Excessive Iron Induces Macrophage Dysfunction in the Liver, Causing Adverse Pregnancy Outcomes in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Maternal Iron Overload Experimental Model
2.3. Effects of Maternal Iron Overload on Inflammatory Cytokines in the Liver and Placenta
2.4. Measurements of Iron, Ferritin, and Inflammatory Cytokines
2.5. Histology of the Liver and Placental Tissue
2.6. Real-Time qPCR
2.7. Cell Culture and in Vitro Experiments
2.8. Determination of Lactate Dehydrogenase (LDH)
2.9. Determination of Iron Uptake
2.10. Maternal Macrophage Reduction Experimental Model
2.11. Statistics
3. Results
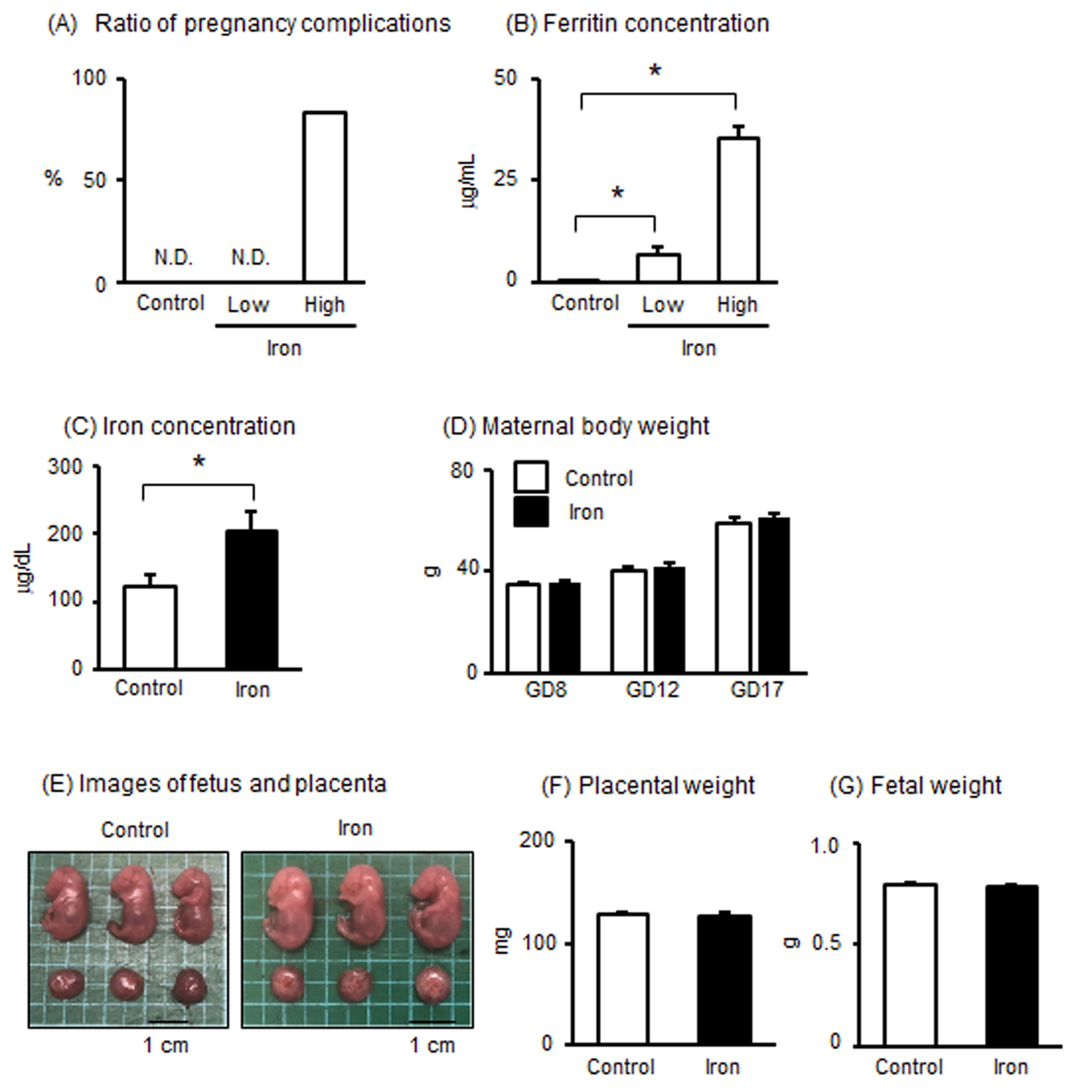
3.1. Effects of Iron Overload in Pregnant Mice
3.2. Effects of Iron Overload in Placenta of Pregnant Mice
3.3. Effects of Iron Overload in the Liver of Pregnant Mice
3.4. Effects of Iron Overload on Blood Pressure and Anti-Angiogenic Factors in Pregnant Mice
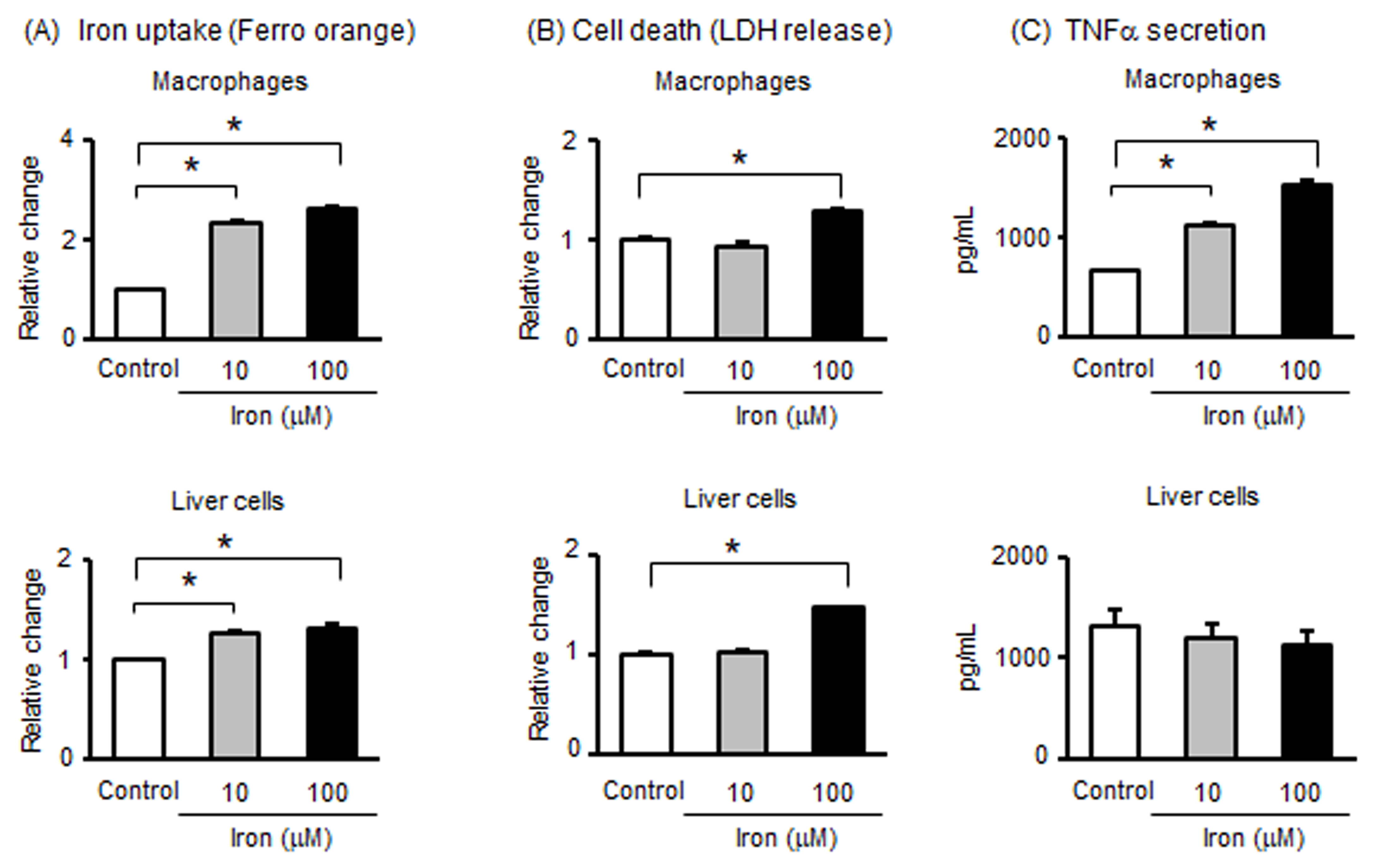
3.5. Effects of Iron on Macrophages and Liver Cells
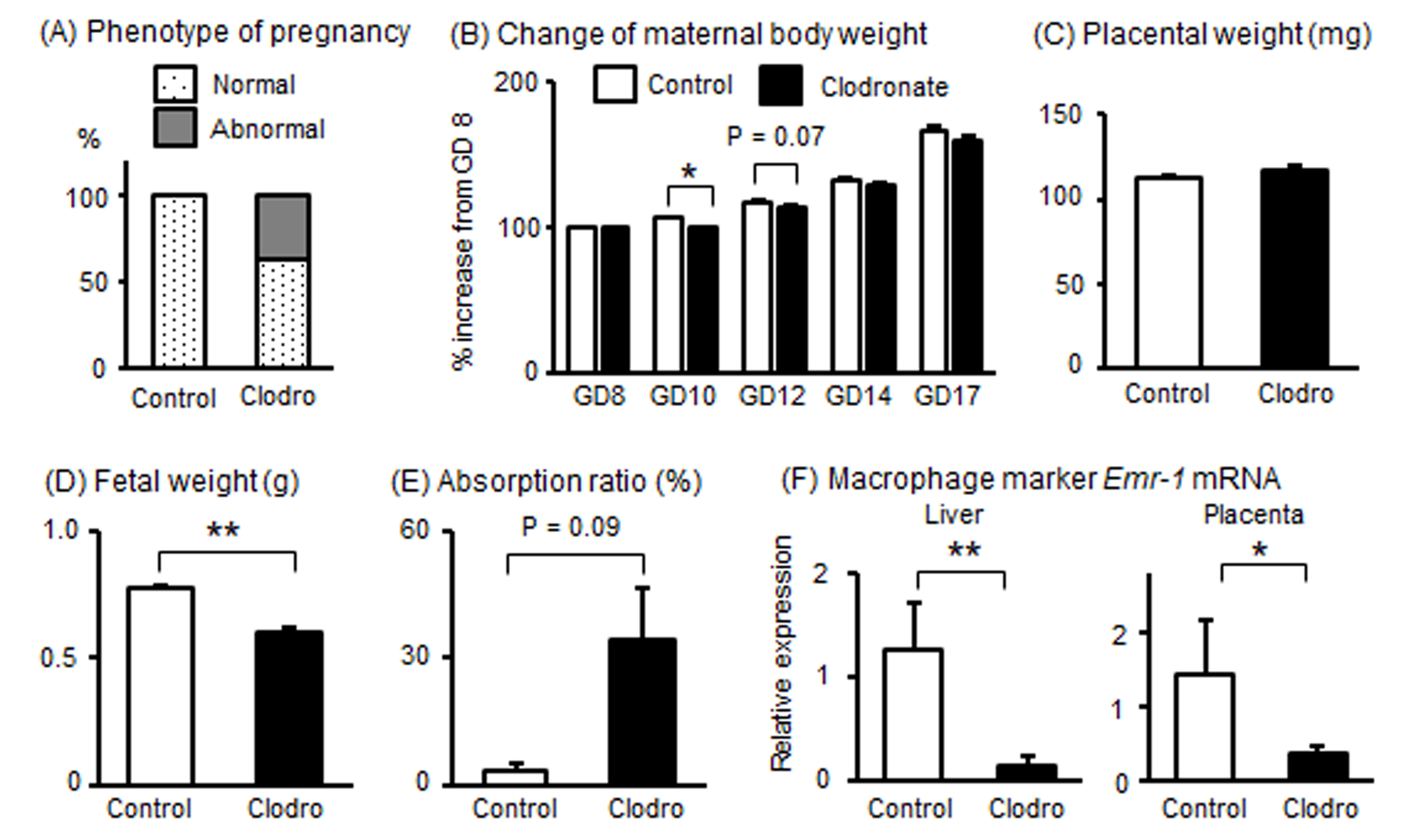
3.6. Effects of Macrophage Reduction in Pregnant Mice
3.7. Effects of Macrophage Reduction in the Liver of Pregnant Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TNFα | tumor necrosis factor-α |
| GD | gestational day |
| IL | interleukin |
| sFlt-1 | soluble fms-like tyrosine kinase |
| Emr1 | EGF-like module-containing mucin-like hormone receptor-like 1 |
| Gapdh | glyceraldehyde 3-phosphate dehydrogenase |
| LDH | lactate dehydrogenase |
| FBXL5 | F box and leucine-rich repeat protein 5 |
| Fth | ferritin heavy chain |
References
- Cazzola, M.; Bergamaschi, G.; Dezza, L.; Arosio, P. Manipulations of cellular iron metabolism for modulating normal and malignant cell proliferation: Achievements and prospects. Blood 1990, 75, 1903–1919. [Google Scholar] [CrossRef] [PubMed]
