Assessment of Metabolic Alterations Induced by Halogenated Additives and Antifungal Activity of Extracts from the Endophytic Fungus Fusarium sp. Associated with Dizygostemon riparius (Plantaginaceae)
Abstract
1. Introduction
2. Materials and Methods
2.1. Endophytic Fungus
2.2. Fermentation and Extraction
2.3. Microculture and Morphological Identification
2.4. Extraction of Crude Extracts and Fractionation of Methanolic Extracts from Control (Czapek) and Media Modified with MnCl2 and NH4Br
2.5. Chromatographic Profile by High-Performance Liquid Chromatography (HPLC) of the Obtained Fractions
2.6. Analysis by Ultra-Performance Liquid Chromatography—Electrospray Ionisation—Quadrupole Time-of-Flight Mass Spectrometry (UPLC-ESI-QTOF-MS)
2.7. In Vitro and In Vivo Tests for Anti-Candida Activity
2.7.1. Candida Strains, Growth Conditions, and Inoculum Preparation
2.7.2. Determination of Minimum Inhibitory Concentration (MIC)
2.7.3. Determination of Minimum Fungicidal Concentration (MFC)
2.7.4. Effect of Extract Against Candida Biofilms
2.7.5. Biofilm Viability Assay
2.7.6. Crystal Violet Staining Analysis of Biofilm
2.8. In Vivo Assay in Tenebrio molitor Larvae
2.8.1. Acute Toxicity Assay Against T. molitor Larvae
2.8.2. Efficacy of Extracts in T. molitor Larvae Infected with C. albicans
2.9. Statistical Analysis
3. Results
3.1. Annotation of Compounds Present in the MeOH Extracts from Mycelium
3.2. In Vitro Anti-Candida Assays
3.2.1. Antifungal Activity of Fusarium sp. Extract Fractions Against Candida Albicans Strains
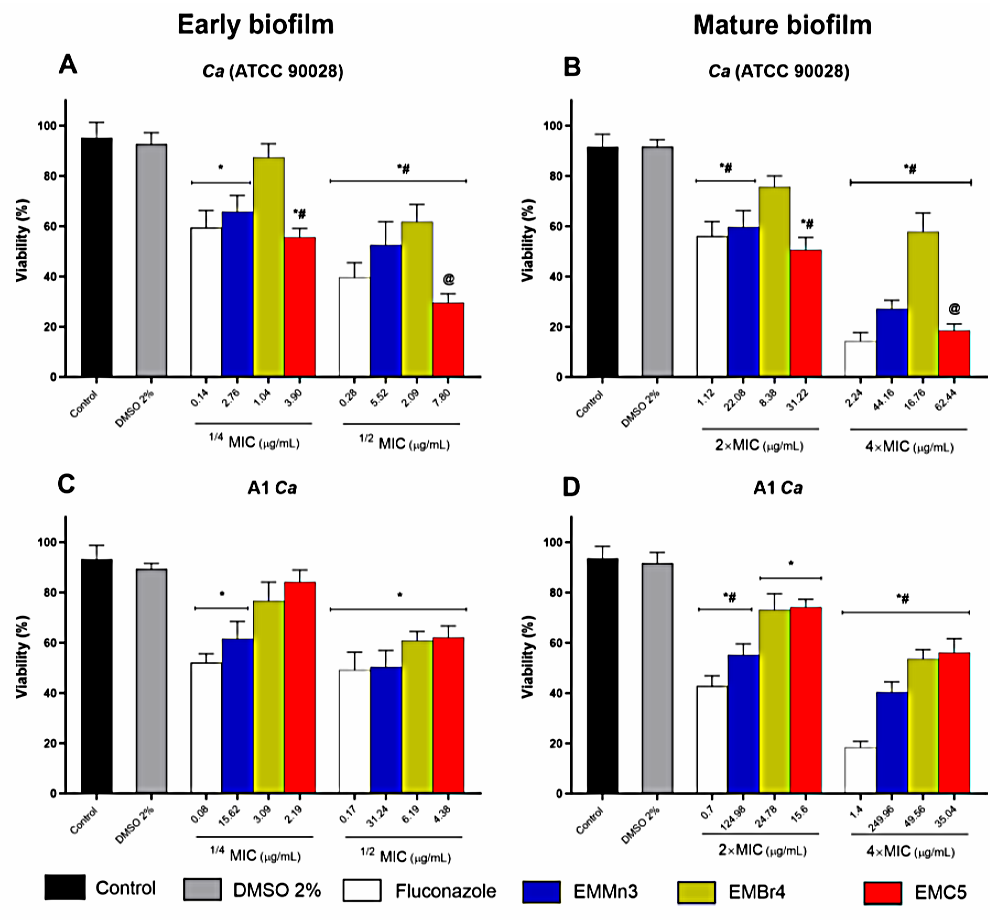
3.2.2. Inhibition of Biofilm Viability by Fusarium sp. Extract Fractions in Candida albicans
3.3. In Vivo Assays
3.3.1. In Vivo Toxicity of Fusarium sp. Extract Fractions in Tenebrio molitor Larvae
3.3.2. Survival of T. molitor Larvae Infected with C. albicans and Treated with Fusarium sp. Fractions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tupaki-Sreepurna, A.; Kindo, A.J. Fusarium: The Versatile Pathogen. Indian J. Med. Microbiol. 2018, 36, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Jankowiak, R.; Stępniewska, H.; Bilański, P.; Hausner, G. Fusarium Species Associated with Naturally Regenerated Fagus Sylvatica Seedlings Affected by Phytophthora. Eur. J. Plant Pathol. 2025, 171, 661–681. [Google Scholar] [CrossRef]
- Todorović, I.; Moënne-Loccoz, Y.; Raičević, V.; Jovičić-Petrović, J.; Muller, D. Microbial Diversity in Soils Suppressive to Fusarium Diseases. Front. Plant Sci. 2023, 14, 1228749. [Google Scholar] [CrossRef] [PubMed]
- Rezende Ferreira, R.; Santos Cruz, J.; Hamerski, L. OSMAC Strategy: An Affordable Method for the Discovery of New Microbial Compounds. Rev. Virtual Química 2022, 14, 853–869. [Google Scholar] [CrossRef]
