In Vitro Metabolism of Doping Agents (Stanozolol, LGD-4033, Anastrozole, GW1516, Trimetazidine) by Human Seminal Vesicle and Liver Fractions
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals, Kits and Reagents
2.2. Preparation of Incubation Buffer
2.3. In Vitro S9 Fraction Metabolic Assays
2.4. Sample Preparation and Extraction Procedure
2.5. LC-HRAM MS Instrumentation and Analytical Conditions
2.6. Cell Culture
2.7. RNA Extraction
2.8. cDNA Reverse Transcription
2.9. Digital PCR for Detecting Gene Expression
3. Results
3.1. In Vitro Assay for Doping Substances
3.2. In Vitro Protein Activity of CYP Enzymes
3.3. Gene Expression of Different CYPs
4. Discussion
4.1. In Vitro Assay for Doping Substances
4.2. In Vitro Protein Activity of Different CYPs
4.3. Gene Expression of Different CYPs
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AAF | Adverse analytical finding |
| LC | Liquid chromatography |
| HRAM MS | High-resolution/accurate mass spectrometry |
| dPCR | digital polymerase chain reaction |
References
- Thevis, M.; Kuuranne, T.; Fedoruk, M.; Geyer, H. Sports drug testing and the athletes’ exposome. Drug Test. Anal. 2021, 13, 1814–1821. [Google Scholar] [CrossRef] [PubMed]
- Breuer, J.; Garzinsky, A.; Thomas, A.; Nieschlag, E.; Kliesch, S.; Fedoruk, M.; Geyer, H.; Thevis, M. Complementary information concerning the suspected interindividual transmission of gw1516, a substance prohibited in sport, through intimate contact: A case report. Forensic Toxicol. 2024, 2, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Breuer, J.; Garzinsky, A.-M.; Thomas, A.; Kliesch, S.; Nieschlag, E.; Wenzel, F.; Georgas, E.; Geyer, H.; Thevis, M. Investigations into the concentrations and metabolite profiles of doping agents and antidepressants in human seminal fluid using liquid chromatography-mass spectrometry. Drug Metab. Dispos. 2024, 52, 1313–1322. [Google Scholar] [CrossRef] [PubMed]
- Kintz, P. Drug transfer during intimate moments: A key issue in doping control that can be documented by hair tests of the athlete and the partner. Med. Sci. Law. 2024, 64, 72–76. [Google Scholar] [CrossRef]
- Richardson, S.J.; Bai, A.; Kulkarni, A.A.; Moghaddam, M.F. Efficiency in drug discovery: Liver s9 fraction assay as a screen for metabolic stability. Drug Metab. Lett. 2016, 10, 83–90. [Google Scholar] [CrossRef]
- Cao, Y.J.; Hendrix, C.W. Male genital tract pharmacology: Developments in quantitative methods to better understand a complex peripheral compartment. Clin. Pharmacol. Ther. 2008, 83, 401–412. [Google Scholar] [CrossRef]
- Ndovi, T.T.; Parsons, T.; Choi, L.; Caffo, B.; Rohde, C.; Hendrix, C.W. A new method to estimate quantitatively seminal vesicle and prostate gland contributions to ejaculate. Br. J. Clin. Pharmacol. 2007, 63, 404–420. [Google Scholar] [CrossRef]
- Bylund, J.; Finnström, N.; Oliw, E.H. Gene expression of a novel cytochrome P450 of the cyp4f subfamily in human seminal vesicles. Biochem. Biophys. Res. Commun. 1999, 261, 169–174. [Google Scholar] [CrossRef]
