Follicular Biochemical Characterization and Fatty Acid Metabolic Signatures of Follicles During Ovulation Process Reveal the Potential Mechanism for Ovarian Cyst Formation in Sows
Abstract
1. Introduction
2. Materials and Methods
2.1. Follicular Fluid Isolation and Follicle Cell Collection
2.2. Detection of Hormone Levels
2.3. Ovarian Tissues Section and Hematoxylin-Eosin (HE) Staining
2.4. Transmission Electron Microscope (TEM) Staining
2.5. Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR)
2.6. Targeted Fatty Acid Metabolomics of Follicular Fluid
2.7. Statistical Analysis
3. Results
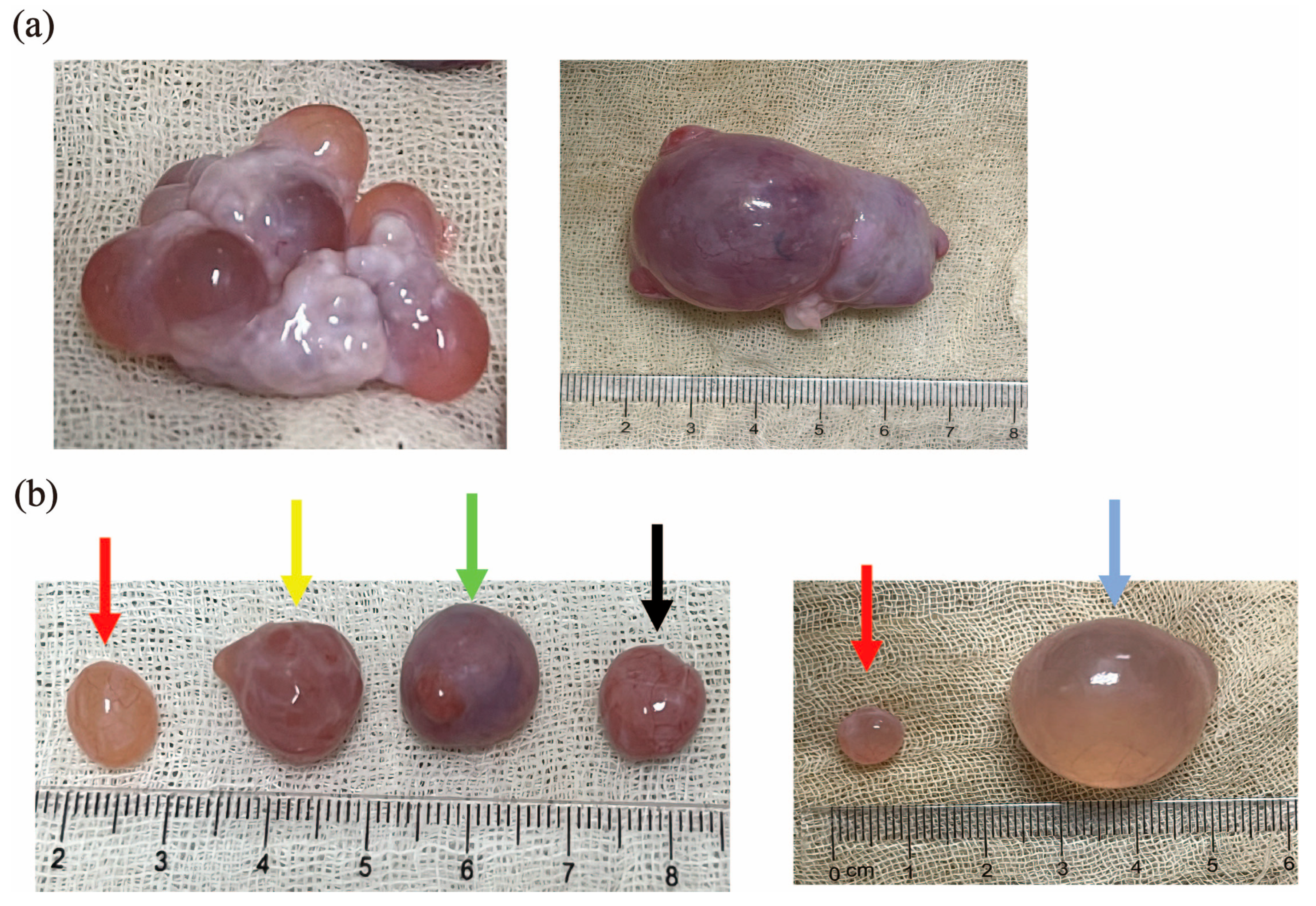
3.1. Classification of Pre-Ovulatory, Peri-Ovulatory, and Cystic Follicles in Sows
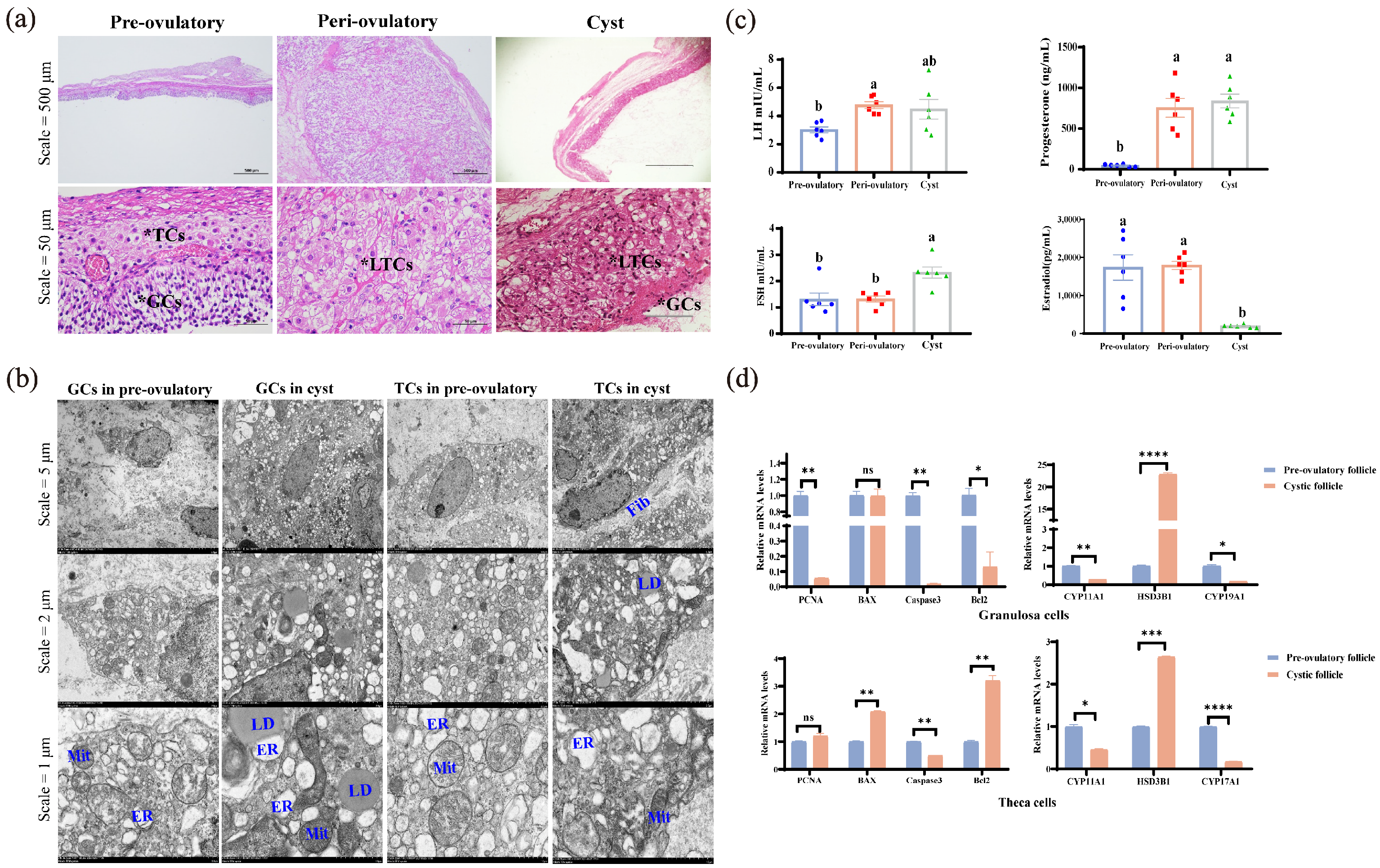
3.2. Biological Characteristics of Pre-Ovulatory, Peri-Ovulatory, and Cystic Follicles Include Histology, Hormonal Assays, and Molecular Biology
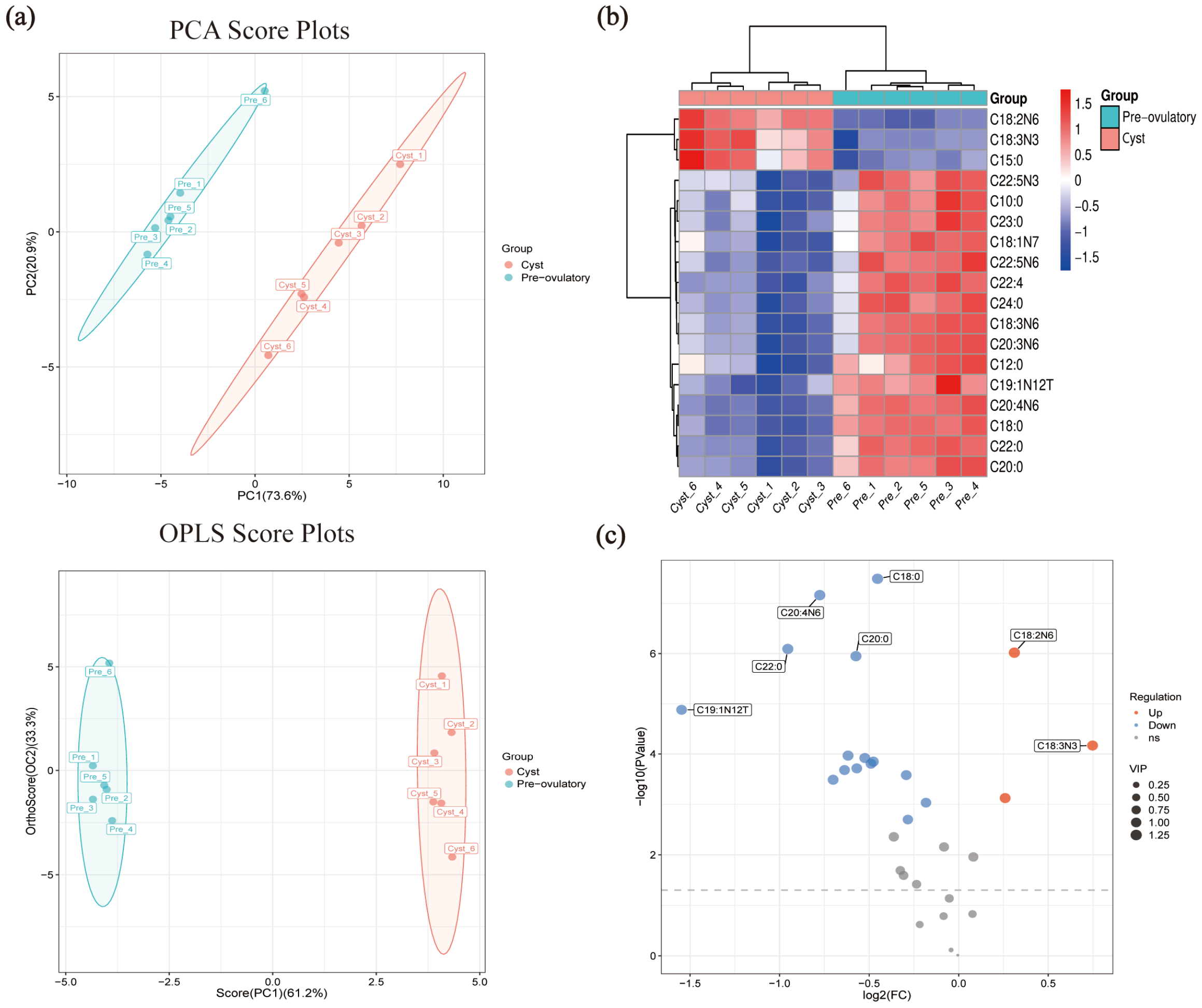
3.3. The Profile of Fatty Acid in FF from Pre-Ovulatory, Peri-Ovulatory, and Cystic Follicles
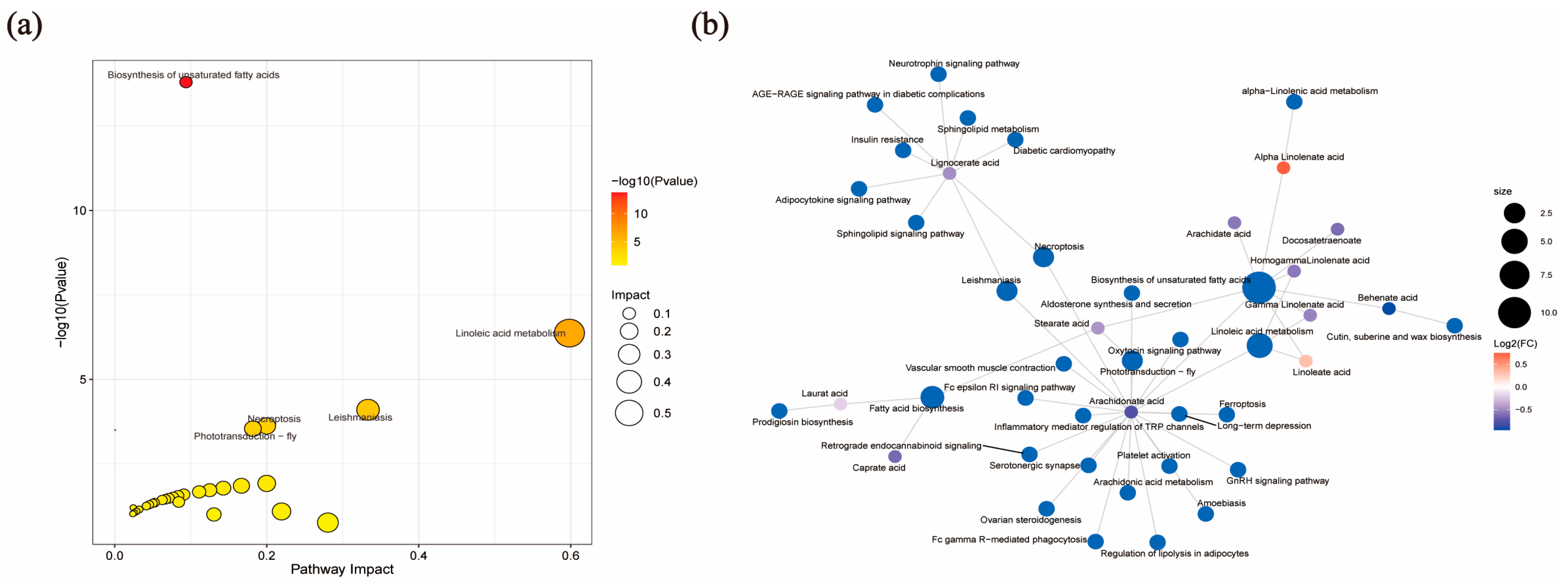
3.4. Analysis of Target Fatty Acid Metabolomics in FF from Pre-Ovulatory and Cystic Follicles
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BAX | Bcl-2-associated x-protein |
| Bcl2 | B-cell lymphoma/leukemia 2 |
| Caspase3 | Cysteine-aspartic acid protease 3 |
| CYP11A1 | Cytochrome P450 Family 11 Subfamily A Member 1 |
| CYP17A1 | Cytochrome P450 Family 17 Subfamily A Member 1 |
| CYP19A1 | Cytochrome P450 Family 19 Subfamily A Member 1 |
| PCNA | Proliferating cell nuclear antigen |
| PGs | Prostaglandins |
| PGE2 | Prostaglandin E2 |
| PGF2α | Prostaglandin F2α |
References
- Diskin, M.G.; Mackey, D.R.; Roche, J.F.; Sreenan, J.M. Effects of nutrition and metabolic status on circulating hormones and ovarian follicle development in cattle. Anim. Reprod. Sci. 2003, 78, 345–370. [Google Scholar] [CrossRef] [PubMed]
- Goodarzi, M.O.; Dumesic, D.A.; Chazenbalk, G.; Azziz, R. Polycystic ovary syndrome: Etiology, pathogenesis and diagnosis. Nat. Rev. Endocrinol. 2011, 7, 219–231. [Google Scholar] [CrossRef]