- Santos, A.K.M.; dos Santos, B.A.; Farias, J.R.; de Morais, S.V.; Vasconcelos, C.C.; Guerra, R.N.M.; Rodrigues-Filho, E.; Lopes, A.J.O.; Cantanhede Filho, A.J. Effect of Mn(II) and Co(II) on Anti-Candida Metabolite Production by Aspergillus sp. an Endophyte Isolated from Dizygostemon Riparius (Plantaginaceae). Pharmaceuticals 2024, 17, 1678. [Google Scholar] [CrossRef]
- Hoyos, L.V.; Vasquez-Muñoz, L.E.; Osorio, Y.; Valencia-Revelo, D.; Devia-Cometa, D.; Große, M.; Charria-Girón, E.; Caicedo-Ortega, N.H. Tailored Culture Strategies to Promote Antimicrobial Secondary Metabolite Production in Diaporthe Caliensis: A Metabolomic Approach. Microb. Cell Fact. 2024, 23, 328. [Google Scholar] [CrossRef]
- Michaliski, L.F.; Ióca, L.P.; Oliveira, L.S.; Crnkovic, C.M.; Takaki, M.; Freire, V.F.; Berlinck, R.G.S. Improvement of Targeted Fungi Secondary Metabolite Production Using a Systematic Experimental Design and Chemometrics Analysis. Methods Protoc. 2023, 6, 77. [Google Scholar] [CrossRef]
- Rodríguez Martín-Aragón, V.; Trigal Martínez, M.; Cuadrado, C.; Daranas, A.H.; Fernández Medarde, A.; Sánchez López, J.M. OSMAC Approach and Cocultivation for the Induction of Secondary Metabolism of the Fungus Pleotrichocladium Opacum. ACS Omega 2023, 8, 39873–39885. [Google Scholar] [CrossRef]
- Karaffa, L.; Fekete, E.; Kubicek, C.P. The Role of Metal Ions in Fungal Organic Acid Accumulation. Microorganisms 2021, 9, 1267. [Google Scholar] [CrossRef]
- Ekwomadu, T.I.; Mwanza, M. Fusarium Fungi Pathogens, Identification, Adverse Effects, Disease Management, and Global Food Security: A Review of the Latest Research. Agriculture 2023, 13, 1810. [Google Scholar] [CrossRef]
- Ibrahim, S.R.M.; Sirwi, A.; Eid, B.G.; Mohamed, S.G.A.; Mohamed, G.A. Bright Side of Fusarium Oxysporum: Secondary Metabolites Bioactivities and Industrial Relevance in Biotechnology and Nanotechnology. J. Fungi 2021, 7, 943. [Google Scholar] [CrossRef]
- Panuthai, T.; Sihanonth, P.; Piapukiew, J.; Sooksai, S.; Sangvanich, P.; Karnchanatat, A. An Extracellular Lipase from the Endophytic Fungi Fusarium Oxysporum Isolated from the Thai Medicinal Plant, Croton Oblongifolius Roxb. Afr. J. Microbiol. Res. 2012, 6, 2622–2638. [Google Scholar] [CrossRef]
- Ortega, L.M.; Kikot, G.E.; Astoreca, A.L.; Alconada, T.M. Screening of Fusarium Graminearum Isolates for Enzymes Extracellular and Deoxynivalenol Production. J. Mycol. 2013, 2013, 358140. [Google Scholar] [CrossRef]
- de Oliveira Santos, G.C.; Vasconcelos, C.C.; Lopes, A.J.; de Sousa Cartágenes, M.D.; Filho, A.K.; do Nascimento, F.R.; Ramos, R.M.; Pires, E.R.; de Andrade, M.S.; Rocha, F.M.; et al. Candida Infections and Therapeutic Strategies: Mechanisms of Action for Traditional and Alternative Agents. Front. Microbiol. 2018, 9, 1351. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.F.; Farias, J.R.; Franco, D.C.; Galiza, A.A.; Motta, E.P.; Oliveira, A.D.; Vasconcelos, C.C.; Cartágenes, M.D.; Rocha, C.Q.; Silva, M.C.; et al. Anti-Candida Albicans Activity of Ononin and Other Secondary Metabolites from Platonia Insignis MART. Metabolites 2022, 12, 1014. [Google Scholar] [CrossRef]
