Analysis of the Acute Cytokine Dynamics Induced in Professional Padel According to the Playing Side of the Court and Sex-Related Differences
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Experimental Approach
2.2.1. First Session
2.2.2. Second Session
2.3. Blood Sampling
2.4. Statistical Analysis
3. Results
3.1. Participants’ Characteristics and Cardiorespiratory Fitness
3.2. Physiological Demands of the Simulated Competition
3.3. Analysis of Inflammatory Responses
3.4. Analysis According to Playing Side on the Court
4. Discussion
4.1. VO2max and Sex-Related Differences
4.2. Impact of the Playing Side on Heart Rate
4.3. Limitations and New Perspectives
5. Conclusions
Clinical and Practical Implications
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BMI | Body mass index |
| ES | Effect size (ES) |
| FLS | Female players on the left side (FLS) |
| FRS | Female players on the right side (FRS) |
| HRmax | Maximum heart rate |
| HRmean | Mean heart rate |
| IFN-γ | Interferon-gamma |
| IL-1 | Interleukin-1 |
| IL-10 | Interleukin-10 |
| IL-13 | Interleukin-13 |
| IL-5 | Interleukin-5 |
| IL-6 | Interleukin-6 |
| LIF | Leukemia inhibitory factor |
| MLS | Male players on the left side (MLS) |
| MRS | Male players on the right side (MRS) |
| SD | Standard deviation |
| TNF-α | Tumor necrosis factor alpha |
| VO2max | maximal oxygen consumption |
References
- Severinsen, M.C.K.; Pedersen, B.K. Muscle–Organ Crosstalk: The Emerging Roles of Myokines. Endocr. Rev. 2020, 41, 594–609. [Google Scholar] [CrossRef] [PubMed]
- de Sousa, C.A.Z.; Sierra, A.P.R.; Martínez Galán, B.S.; de Sousa Maciel, J.F.; Manoel, R.; Barbeiro, H.V.; de Souza, H.P.; Cury-Boaventura, M.F. Time Course and Role of Exercise-Induced Cytokines in Muscle Damage and Repair After a Marathon Race. Front. Physiol. 2021, 12, 752144. [Google Scholar] [CrossRef] [PubMed]
- Rohnejad, B.; Monazzami, A. Effects of high-intensity intermittent training on some inflammatory and muscle damage indices in overweight middle-aged men. Apunt. Sports Med. 2023, 58, 100404. [Google Scholar] [CrossRef]