- Khan, F.A.; Nabi, S.U.; Pande, M.; Das, G.K.; Sarkar, M. Bilateral follicular cysts in a water buffalo. Trop. Anim. Health Prod. 2011, 43, 539–541. [Google Scholar] [CrossRef]
- Eyestone, W.H.; Ax, R.L. A review of ovarian follicular cysts in cows, with comparisons to the condition in women, rats and rabbits. Theriogenology 1984, 22, 109–125. [Google Scholar] [CrossRef] [PubMed]
- Knauf, Y.; Bostedt, H.; Failing, K.; Knauf, S.; Wehrend, A. Gross pathology and endocrinology of ovarian cysts in bitches. Reprod. Domest. Anim. 2014, 49, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Castagna, C.D.; Peixoto, C.H.; Bortolozzo, F.P.; Wentz, I.; Neto, G.B.; Ruschel, F. Ovarian cysts and their consequences on the reproductive performance of swine herds. Anim. Reprod. Sci. 2004, 81, 115–123. [Google Scholar] [CrossRef]
- Ding, Y.; Jiang, Y.; Zhu, M.; Zhu, Q.; He, Y.; Lu, Y.; Wang, Y.; Qi, J.; Feng, Y.; Huang, R.; et al. Follicular fluid lipidomic profiling reveals potential biomarkers of polycystic ovary syndrome: A pilot study. Front. Endocrinol. 2022, 13, 960274. [Google Scholar] [CrossRef]
- Ortega, H.H.; Díaz, P.U.; Salvetti, N.R.; Hein, G.J.; Marelli, B.E.; Rodríguez, F.M.; Stassi, A.F.; Rey, F. Follicular Cysts: A Single Sign and Different Diseases. A View from Comparative Medicine. Curr. Pharm. Design 2016, 22, 5634–5645. [Google Scholar] [CrossRef]
- Jeengar, K.; Chaudhary, V.; Kumar, A.; Raiya, S.; Gaur, M.; Purohit, G.N. Ovarian cysts in dairy cows: Old and new concepts for definition, diagnosis and therapy. Anim. Reprod. 2014, 11, 63–73. [Google Scholar]
- Bor, S.S.; Bor, S.A. Ovarian cysts, an anovulatory condition in dairy cattle. J. Vet. Med. Sci. 2020, 82, 1515–1522. [Google Scholar] [CrossRef]