- Motta, E.P.; Farias, J.R.; Costa, A.A.; Silva, A.F.; Oliveira Lopes, A.J.; Cartágenes, M.D.; Nicolete, R.; Abreu, A.G.; Fernandes, E.S.; Nascimento, F.R.; et al. The Anti-Virulence Effect of Vismia Guianensis against Candida Albicans and Candida Glabrata. Antibiotics 2022, 11, 1834. [Google Scholar] [CrossRef] [PubMed]
- Sakkas, H.; Papadopoulou, C. Antimicrobial Activity of Basil, Oregano, and Thyme Essential Oils. J. Microbiol. Biotechnol. 2017, 27, 429–438. [Google Scholar] [CrossRef]
- Shirling, E.B.; Gottlieb, D. Methods for Characterization of Streptomyces Species. Int. J. Syst. Bacteriol. 1966, 16, 313–340. [Google Scholar] [CrossRef]
- Oliveira, O.W.; Petrovick, P.R. Secagem Por Aspersão (Spray Drying) de Extratos Vegetais: Bases e Aplicações. Rev. Bras. Farmacogn. 2010, 20, 641–650. [Google Scholar] [CrossRef]
- Habler, K.; Rychlik, M. Multi-Mycotoxin Stable Isotope Dilution LC-MS/MS Method for Fusarium Toxins in Cereals. Anal. Bioanal. Chem. 2016, 408, 307–317. [Google Scholar] [CrossRef]
- CLSI. Reference Method For Broth Dilution Antifungal Susceptibility Testing Of Yeasts (Approved Standard. M27-A3), 3rd ed.; Clinical and Laboratory Standards Institute: Wayne, NJ, USA, 2017. [Google Scholar]
- Siddiqui, Z.N.; Farooq, F.; Musthafa, T.N.M.; Ahmad, A.; Khan, A.U. Synthesis, Characterization and Antimicrobial Evaluation of Novel Halopyrazole Derivatives. J. Saudi Chem. Soc. 2013, 17, 237–243. [Google Scholar] [CrossRef]
- da Silva, A.F.; da Rocha, C.Q.; da Silva, L.C.; Carvalho Júnior, A.R.; Mendes, I.N.; de Araruna, A.B.; Motta, E.P.; Silva, R.D.; Campos, C.D.; Farias, J.R.; et al. Antifungal and Antivirulence Activities of Hydroalcoholic Extract and Fractions of Platonia Insignis Leaves against Vaginal Isolates of Candida Species. Pathogens 2020, 9, 84. [Google Scholar] [CrossRef] [PubMed]
- Seneviratne, C.J.; Rajan, S.; Wong, S.S.W.; Tsang, D.N.C.; Lai, C.K.C.; Samaranayake, L.P.; Jin, L. Antifungal Susceptibility in Serum and Virulence Determinants of Candida Bloodstream Isolates from Hong Kong. Front. Microbiol. 2016, 7, 216. [Google Scholar] [CrossRef]
- da Silva, A.R.; de Andrade Neto, J.B.; da Silva, C.R.; Campos, R.D.; Costa Silva, R.A.; Freitas, D.D.; do Nascimento, F.B.; de Andrade, L.N.; Sampaio, L.S.; Grangeiro, T.B.; et al. Berberine Antifungal Activity in Fluconazole-Resistant Pathogenic Yeasts: Action Mechanism Evaluated by Flow Cytometry and Biofilm Growth Inhibition in Candida spp. Antimicrob. Agents Chemother. 2016, 60, 3551–3557. [Google Scholar] [CrossRef] [PubMed]