- Simpson, R.J.; Campbell, J.P.; Gleeson, M.; Krüger, K.; Nieman, D.C.; Pyne, D.B.; Turner, J.E.; Walsh, N.P. Can exercise affect immune function to increase susceptibility to infection? Exerc. Immunol. Rev. 2020, 26, 8–22. [Google Scholar]
- Ziemann, E.; Zembroń-Lacny, A.; Kasperska, A.; Antosiewicz, J.; Grzywacz, T.; Garsztka, T.; Laskowski, R. Exercise training-induced changes in inflammatory mediators and heat shock proteins in young tennis players. J. Sports Sci. Med. 2013, 12, 282–289. [Google Scholar]
- Kozłowska, M.; Zurek, P.; Rodziewicz, E.; Góral, K.; Zmijewski, P.; Lipinska, P.; Laskowski, R.; Walentukiewicz, A.K.; Antosiewicz, J.; Ziemann, E. Immunological response and match performance of professional tennis players of different age groups during a competitive season. J. Strength Cond. Res. 2021, 35, 2255–2262. [Google Scholar] [CrossRef]
- Rossi, F.E.; Maldonado, A.J.; Cholewa, J.M.; Ribeiro, S.L.G.; de Araújo Barros, C.A.; Figueiredo, C.; Reichel, T.; Krüger, K.; Lira, F.S.; Minuzzi, L.G. Exercise training-induced changes in immunometabolic markers in youth badminton athletes. Sci. Rep. 2022, 12, 15539. [Google Scholar] [CrossRef]
- Martín-Miguel, I.; Escudero-Tena, A.; Sánchez-Alcaraz, B.J.; Courel-Ibáñez, J.; Muñoz, D. Physiological, physical and anthropometric parameters in padel: A systematic review. Int. J. Sports Sci. Coach. 2024, 20, 407–424. [Google Scholar] [CrossRef]
- Scheffer, D.d.L.; Latini, A. Exercise-induced immune system response: Anti-inflammatory status on peripheral and central organs. Biochim. Biophys. Acta-Mol. Basis Dis. 2020, 10, 1866. [Google Scholar] [CrossRef]
- Sánchez-Alcaraz, B.J.; Cánovas Martínez, J.; Sánchez Pay, A.; Muñoz, D. Research on padel. A systematic review. Padel Sci. J. 2023, 1, 71–105. [Google Scholar] [CrossRef]
- Pradas, F.; Cadiz, M.P.; Nestares, M.T.; Martinez-Diaz, I.C.; Carrasco, L. Effects of Padel Competition on Brain Health-Related Myokines. Int. J. Environ. Res. Public Health 2021, 18, 6042. [Google Scholar] [CrossRef] [PubMed]
- Ampuero, R.; Mellado-Arbelo, O.; Fuentes-García, J.P.; Baiget, E. Game sides technical-tactical differences between professional male padel players. Int. J. Sports Sci. Coach. 2023, 19, 1332–1338. [Google Scholar] [CrossRef]