- Zeng, X.; Li, S.; Liu, L.; Cai, S.; Ye, Q.; Xue, B.; Wang, X.; Zhang, S.; Chen, F.; Cai, C.; et al. Role of functional fatty acids in modulation of reproductive potential in livestock. J. Anim. Sci. Biotechnol. 2023, 14, 24. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.F.; Qi, J.; Xue, X.L.; Li, X.Y.; Liao, Y.; Sun, Y.; Tao, Y.Z.; Yin, H.Y.; Liu, W.; Li, S.X.; et al. Follicular free fatty acid metabolic signatures and their effects on oocyte competence in non-obese PCOS patients. Reproduction 2022, 164, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Sanz, J.I.; Pérez-Ruiz, I.; Meijide, S.; Ferrando, M.; Larreategui, Z.; Ruiz-Larrea, M.B. Lower follicular n-3 polyunsaturated fatty acid levels are associated with a better response to ovarian stimulation. J. Assist. Reprod. Gen. 2019, 36, 473–482. [Google Scholar] [CrossRef]
- Yao, J.K.; Ryan, R.J.; Dyck, P.J. The porcine ovarian follicle. VI. Comparison of fatty acid composition of serum and follicular fluid at different developmental stages. Biol. Reprod. 1980, 22, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Zeron, Y.; Ocheretny, A.; Kedar, O.; Borochov, A.; Sklan, D.; Arav, A. Seasonal changes in bovine fertility: Relation to developmental competence of oocytes, membrane properties and fatty acid composition of follicles. Reproduction 2001, 121, 447–454. [Google Scholar] [CrossRef]
- Dai, J.; Pang, M.; Cai, J.; Liu, Y.; Qin, Y. Integrated transcriptomic and metabolomic investigation of the genes and metabolites involved in swine follicular cyst formation. Front. Vet. Sci. 2023, 10, 1298132. [Google Scholar] [CrossRef]
- Thomson, A.B.; Keelan, M.; Garg, M.L.; Clandinin, M.T. Intestinal aspects of lipid absorption: In review. Can. J. Physiol. Pharmacol. 1989, 67, 179–191. [Google Scholar] [CrossRef]
- Baddela, V.S.; Sharma, A.; Vanselow, J. Non-esterified fatty acids in the ovary: Friends or foes? Reprod. Biol. Endocrinol. 2020, 18, 60. [Google Scholar] [CrossRef]
- Brantmeier, S.A.; Grummer, R.R.; Ax, R.L. Concentrations of high density lipoproteins vary among follicular sizes in the bovine. J. Dairy Sci. 1987, 70, 2145–2149. [Google Scholar] [CrossRef]