- Zago, C.E.; Silva, S.; Sanitá, P.V.; Barbugli, P.A.; Dias, C.M.I.; Lordello, V.B.; Vergani, C.E. Dynamics of Biofilm Formation and the Interaction between Candida Albicans and Methicillin-Susceptible (MSSA) and -Resistant Staphylococcus Aureus (MRSA). PLoS ONE 2015, 10, e0123206. [Google Scholar] [CrossRef] [PubMed]
- de Souza, P.C.; Morey, A.T.; Castanheira, G.M.; Bocate, K.P.; Panagio, L.A.; Ito, F.A.; Furlaneto, M.C.; Yamada-Ogatta, S.F.; Costa, I.N.; Mora-Montes, H.M.; et al. Tenebrio Molitor (Coleoptera: Tenebrionidae) as an Alternative Host to Study Fungal Infections. J. Microbiol. Methods 2015, 118, 182–186. [Google Scholar] [CrossRef]
- Ogofure, A.G.; Pelo, S.P.; Green, E. Identification and Assessment of Secondary Metabolites from Three Fungal Endophytes of Solanum Mauritianum Against Public Health Pathogens. Molecules 2024, 29, 4924. [Google Scholar] [CrossRef]
- Son, S.W.; Kim, H.Y.; Choi, G.J.; Lim, H.K.; Jang, K.S.; Lee, S.O.; Lee, S.; Sung, N.D.; Kim, J.-C. Bikaverin and Fusaric Acid from Fusarium Oxysporum Show Antioomycete Activity against Phytophthora Infestans. J. Appl. Microbiol. 2008, 104, 692–698. [Google Scholar] [CrossRef]
- Trigos, A.; Reyna, S.; Cervantes, L. Three Diketopiperazines from the Cultivated Fungus Fusarium Oxysporum. Nat. Prod. Lett. 1995, 6, 241–246. [Google Scholar] [CrossRef]
- Alfattani, A.; Marcourt, L.; Hofstetter, V.; Queiroz, E.F.; Leoni, S.; Allard, P.-M.; Gindro, K.; Stien, D.; Perron, K.; Wolfender, J.-L. Combination of Pseudo-LC-NMR and HRMS/MS-Based Molecular Networking for the Rapid Identification of Antimicrobial Metabolites From Fusarium Petroliphilum. Front. Mol. Biosci. 2021, 8, 725691. [Google Scholar] [CrossRef]
- Rodriguez, M.A.; Cabrera, G.; Godeas, A. Cyclosporine A from a Nonpathogenic Fusarium Oxysporum Suppressing Sclerotinia Sclerotiorum. J. Appl. Microbiol. 2006, 100, 575–586. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Bai, X.; Hua, Y.; Zhang, H.; Wang, H. Fusariumins C and D, Two Novel Antimicrobial Agents from Fusarium Oxysporum ZZP-R1 Symbiotic on Rumex Madaio Makino. Fitoterapia 2019, 134, 1–4. [Google Scholar] [CrossRef]
- Starratt, A.N.; Madhosingh, C. Sterol and Fatty Acid Components of Mycelium of Fusarium Oxysporum. Can. J. Microbiol. 1967, 13, 1351–1355. [Google Scholar] [CrossRef] [PubMed]
- Ghule, M.R.; Sawant, I.S.; Oulkar, D.; Hingmire, S.; Shabeer, A.; Holkar, S. Identification of Secondary Metabolites in Mycoparasites Fusarium Strains and Antifungal Activity of Fusaric Acid against Plasmopara Viticola. Arch. Phytopathol. Plant Prot. 2022, 55, 1283–1297. [Google Scholar] [CrossRef]
- Jayasinghe, L.; Abbas, H.K.; Jacob, M.R.; Herath, W.H.M.W.; Nanayakkara, N.P.D. N -Methyl-4-Hydroxy-2-Pyridinone Analogues from Fusarium Oxysporum. J. Nat. Prod. 2006, 69, 439–442. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Yang, X.-Q.; Wan, J.-L.; Han, H.-L.; Zhao, Y.-D.; Cai, L.; Yang, Y.-B.; Ding, Z.-T. The Antifungal Metabolites Isolated from Maize Endophytic Fungus Fusarium Sp. Induced by OSMAC Strategy. Fitoterapia 2023, 171, 105710. [Google Scholar] [CrossRef]
