Effects of Exercise on Gut Microbiome and Serum Metabolomics in Post-Traumatic Osteoarthritis Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
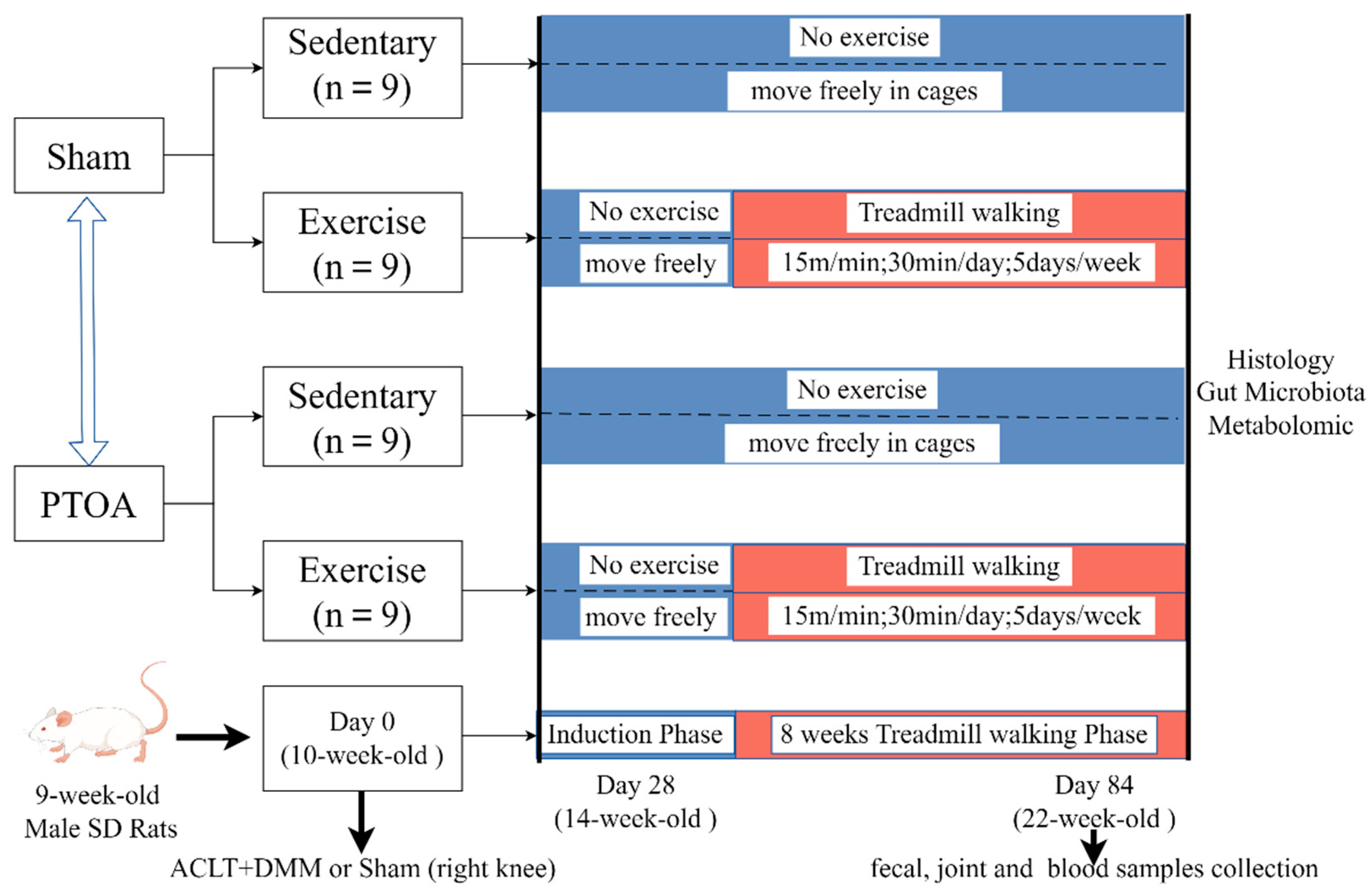
2.2. Study Design and Exercise Protocols
2.3. Micro-Computed Tomography (Micro-CT) Analysis of Subchondral Bone
2.4. Histological Analysis of Articular Cartilage
2.5. DNA Extraction, 16S rRNA Amplification, and Illumina Miseq Sequencing