- Emerit, J.; Beaumont, C.; Trivin, F. Iron metabolism, free radicals, and oxidative injury. Biomed. Pharmacother. 2001, 55, 333–339. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Qian, Z.; Jiang, Y.; Chen, L.; Wu, D.; Liu, L.; Zhang, F.; Ning, X.; Zhang, Y.; Xiao, J. Insights into the pathogenesis of gestational and hepatic diseases: The impact of ferroptosis. Front. Cell Dev. Biol. 2024, 12, 1482838. [Google Scholar] [CrossRef]
- Kanamori, Y.; Tanaka, M.; Itoh, M.; Ochi, K.; Ito, A.; Hidaka, I.; Sakaida, I.; Ogawa, Y.; Suganami, T. Iron-rich Kupffer cells exhibit phenotypic changes during the development of liver fibrosis in NASH. iScience 2021, 24, 102032. [Google Scholar] [CrossRef]
- Altamura, S.; Mudder, K.; Schlotterer, A.; Fleming, T.; Heidenreich, E.; Qiu, R.; Hammes, H.P.; Nawroth, P.; Muckenthaler, M.U. Iron aggravates hepatic insulin resistance in the absence of inflammation in a novel db/db mouse model with iron overload. Mol. Metab. 2021, 51, 101235. [Google Scholar] [CrossRef]
- Atarashi, M.; Izawa, T.; Miyagi, R.; Ohji, S.; Hashimoto, A.; Kuwamura, M.; Yamate, J. Dietary Iron Supplementation Alters Hepatic Inflammation in a Rat Model of Nonalcoholic Steatohepatitis. Nutrients 2018, 10, 175. [Google Scholar] [CrossRef] [PubMed]
- Vinchi, F.; Porto, G.; Simmelbauer, A.; Altamura, S.; Passos, S.T.; Garbowski, M.; Silva, A.M.N.; Spaich, S.; Seide, S.E.; Sparla, R.; et al. Atherosclerosis is aggravated by iron overload and ameliorated by dietary and pharmacological iron restriction. Eur. Heart J. 2020, 41, 2681–2695. [Google Scholar] [CrossRef]