- He, T.; Li, X.; Iacovelli, R.; Hackl, T.; Haslinger, K. Genomic and Metabolomic Analysis of the Endophytic Fungus Fusarium Sp. VM-40 Isolated from the Medicinal Plant Vinca Minor. J. Fungi 2023, 9, 704. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Kinoshita, Y.; Ran Thor, G.; Hasumi, M.; Kinoshita, K.; Koyama, K.; Takahashi, K.; Yoshimura, I. Isofuranonaphthoquinone Derivatives from Cultures of the Lichen Arthonia Cinnabarina (DC.) Wallr. Phytochemistry 2002, 60, 741–745. [Google Scholar] [CrossRef]
- Niemann, G.J.; Liem, J.; van der Kerk-van Hoof, A.; Niessen, W.M.A. Phytoalexins, Benzoxazinones, N-Aroylanthranilates and N-Aroylanilines, from Fusarium-Infected Carnation Stems. Phytochemistry 1992, 31, 3761–3767. [Google Scholar] [CrossRef]
- Mirocha, C.J.; Abbas, H.K.; Kommedahl, T.; Jarvis, B.B. Mycotoxin Production by Fusarium Oxysporum and Fusarium Sporotrichioides Isolated from Baccharis spp. from Brazil. Appl. Environ. Microbiol. 1989, 55, 254–255. [Google Scholar] [CrossRef]
- Miersch, O.; Bohlmann, H.; Wasternack, C. Jasmonates and Related Compounds from Fusarium Oxysporum. Phytochemistry 1999, 50, 517–523. [Google Scholar] [CrossRef]
- Gómez, O.C.; Luiz, J.H.H. Endophytic Fungi Isolated from Medicinal Plants: Future Prospects of Bioactive Natural Products from Tabebuia/Handroanthus Endophytes. Appl. Microbiol. Biotechnol. 2018, 102, 9105–9119. [Google Scholar] [CrossRef]
- Wen, J.; Okyere, S.K.; Wang, S.; Wang, J.; Xie, L.; Ran, Y.; Hu, Y. Endophytic Fungi: An Effective Alternative Source of Plant-Derived Bioactive Compounds for Pharmacological Studies. J. Fungi 2022, 8, 205. [Google Scholar] [CrossRef]
- Omomowo, I.O.; Amao, J.A.; Abubakar, A.; Ogundola, A.F.; Ezediuno, L.O.; Bamigboye, C.O. A Review on the Trends of Endophytic Fungi Bioactivities. Sci. Afr. 2023, 20, e01594. [Google Scholar] [CrossRef]
- Caruso, D.J.; Palombo, E.A.; Moulton, S.E.; Zaferanloo, B. Exploring the Promise of Endophytic Fungi: A Review of Novel Antimicrobial Compounds. Microorganisms 2022, 10, 1990. [Google Scholar] [CrossRef]
- Huang, B.B.; Liu, Y.Y.; Zhu, P.F.; Jiang, Y.C.; Ouyang, M.-A. Concise Total Synthesis and Antifungal Activities of Fusaric Acid, a Natural Product. Molecules 2020, 25, 3859. [Google Scholar] [CrossRef]
- Huang, B.B.; Gao, M.W.; Li, G.; Ouyang, M.-A.; Chen, Q.-J. Design, Synthesis, Structure–Activity Relationship, and Three-Dimensional Quantitative Structure–Activity Relationship of Fusarium Acid Derivatives and Analogues as Potential Fungicides. J. Agric. Food Chem. 2023, 71, 18566–18577. [Google Scholar] [CrossRef]