- Miralles, R.; Guzmán, J.F.; Ramón-Llin, J.; Martínez-Gallego, R. Impact of Playing Position on Competition External Load in Professional Padel Players Using Inertial Devices. Sensors 2025, 25, 800. [Google Scholar] [CrossRef]
- Carbonell-Martínez, J.A.; Ferrándiz-Moreno, J.; Pascual-Verdú, N. Analysis of heart rate in amateur female padel. Retos 2017, 32, 204–207. [Google Scholar]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [PubMed]
- Liguori, G. ACSM’s Guidelines for Exercise Testing and Prescription, 11th ed.; Lippincott Connect-ACSM: Riverwoods, IL, USA, 2021; ISBN 9781975150181. [Google Scholar]
- Acosta, J.R.; Tavira, B.; Douagi, I.; Kulyté, A.; Arner, P.; Rydén, M.; Laurencikiene, J. Human-Specific Function of IL-10 in Adipose Tissue Linked to Insulin Resistance. J. Clin. Endocrinol. Metab. 2019, 104, 4552–4562. [Google Scholar] [CrossRef]
- Sethi, J.K.; Hotamisligil, G.S. Metabolic Messengers: Tumour necrosis factor. Nat. Metab. 2021, 3, 1302–1312. [Google Scholar] [CrossRef]
- Rohm, T.V.; Meier, D.T.; Olefsky, J.M.; Donath, M.Y. Inflammation in obesity, diabetes, and related disorders. Immunity 2022, 55, 31–55. [Google Scholar] [CrossRef]
- Kawai, T.; Autieri, M.V.; Scalia, R. Adipose tissue inflammation and metabolic dysfunction in obesity. Am. J. Physiol. Cell Physiol. 2021, 320, C375–C391. [Google Scholar] [CrossRef]
- Lu, Z.; Wang, Z.; Zhang, X.A.; Ning, K. Myokines May Be the Answer to the Beneficial Immunomodulation of Tailored Exercise—A Narrative Review. Biomolecules 2024, 14, 1205. [Google Scholar] [CrossRef]
- Docherty, S.; Harley, R.; McAuley, J.J.; Crowe, L.A.N.; Pedret, C.; Kirwan, P.D.; Siebert, S.; Millar, N.L. The effect of exercise on cytokines: Implications for musculoskeletal health: A narrative review. BMC Sports Sci. Med. Rehabil. 2022, 14, 5. [Google Scholar] [CrossRef] [PubMed]
