | Proteome Discoverer™ | https://www.thermofisher.com/us/en/home/industrial/mass-spectrometry/liquid-chromatography-mass-spectrometry-lc-ms/lc-ms-software/multi-omics-data-analysis/proteome-discoverer-software.html?erpType=undefined (accessed on 9 April 2025) | Raw Data Processing, Data Normalization, Statistical Analysis, Identification of PTMs |
| MaxQuant | https://www.maxquant.org/ (accessed on 9 April 2025) | Raw Data Processing | |
| Express Analyst | https://www.expressanalyst.ca/ (accessed on 9 April 2025) | Statistical Analysis, Data Visualization | |
| Uniprot | https://www.uniprot.org/ (accessed on 9 April 2025) | Protein and Peptide Description | |
| KEGG | https://www.genome.jp/kegg/ (accessed on 9 April 2025) | Pathway Analysis | |
| ProteomeXchange | https://www.proteomexchange.org/ (accessed on 9 April 2025) | Data Repository | |
| Lipidomics | Lipid Search™ | https://www.thermofisher.com/order/catalog/product/OPTON-30880 (accessed on 9 April 2025) | Raw Data Processing |
| MS-DIAL | https://systemsomicslab.github.io/compms/msdial/main.html (accessed on 9 April 2025) | Raw Data Processing | |
| mzMine | https://mzio.io/mzmine-news/ (accessed on 9 April 2025) | Raw Data Processing | |
| Compound Discoverer™ | https://www.thermofisher.com/us/en/home/industrial/mass-spectrometry/liquid-chromatography-mass-spectrometry-lc-ms/lc-ms-software/multi-omics-data-analysis/compound-discoverer-software.html (accessed on 9 April 2025) | Raw Data Processing, Spectral Identification | |
| MetaboAnalyst 6.0 | https://www.metaboanalyst.ca/ (accessed on 9 April 2025) | Statistical Analysis, Data Visualization | |
| LipidOne | https://lipidone.eu/ (accessed on 9 April 2025) | Statistical Analysis, Data Visualization | |
| LIPEA | https://hyperlipea.org/home (accessed on 9 April 2025) | Pathway Analysis | |
| KEGG | https://www.genome.jp/kegg/ (accessed on 9 April 2025) | Pathway Analysis | |
| Metabolomics Workbench | https://www.metabolomicsworkbench.org/ (accessed on 9 April 2025) | Data Repository | |
| Metabolomics | Compound Discoverer™ | https://www.thermofisher.com/us/en/home/industrial/mass-spectrometry/liquid-chromatography-mass-spectrometry-lc-ms/lc-ms-software/multi-omics-data-analysis/compound-discoverer-software.html (accessed on 9 April 2025) | Raw Data Processing, Spectral Identification |
| MS-DIAL | https://systemsomicslab.github.io/compms/msdial/main.html (accessed on 9 April 2025) | Raw Data Processing | |
| mzMine | https://mzio.io/mzmine-news/ (accessed on 9 April 2025) | Raw Data Processing | |
| MetaboAnalyst 6.0 | https://www.metaboanalyst.ca/ (accessed on 9 April 2025) | Statistical Analysis, Data Visualization | |
| KEGG | https://www.genome.jp/kegg/ (accessed on 9 April 2025) | Pathway Analysis | |
| Metabolomics Workbench | https://www.metabolomicsworkbench.org/ (accessed on 9 April 2025) | Data Repository | |
| Integromics | OmicsNet 2.0 | https://www.omicsnet.ca/ (accessed on 9 April 2025) | Data Processing, Data Visualization |
| OmicsAnalyst 2.0 | https://www.omicsanalyst.ca/ (accessed on 9 April 2025) | Data Processing, Data Visualization | |
5. Discussion
| Microvesicles | ||
|---|---|---|
| Metabolites | Axon Regeneration | Reference (PMIDs) |
| N-acetylglycosamine | CNS, PNS | [116,117] |
| Allantoin | PNS | [118,136] |
| Histidine | CNS, PNS | [119] |
| Lysophosphatidylcholine (LPC) | CNS | [120,121] |
| Lysophosphatidylethanolamine (LPE) | CNS | [122,123] |
| Taurine | CNS | [124,125,126,127] |
| Gangliosides | CNS, PNS | [38] |
| Exosomes | ||
| Metabolites | Axon regeneration | Reference |
| Acetyl-L-Carnitine (ALCAR) | CNS, PNS | [128,129] |
| Carnosine | CNS | [130,131] |
| Dimethylglycine | CNS | [132] |
| Histidine | CNS, PNS | [119] |
| Lysophosphatidylcholine (LPC) | CNS | [120,133] |
| Lysophosphatidylethanolamine (LPE) | CNS | [122,123] |
| Taurine | CNS | [124,125,126,127] |
| Gangliosides | CNS, PNS | [38] |
| Lysophosphatidic acid (LPA) | CNS, PNS | [134] |
| Modified vesicles/exosomes | ||
| Metabolites | Axon regeneration | Reference |
| Sphingolipids | CNS | [92,135] |
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EVs | Extracellular Vesicles |
| CNS | Central Nervous System |
| PNS | Peripheral Nervous System |
| RGCs | Retinal ganglion cells |
| MGCs | Microglial cells |
| PEG | polyethylene glycol |
| IA | Immunoaffinity |
| SEC | Size exclusion chromatography |
| A4F | Asymmetric flow field-flow fractionation |
| TEM | Transmission Electron Microscopy |
| AFM | Atomic Force Microscopy |
| Cryo-EM | Cryo-electron microscopy |
| NTA | Nanoparticle Tracking Analysis |
| DLS | Dynamic Light Scattering |
| MS | Mass Spectrometry |
| LC-MS/MS | Liquid Chromatography-Tandem Mass Spectrometry |
| ESI-MS | Electrospray Ionization |
| HRMS | High-Resolution Mass Spectrometry |
| DDA | Data-Dependent Acquisition |
| DIA | Data-Independent Acquisition |
| MALDI- TOF MS | Matrix-Assisted Laser Desorption/Ionization Time-of-Flight Mass Spectrometry |