- Macías-Rubalcava, M.L.; Lappe-Oliveras, P.; Villanueva-Silva, R. Disruption of Cell Wall and Membrane Integrity as Antioomycete and Antifungal Mode of Action by Fusaric and 9,10-Dehydrofusaric Acids from Endophytic Fungus Fusarium Lactis Strain SME13-2. J. Appl. Microbiol. 2025, 136, lxae301. [Google Scholar] [CrossRef] [PubMed]
- Martín, V.; de la Haba, R.R.; López-Cornejo, P.; López-López, M.; Antonio Lebrón, J.; Bernal, E.; Baeza, N.; Ruiz, S.; José Ostos, F.; Merino-Bohorquez, V.; et al. Synergistic Antifungal Activity against Candida Albicans between Voriconazole and Cyclosporine a Loaded in Polymeric Nanoparticles. Int. J. Pharm. 2024, 664, 124593. [Google Scholar] [CrossRef]
- Li, F.; Lv, Z.; Zhong, Z.; Mao, L.; Chua, L.S.; Xu, L.; Huang, R. The Effect of Cyclosporin A on Aspergillus Niger and the Possible Mechanisms Involved. Foods 2023, 12, 567. [Google Scholar] [CrossRef]
- de Andrade, I.B.; Corrêa-Junior, D.; Alves, V.; Figueiredo-Carvalho, M.H.G.; Santos, M.V.; Almeida, M.A.; Valdez, A.F.; Nimrichter, L.; Almeida-Paes, R.; Frases, S. Cyclosporine Affects the Main Virulence Factors of Cryptococcus Neoformans In Vitro. J. Fungi 2023, 9, 487. [Google Scholar] [CrossRef]
- Li, Y.; Sun, S.; Guo, Q.; Ma, L.; Shi, C.; Su, L.; Li, H. In Vitro Interaction between Azoles and Cyclosporin A against Clinical Isolates of Candida Albicans Determined by the Chequerboard Method and Time-Kill Curves. J. Antimicrob. Chemother. 2008, 61, 577–585. [Google Scholar] [CrossRef]
- Xu, M.; Huang, Z.; Zhu, W.; Liu, Y.; Bai, X.; Zhang, H. Fusarium-Derived Secondary Metabolites with Antimicrobial Effects. Molecules 2023, 28, 3424. [Google Scholar] [CrossRef] [PubMed]
- Wattana-Amorn, P.; Charoenwongsa, W.; Williams, C.; Crump, M.P.; Apichaisataienchote, B. Antibacterial Activity of Cyclo(L-Pro-L-Tyr) and Cyclo(D-Pro-L-Tyr) from Streptomyces Sp. Strain 22-4 against Phytopathogenic Bacteria. Nat. Prod. Res. 2016, 30, 1980–1983. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Xu, Z.; Cao, R.; Li, J.; Wu, C.-J.; Wang, Y.; Zhu, H. Effects of Hydroxyl Group in Cyclo(Pro-Tyr)-like Cyclic Dipeptides on Their Anti-QS Activity and Self-Assembly. iScience 2023, 26, 107048. [Google Scholar] [CrossRef] [PubMed]
- Tayung, K.; Jha, D.K. Antimicrobial Endophytic Fungal Assemblages Inhabiting Bark of Taxus baccata L. of Indo-Burma Mega Biodiversity Hotspot. Indian J. Microbiol. 2010, 50, 74–81. [Google Scholar] [CrossRef]
- Amuzu, P.; Pan, X.; Hou, X.; Sun, J.; Jakada, M.A.; Odigie, E.; Xu, D.; Lai, D.; Zhou, L. Recent Updates on the Secondary Metabolites from Fusarium Fungi and Their Biological Activities (Covering 2019 to 2024). J. Fungi 2024, 10, 778. [Google Scholar] [CrossRef]
- Li, W.-S.; Chen, Y.-C.; Kuo, S.-F.; Chen, F.-J.; Lee, C.-H. The Impact of Biofilm Formation on the Persistence of Candidemia. Front. Microbiol. 2018, 9, 1196. [Google Scholar] [CrossRef]
- Silva, S.; Rodrigues, C.; Araújo, D.; Rodrigues, M.; Henriques, M. Candida Species Biofilms’ Antifungal Resistance. J. Fungi 2017, 3, 8. [Google Scholar] [CrossRef]
