Novel Lipid Biomarkers of Chronic Kidney Disease of Unknown Etiology Based on Urinary Small Extracellular Vesicles: A Pilot Study of Sugar Cane Workers
Abstract
1. Introduction
2. Materials and Methods
2.1. Urine Samples from Male Agricultural Workers in Guatemala
2.2. Purification of Extracellular Vesicles
2.3. Determination of Concentration of the Extracellular Vesicles by Nanoparticle Tracking Analysis
2.4. Small Extracellular Vesicle Characterization by Bead-Based Multiplex Flow Cytometry Assay
2.5. Transmission Electron Microscopy (TEM)
2.6. Lipid Extraction from Small Extracellular Vesicles
2.7. LC-MS/MS Conditions
2.8. Statistical Analysis
3. Results
3.1. Guatemala Participants
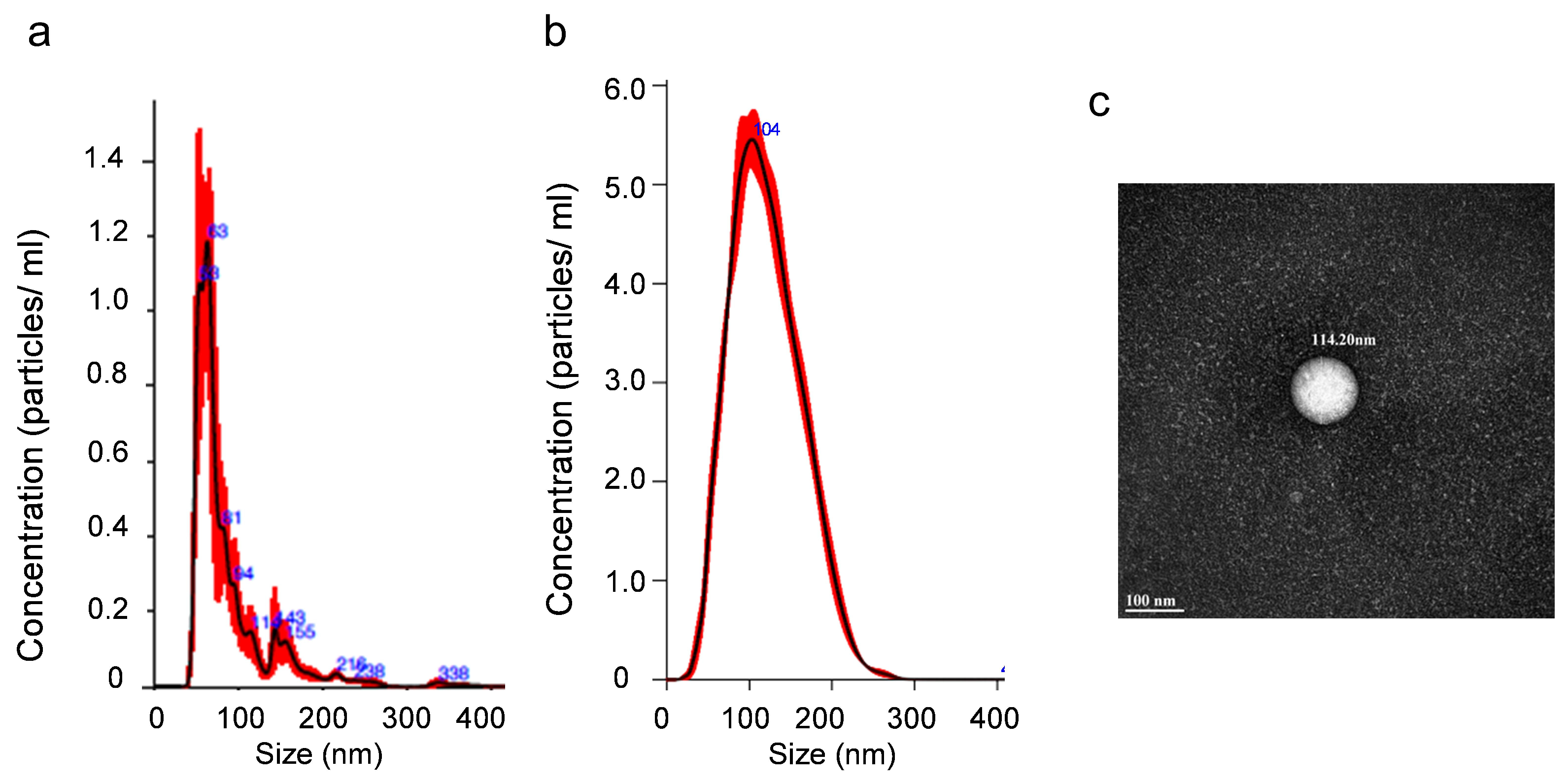
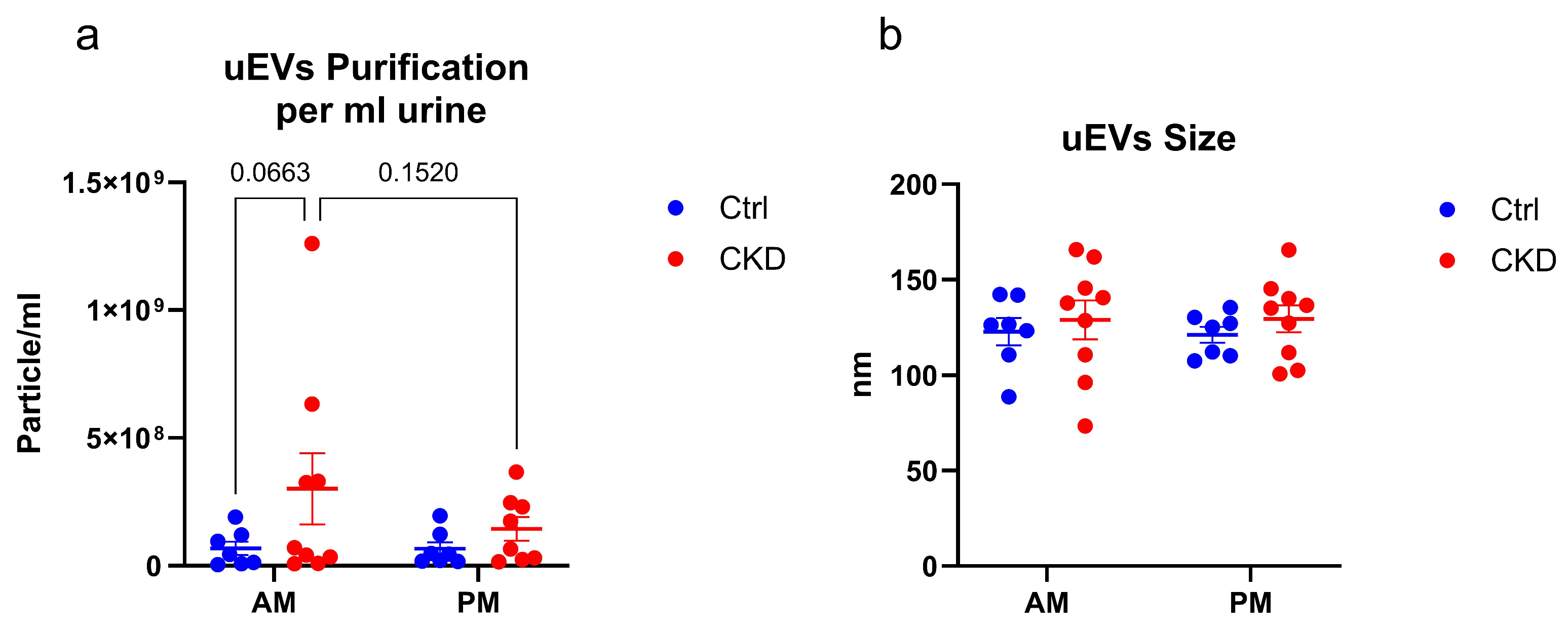
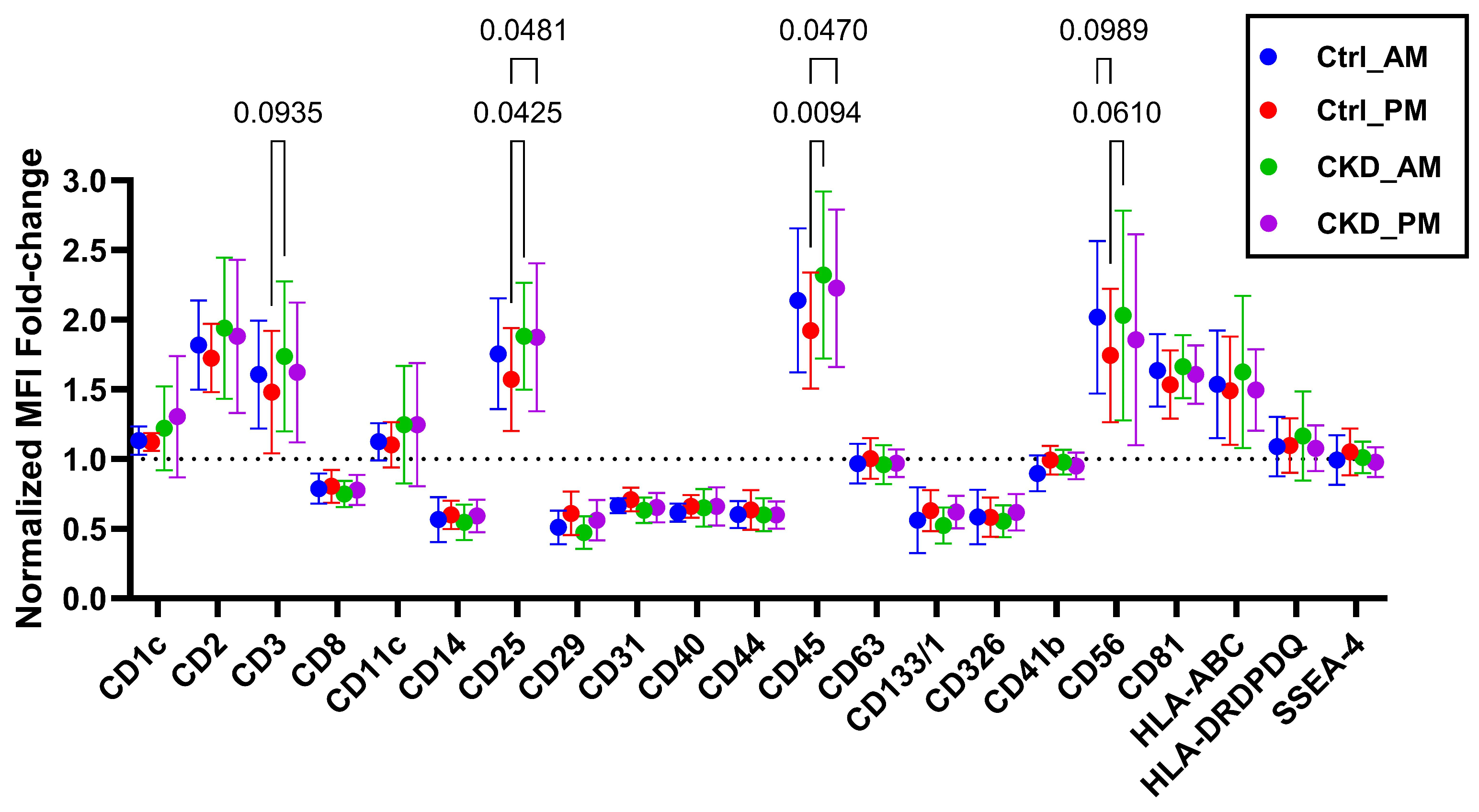
3.2. Characterization of Small Extracellular Vesicles
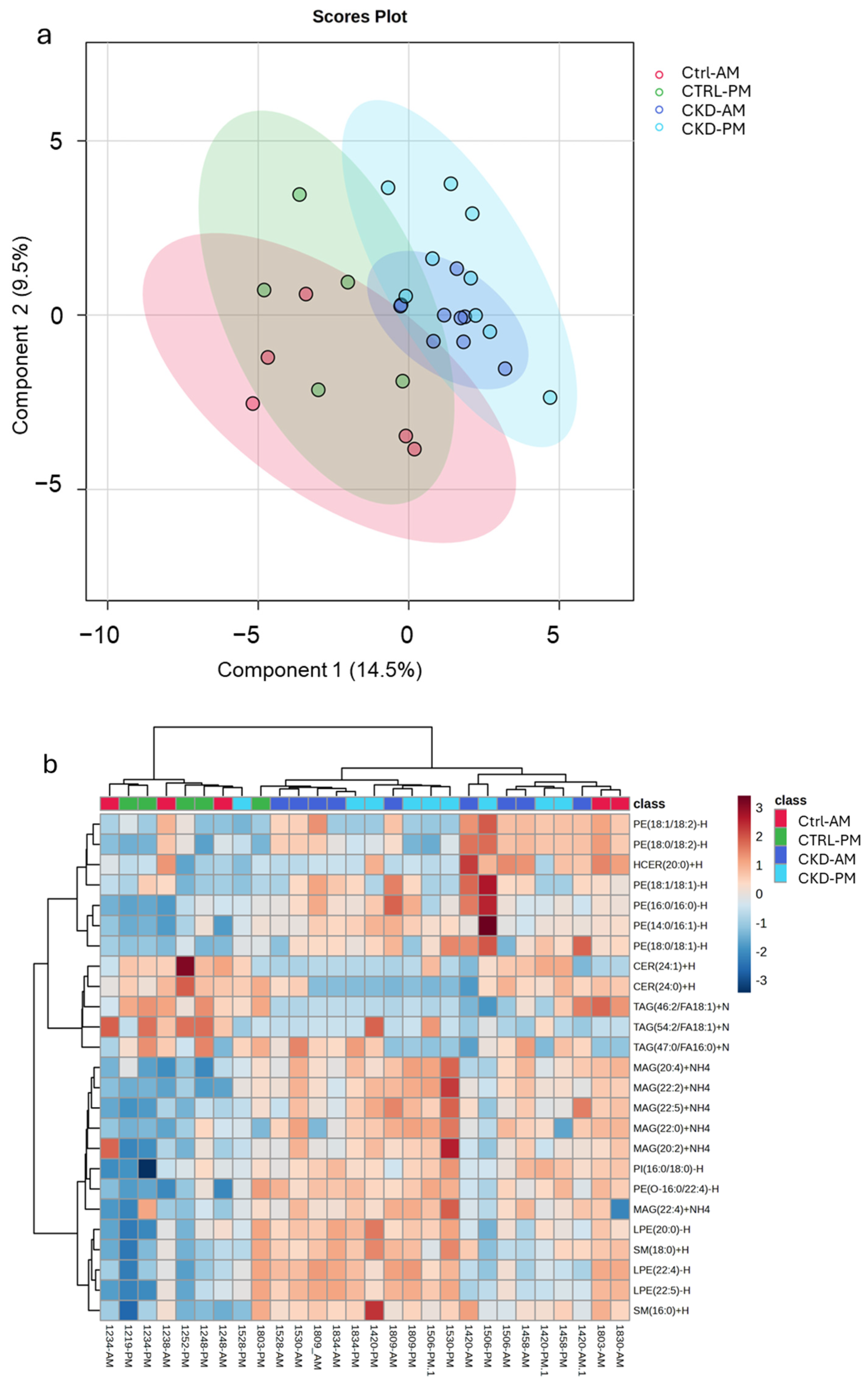
3.3. Lipidomics Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACR | Albumin-to-creatinine ratio |
| AKI | Acute kidney injury |
| ANOVA | Analysis of variance |
| APC | Allophycocyanin |
| ARMMs | Arrestin domain-containing protein 1-mediated microvesicles |
| BMI | Body mass index |
| BUN | Blood urea nitrogen |
| CE | Cholesterol ester |
| CER | Ceramide |
| CKD | Chronic kidney disease |
| CKDu | Chronic kidney disease of unknown etiology |
| CLU | Clusterin |
| COMIRB | Colorado Multiple Institution Review Board |
| Ctrl | Control |
| Cyc-C | Cystatin-C |
| DAG | Diacylglycerol |
| eGFR | Estimated glomerular filtration rate |
| EVs | Extracellular vesicles |
| Fcs | Standard files used to store flow cytometry data |
| HbA1c | Glycated hemoglobin |
| IL-18 | Interleukin 18 |
| IRB | Institutional Review Board |
| KDIGO | Kidney Disease: Improving Global Outcomes |
| KIM-1 | Kidney injury molecule-1 |
| LC MS/MS | Liquid chromatography tandem mass spectrometry |
| LPC | Lysophosphatidylcholine |
| LPE | Lysophosphatidylethanolamine |
| LSD | Least significant difference |
| MACSPlex | ClinMax™ Multiplex Bead Assay Platform for Flow Cytometry |