- Wang, X.; He, B.; Shi, J.; Li, Q.; Zhu, H.-J. Comparative proteomics analysis of human liver microsomes and s9 fractions. Drug Metab. Dispos. 2020, 48, 31–40. [Google Scholar] [CrossRef]
- Zanger, U.M.; Schwab, M. Cytochrome P450 enzymes in drug metabolism: Regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol. Ther. 2013, 138, 103–141. [Google Scholar] [CrossRef]
- Bernhardt, R. Cytochromes P450 as versatile biocatalysts. J. Biotechnol. 2006, 124, 128–145. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Deshmukh, R.S.; Ericksen, S.S.; Tu, Y.; Szklarz, G.D. Preferred binding orientations of phenacetin in cyp1a1 and cyp1a2 are associated with isoform-selective metabolism. Drug Metab. Dispos. 2012, 40, 2324–2331. [Google Scholar] [CrossRef] [PubMed]
- Sevrioukova, I.F.; Poulos, T.L. Structural basis for regiospecific midazolam oxidation by human cytochrome P450 3a4. Proc. Natl. Acad. Sci. USA 2017, 114, 486–491. [Google Scholar] [CrossRef] [PubMed]
- Schänzer, W.; Opfermann, G.; Donike, M. Metabolism of stanozolol: Identification and synthesis of urinary metabolites. J. Steroid Biochem. 1990, 36, 153–174. [Google Scholar] [CrossRef]
- Wagener, F.; Guddat, S.; Görgens, C.; Angelis, Y.S.; Petrou, M.; Lagojda, A.; Kühne, D.; Thevis, M. Investigations into the elimination profiles and metabolite ratios of micro-dosed selective androgen receptor modulator lgd-4033 for doping control purposes. Anal. Bioanal. Chem. 2022, 414, 1151–1162. [Google Scholar] [CrossRef]
- Sigmund, G.; Koch, A.; Orlovius, A.K.; Guddat, S.; Thomas, A.; Schänzer, W.; Thevis, M. Doping control analysis of trimetazidine and characterization of major metabolites using mass spectrometric approaches. Drug Test. Anal. 2014, 6, 1197–1205. [Google Scholar] [CrossRef]
- Breuer, J.; Thomas, A.; Delahaut, P.; Schanzer, W.; Geyer, H.; Thevis, M. Investigations into the concentration and metabolite profiles of stanozolol and lgd-4033 in blood plasma and seminal fluid using liquid chromatography high-resolution mass spectrometry. Anal. Bioanal. Chem. 2023, 415, 669–681. [Google Scholar] [CrossRef]
- Ceelen, L.; De Spiegelaere, W.; David, M.; De Craene, J.; Vinken, M.; Vanhaecke, T.; Rogiers, V. Critical selection of reliable reference genes for gene expression study in the heparg cell line. Biochem. Pharmacol. 2011, 81, 1255–1261. [Google Scholar] [CrossRef][Green Version]
- Luckert, C.; Schulz, C.; Lehmann, N.; Thomas, M.; Hofmann, U.; Hammad, S.; Hengstler, J.G.; Braeuning, A.; Lampen, A.; Hessel, S. Comparative analysis of 3D culture methods on human hepg2 cells. Arch. Toxicol. 2017, 91, 393–406. [Google Scholar] [CrossRef]
- Kamdem, L.K.; Liu, Y.; Stearns, V.; Kadlubar, S.A.; Ramirez, J.; Jeter, S.; Shahverdi, K.; Ward, B.A.; Ogburn, E.; Ratain, M.J. In vitro and in vivo oxidative metabolism and glucuronidation of anastrozole. Br. J. Clin. Pharmacol. 2010, 70, 854–869. [Google Scholar] [CrossRef]