| PCs | phosphatidylcholines |
| SMs | sphingomyelins |
| PEs | phosphatidylethanolamines |
| TMT | Tandem Mass Tag |
| FVEPs | Flash visual-evoked potential |
| UMSCs | Umbilical cord mesenchymal stem cells |
| BMSCs | Bone marrow mesenchymal stem cells |
| LPC | Lysophosphatidylcholine |
| LPE | Lysophosphatidylethanolamine |
| LPA | Lysophosphatidic acid |
References
- Xia, B.; Gao, J.; Li, S.; Huang, L.; Zhu, L.; Ma, T.; Zhao, L.; Yang, Y.; Luo, K.; Shi, X.; et al. Mechanical stimulation of Schwann cells promote peripheral nerve regeneration via extracellular vesicle-mediated transfer of microRNA 23b-3p. Theranostics 2020, 10, 8974–8995. [Google Scholar] [CrossRef] [PubMed]
- Klimovich, P.; Rubina, K.; Sysoeva, V.; Semina, E. New Frontiers in Peripheral Nerve Regeneration: Concerns and Remedies. Int. J. Mol. Sci. 2021, 22, 13380. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Xu, Y.; Ahmad, M.A.; Javed, R.; Hagiwara, H.; Tian, X. Exosomes as a Promising Therapeutic Strategy for Peripheral Nerve Injury. Curr. Neuropharmacol. 2021, 19, 2141–2151. [Google Scholar] [CrossRef] [PubMed]
- Geoffroy, C.G.; Lorenzana, A.O.; Kwan, J.P.; Lin, K.; Ghassemi, O.; Ma, A.; Xu, N.; Creger, D.; Liu, K.; He, Z.; et al. Effects of PTEN and Nogo Codeletion on Corticospinal Axon Sprouting and Regeneration in Mice. J. Neurosci. 2015, 35, 6413. [Google Scholar] [CrossRef]
- van der Merwe, Y.; Steketee, M.B. Extracellular Vesicles: Biomarkers, Therapeutics, and Vehicles in the Visual System. Curr. Ophthalmol. Rep. 2017, 5, 276–282. [Google Scholar] [CrossRef]
- Welsh, J.A.; Goberdhan, D.C.I.; O’Driscoll, L.; Buzas, E.I.; Blenkiron, C.; Bussolati, B.; Cai, H.; Di Vizio, D.; Driedonks, T.A.P.; Erdbrugger, U.; et al. Minimal information for studies of extracellular vesicles (MISEV2023): From basic to advanced approaches. J. Extracell. Vesicles 2024, 13, e12404. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, W.; Guo, M.; Tan, Q.; Zhou, E.; Deng, J.; Li, M.; Chen, J.; Yang, Z.; Jin, Y. Metabolomics of Extracellular Vesicles: A Future Promise of Multiple Clinical Applications. Int. J. Nanomed. 2022, 17, 6113–6129. [Google Scholar] [CrossRef]
- La Russa, V.F.; Mondal, D.; Miller, A.; Safah, H.; Rozans, M.; Curiel, T.; Agrawal, K.; Weiner, R. Neuronal stem cells biology and plasticity. Cancer Invest. 2003, 21, 792–804. [Google Scholar] [CrossRef]
- Innocenti, G.M. Defining neuroplasticity. Handb. Clin. Neurol. 2022, 184, 3–18. [Google Scholar] [CrossRef]
- Kriegstein, A.; Alvarez-Buylla, A. The glial nature of embryonic and adult neural stem cells. Annu. Rev. Neurosci. 2009, 32, 149–184. [Google Scholar] [CrossRef]
- Bradke, F. Mechanisms of Axon Growth and Regeneration: Moving between Development and Disease. J. Neurosci. 2022, 42, 8393–8405. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, M. Proteomic identification of the molecular basis of mammalian CNS growth cones. Neurosci. Res. 2014, 88, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Stevens, C.F. The Neuron. Sci. Am. 1979, 241, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Carroll, S.L. Wallerian Degeneration. In Encyclopedia of Neuroscience; Squire, L.R., Ed.; Academic Press: Oxford, UK, 2009; pp. 485–491. [Google Scholar]
- Conforti, L.; Gilley, J.; Coleman, M.P. Wallerian degeneration: An emerging axon death pathway linking injury and disease. Nat. Rev. Neurosci. 2014, 15, 394–409. [Google Scholar] [CrossRef]
- Chen, P.; Piao, X.; Bonaldo, P. Role of macrophages in Wallerian degeneration and axonal regeneration after peripheral nerve injury. Acta Neuropathol. 2015, 130, 605–618. [Google Scholar] [CrossRef]
- Geoffroy, C.G.; Hilton, B.J.; Tetzlaff, W.; Zheng, B. Evidence for an Age-Dependent Decline in Axon Regeneration in the Adult Mammalian Central Nervous System. Cell Rep. 2016, 15, 238–246. [Google Scholar] [CrossRef]
- Chen, M.; Zheng, B. Axon plasticity in the mammalian central nervous system after injury. Trends Neurosci. 2014, 37, 583–593. [Google Scholar] [CrossRef]
- Fan, L.; Liu, C.; Chen, X.; Zheng, L.; Zou, Y.; Wen, H.; Guan, P.; Lu, F.; Luo, Y.; Tan, G.; et al. Exosomes-Loaded Electroconductive Hydrogel Synergistically Promotes Tissue Repair after Spinal Cord Injury via Immunoregulation and Enhancement of Myelinated Axon Growth. Adv. Sci. 2022, 9, e2105586. [Google Scholar] [CrossRef]
- Fague, L.; Liu, Y.A.; Marsh-Armstrong, N. The basic science of optic nerve regeneration. Ann. Transl. Med. 2021, 9, 1276. [Google Scholar] [CrossRef]
- Steele-Nicholson, L.J.; Andrews, M.R. Axon-Targeting Motifs: Mechanisms and Applications of Enhancing Axonal Localisation of Transmembrane Proteins. Cells 2022, 11, 937. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, G.; Li, S.; Li, J.; Wang, W.; Xue, J.; Wang, Y.; Fang, M.; Zhou, N. Correction: Endothelial cell-derived exosomes boost and maintain repair-related phenotypes of Schwann cells via miR199-5p to promote nerve regeneration. J. Nanobiotechnol. 2024, 22, 81. [Google Scholar] [CrossRef] [PubMed]