| MAG | Monoacylglycerol |
| MFI | Median fluorescence intensity |
| MRM | Multiple reaction monitoring |
| NGAL | Neutrophil gelatinase-associated lipocalin |
| NSAIDS | Nonsteroidal anti-inflammatory drugs |
| NTA | Nanoparticle tracking analysis |
| OPN | Osteopontin |
| PBS | Phosphate-buffered saline |
| PC | Phosphatidylcholine |
| PE | Phosphatidylethanolamine |
| PE(O) | Phosphatidylethanolamine plasmalogen |
| PG | Phosphatidylglycerol |
| PI | Phosphatidylinositol |
| PS | Phosphatidylserine |
| ROC | Receiver operating characteristic |
| sCMOS | Scientific Complementary Metal–Oxide–Semiconductor |
| sEV | Small extracellular vesicle |
| SM | Sphingomyelin |
| TAG | Triacylglycerol |
| TEM | Transmission electron microscopy |
| TFF3 | Trefoil factor 3 |
| VIP | Variable importance in projection |
References
- Abraham, G.; Varughese, S.; Thandavan, T.; Iyengar, A.; Fernando, E.; Naqvi, S.A.; Sheriff, R.; Ur-Rashid, H.; Gopalakrishnan, N.; Kafle, R.K. Chronic kidney disease hotspots in developing countries in South Asia. Clin. Kidney J. 2016, 9, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Claudel, S.E.; Waikar, S.S.; Chronic Kidney Disease of UnceRtain Etiology in Agricultural Communities Research Consortium. Systematic Review of Kidney Injury Biomarkers for the Evaluation of CKD of Uncertain Etiology. Kidney Int. Rep. 2024, 9, 1614–1632. [Google Scholar] [CrossRef]
- Ramirez-Rubio, O.; McClean, M.D.; Amador, J.J.; Brooks, D.R. An epidemic of chronic kidney disease in Central America: An overview. Postgrad. Med. J. 2013, 89, 123–125. [Google Scholar] [CrossRef] [PubMed]
- Laws, R.L.; Brooks, D.R.; Amador, J.J.; Weiner, D.E.; Kaufman, J.S.; Ramirez-Rubio, O.; Riefkohl, A.; Scammell, M.K.; Lopez-Pilarte, D.; Sanchez, J.M.; et al. Changes in kidney function among Nicaraguan sugarcane workers. Int. J. Occup. Environ. Health 2015, 21, 241–250. [Google Scholar] [CrossRef]