- Mu, Y.M.; Yanase, T.; Nishi, Y.; Tanaka, A.; Saito, M.; Jin, C.H.; Mukasa, C.; Okabe, T.; Nomura, M.; Goto, K.; et al. Saturated FFAs, palmitic acid and stearic acid, induce apoptosis in human granulosa cells. Endocrinology 2001, 142, 3590–3597. [Google Scholar] [CrossRef]
- Aardema, H.; Lolicato, F.; van de Lest, C.H.; Brouwers, J.F.; Vaandrager, A.B.; van Tol, H.T.; Roelen, B.A.; Vos, P.L.; Helms, J.B.; Gadella, B.M. Bovine cumulus cells protect maturing oocytes from increased fatty acid levels by massive intracellular lipid storage. Biol. Reprod. 2013, 88, 164. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Wang, W.; Zhang, X.; Ke, H.; Qin, Y.; You, L.; Li, W.; Lu, G.; Chan, W.Y.; Leung, P.C.K.; et al. Palmitic acid causes insulin resistance in granulosa cells via activation of JNK. J. Mol. Endocrinol. 2019, 62, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Yenuganti, V.R.; Viergutz, T.; Vanselow, J. Oleic acid induces specific alterations in the morphology, gene expression and steroid hormone production of cultured bovine granulosa cells. Gen. Comp. Endocrinol. 2016, 232, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Maillard, V.; Desmarchais, A.; Durcin, M.; Uzbekova, S.; Elis, S. Docosahexaenoic acid (DHA) effects on proliferation and steroidogenesis of bovine granulosa cells. Reprod. Biol. Endocrin. 2018, 16, 40. [Google Scholar] [CrossRef]
- Yenuganti, V.R.; Koczan, D.; Vanselow, J. Genome wide effects of oleic acid on cultured bovine granulosa cells: Evidence for the activation of pathways favoring folliculo-luteal transition. BMC Genom. 2021, 22, 486. [Google Scholar] [CrossRef]
- Zhang, N.; Wang, L.; Luo, G.; Tang, X.; Ma, L.; Zheng, Y.; Liu, S.A.; Price, C.; Jiang, Z. Arachidonic Acid Regulation of Intracellular Signaling Pathways and Target Gene Expression in Bovine Ovarian Granulosa Cells. Animals 2019, 9, 374. [Google Scholar] [CrossRef]
- Duffy, D.M.; Ko, C.; Jo, M.; Brannstrom, M.; Curry, T.E. Ovulation: Parallels With Inflammatory Processes. Endocr. Rev. 2019, 40, 369–416. [Google Scholar] [CrossRef]