- Mueller, M.; Leonhard, C.; Wacker, K.; Ringelstein, E.B.; Okabe, M.; Hickey, W.F.; Kiefer, R. Macrophage response to peripheral nerve injury: The quantitative contribution of resident and hematogenous macrophages. Lab. Invest. 2003, 83, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Perry, V.H.; Brown, M.C.; Gordon, S. The macrophage response to central and peripheral nerve injury. A possible role for macrophages in regeneration. J. Exp. Med. 1987, 165, 1218–1223. [Google Scholar] [CrossRef] [PubMed]
- Taskinen, H.S.; Roytta, M. The dynamics of macrophage recruitment after nerve transection. Acta Neuropathol. 1997, 93, 252–259. [Google Scholar] [CrossRef]
- Park, K.K.; Liu, K.; Hu, Y.; Kanter, J.L.; He, Z. PTEN/mTOR and axon regeneration. Exp. Neurol. 2010, 223, 45–50. [Google Scholar] [CrossRef]
- Park, K.K.; Liu, K.; Hu, Y.; Smith, P.D.; Wang, C.; Cai, B.; Xu, B.; Connolly, L.; Kramvis, I.; Sahin, M.; et al. Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science 2008, 322, 963–966. [Google Scholar] [CrossRef]
- Poongodi, R.; Chen, Y.L.; Yang, T.H.; Huang, Y.H.; Yang, K.D.; Lin, H.C.; Cheng, J.K. Bio-Scaffolds as Cell or Exosome Carriers for Nerve Injury Repair. Int. J. Mol. Sci. 2021, 22, 13347. [Google Scholar] [CrossRef]
- Thery, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Lopez-Leal, R.; Court, F.A. Schwann Cell Exosomes Mediate Neuron-Glia Communication and Enhance Axonal Regeneration. Cell. Mol. Neurobiol. 2016, 36, 429–436. [Google Scholar] [CrossRef]
- Zhu, S.; Chen, L.; Wang, M.; Zhang, J.; Chen, G.; Yao, Y.; Song, S.; Li, T.; Xu, S.; Yu, Z.; et al. Schwann cell-derived extracellular vesicles as a potential therapy for retinal ganglion cell degeneration. J. Control. Release 2023, 363, 641–656. [Google Scholar] [CrossRef]
- Massoumi, H.; Amin, S.; Soleimani, M.; Momenaei, B.; Ashraf, M.J.; Guaiquil, V.H.; Hematti, P.; Rosenblatt, M.I.; Djalilian, A.R.; Jalilian, E. Extracellular-Vesicle-Based Therapeutics in Neuro-Ophthalmic Disorders. Int. J. Mol. Sci. 2023, 24, 9006. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Leal, R.; Diaz-Viraque, F.; Catalan, R.J.; Saquel, C.; Enright, A.; Iraola, G.; Court, F.A. Schwann cell reprogramming into repair cells increases miRNA-21 expression in exosomes promoting axonal growth. J. Cell Sci. 2020, 133, jcs239004. [Google Scholar] [CrossRef] [PubMed]
- Royo, F.; Thery, C.; Falcon-Perez, J.M.; Nieuwland, R.; Witwer, K.W. Methods for Separation and Characterization of Extracellular Vesicles: Results of a Worldwide Survey Performed by the ISEV Rigor and Standardization Subcommittee. Cells 2020, 9, 1955. [Google Scholar] [CrossRef] [PubMed]
- Feodorova, Y.; Koch, M.; Bultman, S.; Michalakis, S.; Solovei, I. Quick and reliable method for retina dissociation and separation of rod photoreceptor perikarya from adult mice. MethodsX 2015, 2, 39–46. [Google Scholar] [CrossRef]
- Chintalapudi, S.R.; Patel, N.N.; Goldsmith, Z.K.; Djenderedjian, L.; Wang, X.D.; Marion, T.N.; Jablonski, M.M.; Morales-Tirado, V.M. Isolation of Primary Murine Retinal Ganglion Cells (RGCs) by Flow Cytometry. J. Vis. Exp. 2017, 5, 55785. [Google Scholar] [CrossRef]
- Weng, Y.; Sui, Z.; Shan, Y.; Hu, Y.; Chen, Y.; Zhang, L.; Zhang, Y. Effective isolation of exosomes with polyethylene glycol from cell culture supernatant for in-depth proteome profiling. Analyst 2016, 141, 4640–4646. [Google Scholar] [CrossRef]
- Lopez, P.H.H.; Baez, B.B. Gangliosides in Axon Stability and Regeneration. Prog. Mol. Biol. Transl. Sci. 2018, 156, 383–412. [Google Scholar] [CrossRef]
- Qazi, R.E.M.; Sajid, Z.; Zhao, C.; Hussain, I.; Iftikhar, F.; Jameel, M.; Rehman, F.U.; Mian, A.A. Lyophilization Based Isolation of Exosomes. Int. J. Mol. Sci. 2023, 24, 10477. [Google Scholar] [CrossRef]
- Noboa-Velastegui, J.; Leon, J.C.; Castro, J.; Fletes, A.; Madrigal, P.; Alvarez, I.; Navarro, R. Comparison of Methods for Isolating Exosomes from Plasma Subjects with Normal and High Fat Percentages. Life 2025, 15, 410. [Google Scholar] [CrossRef]
- Martins, T.S.; Catita, J.; Rosa, I.M.; da Cruz e SilvaSilva, O.A.B.; Henriques, A.G. Exosome isolation from distinct biofluids using precipitation and column-based approaches. PLoS ONE 2018, 13, e0198820. [Google Scholar] [CrossRef]
- Veerman, R.E.; Teeuwen, L.; Czarnewski, P.; Gucluler Akpinar, G.; Sandberg, A.; Cao, X.; Pernemalm, M.; Orre, L.M.; Gabrielsson, S.; Eldh, M. Molecular evaluation of five different isolation methods for extracellular vesicles reveals different clinical applicability and subcellular origin. J. Extracell. Vesicles 2021, 10, e12128. [Google Scholar] [CrossRef]