- Foley, R.N.; Wang, C.; Collins, A.J. Cardiovascular risk factor profiles and kidney function stage in the US general population: The NHANES III study. Mayo Clin. Proc. 2005, 80, 1270–1277. [Google Scholar] [CrossRef] [PubMed]
- Lebov, J.F.; Engel, L.S.; Richardson, D.; Hogan, S.L.; Hoppin, J.A.; Sandler, D.P. Pesticide use and risk of end-stage renal disease among licensed pesticide applicators in the Agricultural Health Study. Occup. Environ. Med. 2016, 73, 3–12. [Google Scholar] [CrossRef]
- Pizzorno, J. The Kidney Dysfunction Epidemic, Part 1: Causes. Integr. Med. 2015, 14, 8–13. [Google Scholar]
- Wesseling, C.; van Wendel de Joode, B.; Crowe, J.; Rittner, R.; Sanati, N.A.; Hogstedt, C.; Jakobsson, K. Mesoamerican nephropathy: Geographical distribution and time trends of chronic kidney disease mortality between 1970 and 2012 in Costa Rica. Occup. Environ. Med. 2015, 72, 714–721. [Google Scholar] [CrossRef]
- Johnson, R.J.; Wesseling, C.; Newman, L.S. Chronic Kidney Disease of Unknown Cause in Agricultural Communities. N. Engl. J. Med. 2019, 380, 1843–1852. [Google Scholar] [CrossRef]
- Abdul, K.S.M.; De Silva, P.; Ekanayake, E.; Thakshila, W.; Gunarathna, S.D.; Gunasekara, T.; Jayasinghe, S.S.; Asanthi, H.B.; Chandana, E.P.S.; Chaminda, G.G.T.; et al. Occupational Paraquat and Glyphosate Exposure May Decline Renal Functions among Rural Farming Communities in Sri Lanka. Int. J. Environ. Res. Public Health 2021, 18, 3278. [Google Scholar] [CrossRef]
- Rogers, K.L.; Roncal-Jimenez, C.A.; Leiva, R.; Stem, A.; Wijkstrom, J.; Serpas, L.; Gonzalez-Quiroz, M.A.; Sasai, F.; Wernerson, A.; Schaeffer, J.; et al. Silica Nanoparticles and Mesoamerican Nephropathy: A Case Series. Am. J. Kidney Dis. 2024, 83, 420–423. [Google Scholar] [CrossRef]
- Ben Khadda, Z.; Lahmamsi, H.; El Karmoudi, Y.; Ezrari, S.; El Hanafi, L.; Sqalli Houssaini, T. Chronic Kidney Disease of Unknown Etiology: A Global Health Threat in Rural Agricultural Communities-Prevalence, Suspected Causes, Mechanisms, and Prevention Strategies. Pathophysiology 2024, 31, 761–786. [Google Scholar] [CrossRef] [PubMed]