- Fisher, A.L.; Nemeth, E. Iron homeostasis during pregnancy. Am. J. Clin. Nutr. 2017, 106 (Suppl. S6), 1567S–1574S. [Google Scholar] [CrossRef]
- Fisher, A.L.; Sangkhae, V.; Balusikova, K.; Palaskas, N.J.; Ganz, T.; Nemeth, E. Iron-dependent apoptosis causes embryotoxicity in inflamed and obese pregnancy. Nat. Commun. 2021, 12, 4026. [Google Scholar] [CrossRef]
- Le, C.H. The Prevalence of Anemia and Moderate-Severe Anemia in the US Population (NHANES 2003-2012). PLoS ONE 2016, 11, e0166635. [Google Scholar] [CrossRef]
- Khambalia, A.Z.; Aimone, A.; Nagubandi, P.; Roberts, C.L.; McElduff, A.; Morris, J.M.; Powell, K.L.; Tasevski, V.; Nassar, N. High maternal iron status, dietary iron intake and iron supplement use in pregnancy and risk of gestational diabetes mellitus: A prospective study and systematic review. Diabet. Med. 2016, 33, 1211–1221. [Google Scholar] [CrossRef]
- Hou, J.; Cliver, S.P.; Tamura, T.; Johnston, K.E.; Goldenberg, R. Maternal serum ferritin and fetal growth. Obstet. Gynecol. 2000, 95, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Wang, Q.; Ding, B.; Gong, Y.; Wu, Y.; Sun, J.; Wang, X.; Liu, L.; Zhang, F.; Du, D.; et al. Expression profiles and functions of ferroptosis-related genes in the placental tissue samples of early- and late-onset preeclampsia patients. BMC Pregnancy Childbirth 2022, 22, 87. [Google Scholar] [CrossRef]
- Barke, T.L.; Goldstein, J.A.; Sundermann, A.C.; Reddy, A.P.; Linder, J.E.; Correa, H.; Velez-Edwards, D.R.; Aronoff, D.M. Gestational diabetes mellitus is associated with increased CD163 expression and iron storage in the placenta. Am. J. Reprod. Immunol. 2018, 80, e13020. [Google Scholar] [CrossRef]
- Ganz, T. Macrophages and systemic iron homeostasis. J. Innate Immun. 2012, 4, 446–453. [Google Scholar] [CrossRef]