- Hoving, L.R.; Heijink, M.; van Harmelen, V.; van Dijk, K.W.; Giera, M. GC-MS Analysis of Medium- and Long-Chain Fatty Acids in Blood Samples. Methods Mol. Biol. 2018, 1730, 257–265. [Google Scholar] [CrossRef]
- Beccaria, M.; Franchina, F.A.; Nasir, M.; Mellors, T.; Hill, J.E.; Purcaro, G. Investigation of mycobacteria fatty acid profile using different ionization energies in GC-MS. Anal. Bioanal. Chem. 2018, 410, 7987–7996. [Google Scholar] [CrossRef]
- Jo, M.; Brännström, M.; Akins, J.W.; Curry, T.E., Jr. New insights into the ovulatory process in the human ovary. Hum. Reprod. Update 2025, 31, 21–47. [Google Scholar] [CrossRef]
- Salvetti, N.R.; Panzani, C.G.; Gimeno, E.J.; Neme, L.G.; Alfaro, N.S.; Ortega, H.H. An imbalance between apoptosis and proliferation contributes to follicular persistence in polycystic ovaries in rats. Reprod. Biol. Endocrin. 2009, 7, 68. [Google Scholar] [CrossRef] [PubMed]
- Isobe, N.; Yoshimura, Y. Deficient proliferation and apoptosis in the granulosa and theca interna cells of the bovine cystic follicle. J. Reprod. Dev. 2007, 53, 1119–1124. [Google Scholar] [CrossRef] [PubMed]
- Szulanczyk, K. Histological changes within ovarian cortex, oviductal and uterine mucosa in case of ovarian cysts presence in sows. Folia Histochem. Cytobiol. 2009, 47, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.L.; Zhang, J.; Ping, Z.G.; Wang, C.Q.; Sun, Y.F.; Chen, L.; Li, X.Y.; Li, C.J.; Zhu, X.L.; Liu, Z.; et al. Relationship Between Apoptosis and Proliferation in Granulosa and Theca Cells of Cystic Follicles in Sows. Reprod. Domest. Anim. 2012, 47, 601–608. [Google Scholar] [CrossRef]
- Alfradique, V.A.P.; Netto, D.L.S.; Alves, S.V.P.; Machado, A.F.; Novaes, C.M.; Penitente-Filho, J.M.; Machado-Neves, M.; Lopes, M.S.; Guimarães, S.E.F. The impact of FSH stimulation and age on the ovarian and uterine traits and histomorphometry of prepubertal gilts. Domest. Anim. Endocrinol. 2023, 83, 106786. [Google Scholar] [CrossRef] [PubMed]