- Rius-Pérez, S.; Torres-Cuevas, I.; Millán, I.; Ortega, Á.L.; Pérez, S.; Sandhu, M.A. PGC-1 α, Inflammation, and Oxidative Stress: An Integrative View in Metabolism. Oxid. Med. Cell. Longev. 2020, 2020, 1452696. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Zhu, Y.; Deng, C.; Liang, Z.; Chen, J.; Chen, Y.; Wang, X.; Liu, Y.; Tian, Y.; Yang, Y. Peroxisome proliferator-activated receptor gamma coactivator-1 (PGC-1) family in physiological and pathophysiological process and diseases. Signal Transduct. Target. Ther. 2024, 9, 50. [Google Scholar] [CrossRef]
- Balakrishnan, R.; Thurmond, D.C. Mechanisms by Which Skeletal Muscle Myokines Ameliorate Insulin Resistance. Int. J. Mol. Sci. 2022, 23, 4636. [Google Scholar] [CrossRef]
- Ramón-Llin, J.; Guzman-Lujan, J.F.; Martinez-Gallego, R. Comparison of heartrate between elite and national paddle players during competition. Retos 2018, 33, 91–95. [Google Scholar] [CrossRef]
- Witek, K.; Zurek, P.; Zmijewski, P.; Jaworska, J.; Lipińska, P.; Dzedzej-Gmiat, A.; Antosiewicz, J.; Ziemann, E. Myokines in response to a tournament season among young tennis players. BioMed Res. Int. 2016, 2016, 1460892. [Google Scholar] [CrossRef] [PubMed]
- Ramón-Llin, J.; Guzmán, J.; Martínez-Gallego, R.; Muñoz, D.; Sánchez-Pay, A.; Sánchez-Alcaraz, B.J. Stroke analysis in padel according to match outcome and game side on court. Int. J. Environ. Res. Public Health 2020, 17, 7838. [Google Scholar] [CrossRef]
- Pradas, F.; García-Giménez, A.; Toro-Román, V.; Sánchez-Alcaraz, B.J.; Ochiana, N.; Castellar, C. Effect of a padel match on biochemical and haematological parameters in professional players with regard to gender-related differences. Sustainability 2020, 12, 8633. [Google Scholar] [CrossRef]
- Hoppe, M.W.; Hotfiel, T.; Stückradt, A.; Grim, C.; Ueberschär, O.; Freiwald, J.; Baumgart, C. Effects of passive, active and mixed playing strategies on external and internal loads in female tennis players. PLoS ONE 2020, 15, e0239463. [Google Scholar] [CrossRef]
- Deka, P.; Berg, K.; Harder, J.; Batelaan, H.; McGRATH, M. Oxygen cost and physiological responses of recreational badminton match play. J. Sports Med. Phys. Fit. 2017, 57, 760–765. [Google Scholar] [CrossRef]
- García-Benítez, S.; Courel-Ibáñez, J.; Pérez-Bilbao, T.; Felipe, J.L. Game Responses During Young Padel Match Play: Age and Sex Comparisons. J. Strength Cond. Res. 2018, 32, 1144–1149. [Google Scholar] [CrossRef] [PubMed]