- Edelstein, C.L. Biomarkers of acute kidney injury. Adv. Chronic Kidney Dis. 2008, 15, 222–234. [Google Scholar] [CrossRef] [PubMed]
- Alli, A.A. Extracellular Vesicles: Investigating the Pathophysiology of Diabetes-Associated Hypertension and Diabetic Nephropathy. Biology 2023, 12, 1138. [Google Scholar] [CrossRef]
- Erdbrugger, U.; Blijdorp, C.J.; Bijnsdorp, I.V.; Borras, F.E.; Burger, D.; Bussolati, B.; Byrd, J.B.; Clayton, A.; Dear, J.W.; Falcon-Perez, J.M.; et al. Urinary extracellular vesicles: A position paper by the Urine Task Force of the International Society for Extracellular Vesicles. J. Extracell. Vesicles 2021, 10, e12093. [Google Scholar] [CrossRef] [PubMed]
- Saint-Pol, J.; Culot, M. Minimum information for studies of extracellular vesicles (MISEV) as toolbox for rigorous, reproducible and homogeneous studies on extracellular vesicles. Toxicol. In Vitro 2025, 106, 106049. [Google Scholar] [CrossRef]
- DiStefano, J.K.; Ravi, R.K.; Khosroheidari, M. Urinary Exosomes as Potential Source for Identification of Biomarkers for Kidney Damage: Comparing Methodologies. In General Methods in Biomarker Research and their Applications; Preedy, V., Patel, V.B., Eds.; Springer Science Business Media: Dordrecht, The Netherlands, 2015; pp. 939–954. [Google Scholar]
- Butler-Dawson, J.; Barnoya, J.; Brindley, S.; Krisher, L.; Fan, W.; Asensio, C.; Newman, L.S. Cross-sectional study examining the accuracy of self-reported smoking status as compared to urinary cotinine levels among workers at risk for chronic kidney disease of unknown origin in Guatemala. BMJ Open 2021, 11, e050374. [Google Scholar] [CrossRef]
- KDIGO Kidney Disease: Improving Global Outcomes, C.K.D.W.G. KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2024, 105, S117–S314. [Google Scholar]
- Levey, A.S.; Stevens, L.A. Estimating GFR using the CKD Epidemiology Collaboration (CKD-EPI) creatinine equation: More accurate GFR estimates, lower CKD prevalence estimates, and better risk predictions. Am. J. Kidney Dis. 2010, 55, 622–627. [Google Scholar] [CrossRef]