- Sindrilaru, A.; Peters, T.; Wieschalka, S.; Baican, C.; Baican, A.; Peter, H.; Hainzl, A.; Schatz, S.; Qi, Y.; Schlecht, A.; et al. An unrestrained proinflammatory M1 macrophage population induced by iron impairs wound healing in humans and mice. J. Clin. Investig. 2011, 121, 985–997. [Google Scholar] [CrossRef] [PubMed]
- Care, A.S.; Diener, K.R.; Jasper, M.J.; Brown, H.M.; Ingman, W.V.; Robertson, S.A. Macrophages regulate corpus luteum development during embryo implantation in mice. J. Clin. Investig. 2013, 123, 3472–3487. [Google Scholar] [CrossRef]
- Gomez-Lopez, N.; Garcia-Flores, V.; Chin, P.Y.; Groome, H.M.; Bijland, M.T.; Diener, K.R.; Romero, R.; Robertson, S.A. Macrophages exert homeostatic actions in pregnancy to protect against preterm birth and fetal inflammatory injury. JCI Insight 2021, 6, e146089. [Google Scholar] [CrossRef]
- Ono, Y.; Yoshino, O.; Hiraoka, T.; Sato, E.; Fukui, Y.; Ushijima, A.; Nawaz, A.; Hirota, Y.; Wada, S.; Tobe, K.; et al. CD206+ M2-Like Macrophages Are Essential for Successful Implantation. Front. Immunol. 2020, 11, 557184. [Google Scholar] [CrossRef]
- Moon, S.N.; Han, J.W.; Hwang, H.S.; Kim, M.J.; Lee, S.J.; Lee, J.Y.; Oh, C.K.; Jeong, D.C. Establishment of secondary iron overloaded mouse model: Evaluation of cardiac function and analysis according to iron concentration. Pediatr. Cardiol. 2011, 32, 947–952. [Google Scholar] [CrossRef]
- Shirasuna, K.; Karasawa, T.; Usui, F.; Kobayashi, M.; Komada, T.; Kimura, H.; Kawashima, A.; Ohkuchi, A.; Taniguchi, S.; Takahashi, M. NLRP3 Deficiency Improves Angiotensin II-Induced Hypertension But Not Fetal Growth Restriction During Pregnancy. Endocrinology 2015, 156, 4281–4292. [Google Scholar] [CrossRef] [PubMed]