- Killit, B.; Arslan, E.; Soylu, Y. Time-motion characteristics, notational analysis and physiological demands of tennis match play: A review. Acta Kinesiol. 2018, 12, 22–23. [Google Scholar]
- Picabea, J.M.; Cámara, J.; Yanci, J. Heart Rate Response, Temporal Structure and the Stroke Technique Distribution in Table Tennis National Category Matches. Int. J. Environ. Res. Public Health 2023, 20, 739. [Google Scholar] [CrossRef] [PubMed]
- Savarirajan, R. Result of Heart Rate, Playing Time and Performance of Tamilnadu Badminton Senior Ranking Players. Int. J. Sports Sci. Fit. 2016, 6, 43–57. [Google Scholar]
- Díaz-García, J.; Grijota, F.J.; Robles, M.C.; Maynar, M.; Muñoz, D. Study of internal load in amateur padel through heart rate. Apunt. Educ. Fis. Deport. 2017, 1, 75–81. [Google Scholar] [CrossRef]
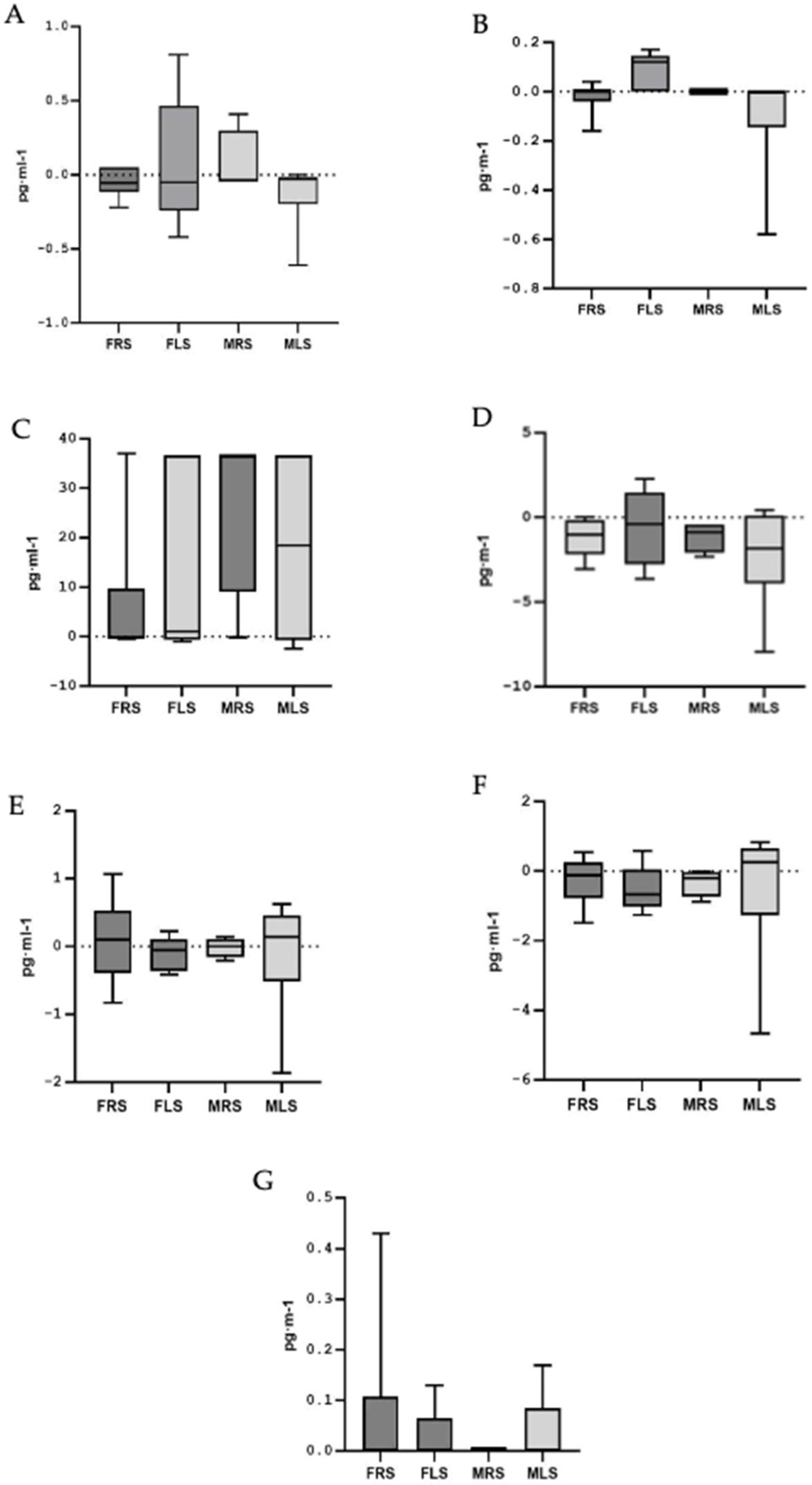
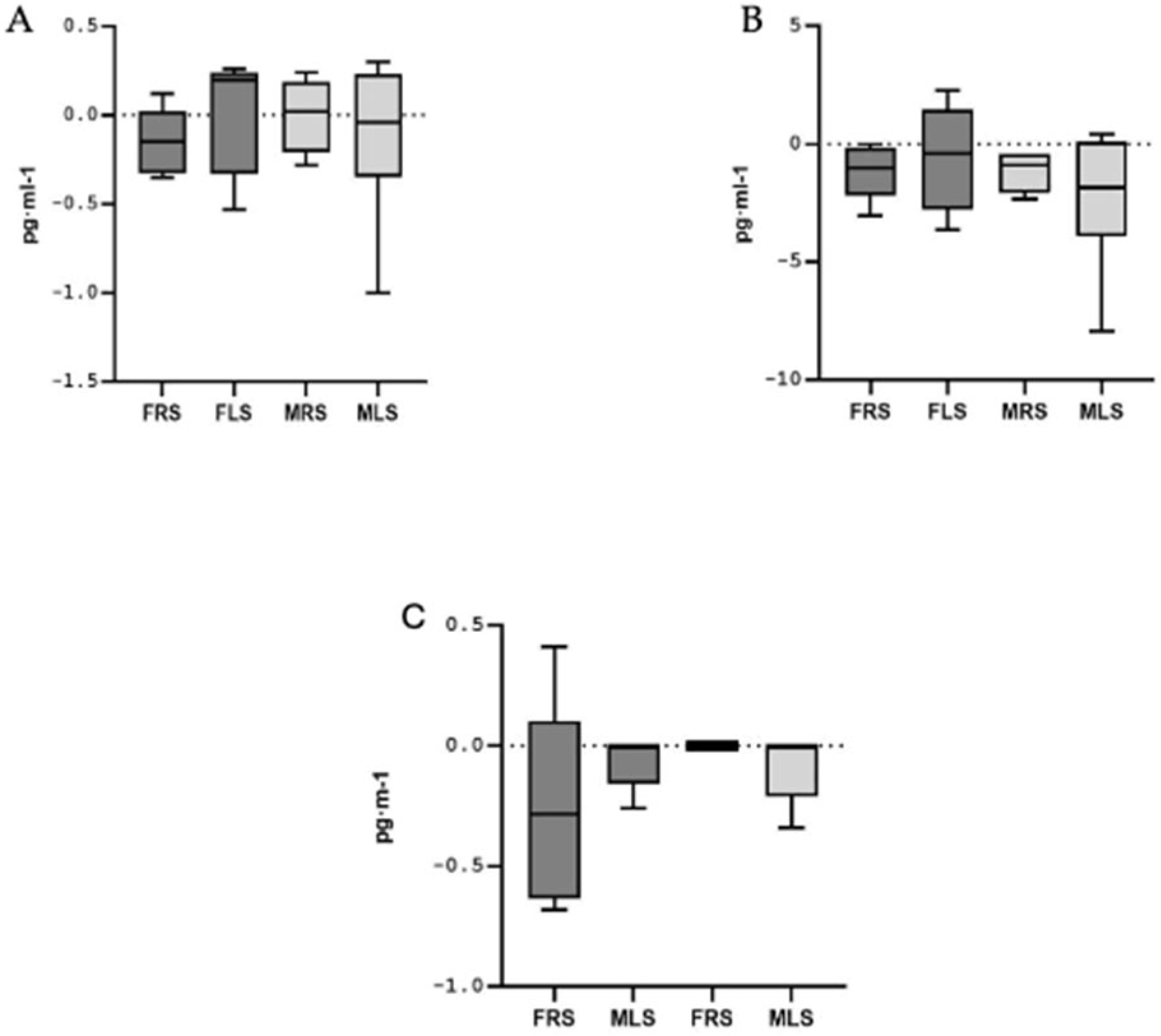
| Female | Male | Total | p | ES | |
|---|---|---|---|---|---|
| Age (years) | 29 ± 3.8 | 26.3 ± 8.2 | 27.7 ± 6.3 | 0.323 | −0.42 |
| BMI (kg·m−2) | 21.7 ± 1 | 24.4 ± 1.8 | 23 ± 1.9 | 0.001 | 1.85 |
| Weight (kg) | 60.6 ± 4.5 | 76.6 ± 6.2 | 68.2 ± 9.7 | <0.001 | 2.95 |
| Height (cm) | 167.1 ± 5.6 | 177.1 ± 2.8 | 171.8 ± 6.7 | <0.001 | 2.25 |
| Body fat (%) | 20 ± 2.1 | 13.3 ± 5.1 | 16.9 ± 5.1 | 0.001 | −1.71 |
| Muscle mass (%) | 37 ± 2.9 | 43.3 ± 2.2 | 27.5 ± 5.8 | <0.001 | 2.44 |
| VO2max (mL·kg−1·min−1) | 47.4 ± 4.8 | 57.5 ± 5.7 | 52.81 ± 7.2 | <0.001 | 1.91 |
| HRmax (bpm) | 186.2 ± 7.7 | 188.2 ± 10.6 | 187.2 ± 0.9 | 0.639 | 0.22 |
| IL-1ß | IL-2 | IL-5 | IL-6 | IL-7 | IL-8 | IL-10 | IL-12 | IL-13 | TNF-α | IFN-γ | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BMI | r | −0.33 | 0.40 | −0.33 | −0.39 | 0.75 | 0.10 | −0.40 | −0.21 | −0.27 | −0.44 * | −0.33 |
| p | 0.13 | 0.86 | 0.88 | 0.07 | 0.74 | 0.65 | 0.07 | 0.34 | 0.22 | 0.043 * | 0.13 | |
| Age | r | 0.11 | 0.08 + | −0.27 | 0.26 | 0.57 | 0.02 + | 0.21 | 0.29 | 0.01 | 0.71 | 0.22 |
| p | 0.61 | 0.70 | 0.23 | 0.25 | 0.80 | 0.92 | 0.34 | 0.20 | 0.96 | 0.75 | 0.33 |
| Cytokines (pg·mL−1) | Pre | Post | Intragroup Contrast | ||||
|---|---|---|---|---|---|---|---|