- Richards, J.S.; Ascoli, M. Endocrine, Paracrine, and Autocrine Signaling Pathways That Regulate Ovulation. Trends Endocrinol. Met. 2018, 29, 313–325. [Google Scholar] [CrossRef]
- Findlay, J.K.; Britt, K.; Kerr, J.B.; O’Donnell, L.; Jones, M.E.; Drummond, A.E.; Simpson, E.R. The road to ovulation: The role of oestrogens. Reprod. Fertil. Dev. 2001, 13, 543–547. [Google Scholar] [CrossRef] [PubMed]
- King, S.R.; LaVoie, H.A. Gonadal transactivation of STARD1, CYP11A1 and HSD3B. Front. Biosci. 2012, 17, 824–846. [Google Scholar] [CrossRef]
- Sun, Y.L.; Ping, Z.G.; Li, C.J.; Sun, Y.F.; Yi, K.L.; Chen, L.; Li, X.Y.; Wang, X.L.; Zhou, X. Comparative proteomic analysis of follicular fluids from normal and cystic follicles in sows. Reprod. Domest. Anim. 2011, 46, 889–895. [Google Scholar] [CrossRef]
- Mo, J.; Sun, L.; Cheng, J.; Lu, Y.; Wei, Y.; Qin, G.; Liang, J.; Lan, G. Non-targeted Metabolomics Reveals Metabolic Characteristics of Porcine Atretic Follicles. Front. Vet. Sci. 2021, 8, 679947. [Google Scholar] [CrossRef]
- Cheng, J.; Pan, Y.; Yang, S.; Wei, Y.; Lv, Q.; Xing, Q.; Zhang, R.; Sun, L.; Qin, G.; Shi, D.; et al. Integration of transcriptomics and non-targeted metabolomics reveals the underlying mechanism of follicular atresia in Chinese buffalo. J. Steroid Biochem. Mol. Biol. 2021, 212, 105944. [Google Scholar] [CrossRef] [PubMed]
- Shemesh, M.; Bensadoun, A.; Hansel, W. Lipoprotein lipase activity in the bovine corpus luteum during the estrous cycle and early pregnancy. Proc. Soc. Exp. Biol. Med. 1976, 151, 667–669. [Google Scholar] [CrossRef] [PubMed]
- Stoffel, W.; Binczek, E.; Schmidt-Soltau, I.; Brodesser, S.; Wegner, I. High fat/high cholesterol diet does not provoke atherosclerosis in the ω3-and ω6-polyunsaturated fatty acid synthesis–inactivated Δ6-fatty acid desaturase–deficient mouse. Mol. Metab. 2021, 54, 101335. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, Y.; Sun, J.; Zhang, W.; Guo, Z.; Ma, Q. Arachidonic acid metabolism in health and disease. MedComm 2023, 4, e363. [Google Scholar] [CrossRef]