- Usui, F.; Shirasuna, K.; Kimura, H.; Tatsumi, K.; Kawashima, A.; Karasawa, T.; Yoshimura, K.; Aoki, H.; Tsutsui, H.; Noda, T.; et al. Inflammasome activation by mitochondrial oxidative stress in macrophages leads to the development of angiotensin II-induced aortic aneurysm. Arter. Thromb. Vasc. Biol. 2015, 35, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Ozeki, A.; Tani, K.; Takahashi, H.; Suzuki, H.; Nagayama, S.; Hirashima, C.; Iwata, H.; Kuwayama, T.; Ohkuchi, A.; Shirasuna, K. Preeclamptic patient-derived circulating cell-free DNA activates the production of inflammatory cytokines via toll-like receptor 9 signalling in the human placenta. J. Hypertens. 2019, 7, 2452–2460. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Hobbs, G.L.; Sanford, K.K.; Evans, V.J.; Earle, W.R. Establishment of a clone of mouse liver cells from a single isolated cell. J. Natl. Cancer Inst. 1957, 18, 701–707. [Google Scholar]
- Dutra, F.F.; Alves, L.S.; Rodrigues, D.; Fernandez, P.L.; de Oliveira, R.B.; Golenbock, D.T.; Zamboni, D.S.; Bozza, M.T. Hemolysis-induced lethality involves inflammasome activation by heme. Proc. Natl. Acad. Sci. USA 2014, 111, E4110–E4118. [Google Scholar] [CrossRef] [PubMed]
- Hubel, C.A.; Kozlov, A.V.; Kagan, V.E.; Evans, R.W.; Davidge, S.T.; McLaughlin, M.K.; Roberts, J.M. Decreased transferrin and increased transferrin saturation in sera of women with preeclampsia: Implications for oxidative stress. Am. J. Obstet. Gynecol. 1996, 175, 692–700. [Google Scholar] [CrossRef]
- Maynard, S.E.; Min, J.Y.; Merchan, J.; Lim, K.H.; Li, J.; Mondal, S.; Libermann, T.A.; Morgan, J.P.; Sellke, F.W.; Stillman, I.E.; et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J. Clin. Investig. 2003, 111, 649–658. [Google Scholar] [CrossRef]