- Chen, J.; Li, P.; Zhang, T.; Xu, Z.; Huang, X.; Wang, R.; Du, L. Review on Strategies and Technologies for Exosome Isolation and Purification. Front. Bioeng. Biotechnol. 2021, 9, 811971. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Lee, K.; Park, I.B.; Kim, N.H.; Cho, S.; Rhee, W.J.; Oh, Y.; Choi, J.; Nam, S.; Lee, D.H. The profiles of microRNAs from urinary extracellular vesicles (EVs) prepared by various isolation methods and their correlation with serum EV microRNAs. Diabetes Res. Clin. Pract. 2020, 160, 108010. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Zhao, M.; Hu, Y.; Guo, H.; Zhang, Y.; Huang, Y.; Zhao, L.; Chai, Y.; Wang, Z. Blockade of exosome generation by GW4869 inhibits the education of M2 macrophages in prostate cancer. BMC Immunol. 2022, 23, 37. [Google Scholar] [CrossRef] [PubMed]
- Muraoka, S.; Jedrychowski, M.P.; Yanamandra, K.; Ikezu, S.; Gygi, S.P.; Ikezu, T. Proteomic Profiling of Extracellular Vesicles Derived from Cerebrospinal Fluid of Alzheimer’s Disease Patients: A Pilot Study. Cells 2020, 9, 1959. [Google Scholar] [CrossRef]
- Konoshenko, M.Y.; Lekchnov, E.A.; Vlassov, A.V.; Laktionov, P.P. Isolation of Extracellular Vesicles: General Methodologies and Latest Trends. Biomed Res. Int. 2018, 2018, 8545347. [Google Scholar] [CrossRef]
- Gardiner, C.; Di Vizio, D.; Sahoo, S.; Thery, C.; Witwer, K.W.; Wauben, M.; Hill, A.F. Techniques used for the isolation and characterization of extracellular vesicles: Results of a worldwide survey. J. Extracell. Vesicles 2016, 5, 32945. [Google Scholar] [CrossRef]
- Livshits, M.A.; Khomyakova, E.; Evtushenko, E.G.; Lazarev, V.N.; Kulemin, N.A.; Semina, S.E.; Generozov, E.V.; Govorun, V.M. Isolation of exosomes by differential centrifugation: Theoretical analysis of a commonly used protocol. Sci. Rep. 2015, 5, 17319. [Google Scholar] [CrossRef]
- Momen-Heravi, F.; Balaj, L.; Alian, S.; Mantel, P.Y.; Halleck, A.E.; Trachtenberg, A.J.; Soria, C.E.; Oquin, S.; Bonebreak, C.M.; Saracoglu, E.; et al. Current methods for the isolation of extracellular vesicles. Biol. Chem. 2013, 394, 1253–1262. [Google Scholar] [CrossRef]
- Cantin, R.; Diou, J.; Belanger, D.; Tremblay, A.M.; Gilbert, C. Discrimination between exosomes and HIV-1: Purification of both vesicles from cell-free supernatants. J. Immunol. Methods 2008, 338, 21–30. [Google Scholar] [CrossRef]
- Boing, A.N.; van der Pol, E.; Grootemaat, A.E.; Coumans, F.A.; Sturk, A.; Nieuwland, R. Single-step isolation of extracellular vesicles by size-exclusion chromatography. J. Extracell. Vesicles 2014, 3, 23430. [Google Scholar] [CrossRef]
- Van Deun, J.; Mestdagh, P.; Sormunen, R.; Cocquyt, V.; Vermaelen, K.; Vandesompele, J.; Bracke, M.; De Wever, O.; Hendrix, A. The impact of disparate isolation methods for extracellular vesicles on downstream RNA profiling. J. Extracell. Vesicles 2014, 3, 24858. [Google Scholar] [CrossRef] [PubMed]
- Greening, D.W.; Xu, R.; Ji, H.; Tauro, B.J.; Simpson, R.J. A protocol for exosome isolation and characterization: Evaluation of ultracentrifugation, density-gradient separation, and immunoaffinity capture methods. Methods Mol. Biol. 2015, 1295, 179–209. [Google Scholar] [CrossRef] [PubMed]
- Kowal, J.; Arras, G.; Colombo, M.; Jouve, M.; Morath, J.P.; Primdal-Bengtson, B.; Dingli, F.; Loew, D.; Tkach, M.; Thery, C. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc. Natl. Acad. Sci. USA 2016, 113, E968–E977. [Google Scholar] [CrossRef] [PubMed]
- Tauro, B.J.; Greening, D.W.; Mathias, R.A.; Ji, H.; Mathivanan, S.; Scott, A.M.; Simpson, R.J. Comparison of ultracentrifugation, density gradient separation, and immunoaffinity capture methods for isolating human colon cancer cell line LIM1863-derived exosomes. Methods 2012, 56, 293–304. [Google Scholar] [CrossRef]
- Ayala-Mar, S.; Donoso-Quezada, J.; Gallo-Villanueva, R.C.; Perez-Gonzalez, V.H.; Gonzalez-Valdez, J. Recent advances and challenges in the recovery and purification of cellular exosomes. Electrophoresis 2019, 40, 3036–3049. [Google Scholar] [CrossRef]
- Zarovni, N.; Corrado, A.; Guazzi, P.; Zocco, D.; Lari, E.; Radano, G.; Muhhina, J.; Fondelli, C.; Gavrilova, J.; Chiesi, A. Integrated isolation and quantitative analysis of exosome shuttled proteins and nucleic acids using immunocapture approaches. Methods 2015, 87, 46–58. [Google Scholar] [CrossRef]
- Gonzalez-Felix, M.A.; Mejia-Manzano, L.A.; Gonzalez-Valdez, J. Biological nanoparticles: Relevance as novel target drug delivery systems and leading chromatographic isolation approaches. Electrophoresis 2022, 43, 109–118. [Google Scholar] [CrossRef]
- Sidhom, K.; Obi, P.O.; Saleem, A. A Review of Exosomal Isolation Methods: Is Size Exclusion Chromatography the Best Option? Int. J. Mol. Sci. 2020, 21, 6466. [Google Scholar] [CrossRef]
- Wu, X.; Showiheen, S.A.A.; Sun, A.R.; Crawford, R.; Xiao, Y.; Mao, X.; Prasadam, I. Exosomes Extraction and Identification. Methods Mol. Biol. 2019, 2054, 81–91. [Google Scholar] [CrossRef]
- Karimi, N.; Cvjetkovic, A.; Jang, S.C.; Crescitelli, R.; Hosseinpour Feizi, M.A.; Nieuwland, R.; Lotvall, J.; Lasser, C. Detailed analysis of the plasma extracellular vesicle proteome after separation from lipoproteins. Cell Mol. Life Sci. 2018, 75, 2873–2886. [Google Scholar] [CrossRef]