- Möller, I.; Thomas, A.; Beuck, S.; Rodchenkov, G.; Bornatsch, W.; Geyer, H.; Schänzer, W.; Thevis, M. In-vitro metabolism of gw1516 and implementation of its major metabolites in routine doping controls. In Cologne Workshop on Dope Analysis; Sportverlag Strauß: Hellenthal, Germany, 2010; pp. 17–26. [Google Scholar]
- Thevis, M.; Möller, I.; Thomas, A.; Beuck, S.; Rodchenkov, G.; Bornatsch, W.; Geyer, H.; Schänzer, W. Characterization of two major urinary metabolites of the pparδ-agonist gw1516 and implementation of the drug in routine doping controls. Anal. Bioanal. Chem. 2010, 396, 2479–2491. [Google Scholar] [CrossRef] [PubMed]
- Fragkaki, A.G.; Sakellariou, P.; Kiousi, P.; Kioukia-Fougia, N.; Tsivou, M.; Petrou, M.; Angelis, Y. Human in vivo metabolism study of lgd-4033. Drug Test. Anal. 2018, 10, 1635–1645. [Google Scholar] [CrossRef]
- Geldof, L.; Pozo, O.J.; Lootens, L.; Morthier, W.; Van Eenoo, P.; Deventer, K. In vitro metabolism study of a black market product containing sarm lgd-4033. Drug Test. Anal. 2017, 9, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Thevis, M.; Lagojda, A.; Kuehne, D.; Thomas, A.; Dib, J.; Hansson, A.; Hedeland, M.; Bondesson, U.; Wigger, T.; Karst, U. Characterization of a non-approved selective androgen receptor modulator drug candidate sold via the internet and identification of in vitro generated phase-i metabolites for human sports drug testing. Rapid Commun. Mass. Spectrom. 2015, 29, 991–999. [Google Scholar] [CrossRef]
- Mazzarino, M.; de la Torre, X.; Fiacco, I.; Botre, F. Drug-drug interaction and doping, part 2: An in vitro study on the effect of non-prohibited drugs on the phase i metabolic profile of stanozolol. Drug Test. Anal. 2014, 6, 969–977. [Google Scholar] [CrossRef]
- Pozo, O.J.; Van Eenoo, P.; Deventer, K.; Lootens, L.; Grimalt, S.; Sancho, J.V.; Hernandez, F.; Meuleman, P.; Leroux-Roels, G.; Delbeke, F.T. Detection and structural investigation of metabolites of stanozolol in human urine by liquid chromatography tandem mass spectrometry. Steroids 2009, 74, 837–852. [Google Scholar] [CrossRef]
- Nalakath, J.; Pt, R.; Kadry, A.; Palathinkal, A.B.; Praseen, O.K.; Nelliyott, I.; Hebel, C.; Selvapalam, N.; Nagarajan, E.R. Comprehensive metabolite profiling of trimetazidine in camels using high-resolution accurate mass spectrometry: Implications for doping control. Rapid Commun. Mass. Spectrom. 2023, 37, e9626. [Google Scholar] [CrossRef]
- Feidt, D.M.; Klein, K.; Hofmann, U.; Riedmaier, S.; Knobeloch, D.; Thasler, W.E.; Weiss, T.S.; Schwab, M.; Zanger, U.M. Profiling induction of cytochrome P450 enzyme activity by statins using a new liquid chromatography-tandem mass spectrometry cocktail assay in human hepatocytes. Drug Metab. Dispos. 2010, 38, 1589–1597. [Google Scholar] [CrossRef]
- Walsky, R.L.; Obach, R.S. Validated assays for human cytochrome P450 activities. Drug Metab. Dispos. 2004, 32, 647–660. [Google Scholar] [CrossRef]
- Taxak, N.; Prasad, K.C.; Bharatam, P.V. Mechanistic insights into the bioactivation of phenacetin to reactive metabolites: A dft study. Comput. Theor. Chem. 2013, 1007, 48–56. [Google Scholar] [CrossRef]