- Liu, R.; Bai, S.; Zheng, S.; Zhu, X.; Zhang, Y.; Xu, B.; Zhao, W. Identification of the Metabolomics Signature of Human Follicular Fluid from PCOS Women with Insulin Resistance. Dis. Markers 2022, 2022, 6877541. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, B.; Cai, S.; Zeng, X.; Ye, Q.; Mao, X.; Zhang, S.; Zeng, X.; Ye, C.; Qiao, S. Metabolic disorder of amino acids, fatty acids and purines reflects the decreases in oocyte quality and potential in sows. J. Proteom. 2019, 200, 134–143. [Google Scholar] [CrossRef]

| Content (µg mL−1) | LA C18:2N6 | OA C18:1N9C | PA C16:0 | SA C18:0 | AA C20:4N6 | |||
|---|---|---|---|---|---|---|---|---|
| Pre-ovulatory | 77.31 ± 0.495 c | 61.45 ± 0.47 b | 60.54 ± 0.54 b | 53.55 ± 0.54 a | 42.83 ± 0.92 a | |||
| Peri-ovulatory | 91.41 ± 1.66 b | 105.32 ± 2.12 a | 75.02 ± 1.89 a | 50.05 ± 1.70 a | 31.21 ± 1.34 b | |||
| Cyst | 95.91 ± 1.07 a | 59.25 ± 0.94 c | 57.19 ± 0.79 b | 39.12 ± 0.69 b | 25.02 ± 0.65 c | |||
| Composition | SFAs | MUFAs | PUFAs | |||||
| Pre-ovulatory | 35.9% | 22.4% | 41.7% | |||||
| Peri-ovulatory | 34.1% | 30.7% | 35.2% | |||||
| Cyst | 33.1% | 23% | 43.9% | |||||
| Abbreviation | Name | VIP Value | Fold Change | log2 (FC_cyst/pre) | p Value |
|---|---|---|---|---|---|
| C18:2N6 | Linoleic | 1.257 | 1.240 | 0.31 | 9.6 × 107 |
| C20:4N6 | Arachidonic | 1.252 | 0.584 | −0.78 | 6.8 × 108 |
| C18:0 | Stearate | 1.250 | 0.730 | −0.45 | 3.2 × 108 |
| C22:0 | Behenate | 1.223 | 0.516 | −0.95 | 8.1 × 107 |
| C20:0 | Arachidate | 1.217 | 0.672 | −0.57 | 1.1 × 106 |
| C22:4 | Docosatetraenoate | 1.180 | 0.652 | −0.62 | 1.1 × 104 |
| C18:3N3 | Alpha Linolenate | 1.156 | 1.679 | 0.75 | 6.8 × 105 |
| C24:0 | Lignocerate | 1.148 | 0.719 | −0.48 | 1.4 ×104 |
| C15:0 | Pentadecanoate | 1.146 | 1.197 | 0.26 | 7.5 × 104 |
| C22:5N6 | DPA | 1.144 | 0.615 | −0.70 | 3.3 × 104 |
| C18:3N6 | Gamma Linolenate | 1.139 | 0.695 | −0.52 | 1.2 × 104 |
| C10:0 | Caprate | 1.118 | 0.643 | −0.64 | 2.1 × 104 |
| C20:3N6 | HomogammaLinolenate | 1.117 | 0.674 | −0.57 | 1.9 × 104 |