- Welsh, J.A.; Tang, V.A.; van der Pol, E.; Gorgens, A. MIFlowCyt-EV: The Next Chapter in the Reporting and Reliability of Single Extracellular Vesicle Flow Cytometry Experiments. Cytom. A 2021, 99, 365–368. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Glover, S.C.; Nouri, M.Z.; Tuna, K.M.; Mendoza Alvarez, L.B.; Ryan, L.K.; Shirley, J.F.; Tang, Y.; Denslow, N.D.; Alli, A.A. Lipidomic analysis of urinary exosomes from hereditary alpha-tryptasemia patients and healthy volunteers. FASEB Bioadv. 2019, 1, 624–638. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.; Lu, Y.; Zhou, G.; Hui, F.; Xu, L.; Viau, C.; Spigelman, A.F.; MacDonald, P.E.; Wishart, D.S.; Li, S.; et al. MetaboAnalyst 6.0: Towards a unified platform for metabolomics data processing, analysis and interpretation. Nucleic Acids Res. 2024, 52, W398–W406. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Psychogios, N.; Young, N.; Wishart, D.S. MetaboAnalyst: A web server for metabolomic data analysis and interpretation. Nucleic Acids Res. 2009, 37, W652–W660. [Google Scholar] [CrossRef] [PubMed]
- Ciferri, M.C.; Quarto, R.; Tasso, R. Extracellular Vesicles as Biomarkers and Therapeutic Tools: From Pre-Clinical to Clinical Applications. Biology 2021, 10, 359. [Google Scholar] [CrossRef]
- Doyle, L.M.; Wang, M.Z. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef]
- Almsherqi, Z.A. Potential Role of Plasmalogens in the Modulation of Biomembrane Morphology. Front. Cell Dev. Biol. 2021, 9, 673917. [Google Scholar] [CrossRef]
- Lopez, J.P.; Nouri, M.Z.; Ebrahim, A.; Chacko, K.M.; Schramm, W.C.; Gholam, M.F.; Ozrazgat-Baslanti, T.; Denslow, N.D.; Alli, A.A. Lipid Profiles of Urinary Extracellular Vesicles Released during the Inactive and Active Phases of Aged Male Mice with Spontaneous Hypertension. Int. J. Mol. Sci. 2022, 23, 15397. [Google Scholar] [CrossRef]
- Pike, L.J.; Han, X.; Chung, K.N.; Gross, R.W. Lipid rafts are enriched in arachidonic acid and plasmenylethanolamine and their composition is independent of caveolin-1 expression: A quantitative electrospray ionization/mass spectrometric analysis. Biochemistry 2002, 41, 2075–2088. [Google Scholar] [CrossRef]