- Zhang, H.; Freitas, D.; Kim, H.S.; Fabijanic, K.; Li, Z.; Chen, H.; Mark, M.T.; Molina, H.; Martin, A.B.; Bojmar, L.; et al. Identification of distinct nanoparticles and subsets of extracellular vesicles by asymmetric flow field-flow fractionation. Nat. Cell Biol. 2018, 20, 332–343. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Lyden, D. Asymmetric-flow field-flow fractionation technology for exomere and small extracellular vesicle separation and characterization. Nat. Protoc. 2019, 14, 1027–1053. [Google Scholar] [CrossRef] [PubMed]
- Gimondi, S.; Ferreira, H.; Reis, R.L.; Neves, N.M. Microfluidic Devices: A Tool for Nanoparticle Synthesis and Performance Evaluation. ACS Nano 2023, 17, 14205–14228. [Google Scholar] [CrossRef] [PubMed]
- Jayachandran, M.; Miller, V.M.; Heit, J.A.; Owen, W.G. Methodology for isolation, identification and characterization of microvesicles in peripheral blood. J. Immunol. Methods 2012, 375, 207–214. [Google Scholar] [CrossRef]
- Ahmadian, S.; Jafari, N.; Tamadon, A.; Ghaffarzadeh, A.; Rahbarghazi, R.; Mahdipour, M. Different storage and freezing protocols for extracellular vesicles: A systematic review. Stem Cell Res. Ther. 2024, 15, 453. [Google Scholar] [CrossRef]
- Bosch, S.; de Beaurepaire, L.; Allard, M.; Mosser, M.; Heichette, C.; Chretien, D.; Jegou, D.; Bach, J.M. Trehalose prevents aggregation of exosomes and cryodamage. Sci. Rep. 2016, 6, 36162. [Google Scholar] [CrossRef]
- Gorgens, A.; Corso, G.; Hagey, D.W.; Jawad Wiklander, R.; Gustafsson, M.O.; Felldin, U.; Lee, Y.; Bostancioglu, R.B.; Sork, H.; Liang, X.; et al. Identification of storage conditions stabilizing extracellular vesicles preparations. J. Extracell. Vesicles 2022, 11, e12238. [Google Scholar] [CrossRef]
- Lotvall, J.; Hill, A.F.; Hochberg, F.; Buzas, E.I.; Di Vizio, D.; Gardiner, C.; Gho, Y.S.; Kurochkin, I.V.; Mathivanan, S.; Quesenberry, P.; et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: A position statement from the International Society for Extracellular Vesicles. J. Extracell. Vesicles 2014, 3, 26913. [Google Scholar] [CrossRef]
- Thery, C.; Amigorena, S.; Raposo, G.; Clayton, A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr. Protoc. Cell Biol. 2006, 30, 3–22. [Google Scholar] [CrossRef]
- Jung, M.K.; Mun, J.Y. Sample Preparation and Imaging of Exosomes by Transmission Electron Microscopy. J. Vis. Exp. 2018, e56482. [Google Scholar] [CrossRef]
- Mueller, A.; Anter, A.; Edwards, G.; Junk, A.K.; Liu, Y.; Ziebarth, N.; Bhattacharya, S.K. Glaucomatous aqueous humor vesicles are smaller and differ in composition compared to controls. Exp. Eye Res. 2023, 234, 109562. [Google Scholar] [CrossRef] [PubMed]
- Skliar, M.; Chernyshev, V.S. Imaging of Extracellular Vesicles by Atomic Force Microscopy. J. Vis. Exp. 2019, e59254. [Google Scholar] [CrossRef]
- Kowkabany, G.; Bao, Y. Nanoparticle Tracking Analysis: An Effective Tool to Characterize Extracellular Vesicles. Molecules 2024, 29, 4672. [Google Scholar] [CrossRef] [PubMed]
- Soo, C.Y.; Song, Y.; Zheng, Y.; Campbell, E.C.; Riches, A.C.; Gunn-Moore, F.; Powis, S.J. Nanoparticle tracking analysis monitors microvesicle and exosome secretion from immune cells. Immunology 2012, 136, 192–197. [Google Scholar] [CrossRef]
- Kobayashi, H.; Shiba, T.; Yoshida, T.; Bolidong, D.; Kato, K.; Sato, Y.; Mochizuki, M.; Seto, T.; Kawashiri, S.; Hanayama, R. Precise analysis of single small extracellular vesicles using flow cytometry. Sci. Rep. 2024, 14, 7465. [Google Scholar] [CrossRef]
- Starostina, N.; Brodsky, M.; Prikhodko, S.; Hoo, C.M.; Mecartney, M.L.; West, P. AFM capabilities in characterization of particles and surfaces: From angstroms to microns. J. Cosmet. Sci. 2008, 59, 225–232. [Google Scholar]
- Yuana, Y.; Koning, R.I.; Kuil, M.E.; Rensen, P.C.; Koster, A.J.; Bertina, R.M.; Osanto, S. Cryo-electron microscopy of extracellular vesicles in fresh plasma. J. Extracell. Vesicles 2013, 2, 21494. [Google Scholar] [CrossRef]
- Boxer, S.G.; Kraft, M.L.; Weber, P.K. Advances in imaging secondary ion mass spectrometry for biological samples. Annu. Rev. Biophys. 2009, 38, 53–74. [Google Scholar] [CrossRef]
- Chamberlain, C.A.; Hatch, M.; Garrett, T.J. Extracellular Vesicle Analysis by Paper Spray Ionization Mass Spectrometry. Metabolites 2021, 11, 308. [Google Scholar] [CrossRef]
- Tatischeff, I.; Larquet, E.; Falcon-Perez, J.M.; Turpin, P.Y.; Kruglik, S.G. Fast characterisation of cell-derived extracellular vesicles by nanoparticles tracking analysis, cryo-electron microscopy, and Raman tweezers microspectroscopy. J. Extracell. Vesicles 2012, 1, 19179. [Google Scholar] [CrossRef]