| Mean ± SD | Range | Mean ± SD | Range | p | ES | Z | |
| IL1-ß | 0.41 ± 0.23 | 0.06–1.01 | 0.40 ± 0.09 | 0.20–0.49 | 0.32 | 0.34 | −1.721 a |
| IL-2 | 0.70 ± 0.40 | 0.09–1.52 | 0.76 ± 0.29 | 0.09–1.11 | 0.38 | 0.26 | 0.866 a |
| IL-5 | 0.81 ± 0.53 | 0.00–1.99 | 0.88 ± 0.49 | 0.17–1.87 | 0.32 | 0.24 | −0.798 a |
| IL-6 | 0.39 ± 0.70 | 0.18–2.50 | 0.16 ± 0.82 | 0.01–0.34 | 0.22 | 0.37 | −1.214 a |
| IL-7 | 10.67 ± 16.91 | 0.16–37 | 0.72 ± 0.41 | 0.00–1.46 | 0.28 | 0.32 | −1.071 b |
| IL-8 | 1.20 ± 0.80 | 0.34–3.28 | 1.40 ± 1.03 | 0.00–3.93 | 0.09 | 0.50 | −1.682 a |
| IL-10 | 4.11 ± 2.48 | 0.31–6.67 | 5.04 ± 2.68 | 0.99–10.30 | 0.11 | 0.47 | −1.580 a |
| IL-12 | 0.91 ± 0.52 | 0.36–1.68 | 0.92 ± 0.63 | 0.23–2.19 | 0.76 | 0.89 | −2.96 a |
| IL-13 | 0.57 ± 0.42 | 0.24–1.43 | 0.73 ± 0.57 | 0.24–1.60 | 0.09 | 0.51 | −1.690 a |
| IFN-γ | 3.17 ± 1.84 | 0.47–7.14 | 3.54 ± 1.76 | 0.61–6.56 | 0.13 | 0.47 | −1.511 a |
| TNF-α | 0.43 ± 0.00 | 0.43–0.43 | 0.37 ± 0.13 | 0.00–0.43 | 0.18 | 0.40 | −1.342 b |
| Cytokines (pg·mL−1) | Pre | Post | Intragroup Contrast | ||||
|---|---|---|---|---|---|---|---|
| Mean ± SD | Range | Mean ± SD | Range | p | ES | Z | |
| IL1-ß | 0.24 ± 0.21 | 0.00–0.62 | 0.28 ± 0.35 | 0.06–1.23 | 0.08 | 0.54 | −1.721 a |
| IL-2 | 0.55 ± 0.62 | 0.03–2.11 | 0.57 ± 0.78 | 0.09–2.74 | 0.95 | 0.16 | 0.59 a |
| IL-5 | 0.54 ± 0.34 | 0.17–1.31 | 0.61 ± 0.63 | 0.09–1.87 | 0.88 | 0.04 | −0.14 a |
| IL-6 | 0.20 ± 0.06 | 0.18–0.38 | 0.25 ± 0.24 | 0.18–0.96 | 0.31 | 0.31 | −1.000 a |
| IL-7 | 22.51 ± 18.70 | 0.16–37 | 0.78 ± 1.35 | 0.29–4.64 | 0.03 * | 0.67 | −2.140 a |
| IL-8 | 0.61 ± 0.24 | 0.22–0.98 | 0.81 ± 0.40 | 0.30–1.75 | ≤0.00 * | 0.82 | −2.599 a |
| IL-10 | 2.37 ± 2.44 | 0.54–8.70 | 4.20 ± 4.45 | 1.44–16.62 | 0.01 * | 0.80 | −2.547 a |
| IL-12 | 0.68 ± 0.69 | 0.15–2.39 | 0.75 ± 1.25 | 0.07–4.26 | 0.61 | 1.60 | −5.07 b |
| IL-13 | 0.44 ± 0.53 | 0.24–1.89 | 0.62 ± 0.98 | 0.24–3.34 | 0.18 | 0.42 | −1.342 a |
| IFN-γ | 2.23 ± 2.25 | 0.13–6.27 | 2.64 ± 3.21 | 0.13–10.48 | 0.08 | 0.21 | 0.67 a |
| TNF-α | 0.43 ± 0.00 | 0.43–0.43 | 0.44 ± 0.05 | 0.43–0.60 | 0.317 | 0.82 | −2.599 a |