- Venkatesha, S.; Toporsian, M.; Lam, C.; Hanai, J.; Mammoto, T.; Kim, Y.M.; Bdolah, Y.; Lim, K.H.; Yuan, H.T.; Libermann, T.A.; et al. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat. Med. 2006, 12, 642–649. [Google Scholar] [CrossRef]
- Redman, C.W.; Sargent, I.L. Pre-eclampsia, the placenta and the maternal systemic inflammatory respons—A review. Placenta 2003, 24 (Suppl. SA), S21–S27. [Google Scholar] [CrossRef]
- Laresgoiti-Servitje, E. A leading role for the immune system in the pathophysiology of preeclampsia. J. Leukoc. Biol. 2013, 94, 247–257. [Google Scholar] [CrossRef]
- Guo, L.; Zhang, D.; Liu, S.; Dong, Z.; Zhou, J.; Yin, Y.; Wan, D. Maternal iron supplementation during pregnancy affects placental function and iron status in offspring. J. Trace Elem. Med. Biol. 2022, 71, 126950. [Google Scholar] [CrossRef] [PubMed]
- Moroishi, T.; Nishiyama, M.; Takeda, Y.; Iwai, K.; Nakayama, K.I. The FBXL5-IRP2 axis is integral to control of iron metabolism In Vivo. Cell. Metab. 2011, 14, 339–351. [Google Scholar] [CrossRef]
- Nakamura, K.; Kawakami, T.; Yamamoto, N.; Tomizawa, M.; Fujiwara, T.; Ishii, T.; Harigae, H.; Ogasawara, K. Activation of the NLRP3 inflammasome by cellular labile iron. Exp. Hematol. 2016, 44, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Yang, J.; Sun, G.; Hu, J.; Zhang, Q.; Cai, J.; Yuan, D.; Li, H.; Hei, Z.; Yao, W. Macrophage extracellular traps aggravate iron overload-related liver ischaemia/reperfusion injury. Br. J. Pharmacol. 2021, 178, 3783–3796. [Google Scholar] [CrossRef]
- Wang, H.; An, P.; Xie, E.; Wu, Q.; Fang, X.; Gao, H.; Zhang, Z.; Li, Y.; Wang, X.; Zhang, J.; et al. Characterization of ferroptosis in murine models of hemochromatosis. Hepatology 2017, 66, 449–465. [Google Scholar] [CrossRef] [PubMed]