- Mihaly, J.; Deak, R.; Szigyarto, I.C.; Bota, A.; Beke-Somfai, T.; Varga, Z. Characterization of extracellular vesicles by IR spectroscopy: Fast and simple classification based on amide and CH stretching vibrations. Biochim. Biophys. Acta Biomembr. 2017, 1859, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Willms, E.; Johansson, H.J.; Mager, I.; Lee, Y.; Blomberg, K.E.; Sadik, M.; Alaarg, A.; Smith, C.I.; Lehtio, J.; El Andaloussi, S.; et al. Cells release subpopulations of exosomes with distinct molecular and biological properties. Sci. Rep. 2016, 6, 22519. [Google Scholar] [CrossRef] [PubMed]
- Maekawa, M.; Mano, N. Cutting-edge LC-MS/MS applications in clinical mass spectrometry: Focusing on analysis of drugs and metabolites. Biomed. Chromatogr. 2022, 36, e5347. [Google Scholar] [CrossRef] [PubMed]
- Abyadeh, M.; Mirshahvaladi, S.; Kashani, S.A.; Paulo, J.A.; Amirkhani, A.; Mehryab, F.; Seydi, H.; Moradpour, N.; Jodeiryjabarzade, S.; Mirzaei, M.; et al. Proteomic profiling of mesenchymal stem cell-derived extracellular vesicles: Impact of isolation methods on protein cargo. J. Extracell. Biol. 2024, 3, e159. [Google Scholar] [CrossRef]
- Gillet, L.C.; Navarro, P.; Tate, S.; Rost, H.; Selevsek, N.; Reiter, L.; Bonner, R.; Aebersold, R. Targeted data extraction of the MS/MS spectra generated by data-independent acquisition: A new concept for consistent and accurate proteome analysis. Mol. Cell. Proteom. 2012, 11, O111-016717. [Google Scholar] [CrossRef]
- Fernandez-Costa, C.; Martinez-Bartolome, S.; McClatchy, D.B.; Saviola, A.J.; Yu, N.K.; Yates, J.R., 3rd. Impact of the Identification Strategy on the Reproducibility of the DDA and DIA Results. J. Proteome Res. 2020, 19, 3153–3161. [Google Scholar] [CrossRef]
- Sung, S.E.; Lim, J.H.; Kang, K.K.; Choi, J.H.; Lee, S.; Sung, M.; Park, W.T.; Kim, Y.I.; Seo, M.S.; Lee, G.W. Proteomic profiling of extracellular vesicles derived from human serum for the discovery of biomarkers in Avascular necrosis. Clin. Proteom. 2024, 21, 39. [Google Scholar] [CrossRef]
- Keerthikumar, S.; Chisanga, D.; Ariyaratne, D.; Al Saffar, H.; Anand, S.; Zhao, K.; Samuel, M.; Pathan, M.; Jois, M.; Chilamkurti, N.; et al. ExoCarta: A Web-Based Compendium of Exosomal Cargo. J. Mol. Biol. 2016, 428, 688–692. [Google Scholar] [CrossRef]
- Yanez-Mo, M.; Siljander, P.R.; Andreu, Z.; Zavec, A.B.; Borras, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef]
- Skotland, T.; Sandvig, K.; Llorente, A. Lipids in exosomes: Current knowledge and the way forward. Prog. Lipid Res. 2017, 66, 30–41. [Google Scholar] [CrossRef]
- Kalra, H.; Drummen, G.P.; Mathivanan, S. Focus on Extracellular Vesicles: Introducing the Next Small Big Thing. Int. J. Mol. Sci. 2016, 17, 170. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.; Mann, M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 2008, 26, 1367–1372. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.; Schafer, J.; Kuhn, K.; Kienle, S.; Schwarz, J.; Schmidt, G.; Neumann, T.; Johnstone, R.; Mohammed, A.K.; Hamon, C. Tandem mass tags: A novel quantification strategy for comparative analysis of complex protein mixtures by MS/MS. Anal. Chem. 2003, 75, 1895–1904. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Sinelnikov, I.V.; Han, B.; Wishart, D.S. MetaboAnalyst 3.0--making metabolomics more meaningful. Nucleic Acids Res. 2015, 43, W251–W257. [Google Scholar] [CrossRef]
- Wang, T.; Li, Y.; Guo, M.; Dong, X.; Liao, M.; Du, M.; Wang, X.; Yin, H.; Yan, H. Exosome-Mediated Delivery of the Neuroprotective Peptide PACAP38 Promotes Retinal Ganglion Cell Survival and Axon Regeneration in Rats With Traumatic Optic Neuropathy. Front. Cell. Dev. Biol. 2021, 9, 659783. [Google Scholar] [CrossRef]
- Chien, J.Y.; Sheu, J.H.; Wen, Z.H.; Tsai, R.K.; Huang, S.P. Neuroprotective effect of 4-(Phenylsulfanyl)butan-2-one on optic nerve crush model in rats. Exp. Eye Res. 2016, 143, 148–157. [Google Scholar] [CrossRef]
- Pojda-Wilczek, D.; Maruszczyk, W.; Sirek, S. Flash visual evoked potentials (FVEP) in various stimulation conditions. Doc. Ophthalmol. 2019, 138, 35–42. [Google Scholar] [CrossRef]
- Fix, S.T.; Arruda, J.E.; Andrasik, F.; Beach, J.; Groom, K. Using visual evoked potentials for the early detection of amnestic mild cognitive impairment: A pilot investigation. Int. J. Geriatr. Psychiatry 2015, 30, 72–79. [Google Scholar] [CrossRef]
- Ding, D.C.; Shyu, W.C.; Lin, S.Z. Mesenchymal stem cells. Cell Transpl. 2011, 20, 5–14. [Google Scholar] [CrossRef]
- Pan, D.; Chang, X.; Xu, M.; Zhang, M.; Zhang, S.; Wang, Y.; Luo, X.; Xu, J.; Yang, X.; Sun, X. UMSC-derived exosomes promote retinal ganglion cells survival in a rat model of optic nerve crush. J. Chem. Neuroanat. 2019, 96, 134–139. [Google Scholar] [CrossRef]
- Nadal-Nicolas, F.M.; Jimenez-Lopez, M.; Sobrado-Calvo, P.; Nieto-Lopez, L.; Canovas-Martinez, I.; Salinas-Navarro, M.; Vidal-Sanz, M.; Agudo, M. Brn3a as a marker of retinal ganglion cells: Qualitative and quantitative time course studies in naive and optic nerve-injured retinas. Invest. Ophthalmol. Vis. Sci. 2009, 50, 3860–3868. [Google Scholar] [CrossRef] [PubMed]