| C23:0 | Tricosanoate | 1.116 | 0.712 | −0.49 | 1.6 × 104 |
| C19:1N12T | 7-Transnonadecenoate | 1.115 | 0.342 | −1.55 | 1.3 × 105 |
| C18:1N7 | Vaccenate | 1.105 | 0.816 | −0.29 | 2.6 × 104 |
| C12:0 | Laurate | 1.060 | 0.881 | −0.18 | 9.3 × 104 |
| C22:5N3 | DPA | 1.019 | 0.821 | −0.28 | 2.0 × 103 |
| KEGG Metabolic Pathway | p Value | Impact |
|---|---|---|
| Biosynthesis of unsaturated fatty acids | <0.0001 | 0.0938 |
| Linoleic acid metabolism | <0.0001 | 0.5982 |
| Leishmaniasis | <0.0001 | 0.3333 |
| Necroptosis | 0.0001 | 0.2 |
| Fatty acid biosynthesis | 0.0002 | 0.0006 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, J.; Deng, Y.; Fu, S.; Cheng, J.; Zhang, R.; Shi, D.; Pan, Y.; Yang, S. Follicular Biochemical Characterization and Fatty Acid Metabolic Signatures of Follicles During Ovulation Process Reveal the Potential Mechanism for Ovarian Cyst Formation in Sows. Metabolites 2025, 15, 421. https://doi.org/10.3390/metabo15070421
Liang J, Deng Y, Fu S, Cheng J, Zhang R, Shi D, Pan Y, Yang S. Follicular Biochemical Characterization and Fatty Acid Metabolic Signatures of Follicles During Ovulation Process Reveal the Potential Mechanism for Ovarian Cyst Formation in Sows. Metabolites. 2025; 15(7):421. https://doi.org/10.3390/metabo15070421
Chicago/Turabian StyleLiang, Jingyuan, Yanfei Deng, Song Fu, Juanru Cheng, Ruimen Zhang, Deshun Shi, Yu Pan, and Sufang Yang. 2025. "Follicular Biochemical Characterization and Fatty Acid Metabolic Signatures of Follicles During Ovulation Process Reveal the Potential Mechanism for Ovarian Cyst Formation in Sows" Metabolites 15, no. 7: 421. https://doi.org/10.3390/metabo15070421
APA StyleLiang, J., Deng, Y., Fu, S., Cheng, J., Zhang, R., Shi, D., Pan, Y., & Yang, S. (2025). Follicular Biochemical Characterization and Fatty Acid Metabolic Signatures of Follicles During Ovulation Process Reveal the Potential Mechanism for Ovarian Cyst Formation in Sows. Metabolites, 15(7), 421. https://doi.org/10.3390/metabo15070421