| Cytokines (pg·mL−1) | Pre | Post | Intragroup Contrast | Sex Intergroup | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Range | Mean ± SD | Range | p | ES | Z | p | ES | p | ES | |
| IL1-ß | 0.33 ± 0.23 | 0.0–1.01 | 0.34 ± 0.25 | 0.06–1.23 | 0.15 | 0.25 | −1.149 a | 0.13 | 0.71 | 0.02 | 1.12 |
| IL-2 | 0.63 ± 0.51 | 0.03–2.11 | 0.67 ± 0.57 | 0.09–2.74 | 0.49 | 0.14 | −0.684 a | 0.25 | 0.54 | 0.01 | 1.20 |
| IL-5 | 0.68 ± 0.46 | 0.0–1.99 | 0.75 ± 0.56 | 0.09–1.87 | 0.38 | 0.19 | −0.865 a | 0.19 | 0.61 | 0.13 | 0.71 |
| IL-6 | 0.30 ± 0.50 | 0.18–2.5 | 0.20 ± 0.18 | 0.01–0.96 | 0.60 | 0.17 | −0.524 b | 1.00 | 0 | 0.19 | 0.61 |
| IL-7 | 16.31 ± 18.36 | 0.16–37 | 0.75 ± 0.95 | 0.0–4.64 | 0.02 * | 0.48 | −2.219 b | 0.31 | 0.47 | 0.05 | 0.95 |
| IL-8 | 0.92 ± 0.66 | 0.22–3.28 | 1.12 ± 0.83 | 0.0–3.93 | ≤0.00 * | 0.64 | −2.949 a | 0.01 | 1.22 | 0.08 | 0.83 |
| IL-10 | 3.28 ± 2.55 | 0.31–8.7 | 4.64 ± 3.56 | 0.99–16.62 | 0.00 * | 0.61 | −2.817 a | 0.11 | 0.73 | 0.11 | 0.75 |
| IL-12 | 0.80 ± 0.60 | 0.15–2.39 | 0.83 ± 0.95 | 0.07–4.26 | 0.91 | 0.02 | −0.103 b | 0.28 | 0.49 | 0.04 | 0.97 |
| IL-13 | 0.30 ± 0.46 | 0.24–1.89 | 0.68 ± 0.77 | 0.24–3.34 | 0.38 | 0.45 | −2.073 a | 0.15 | 0.66 | 0.15 | 0.68 |
| IFN-γ | 2.72 ± 2.05 | 0.13–7.14 | 3.11 ± 2.53 | 0.13–10.48 | 0.15 | 0.30 | −1.419 a | 0.19 | 0.61 | 0.19 | 0.74 |
| TNF-α | 0.43 ± 0.00 | 0.43–0.43 | 0.41 ± 0.10 | 0.0–0.6 | 0.59 | 0.12 | −0.535 a | 1.00 | 0 | 0.31 | 0.46 |
| Pre Test | Post Test | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytokines (pg·mL−1) | Female Mean ± SD | Male Mean ± SD | t/U † | df/Z ‡ | p | ES | Female Mean ± SD | Male Mean ± SD | t/U † | df/Z ‡ | p | ES |
| IL1-ß | 0.41 ± 0.23 | 0.24 ± 0.21 | 0.0 | 19 | 1 | 0.77 | 0.40 ± 0.91 | 0.28 ± 0.35 | 1.10 | 19 | 0.28 | −0.17 |
| IL-2 | 0.70 ± 0.40 | 0.55 ± 0.62 | 38 † | −1.19 ‡ | 0.23 † | 0.28 | 0.76 ± 0.29 | 0.57 ± 0.78 | 0.78 | 19 | 0.44 | −0.32 |
| IL-5 | 0.81 ± 0.53 | 0.54 ± 0.34 | 36 † | −1.34 ‡ | 0.17 † | 0.60 | 0.88 ± 0.49 | 0.61 ± 0.63 | 33 † | −1.55 ‡ | 0.12 † | −0.47 |
| IL-6 | 0.19 ± 0.036 | 0.20 ± 0.64 | −0.41 | 19 | 0.68 | 0.02 | 0.16 ± 0.82 | 0.25 ± 0.24 | −1.18 | 19 | 0.25 | 0.14 |