- Wang, Q.; Yu, J.; Kadungure, T.; Beyene, J.; Zhang, H.; Lu, Q. ARMMs as a versatile platform for intracellular delivery of macromolecules. Nat. Commun. 2018, 9, 960. [Google Scholar] [CrossRef]
- Gelibter, S.; Marostica, G.; Mandelli, A.; Siciliani, S.; Podini, P.; Finardi, A.; Furlan, R. The impact of storage on extracellular vesicles: A systematic study. J. Extracell. Vesicles 2022, 11, e12162. [Google Scholar] [CrossRef] [PubMed]
- Blijdorp, C.J.; Tutakhel, O.A.Z.; Hartjes, T.A.; van den Bosch, T.P.P.; van Heugten, M.H.; Rigalli, J.P.; Willemsen, R.; Musterd-Bhaggoe, U.M.; Barros, E.R.; Carles-Fontana, R.; et al. Comparing Approaches to Normalize, Quantify, and Characterize Urinary Extracellular Vesicles. J. Am. Soc. Nephrol. 2021, 32, 1210–1226. [Google Scholar] [CrossRef] [PubMed]
- Noessner, E.; Lindenmeyer, M.; Nelson, P.J.; Segerer, S. Dendritic cells in human renal inflammation—Part II. Nephron Exp. Nephrol. 2011, 119, e91–e98. [Google Scholar] [CrossRef] [PubMed]
- Tecklenborg, J.; Clayton, D.; Siebert, S.; Coley, S.M. The role of the immune system in kidney disease. Clin. Exp. Immunol. 2018, 192, 142–150. [Google Scholar] [CrossRef]
- Beik, A.I.; Morris, A.G.; Higgins, R.M.; Lam, F.T. Serial flow cytometric analysis of T-cell surface markers can be useful in differential diagnosis of renal allograft dysfunction. Clin. Transplant. 1998, 12, 24–29. [Google Scholar] [CrossRef]
- Ge, F.; Wang, F.; Yan, X.; Li, Z.; Wang, X. Association of BAFF with PI3K/Akt/mTOR signaling in lupus nephritis. Mol. Med. Rep. 2017, 16, 5793–5798. [Google Scholar] [CrossRef]
- Grzelczyk, A.; Gendaszewska-Darmach, E. Novel bioactive glycerol-based lysophospholipids: New data—New insight into their function. Biochimie 2013, 95, 667–679. [Google Scholar] [CrossRef]
- Meyer zu Heringdorf, D.; Jakobs, K.H. Lysophospholipid receptors: Signalling, pharmacology and regulation by lysophospholipid metabolism. Biochim. Biophys. Acta 2007, 1768, 923–940. [Google Scholar] [CrossRef]