- Zhang, Y.; Vermeulen, N.P.; Commandeur, J.N. Characterization of human cytochrome P450 mediated bioactivation of amodiaquine and its major metabolite n-desethylamodiaquine. Br. J. Clin. Pharmacol. 2017, 83, 572–583. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.R.; Pieper, J.A.; Frye, R.F.; Hinderliter, A.L.; Blaisdell, J.A.; Goldstein, J.A. Tolbutamide, flurbiprofen, and losartan as probes of cyp2c9 activity in humans. J. Clin. Pharmacol. 2003, 43, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Vijayabhaskar, V.; Srivastava, P.; Rajagopal, S. Breaking the sensitivity limitations of cytochrome P450 oxidation product: Dansyl chloride derivatisation of 4-oh mephenytoin, a cyp2c19 metabolite and its application to in vitro cyp inhibition assay. J. Chromatogr. B Analyt Technol. Biomed. Life Sci. 2015, 989, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Andy, A.; Kallem, R.R.; Mullangi, R.; Andy, D.; Seshagiri Rao, J. Highly sensitive uhplc-ms/ms method for the simultaneous estimation of propafenone and its metabolites 5-hydroxypropafenone and n-depropylpropafenone on human dried blood spots technique and application to a pharmacokinetic study. J. Pharm. Biomed. Anal. 2017, 142, 328–336. [Google Scholar] [CrossRef]
- Von Ahlfen, S.; Schlumpberger, M. Effects of low A260/A230 ratios in rna preparations on downstream applications. Qiagen Gene Expr. Newsl. 2010, 15, 6–7. [Google Scholar]
- Kuuranne, T.; Leinonen, A.; Thevis, M.; Schänzer, W.; Pystynen, K.; Kostiainen, R. Metabolism of ‘new’anabolic steroids: Development of in vitro methodology in metabolite production and analytical techniques. Recent. Adv. Doping Anal. 2006, 14, 161–168. [Google Scholar]
- Göschl, L.; Gmeiner, G.; Gärtner, P.; Stadler, G.; Enev, V.; Thevis, M.; Schänzer, W.; Guddat, S.; Forsdahl, G. Stanozolol-n-glucuronide metabolites in human urine samples as suitable targets in terms of routine anti-doping analysis. Drug Test. Anal. 2021, 13, 1668–1677. [Google Scholar] [CrossRef]
- Schänzer, W.; Guddat, S.; Thomas, A.; Opfermann, G.; Geyer, H.; Thevis, M. Expanding analytical possibilities concerning the detection of stanozolol misuse by means of high resolution/high accuracy mass spectrometric detection of stanozolol glucuronides in human sports drug testing. Drug Test. Anal. 2013, 5, 810–818. [Google Scholar] [CrossRef]
- Wang, W.Y.; Lin, L.; Boone, E.C.; Stevens, J.; Gaedigk, A. Cyp2d6 copy number determination using digital pcr. Front. Pharmacol. 2024, 15, 1429286. [Google Scholar] [CrossRef]
- Hekim, N.; Gure, M.A.; Metin Mahmutoglu, A.; Gunes, S.; Asci, R.; Henkel, R. Snp’s in xenobiotic metabolism and male infertility. Xenobiotica 2020, 50, 363–370. [Google Scholar] [CrossRef]