- Egashira, M.; Hirota, Y.; Shimizu-Hirota, R.; Saito-Fujita, T.; Haraguchi, H.; Matsumoto, L.; Matsuo, M.; Hiraoka, T.; Tanaka, T.; Akaeda, S.; et al. F4/80+ Macrophages Contribute to Clearance of Senescent Cells in the Mouse Postpartum Uterus. Endocrinology 2017, 158, 2344–2353. [Google Scholar] [CrossRef]
- Ikeda, Y.; Watanabe, H.; Shiuchi, T.; Hamano, H.; Horinouchi, Y.; Imanishi, M.; Goda, M.; Zamami, Y.; Takechi, K.; Izawa-Ishizawa, Y.; et al. Deletion of H-ferritin in macrophages alleviates obesity and diabetes induced by high-fat diet in mice. Diabetologia 2020, 63, 1588–1602. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shimazaki, S.; Ozawa, R.; Isobe, A.; Kuribayashi, S.; Iwata, H.; Shirasuna, K. Excessive Iron Induces Macrophage Dysfunction in the Liver, Causing Adverse Pregnancy Outcomes in Mice. Metabolites 2025, 15, 431. https://doi.org/10.3390/metabo15070431
Shimazaki S, Ozawa R, Isobe A, Kuribayashi S, Iwata H, Shirasuna K. Excessive Iron Induces Macrophage Dysfunction in the Liver, Causing Adverse Pregnancy Outcomes in Mice. Metabolites. 2025; 15(7):431. https://doi.org/10.3390/metabo15070431
Chicago/Turabian StyleShimazaki, Sayaka, Ren Ozawa, Akari Isobe, Sohei Kuribayashi, Hisataka Iwata, and Koumei Shirasuna. 2025. "Excessive Iron Induces Macrophage Dysfunction in the Liver, Causing Adverse Pregnancy Outcomes in Mice" Metabolites 15, no. 7: 431. https://doi.org/10.3390/metabo15070431
APA StyleShimazaki, S., Ozawa, R., Isobe, A., Kuribayashi, S., Iwata, H., & Shirasuna, K. (2025). Excessive Iron Induces Macrophage Dysfunction in the Liver, Causing Adverse Pregnancy Outcomes in Mice. Metabolites, 15(7), 431. https://doi.org/10.3390/metabo15070431