- Nguyen, V.D.; Cieslinski, D.A.; Humes, H.D. Importance of adenosine triphosphate in phospholipase A2-induced rabbit renal proximal tubule cell injury. J. Clin. Investig. 1988, 82, 1098–1105. [Google Scholar] [CrossRef]
- Saulnier-Blache, J.S.; Feigerlova, E.; Halimi, J.M.; Gourdy, P.; Roussel, R.; Guerci, B.; Dupuy, A.; Bertrand-Michel, J.; Bascands, J.L.; Hadjadj, S.; et al. Urinary lysophopholipids are increased in diabetic patients with nephropathy. J. Diabetes Complicat. 2017, 31, 1103–1108. [Google Scholar] [CrossRef]
- d’Alessandro, M.; Soccio, P.; Bergantini, L.; Cameli, P.; Scioscia, G.; Foschino Barbaro, M.P.; Lacedonia, D.; Bargagli, E. Extracellular Vesicle Surface Signatures in IPF Patients: A Multiplex Bead-Based Flow Cytometry Approach. Cells 2021, 10, 1045. [Google Scholar] [CrossRef]
- Gobe, G.C.; Coombes, J.S.; Fassett, R.G.; Endre, Z.H. Biomarkers of drug-induced acute kidney injury in the adult. Expert Opin. Drug Metab. Toxicol. 2015, 11, 1683–1694. [Google Scholar] [CrossRef]
- Vlasakova, K.; Erdos, Z.; Troth, S.P.; McNulty, K.; Chapeau-Campredon, V.; Mokrzycki, N.; Muniappa, N.; Gu, Y.Z.; Holder, D.; Bailey, W.J.; et al. Evaluation of the relative performance of 12 urinary biomarkers for renal safety across 22 rat sensitivity and specificity studies. Toxicol. Sci. 2014, 138, 3–20. [Google Scholar] [CrossRef]
- Wunnapuk, K.; Gobe, G.; Endre, Z.; Peake, P.; Grice, J.E.; Roberts, M.S.; Buckley, N.A.; Liu, X. Use of a glyphosate-based herbicide-induced nephrotoxicity model to investigate a panel of kidney injury biomarkers. Toxicol. Lett. 2014, 225, 192–200. [Google Scholar] [CrossRef]
- Wang, H.; Gao, L.; Zhao, C.; Fang, F.; Liu, J.; Wang, Z.; Zhong, Y.; Wang, X. The role of PI3K/Akt signaling pathway in chronic kidney disease. Int. Urol. Nephrol. 2024, 56, 2623–2633. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.T.; Ramesh, T.; Toh, X.R.; Nguyen, L.N. Emerging roles of lysophospholipids in health and disease. Prog. Lipid Res. 2020, 80, 101068. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Yuen, P.S.; Pisitkun, T.; Gonzales, P.A.; Yasuda, H.; Dear, J.W.; Gross, P.; Knepper, M.A.; Star, R.A. Collection, storage, preservation, and normalization of human urinary exosomes for biomarker discovery. Kidney Int. 2006, 69, 1471–1476. [Google Scholar] [CrossRef] [PubMed]