- Taylor, C.; Crosby, I.; Yip, V.; Maguire, P.; Pirmohamed, M.; Turner, R.M. A review of the important role of cyp2d6 in pharmacogenomics. Genes 2020, 11, 1295. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Antona, C.; Donato, M.T.; Pareja, E.; Gómez-Lechón, M.-J.; Castell, J.V. Cytochrome p-450 mrna expression in human liver and its relationship with enzyme activity. Arch. Biochem. Biophys. 2001, 393, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.; Modi, S.; Pillai, I.; Lian, L.-Y.; Sutcliffe, M.J.; Pritchard, M.P.; Friedberg, T.; Roberts, G.C.; Wolf, C.R. Determinants of the substrate specificity of human cytochrome p-450 cyp2d6: Design and construction of a mutant with testosterone hydroxylase activity. Biochem. J. 1998, 331, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Finnstrom, N.; Bjelfman, C.; Soderstrom, T.G.; Smith, G.; Egevad, L.; Norlen, B.J.; Wolf, C.R.; Rane, A. Detection of cytochrome P450 mrna transcripts in prostate samples by rt-pcr. Eur. J. Clin. Investig. 2001, 31, 880–886. [Google Scholar] [CrossRef]
- Lu, Y.; Cederbaum, A.I. Cyp2e1 and oxidative liver injury by alcohol. Free Radic. Biol. Med. 2008, 44, 723–738. [Google Scholar] [CrossRef]
- Tanaka, E.; Terada, M.; Misawa, S. Cytochrome P450 2e1: Its clinical and toxicological role. J. Clin. Pharm. Ther. 2000, 25, 165–175. [Google Scholar] [CrossRef]
- Shayakhmetova, G.M.; Bondarenko, L.B.; Kovalenko, V.M.; Ruschak, V.V. Cyp2e1 testis expression and alcohol-mediated changes of rat spermatogenesis indices and type i collagen. Arh. Hig. Rada Toksikol. 2013, 64, 51–60. [Google Scholar] [CrossRef]
- Powell, H.; Kitteringham, N.R.; Pirmohamed, M.; Smith, D.A.; Park, B.K. Expression of cytochrome p4502e1 in human liver: Assessment by mrna, genotype and phenotype. Pharmacogenetics 1998, 8, 411–421. [Google Scholar] [CrossRef]
- Kocarek, T.A.; Zangar, R.C.; Novak, R.F. Post-transcriptional regulation of rat cyp2e1 expression: Role of cyp2e1 mrna untranslated regions in control of translational efficiency and message stability. Arch. Biochem. Biophys. 2000, 376, 180–190. [Google Scholar] [CrossRef]
- Daly, A.K.; Rettie, A.E.; Fowler, D.M.; Miners, J.O. Pharmacogenomics of cyp2c9: Functional and clinical considerations. J. Pers. Med. 2017, 8, 1. [Google Scholar] [CrossRef]
- Sangkuhl, K.; Claudio-Campos, K.; Cavallari, L.H.; Agundez, J.A.G.; Whirl-Carrillo, M.; Duconge, J.; Del Tredici, A.L.; Wadelius, M.; Rodrigues Botton, M.; Woodahl, E.L.; et al. Pharmvar genefocus: Cyp2c9. Clin. Pharmacol. Ther. 2021, 110, 662–676. [Google Scholar] [CrossRef] [PubMed]
- Klose, T.S.; Blaisdell, J.A.; Goldstein, J.A. Gene structure of cyp2c8 and extrahepatic distribution of the human cyp2cs. J. Biochem. Mol. Toxicol. 1999, 13, 289–295. [Google Scholar] [CrossRef]
- Wolbold, R.; Klein, K.; Burk, O.; Nüssler, A.K.; Neuhaus, P.; Eichelbaum, M.; Schwab, M.; Zanger, U.M. Sex is a major determinant of cyp3a4 expression in human liver. Hepatology 2003, 38, 978–988. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lu, Y.F.; Corton, J.C.; Klaassen, C.D. Expression of cytochrome P450 isozyme transcripts and activities in human livers. Xenobiotica 2021, 51, 279–286. [Google Scholar] [CrossRef]
- Afshar, M.; Thormann, W. Validated capillary electrophoresis assay for the simultaneous enantioselective determination of propafenone and its major metabolites in biological samples. Electrophoresis 2006, 27, 1517–1525. [Google Scholar] [CrossRef]