- Canteri de Souza, P.; Custódio Caloni, C.; Wilson, D.; Sergio Almeida, R. An Invertebrate Host to Study Fungal Infections, Mycotoxins and Antifungal Drugs: Tenebrio Molitor. J. Fungi 2018, 4, 125. [Google Scholar] [CrossRef]
- Rocha, F.M.G.; Rocha, C.H.L.; Silva, L.C.N.; Pinheiro, A.J.M.C.R.; Mendonça, A.M.S.; Cantanhede Filho, A.J.; Sousa, E.M.; Rocha, C.Q.; Assuncao Holanda, R.; Santos, J.R.A.; et al. N -Butanol Fraction of Terminalia Catappa Possesses Anti-Candida Albicans Properties and in Vivo Action on Tenebrio Molitor Alternative Infection Model. Microb. Pathog. 2025, 198, 107133. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.K.; Chandra, J.; Kuhn, D.M.; Ghannoum, M.A. Mechanism of Fluconazole Resistance in Candida Albicans Biofilms: Phase-Specific Role of Efflux Pumps and Membrane Sterols. Infect. Immun. 2003, 71, 4333–4340. [Google Scholar] [CrossRef] [PubMed]
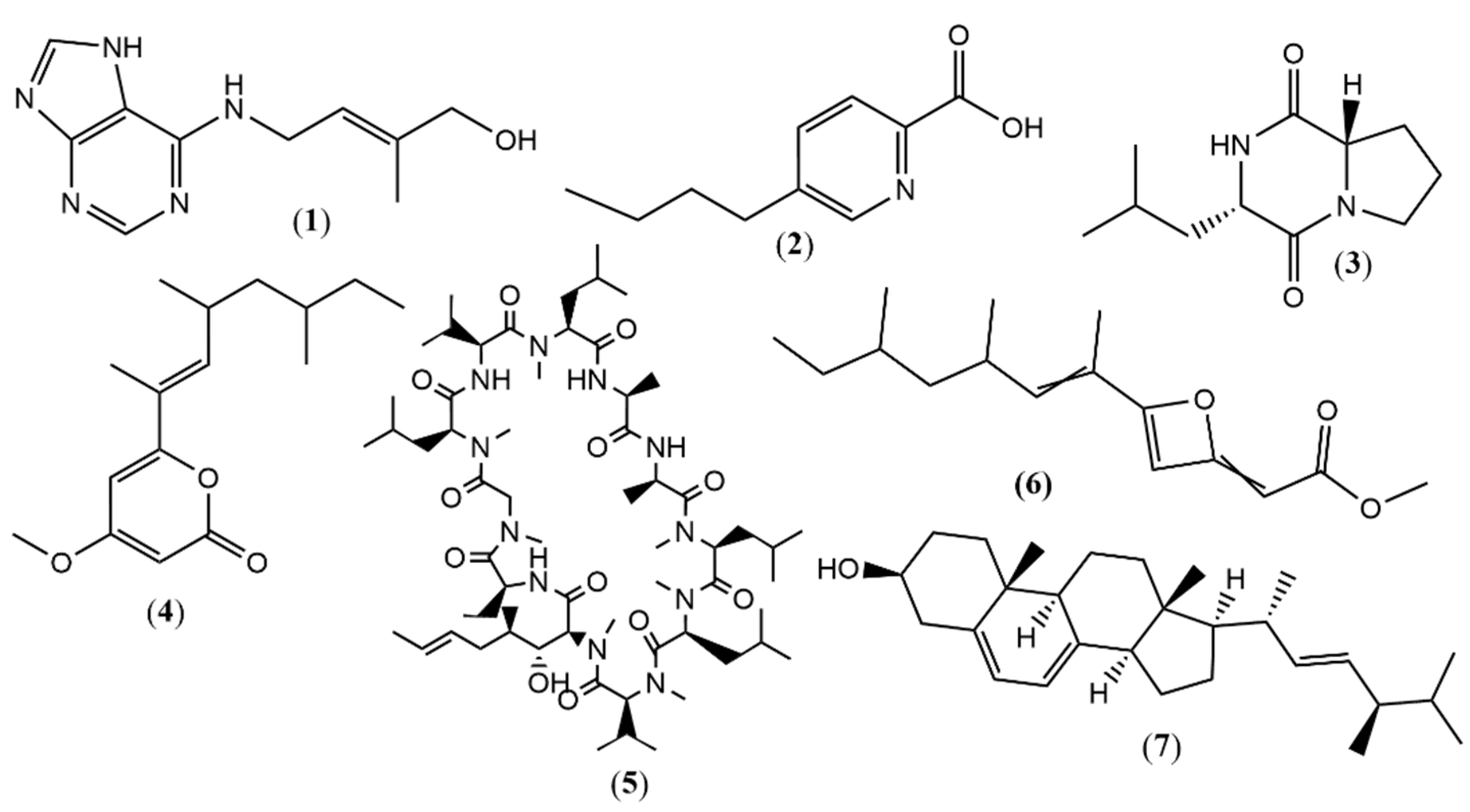
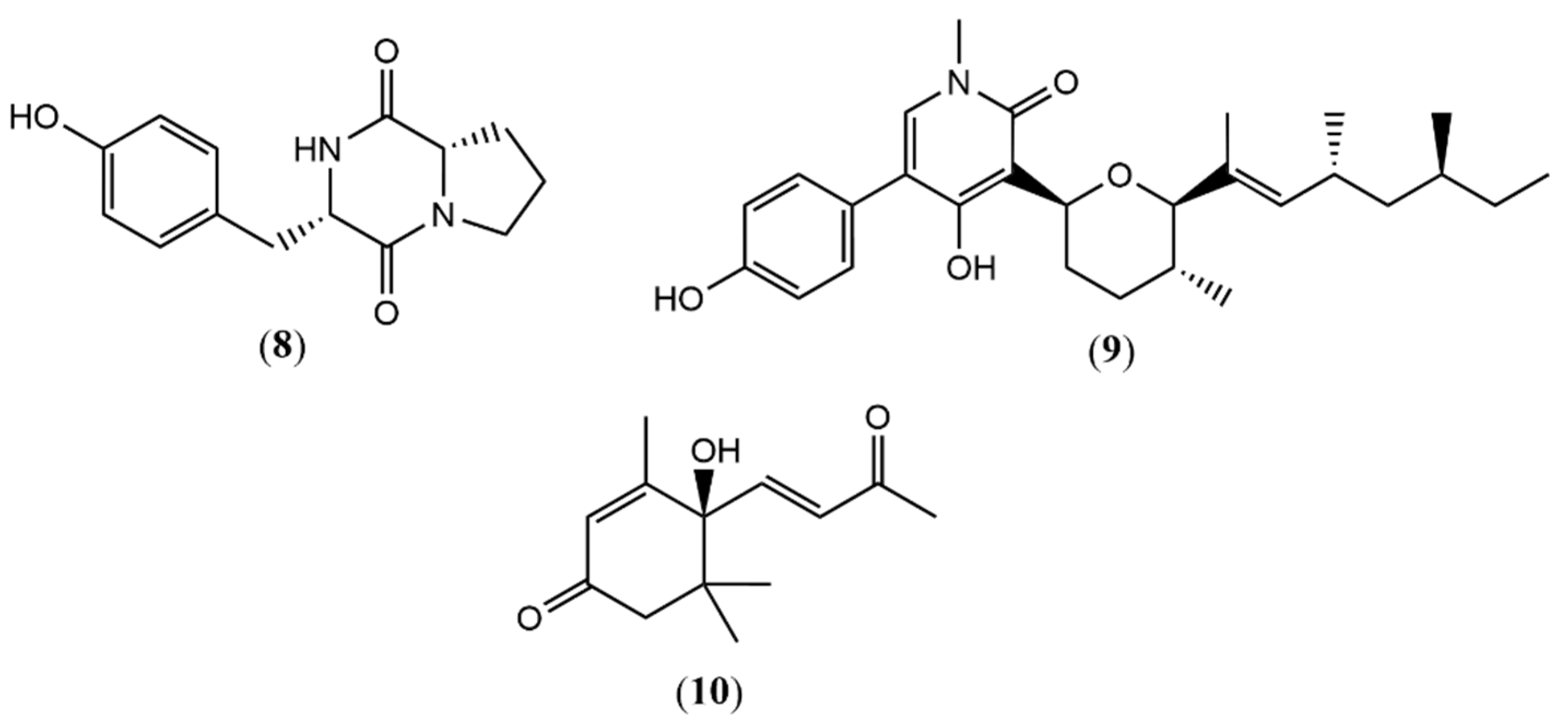
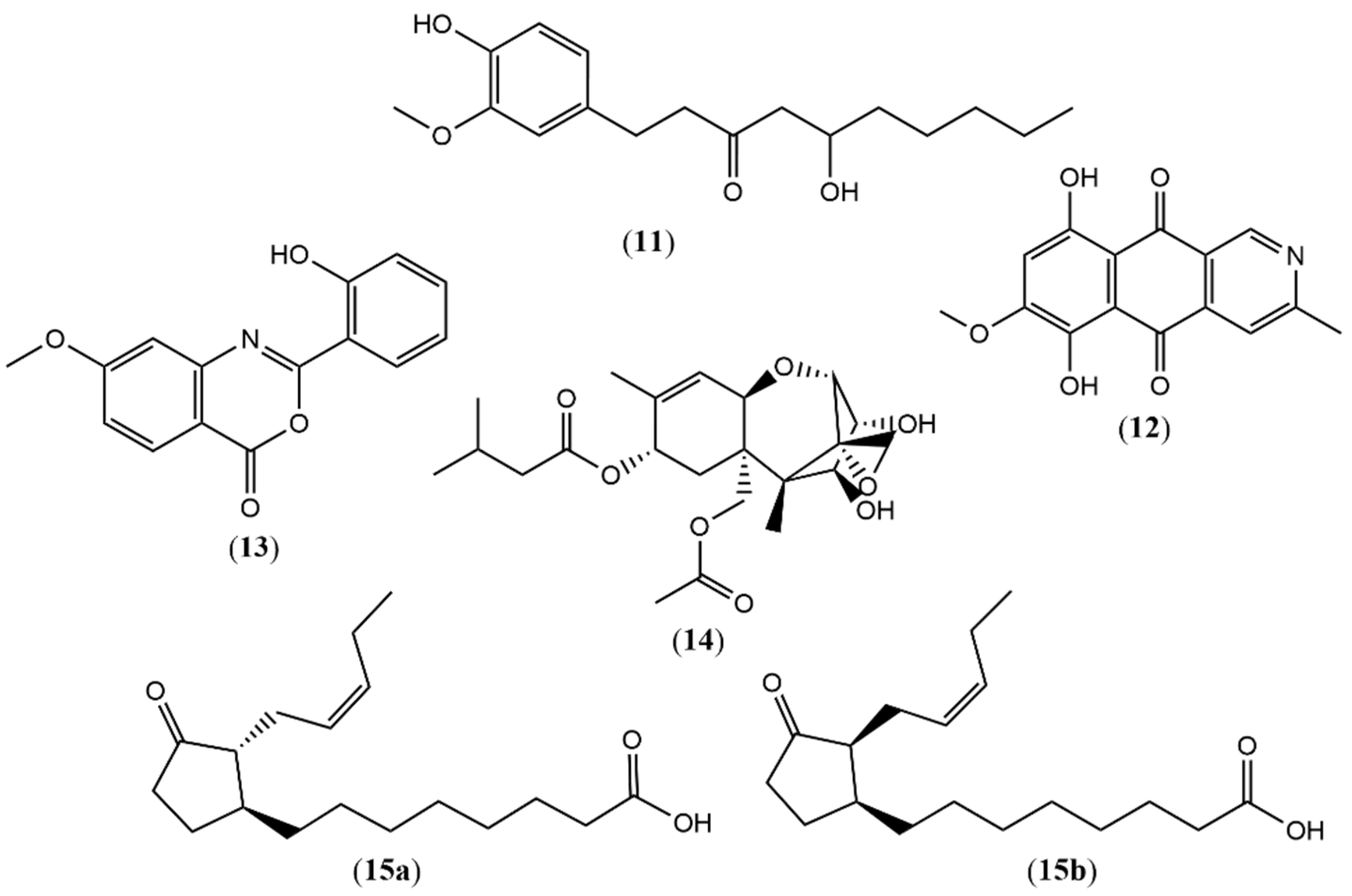
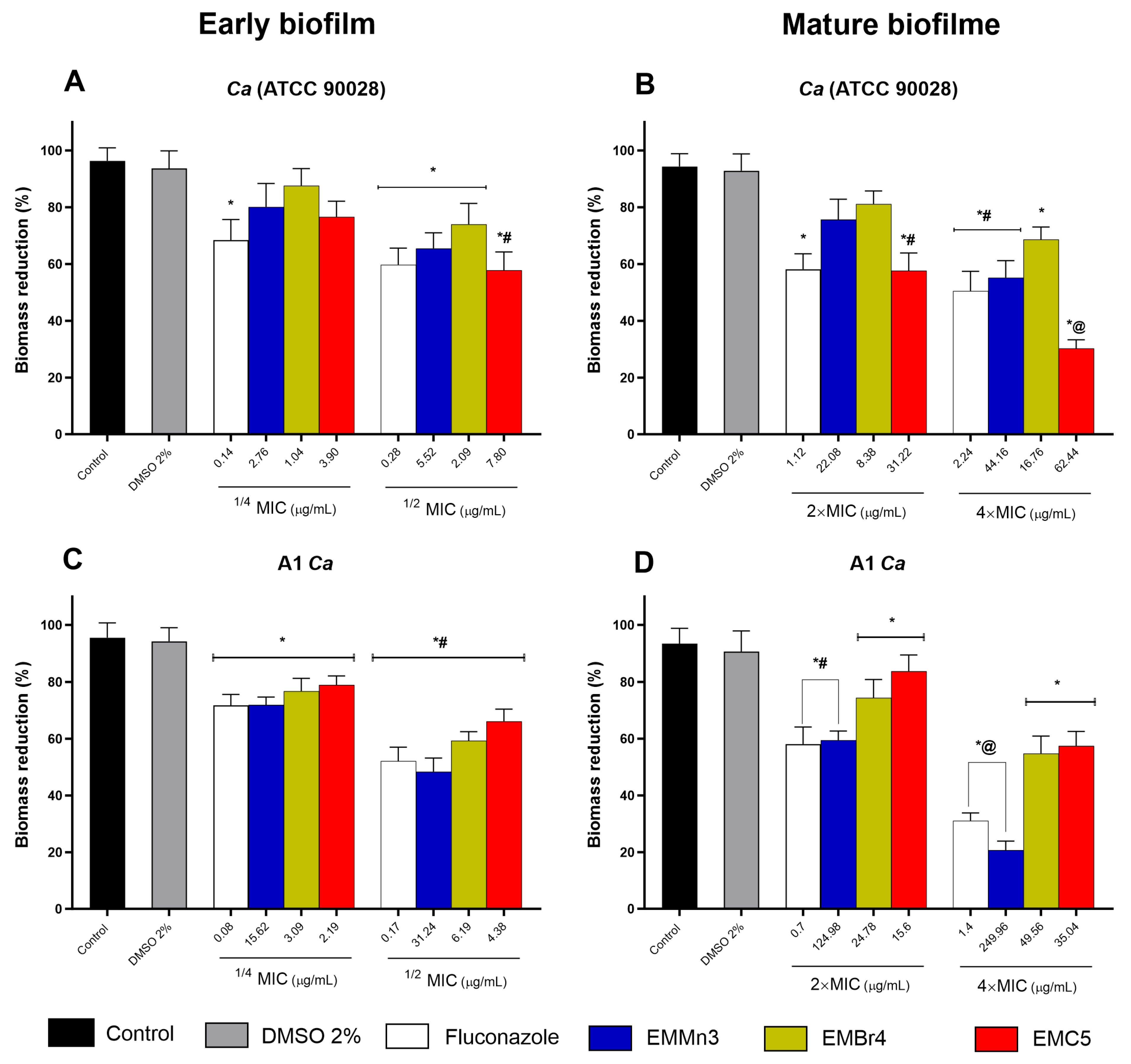
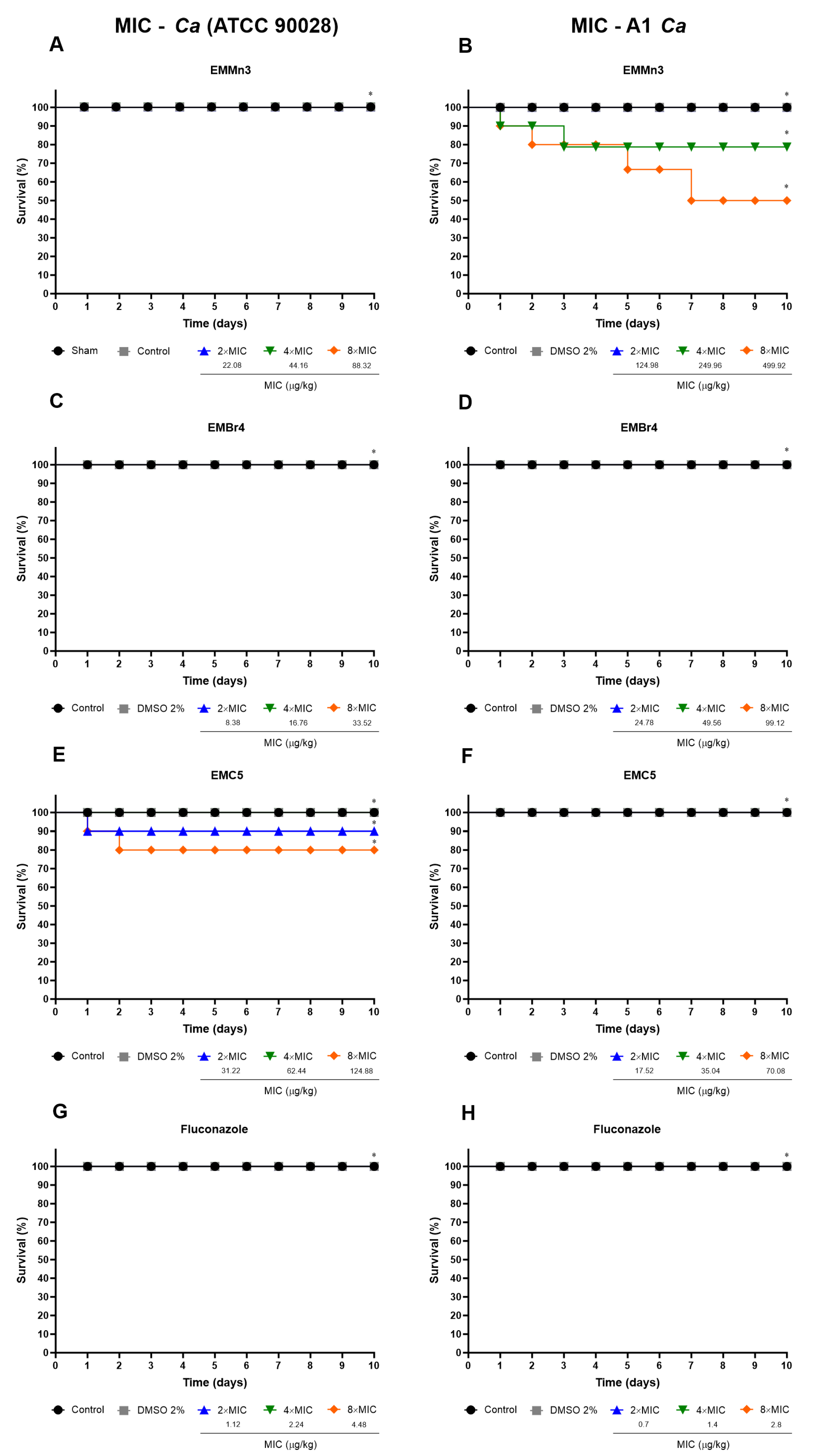
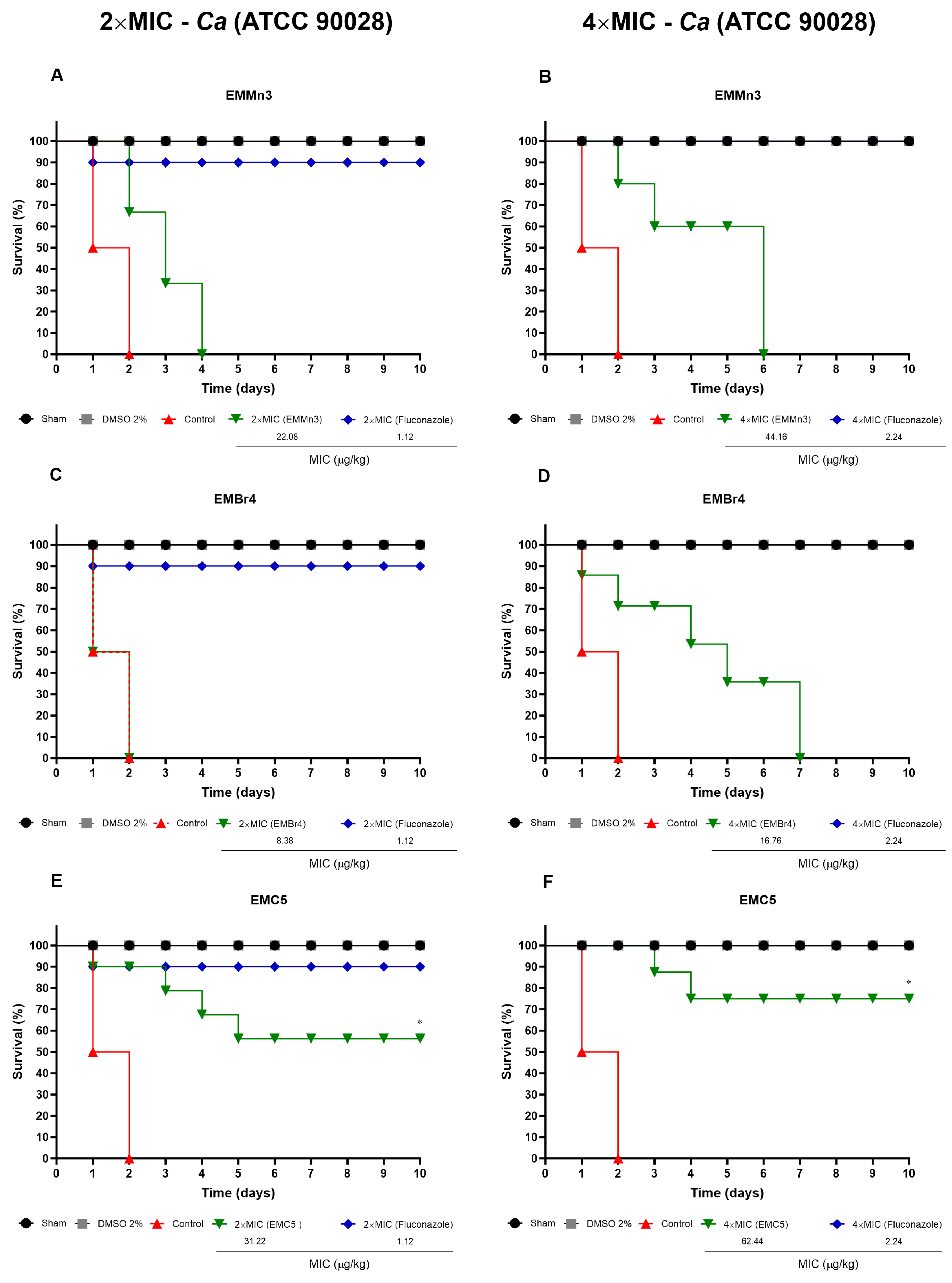
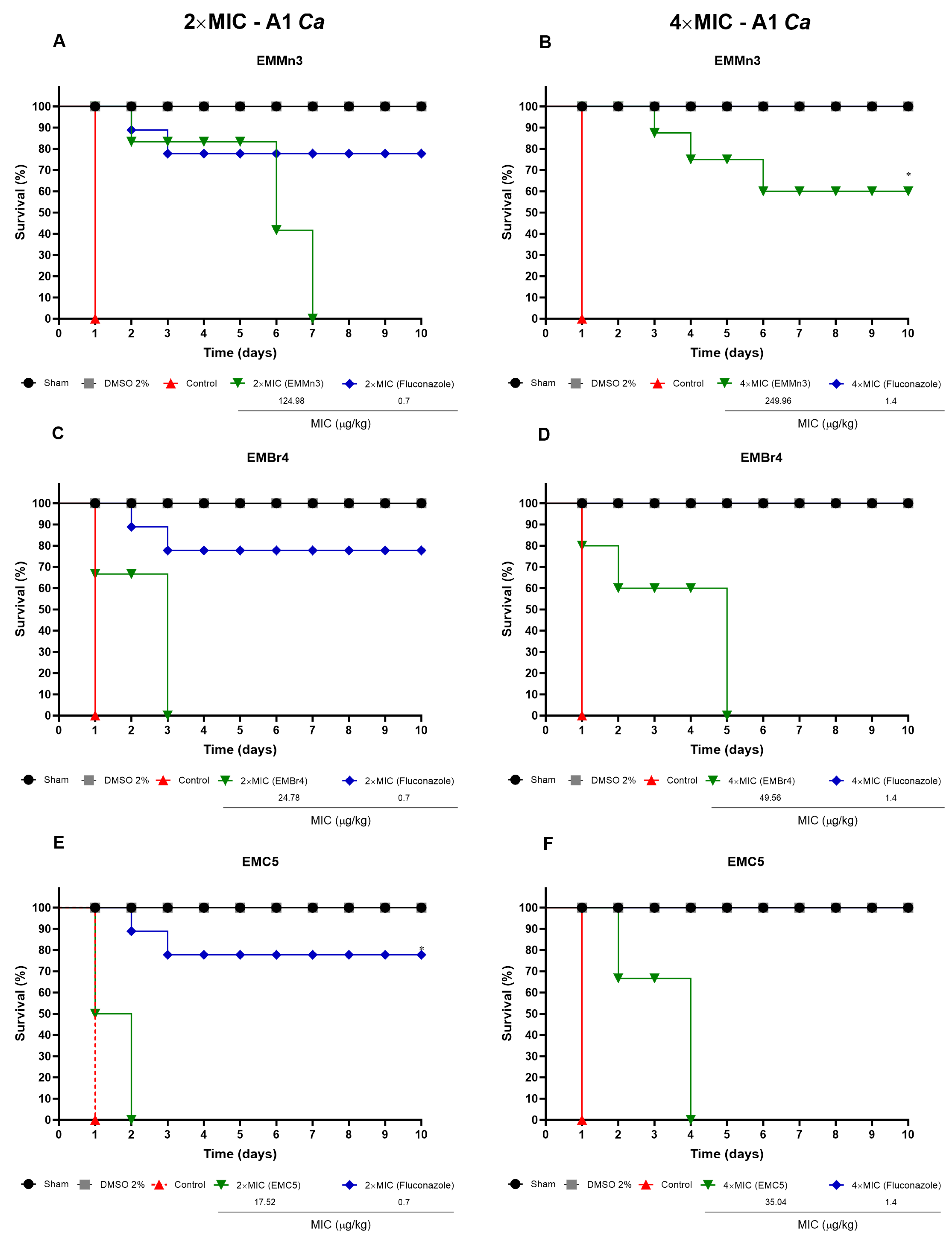
| Nª | RT (min) | Observed Mass (Da) | Calculated Mass (Da) | ∆ (ppm) | Annotated | EMC5 | EMBr4 | EMMn3 | Ref |
|---|---|---|---|---|---|---|---|---|---|
| (Control) | (NH4Br) | (MnCl2) | |||||||
| 1 | 2.4 | 220.1182 a | 220.1193 | −4.99 | Zeatin (cytokinins) | X | -- | -- | [28] |
| 2 | 3.32 | 180.1026 a | 180.1019 | 3.88 | Fusaric acid | X | -- | -- | [29] |
| 3 | 3.83 | 211.1441 a | 211.1441 | 0.00 | L-Leucyl-L-proline lactam | X | -- | -- | [30] |
| 4 | 8.93 | 265.1805 a | 265.1798 | 2.64 | 4-methoxy-6-((E)-4,6- dimethyloct-2-en-2-yl)- 2H-pyran-2-one | X | -- | -- | [31] |
| 5 | 11.09 | 1202.8492 a | 1202.8486 | −0.58 | Cyclosporin A | X | -- | -- | [32] |
| 6 | 8.94 | 265.1805 a | 265.1798 | 2.63 | Fusariumin D | X | -- | -- | [33] |
| 7 | 10.97 | 397.3470 a | 397.3465 | 1.25 | Ergosterol | X | -- | -- | [34] |
| 8 | 4.29 | 261.1234 a | 261.1234 | 0.00 | Cyclo(L-Pro-L-Tyr) | -- | X | -- | [35] |
| 9 | 8.78 | 454.2937 a | 454.2952 | −3.30 | (−)-Sambutoxin | -- | X | -- | [36] |
| 10 | 6.55 | 221.1168 b | 221.1183 | −2.26 | Dehydrovomifoliol | -- | X | -- | [37] |
| 11 | 7.3 | 293.1781 b | 293.1758 | 7.84 | 5-hydroxy-1-(4-hydroxy-3-methoxyphenyl)decan-3-one | -- | -- | X | [38] |
| 12 | 7.3 | 286.0704 a | 286.0710 | −2.09 | Bostrycoidin | -- | -- | X | [39] |
| 13 | 7.72 | 270.0767 a | 270.0761 | 2.22 | Methoxydianthalexin S | -- | -- | X | [40] |
| 14 | 10.29 | 425.2144 a | 425.2170 | −6.11 | HT-2 Toxin | -- | -- | X | [41] |
| 15a | 6.53 | 295.2267 a | 295.2268 | −0.33 | (1S,2R)-3-Oxo-2-(2Z-pentenyl) cyclopentane-1-octanoic acid | -- | -- | X | [42] |