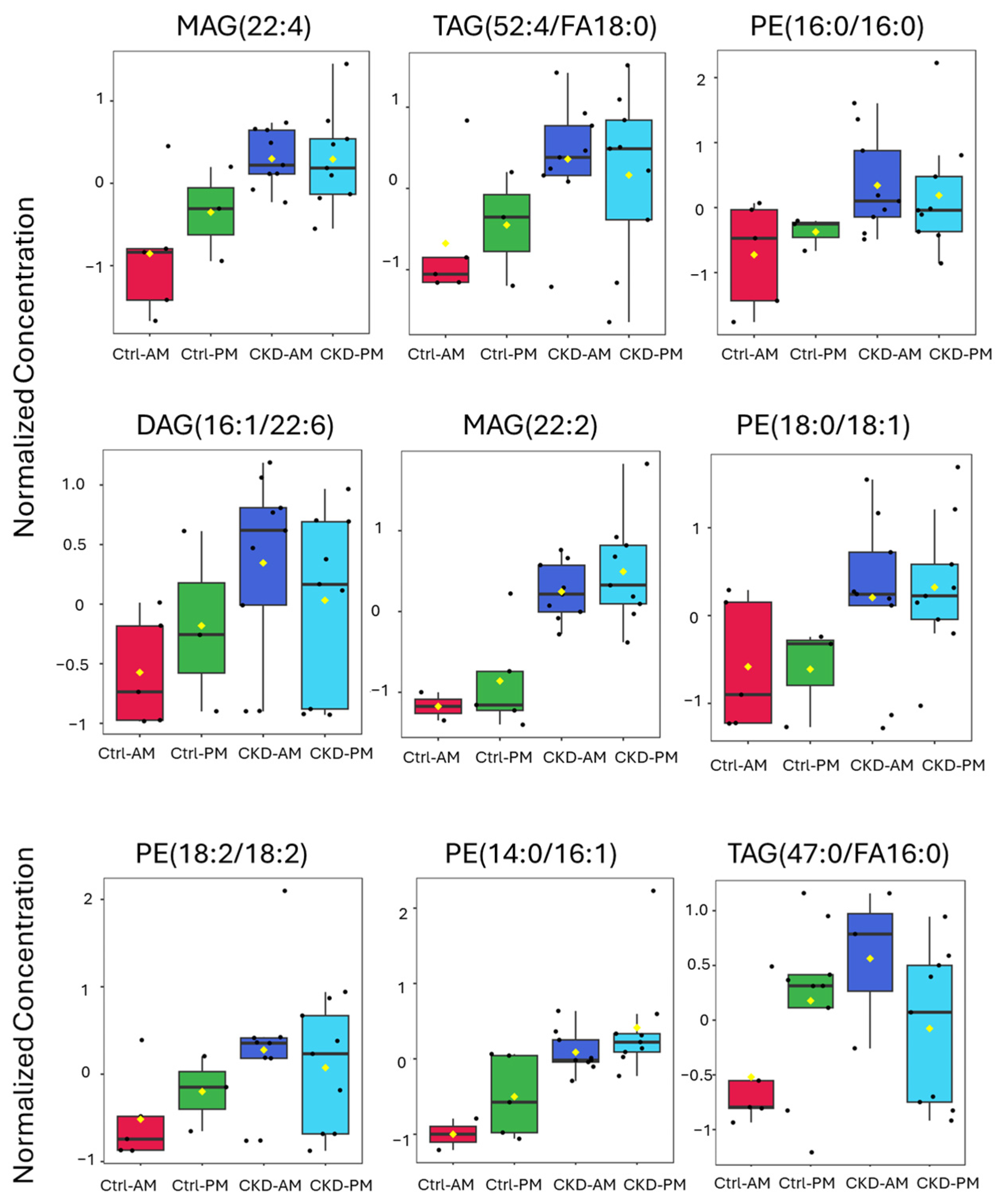
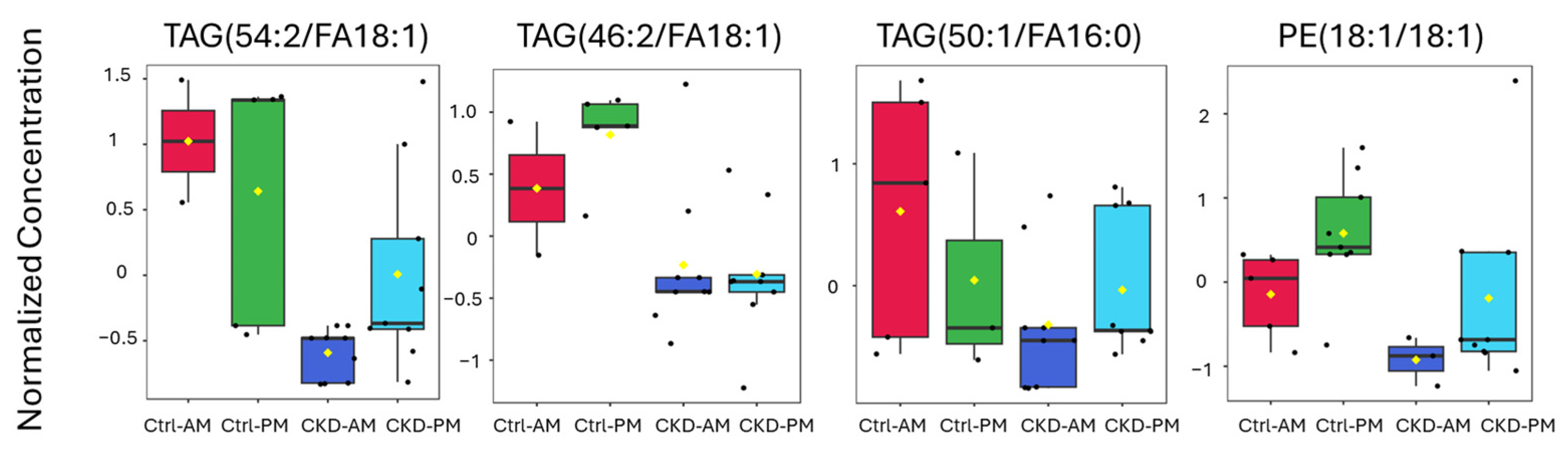
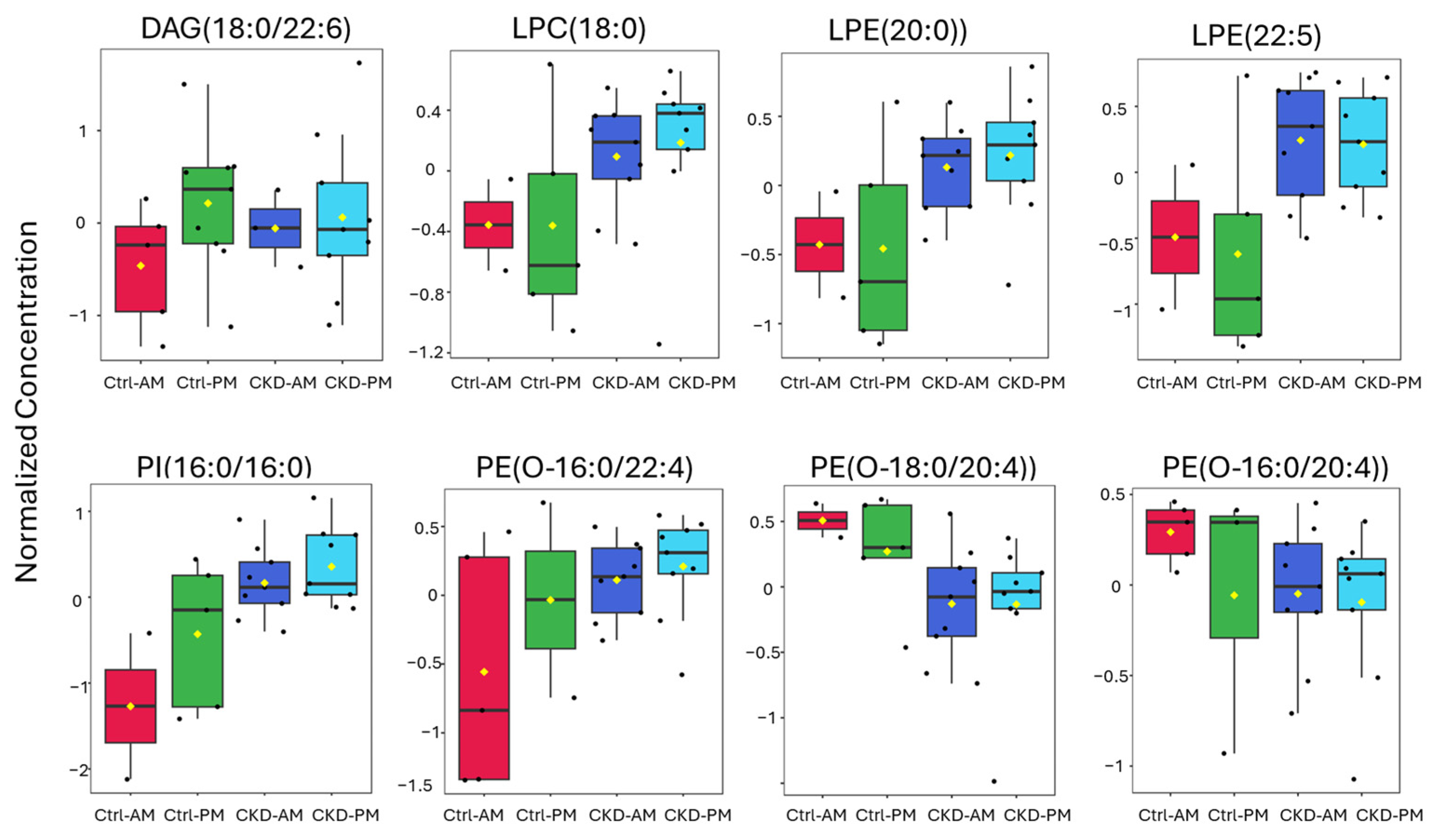
| Groups | n | eGFR mL·min−1·1.73 m2−1 | Serum Creatinine (mg·dL−1) | Change in Creatinine (mg·dL−1) |
|---|---|---|---|---|
| Average ± SD | Average ± SD | Average ± SD | ||
| Ctrl | 6 | 120 ± 22 | 0.81 ± 0.21 | 0.11 ± 0.09 |
| CKDu | 6 | 40 ± 9 | 2.16 ± 0.40 | 0.32 ± 0.17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, J.; Kroll, K.J.; Butler-Dawson, J.; Krisher, L.; Alli, A.A.; Vulpe, C.; Denslow, N.D. Novel Lipid Biomarkers of Chronic Kidney Disease of Unknown Etiology Based on Urinary Small Extracellular Vesicles: A Pilot Study of Sugar Cane Workers. Metabolites 2025, 15, 523. https://doi.org/10.3390/metabo15080523
Zhou J, Kroll KJ, Butler-Dawson J, Krisher L, Alli AA, Vulpe C, Denslow ND. Novel Lipid Biomarkers of Chronic Kidney Disease of Unknown Etiology Based on Urinary Small Extracellular Vesicles: A Pilot Study of Sugar Cane Workers. Metabolites. 2025; 15(8):523. https://doi.org/10.3390/metabo15080523
Chicago/Turabian StyleZhou, Jie, Kevin J. Kroll, Jaime Butler-Dawson, Lyndsay Krisher, Abdel A. Alli, Chris Vulpe, and Nancy D. Denslow. 2025. "Novel Lipid Biomarkers of Chronic Kidney Disease of Unknown Etiology Based on Urinary Small Extracellular Vesicles: A Pilot Study of Sugar Cane Workers" Metabolites 15, no. 8: 523. https://doi.org/10.3390/metabo15080523
APA StyleZhou, J., Kroll, K. J., Butler-Dawson, J., Krisher, L., Alli, A. A., Vulpe, C., & Denslow, N. D. (2025). Novel Lipid Biomarkers of Chronic Kidney Disease of Unknown Etiology Based on Urinary Small Extracellular Vesicles: A Pilot Study of Sugar Cane Workers. Metabolites, 15(8), 523. https://doi.org/10.3390/metabo15080523