- Mead, B.; Tomarev, S. Bone Marrow-Derived Mesenchymal Stem Cells-Derived Exosomes Promote Survival of Retinal Ganglion Cells Through miRNA-Dependent Mechanisms. Stem Cells Transl. Med. 2017, 6, 1273–1285. [Google Scholar] [CrossRef] [PubMed]
- Mead, B.; Logan, A.; Berry, M.; Leadbeater, W.; Scheven, B.A. Intravitreally transplanted dental pulp stem cells promote neuroprotection and axon regeneration of retinal ganglion cells after optic nerve injury. Invest. Ophthalmol. Vis. Sci. 2013, 54, 7544–7556. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Kang, X.; Lu, S.; Liu, L. The therapeutic effects of bone marrow mesenchymal stem cells after optic nerve damage in the adult rat. Clin. Interv. Aging 2015, 10, 487–490. [Google Scholar] [CrossRef][Green Version]
- Mesentier-Louro, L.A.; Zaverucha-do-Valle, C.; da Silva-Junior, A.J.; Nascimento-Dos-Santos, G.; Gubert, F.; de Figueiredo, A.B.; Torres, A.L.; Paredes, B.D.; Teixeira, C.; Tovar-Moll, F.; et al. Distribution of mesenchymal stem cells and effects on neuronal survival and axon regeneration after optic nerve crush and cell therapy. PLoS ONE 2014, 9, e110722. [Google Scholar] [CrossRef]
- Jha, D.; Blennow, K.; Zetterberg, H.; Savas, J.N.; Hanrieder, J. Spatial neurolipidomics-MALDI mass spectrometry imaging of lipids in brain pathologies. J. Mass Spectrom. 2024, 59, e5008. [Google Scholar] [CrossRef]
- Stark, D.T.; Anderson, D.M.G.; Kwong, J.M.K.; Patterson, N.H.; Schey, K.L.; Caprioli, R.M.; Caprioli, J. Optic Nerve Regeneration After Crush Remodels the Injury Site: Molecular Insights From Imaging Mass Spectrometry. Invest. Ophthalmol. Vis. Sci. 2018, 59, 212–222. [Google Scholar] [CrossRef]
- Sekera, E.R.; Saraswat, D.; Zemaitis, K.J.; Sim, F.J.; Wood, T.D. MALDI Mass Spectrometry Imaging in a Primary Demyelination Model of Murine Spinal Cord. J. Am. Soc. Mass Spectrom. 2020, 31, 2462–2468. [Google Scholar] [CrossRef]
- Trzeciecka, A.; Stark, D.T.; Kwong, J.M.K.; Piqueras, M.; Bhattacharya, S.K.; Caprioli, J. Comparative lipid profiling dataset of the inflammation-induced optic nerve regeneration. Data Brief 2019, 24, 103950. [Google Scholar] [CrossRef]
- Jauregui, A.M.; Liu, Y.; Bhattacharya, S.K.; Lee, R.K. Metabolomics dataset of mouse optogenetic axon regeneration after optic nerve crush. Data Brief 2022, 42, 108306. [Google Scholar] [CrossRef]
- Li, F.; Sami, A.; Noristani, H.N.; Slattery, K.; Qiu, J.; Groves, T.; Wang, S.; Veerasammy, K.; Chen, Y.X.; Morales, J.; et al. Glial Metabolic Rewiring Promotes Axon Regeneration and Functional Recovery in the Central Nervous System. Cell Metab. 2020, 32, 767–785.e7. [Google Scholar] [CrossRef] [PubMed]
- Serger, E.; Luengo-Gutierrez, L.; Chadwick, J.S.; Kong, G.; Zhou, L.; Crawford, G.; Danzi, M.C.; Myridakis, A.; Brandis, A.; Bello, A.T.; et al. The gut metabolite indole-3 propionate promotes nerve regeneration and repair. Nature 2022, 607, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, Q.; Xie, C.; Cai, Y.; Chen, X.; Hou, Y.; He, L.; Li, J.; Yao, M.; Chen, S.; et al. Peptide ligands targeting FGF receptors promote recovery from dorsal root crush injury via AKT/mTOR signaling. Theranostics 2021, 11, 10125–10147. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, M.; Soler, R.M.; Ribera, J.; Esquerda, J.E.; Comella, J.X. The carbohydrate N-acetylglucosamine is involved in the guidance of neurites from chick ciliary ganglion neurons through the extracellular matrix of rat skeletal muscle fiber. Neurosci. Lett. 1996, 207, 81–84. [Google Scholar] [CrossRef]
- Ito, Z.; Sakamoto, K.; Imagama, S.; Matsuyama, Y.; Zhang, H.; Hirano, K.; Ando, K.; Yamashita, T.; Ishiguro, N.; Kadomatsu, K. N-acetylglucosamine 6-O-sulfotransferase-1-deficient mice show better functional recovery after spinal cord injury. J. Neurosci. 2010, 30, 5937–5947. [Google Scholar] [CrossRef]
- Loots, J.M.; Loots, G.P.; Joubert, W.S. The effect of allantoin on cellular multiplication in degenerating and regenerating nerves. S. Afr. Med. J. 1979, 55, 53–56. [Google Scholar]
- Sakai, Y.; Hanafusa, H.; Hisamoto, N.; Matsumoto, K. Histidine dephosphorylation of the Gbeta protein GPB-1 promotes axon regeneration in C. elegans. EMBO Rep. 2022, 23, e55076. [Google Scholar] [CrossRef]
- Ousman, S.S.; David, S. MIP-1alpha, MCP-1, GM-CSF, and TNF-alpha control the immune cell response that mediates rapid phagocytosis of myelin from the adult mouse spinal cord. J. Neurosci. 2001, 21, 4649–4656. [Google Scholar] [CrossRef]
- Diehl, P.; Nienaber, F.; Zaldivia, M.T.K.; Stamm, J.; Siegel, P.M.; Mellett, N.A.; Wessinger, M.; Wang, X.; McFadyen, J.D.; Bassler, N.; et al. Lysophosphatidylcholine is a Major Component of Platelet Microvesicles Promoting Platelet Activation and Reporting Atherosclerotic Plaque Instability. Thromb. Haemost. 2019, 119, 1295–1310. [Google Scholar] [CrossRef]