- Fekete, F.; Mangó, K.; Minus, A.; Tóth, K.; Monostory, K. Cyp1a2 mrna expression rather than genetic variants indicate hepatic cyp1a2 activity. Pharmaceutics 2022, 14, 532. [Google Scholar] [CrossRef]
- Kim, S.; Park, J.Y.; Lee, H.W.; Bae, S.U.; Kim, K.E.; Byun, S.J.; Seo, I. Ywhaz and tbp are potential reference gene candidates for qpcr analysis of response to radiation therapy in colorectal cancer. Sci. Rep. 2023, 13, 12902. [Google Scholar] [CrossRef]
- Kaitu’u-Lino, T.J.; Hastie, R.; Cannon, P.; Lee, S.; Stock, O.; Hannan, N.J.; Hiscock, R.; Tong, S. Stability of absolute copy number of housekeeping genes in preeclamptic and normal placentas, as measured by digital pcr. Placenta 2014, 35, 1106–1109. [Google Scholar] [CrossRef]
- Lippert, T.H.; Seeger, H.; Schieferstein, G.; Voelter, W. Immunoreactive ubiquitin in human seminal plasma. J. Androl. 1993, 14, 130–131. [Google Scholar] [CrossRef]
- Kumar, N.; Singh, N.K. Emerging role of novel seminal plasma bio-markers in male infertility: A review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 253, 170–179. [Google Scholar] [CrossRef]
- Fu, L.-Y.; Jia, H.-L.; Dong, Q.-Z.; Wu, J.-C.; Zhao, Y.; Zhou, H.-J.; Ren, N.; Ye, Q.-H.; Qin, L.-X. Suitable reference genes for real-time pcr in human hbv-related hepatocellular carcinoma with different clinical prognoses. BMC Cancer 2009, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bruce, K.D.; Sihota, K.K.; Byrne, C.D.; Cagampang, F.R. The housekeeping gene ywhaz remains stable in a model of developmentally primed non-alcoholic fatty liver disease. Liver Int. 2012, 32, 1315–1321. [Google Scholar] [CrossRef] [PubMed]
- Breuer, J.; Thomas, A.; Geyer, H.; Thevis, M. Probing for the presence of semenogelin in human urine by immunological and chromatographic-mass spectrometric methods in the context of sports drug testing. Anal. Sci. Adv. 2022, 3, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Kintz, P. Reply to the paper of breuer et al.: Complementary information concerning the suspected interindividual transmission of gw1516, a substance prohibited in sport, through intimate contact—A case report. Forensic Toxicol. 2024, 43, 176–178. [Google Scholar] [CrossRef]
- Tang, Y.; Xu, L.; Zhao, J.; Qian, X.; Qiang, H.; Xiang, P.; Yan, H. Metabolic profile of etomidate and its three analogs in zebrafish, human liver microsomes, human urine and hair samples using uhplc-q exactive orbitrap-hrms. Drug Test. Anal. 2025. [Google Scholar] [CrossRef]
- Wagener, F.; Naumann, N.; Göldner, V.; Görgens, C.; Guddat, S.; Karst, U.; Thevis, M. Comparison of in vitro approaches for predicting the metabolism of the selective androgen receptor modulator rad140. Anal. Bioanal. Chem. 2023, 415, 5657–5669. [Google Scholar] [CrossRef]


| Primer | Sequence (Forward) | Sequence (Reverse) |
|---|---|---|
| CYP1A2 | 5′-ctc ctc ctt ctt gcc ctt ca-3′ | 5′-gta gaa gcc att cag cgt tgt g-3′ |
| CYP2B6 | 5′-ttc cta ctg ctt ccg tct atc aaa-3′ | 5′-gtg cag aat ccc aca gct ca-3′ |