| IL-7 | 10.45 ± 17.04 | 22.37 ± 18.88 | −1.52 | 19 | 0.14 | 0.66 | 0.72 ± 0.41 | 0.39 ± 0.12 | 2.47 | 12.06 | 0.02 * | −1.02 |
| IL-8 | 1.20 ± 0.80 | 0.61 ± 0.24 | 2.22 | 19 | 0.03 * | 0.99 | 1.40 ± 1.03 | 0.81 ± 0.40 | 30 † | −1.76 ‡ | 0.78 † | −0.75 |
| IL-10 | 4.11 ± 2.48 | 2.37 ± 2.44 | 1.16 | 19 | 0.12 | 0.70 | 5.04 ± 2.68 | 4.20 ± 4.45 | 0.52 | 19 | 0.60 | −0.22 |
| IL-12 | 0.91 ± 0.52 | 0.68 ± 0.69 | 0.87 | 19 | 0.39 | 0.37 | 0.92 ± 0.63 | 0.75 ± 1.25 | 0.39 | 19 | 0.69 | −0.17 |
| IL-13 | 0.57 ± 0.42 | 0.30 ± 0.14 | 1.95 | 19 | 0.07 | 0.86 | 0.73 ± 0.57 | 0.35 ± 0.26 | 30 † | −1.95 ‡ | 0.05 *† | 0.85 |
| IFN-γ | 3.54 ± 1.76 | 2.64 ± 3.21 | 36 † | −133 ‡ | 0.19 † | 0.34 | 3.54 ± 1.76 | 2.64 ± 3.32 | 32 | −1.58 | 0.11 | 0.33 |
| TNF-α | 0.43 ± 0.0 | 0.43 ± 0.0 | 0.0 † | 18.9 ‡ | 1 † | 0.0 | 0.37 ± 0.13 | 0.44 ± 0.54 | −1.51 | 19 | 0.14 | 0.96 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cádiz-Gallardo, M.P.; Pradas, F.; Patanè, P.; García-Giménez, A.; Lecina, M.; Carrasco, L. Analysis of the Acute Cytokine Dynamics Induced in Professional Padel According to the Playing Side of the Court and Sex-Related Differences. Metabolites 2025, 15, 368. https://doi.org/10.3390/metabo15060368
Cádiz-Gallardo MP, Pradas F, Patanè P, García-Giménez A, Lecina M, Carrasco L. Analysis of the Acute Cytokine Dynamics Induced in Professional Padel According to the Playing Side of the Court and Sex-Related Differences. Metabolites. 2025; 15(6):368. https://doi.org/10.3390/metabo15060368
Chicago/Turabian StyleCádiz-Gallardo, María Pía, Francisco Pradas, Pamela Patanè, Alejandro García-Giménez, Miguel Lecina, and Luis Carrasco. 2025. "Analysis of the Acute Cytokine Dynamics Induced in Professional Padel According to the Playing Side of the Court and Sex-Related Differences" Metabolites 15, no. 6: 368. https://doi.org/10.3390/metabo15060368
APA StyleCádiz-Gallardo, M. P., Pradas, F., Patanè, P., García-Giménez, A., Lecina, M., & Carrasco, L. (2025). Analysis of the Acute Cytokine Dynamics Induced in Professional Padel According to the Playing Side of the Court and Sex-Related Differences. Metabolites, 15(6), 368. https://doi.org/10.3390/metabo15060368