- Hisano, K.; Kawase, S.; Mimura, T.; Yoshida, H.; Yamada, H.; Haniu, H.; Tsukahara, T.; Kurihara, T.; Matsuda, Y.; Saito, N.; et al. Structurally different lysophosphatidylethanolamine species stimulate neurite outgrowth in cultured cortical neurons via distinct G-protein-coupled receptors and signaling cascades. Biochem. Biophys. Res. Commun. 2021, 534, 179–185. [Google Scholar] [CrossRef]
- Miranda, A.M.; Lasiecka, Z.M.; Xu, Y.; Neufeld, J.; Shahriar, S.; Simoes, S.; Chan, R.B.; Oliveira, T.G.; Small, S.A.; Di Paolo, G. Neuronal lysosomal dysfunction releases exosomes harboring APP C-terminal fragments and unique lipid signatures. Nat. Commun. 2018, 9, 291. [Google Scholar] [CrossRef] [PubMed]
- Mersman, B.; Zaidi, W.; Syed, N.I.; Xu, F. Taurine Promotes Neurite Outgrowth and Synapse Development of Both Vertebrate and Invertebrate Central Neurons. Front. Synaptic. Neurosci. 2020, 12, 29. [Google Scholar] [CrossRef] [PubMed]
- Sobrido-Camean, D.; Fernandez-Lopez, B.; Pereiro, N.; Lafuente, A.; Rodicio, M.C.; Barreiro-Iglesias, A. Taurine Promotes Axonal Regeneration after a Complete Spinal Cord Injury in Lampreys. J. Neurotrauma 2020, 37, 899–903. [Google Scholar] [CrossRef] [PubMed]
- Lima, L.; Obregon, F.; Matus, P. Taurine, glutamate and GABA modulate the outgrowth from goldfish retinal explants and its concentrations are affected by the crush of the optic nerve. Amino Acids 1998, 15, 195–209. [Google Scholar] [CrossRef]
- Wu, J.Y.; Prentice, H. Role of taurine in the central nervous system. J. Biomed. Sci. 2010, 17 (Suppl. 1), S1. [Google Scholar] [CrossRef]
- Kronman, H.; Singh, A.; Azam, S.; Guzman, A.S.; Zelli, D.; Lau, T.; Dobbin, J.; Bigio, B.; Nasca, C. Multidimensional Effects of Stress on Neuronal Exosome Levels and Simultaneous Transcriptomic Profiles. Biol. Psychiatry Glob. Open Sci. 2025, 5, 100401. [Google Scholar] [CrossRef]
- Pourshahidi, S.; Shamshiri, A.R.; Derakhshan, S.; Mohammadi, S.; Ghorbani, M. The Effect of Acetyl-L-Carnitine (ALCAR) on Peripheral Nerve Regeneration in Animal Models: A Systematic Review. Neurochem. Res. 2023, 48, 2335–2344. [Google Scholar] [CrossRef]
- Mirzakhani, N.; Farshid, A.A.; Tamaddonfard, E.; Imani, M.; Erfanparast, A.; Noroozinia, F. Carnosine improves functional recovery and structural regeneration after sciatic nerve crush injury in rats. Life Sci. 2018, 215, 22–30. [Google Scholar] [CrossRef]
- Sugihara, Y.; Onoue, S.; Tashiro, K.; Sato, M.; Hasegawa, T.; Katakura, Y. Carnosine induces intestinal cells to secrete exosomes that activate neuronal cells. PLoS ONE 2019, 14, e0217394. [Google Scholar] [CrossRef]
- Zhang, J.; Jiang, C.; Liu, X.; Jiang, C.X.; Cao, Q.; Yu, B.; Ni, Y.; Mao, S. The metabolomic profiling identifies N, N-dimethylglycine as a facilitator of dorsal root ganglia neuron axon regeneration after injury. FASEB J. 2022, 36, e22305. [Google Scholar] [CrossRef] [PubMed]
- Subra, C.; Grand, D.; Laulagnier, K.; Stella, A.; Lambeau, G.; Paillasse, M.; De Medina, P.; Monsarrat, B.; Perret, B.; Silvente-Poirot, S.; et al. Exosomes account for vesicle-mediated transcellular transport of activatable phospholipases and prostaglandins. J. Lipid Res. 2010, 51, 2105–2120. [Google Scholar] [CrossRef] [PubMed]
- Jalink, K.; van Corven, E.J.; Hengeveld, T.; Morii, N.; Narumiya, S.; Moolenaar, W.H. Inhibition of lysophosphatidate- and thrombin-induced neurite retraction and neuronal cell rounding by ADP ribosylation of the small GTP-binding protein Rho. J. Cell Biol. 1994, 126, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, A.; Singh, R. Extracellular vesicles: An emerging player in retinal homeostasis. Front. Cell Dev. Biol. 2023, 11, 1059141. [Google Scholar] [CrossRef]
- Delibas, B.; Kuruoglu, E.; Bereket, M.C.; Onger, M.E. Allantoin, a purine metabolite, enhances peripheral nerve regeneration following sciatic nerve injury in rats: A stereological and immunohistochemical study. J. Chem. Neuroanat. 2021, 117, 102002. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cabrera Gonzalez, M.D.; Watson, J.; Leal, L.; Moceri, I.; Plummer, C.; Mahato, B.; Fouda, A.Y.; Bhattacharya, S.K. Extracellular Vesicle Metabolomics Holds Promise for Adult Axon Regeneration. Metabolites 2025, 15, 454. https://doi.org/10.3390/metabo15070454
Cabrera Gonzalez MD, Watson J, Leal L, Moceri I, Plummer C, Mahato B, Fouda AY, Bhattacharya SK. Extracellular Vesicle Metabolomics Holds Promise for Adult Axon Regeneration. Metabolites. 2025; 15(7):454. https://doi.org/10.3390/metabo15070454
Chicago/Turabian StyleCabrera Gonzalez, Maria D., Jackson Watson, Laura Leal, Isabella Moceri, Camille Plummer, Biraj Mahato, Abdelrahman Y. Fouda, and Sanjoy K. Bhattacharya. 2025. "Extracellular Vesicle Metabolomics Holds Promise for Adult Axon Regeneration" Metabolites 15, no. 7: 454. https://doi.org/10.3390/metabo15070454
APA StyleCabrera Gonzalez, M. D., Watson, J., Leal, L., Moceri, I., Plummer, C., Mahato, B., Fouda, A. Y., & Bhattacharya, S. K. (2025). Extracellular Vesicle Metabolomics Holds Promise for Adult Axon Regeneration. Metabolites, 15(7), 454. https://doi.org/10.3390/metabo15070454