| CYP2C9 | 5′-aag gag atc cgg cgt ttc tc-3′ | 5′-cgg tcc tca atg ctc ctc ttc-3′ |
| CYP2D6 | 5′-gac cag aga tgg gtg acc ag-3′ | 5′-cga tgt cac ggg atg tca ta-3′ |
| CYP2E1 | 5′-cat gag att cag cgg ttc atc-3′ | 5′-ggt gtc tcg ggt tgc ttc a-3′ |
| CYP3A4 | 5′-tca gcc tgg tgc tcc tct atc tat-3′ | 5′-aag ccc tta tgg tag gac aaa ata ttt-3′ |
| TBP | 5′-gag agt tct ggg att gta ccg-3′ | 5′-atc ctc atg att acc gca gc-3′ |
| UBC | 5′-gcc tta gaa ccc cag tat cag-3′ | 5′-aag aaa acc agt gcc cta gag-3′ |
| YHWAZ | 5′-atg caa cca aca cat cct atc-3′ | 5′-gca tta tta gcg tgc tgt ctt-3′ |
| Analyte | Exact Mass [m/z] | Formula | Retention Time [min] | NCE [%] | Product Ions [m/z] | Formation In Vitro/In Vivo | Literature |
|---|---|---|---|---|---|---|---|
| Anastrozole | 294.1713 | C17H19N5 | 7.13 | 30 | 225.1386 | ||
| 266.1652 | |||||||
| A-M3 | 310.1662 | C17H19N5O | 5.84 | 30 | 241.1335 | HL-S9 | [3,20] |
| 214.1226 | |||||||
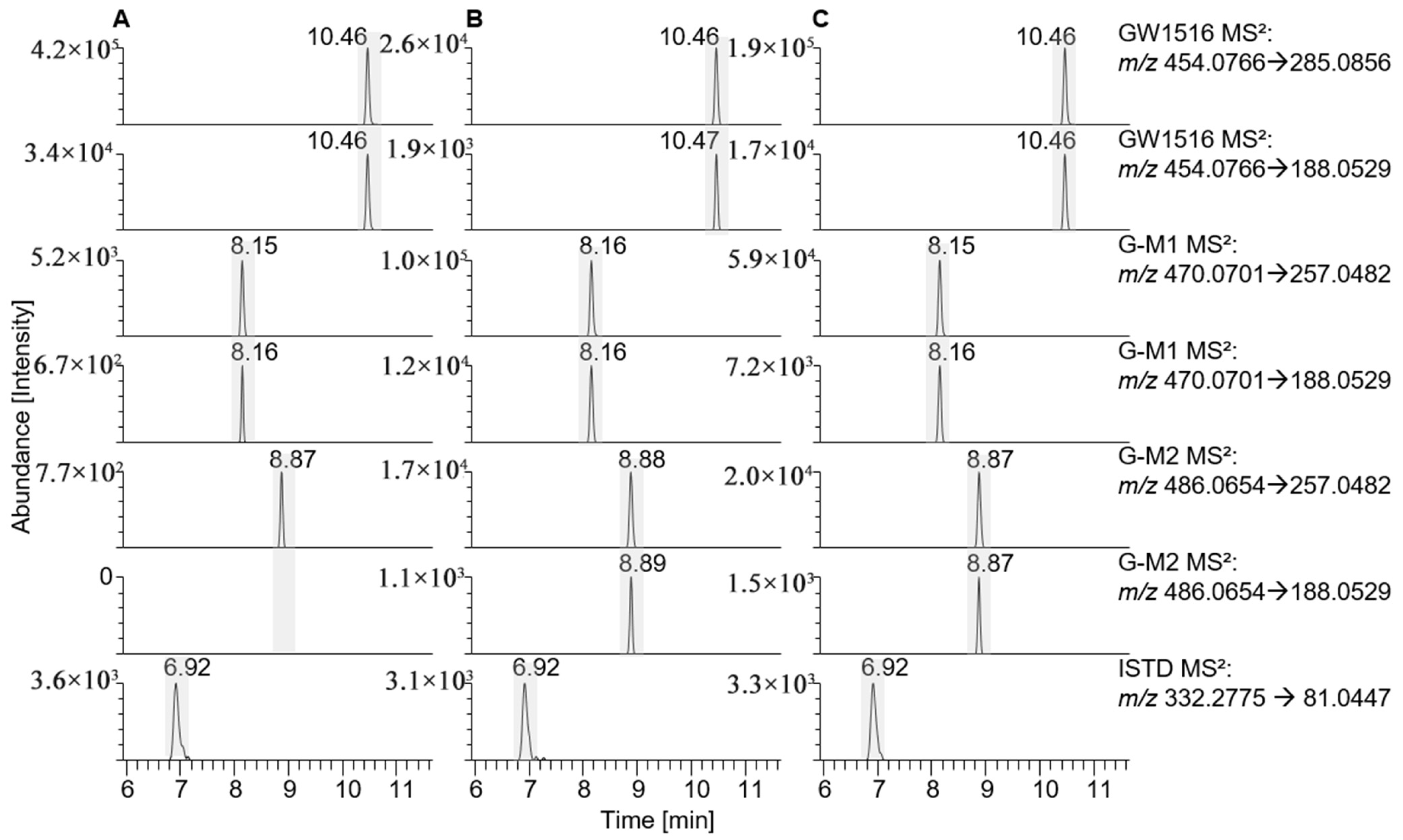
| GW1516 | 454.0766 | C21H19O3NF3S2 | 10.44 | 50 | 257.0482 | ||
| 188.0529 | |||||||
| G-M1-a | 470.0701 | C21H19O4NF3S2 | 8.10 | 50 | 274.0508 | HL-S9, SV-S9, in vivo | [21,22] |
| 257.0482 | |||||||
| G-M1-b | 8.98 | 50 | 274.0508 | in vivo | [21] | ||
| 257.0482 | |||||||
| G-M2 | 486.0654 | C21H19O5NF3S2 | 8.83 | 50 | 272.0346 | HL-S9, SV-S9, in vivo | [21,22] |
| 257.0482 | |||||||
| LGD-4033 | 337.0781 | C14H11F6N2O- | 7.05 | 30 | 267.0751 | ||
| 170.0225 | |||||||
| L-M1 | 7.18 | 267.0751 | HL-S9, in vivo | [15,23,24] | |||
| 239.0438 | |||||||
| L-M3 | 351.0574 | C14H9F6N2O2- | 5.82 | 30 | 237.0645 | SV-S9, HL-S9, in vivo | [15,24,25] |
| 253.0219 | |||||||
| L-M4 | 353.073 | C14H11F6N2O2- | 6.24 | 30 | 255.0751 | HL-S9, in vivo | [15,23,24] |
| 199.0492 | |||||||
| L-M5 | 355.0887 | C14H13F6N2O2- | 5.59 | 30 | 285.0856 | HL-S9, SV-S9, in vivo | [15,23,24] |
| 257.0907 | |||||||
| L-M6 | 369.0679 | C14H11F6N2O3- | 5.36 | 30 | 281.0543 | HL-S9, SV-S9, in vivo | [15,23,24,25] |
| 170.0212 | |||||||
| L-M7 | 385.0628 | C14H11F6N2O4- | 4.68 | 30 | 225.0645 | HL-S9 | [15,23] |
| 227.0427 | |||||||
| Stanozolol | 329.2598 | C21H33N2O+ | 6.92 | 65 | 81.0447 | - | |
| 107.0855 | |||||||
| S-M3-a | 345.2537 | C21H33N2O2+ | 4.9 | 65 | 81.0447 | HL-S9 | [14,26,27] |
| 95.0855 | |||||||
| S-M3-b | 5.01 | 81.0447 | HL-S9 | ||||
| 95.0604 | |||||||
| S-M3-c | 5.58 | 81.0447 | HL-S9 | ||||
| 95.0604 | |||||||
| S-M3-d | 5.74 | 81.0447 | HL-S9 | ||||
| 95.0604 | |||||||
| S-M3-e | 6.08 | 97.0396 | HL-S9 | ||||
| 121.1012 | |||||||
| S-M3-f | 6.27 | 145.076 | HL-S9 | [14,26] | |||
| 95.0855 | |||||||
| S-M4-a | 361.2486 | C21H33N2O3+ | 4.4 | 65 | 81.0447 | HL-S9 | [14,27] |
| 95.0604 | |||||||
| S-M4-b | 4.52 | 81.0447 | HL-S9 | ||||
| 361.2486 | |||||||
| S-M4-c | 4.89 | 81.0447 | HL-S9 | ||||
| 361.2486 | |||||||
| S-M4-d | 5.05 | 97.0396 | HL-S9 | ||||
| 95.0855 | |||||||
| S-M4-e | 5.36 | 145.076 | HL-S9 | [14] | |||
| 95.0604 | |||||||
| S-M5 | 343.238 | C21H31N2O2+ | 5.68 | 65 | 81.0447 | HL-S9 | [26,27] |
| 257.2012 | |||||||