| 15b | 6.53 | 295.2267 a | 295.2268 | −0.33 | (1S,2S)-3-Oxo-2-(2Z-pentenyl) cyclopentane-1-octanoic acid | -- | -- | X | [42] |
| Extract Fractions from the Endophytic Fungus Fusarium sp. | Antifungal | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| EMMn3 | EMBr4 | EMC5 | Fluconazole | |||||||
| Candida albicans Strain | MIC a | MFC a | MFC/MIC Ratio | MIC | MFC | MFC/MIC Ratio | MIC | MFC | MFC/MIC Ratio | MIC |
| Ca (ATCC 90028) B | 11.04 | 44.19 | 4.00 | 4.19 | 15.62 | 3.17 | 15.61 | 55.67 | 3.56 | 0.56 |
| Ca (ATCC 10231) B | 15.62 | 44.19 | 2.82 | 99.21 | 353.55 | 3.56 | 111.36 | 445.44 | 4 | 1.41 |
| A1 C Ca | 62.49 | 157.49 | 2.52 | 12.39 | 27.83 | 4.00 | 8.76 | 24.80 | 2.83 | 0.35 |
| A2 Ca | 31.25 | 124.91 | 3.99 | 31.24 | 78.74 | 2.52 | 35.89 | 62.5 | 1.74 | 0.89 |
| A3 Ca | 280.61 | 561.23 | 0.49 | 35.07 | 140.30 | 4.00 | 55.67 | 125 | 2.24 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Jesus Freitas Sá, H.; Santos, A.K.M.; Fonseca, A.S.; da Silva Santos, L.; Farias, J.R.; Guerra, R.N.M.; Rodrigues-Filho, E.; da Silva, G.S.; Vasconcelos, C.C.; Lopes, A.J.O.; et al. Assessment of Metabolic Alterations Induced by Halogenated Additives and Antifungal Activity of Extracts from the Endophytic Fungus Fusarium sp. Associated with Dizygostemon riparius (Plantaginaceae). Metabolites 2025, 15, 451. https://doi.org/10.3390/metabo15070451
de Jesus Freitas Sá H, Santos AKM, Fonseca AS, da Silva Santos L, Farias JR, Guerra RNM, Rodrigues-Filho E, da Silva GS, Vasconcelos CC, Lopes AJO, et al. Assessment of Metabolic Alterations Induced by Halogenated Additives and Antifungal Activity of Extracts from the Endophytic Fungus Fusarium sp. Associated with Dizygostemon riparius (Plantaginaceae). Metabolites. 2025; 15(7):451. https://doi.org/10.3390/metabo15070451
Chicago/Turabian Stylede Jesus Freitas Sá, Hilzimar, Anne Karoline Maiorana Santos, Adriano Souza Fonseca, Lourivaldo da Silva Santos, Josivan Regis Farias, Rosane Nassar Meireles Guerra, Edson Rodrigues-Filho, Gilmar Silverio da Silva, Cleydlenne Costa Vasconcelos, Alberto Jorge Oliveira Lopes, and et al. 2025. "Assessment of Metabolic Alterations Induced by Halogenated Additives and Antifungal Activity of Extracts from the Endophytic Fungus Fusarium sp. Associated with Dizygostemon riparius (Plantaginaceae)" Metabolites 15, no. 7: 451. https://doi.org/10.3390/metabo15070451
APA Stylede Jesus Freitas Sá, H., Santos, A. K. M., Fonseca, A. S., da Silva Santos, L., Farias, J. R., Guerra, R. N. M., Rodrigues-Filho, E., da Silva, G. S., Vasconcelos, C. C., Lopes, A. J. O., & Cantanhede Filho, A. J. (2025). Assessment of Metabolic Alterations Induced by Halogenated Additives and Antifungal Activity of Extracts from the Endophytic Fungus Fusarium sp. Associated with Dizygostemon riparius (Plantaginaceae). Metabolites, 15(7), 451. https://doi.org/10.3390/metabo15070451