| S-M6 | 359.2329 | C21H31N2O3+ | 5.05 | 65 | 97.0396 | HL-S9 | [26,27] |
| 273.1961 | |||||||
| Trimetazidine | 267.1703 | C14H22N2O3 | 5.06 | 30 | 181.0858 | ||
| 166.0629 | |||||||
| T-M1 | 283.1649 | C14H23N2O4 | 4.50 | 30 | 181.0858 | HL-S9, SV-S9 | [16,28] |
| 166.0629 | |||||||
| Paroxetine-d6 | 336.1876 | 6.67 | 45 | 76.0995 | |||
| S-24 | 381.0868 | C18H13O3N2F4- | 7.00 | 30 | 241.0594 | ||
| Stanozolol-d3 | 332.2275 | 6.97 | 65 | 81.047 |
| Analyte | Formula | Retention Time [min] | [m/z] | Formation In Vitro | |
|---|---|---|---|---|---|
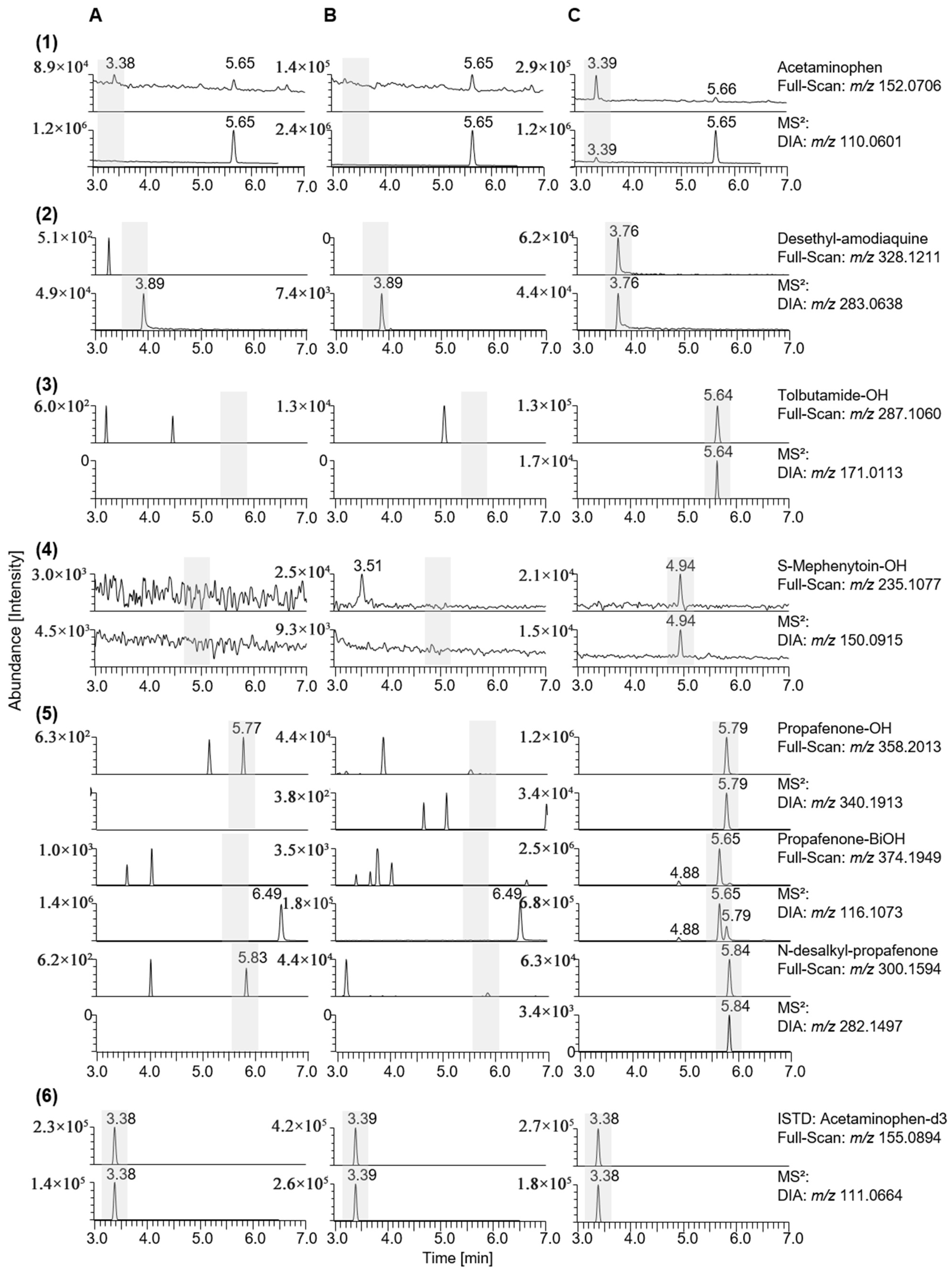
| Phenacetin | C10H13NO | 5.65 | Full-Scan | 180.1019 | - |
| MS2 | 138.0915 | ||||
| Acetaminophen (CYP1A2) | C8H9NO2 | 3.39 | Full-Scan | 152.0706 | HL-S9 |
| MS2 | 110.0601 | ||||
| Amodiaquine | C20H22ClN3O | 3.89 | Full-Scan | 356.1524 | - |
| MS2 | 283.0638 | ||||
| desethyl-amodiaquine (CYP2C8) | C18ClN3O | 3.76 | Full-Scan | 328.1211 | HL-S9 |
| MS2 | 283.0638 | ||||
| S-mephenytoin | C12H14N2O2 | 6.17 | Full-Scan | 219.1128 | - |
| MS2 | 134.0967 | ||||
| OH-mephenytoin (CYP2C19) | C12H14N2O3 | 4.94 | Full-Scan | 235.1077 | HL-S9 |
| MS2 | 150.0915 | ||||
| Tolbutamide | C12H20N2O3S | 7.44 | Full-Scan | 271.1110 | - |
| MS2 | 155.0165 | ||||
| OH-tolbutamide (CYP2C9) | C12H20N2O4S | 5.64 | Full-Scan | 287.1060 | HL-S9 |
| MS2 | 171.0113 | ||||
| Propafenone | C21H27NO3 | 6.49 | Full-Scan | 342.2063 | - |
| MS2 | 116.1073 | ||||
| OH-propafenone (CYP2D6) | C21H27NO4 | 5.79 | Full-Scan | 358.2013 | HL-S9 |
| MS2 | 340.1913 | ||||
| Bi-OH-propafenone | C21H27NO5 | 5.65 | Full-Scan | 374.1949 | HL-S9 |
| MS2 | 116.1069 | ||||
| N-desalkyl-propafenone (CYP3A4/CYP1A2) | C18H22NO3 | 5.82 | Full-Scan | 300.1594 | HL-S9 |
| MS2 | 282.1497 | ||||
| Acetaminophen-d3 | - | 3.38 | Full-Scan | 155.0894 | - |
| MS2 | 111.0664 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sternberg, J.; Peters, I.; Naumann, N.; Thomas, A.; Thevis, M. In Vitro Metabolism of Doping Agents (Stanozolol, LGD-4033, Anastrozole, GW1516, Trimetazidine) by Human Seminal Vesicle and Liver Fractions. Metabolites 2025, 15, 452. https://doi.org/10.3390/metabo15070452
Sternberg J, Peters I, Naumann N, Thomas A, Thevis M. In Vitro Metabolism of Doping Agents (Stanozolol, LGD-4033, Anastrozole, GW1516, Trimetazidine) by Human Seminal Vesicle and Liver Fractions. Metabolites. 2025; 15(7):452. https://doi.org/10.3390/metabo15070452
Chicago/Turabian StyleSternberg, Johanna, Insa Peters, Nana Naumann, Andreas Thomas, and Mario Thevis. 2025. "In Vitro Metabolism of Doping Agents (Stanozolol, LGD-4033, Anastrozole, GW1516, Trimetazidine) by Human Seminal Vesicle and Liver Fractions" Metabolites 15, no. 7: 452. https://doi.org/10.3390/metabo15070452
APA StyleSternberg, J., Peters, I., Naumann, N., Thomas, A., & Thevis, M. (2025). In Vitro Metabolism of Doping Agents (Stanozolol, LGD-4033, Anastrozole, GW1516, Trimetazidine) by Human Seminal Vesicle and Liver Fractions. Metabolites, 15(7), 452. https://doi.org/10.3390/metabo15070452