2.6. Bioinformatics Analysis of Sequencing Data
2.7. Untargeted Metabolomic Profiling of Serum Samples
2.8. Statistical Analysis of Metabolite Profiles
2.9. Statistical Analysis
3. Results
3.1. Exercise Attenuates PTOA-Relevant Phenotypes of Cartilage–Subchondral Bone Unit
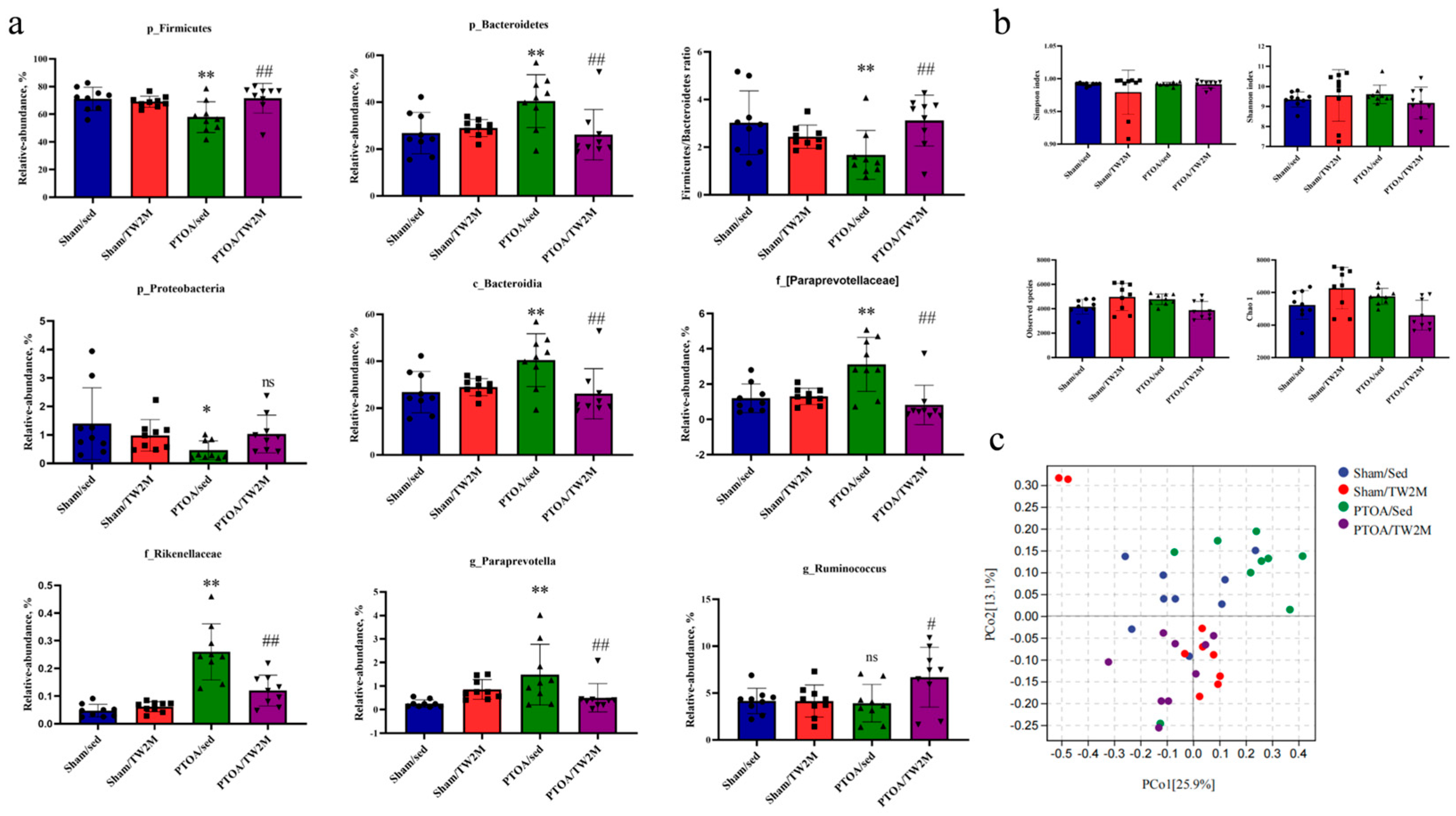
3.2. Exercise Modifies the PTOA-Relevant Gut Dysbiosis
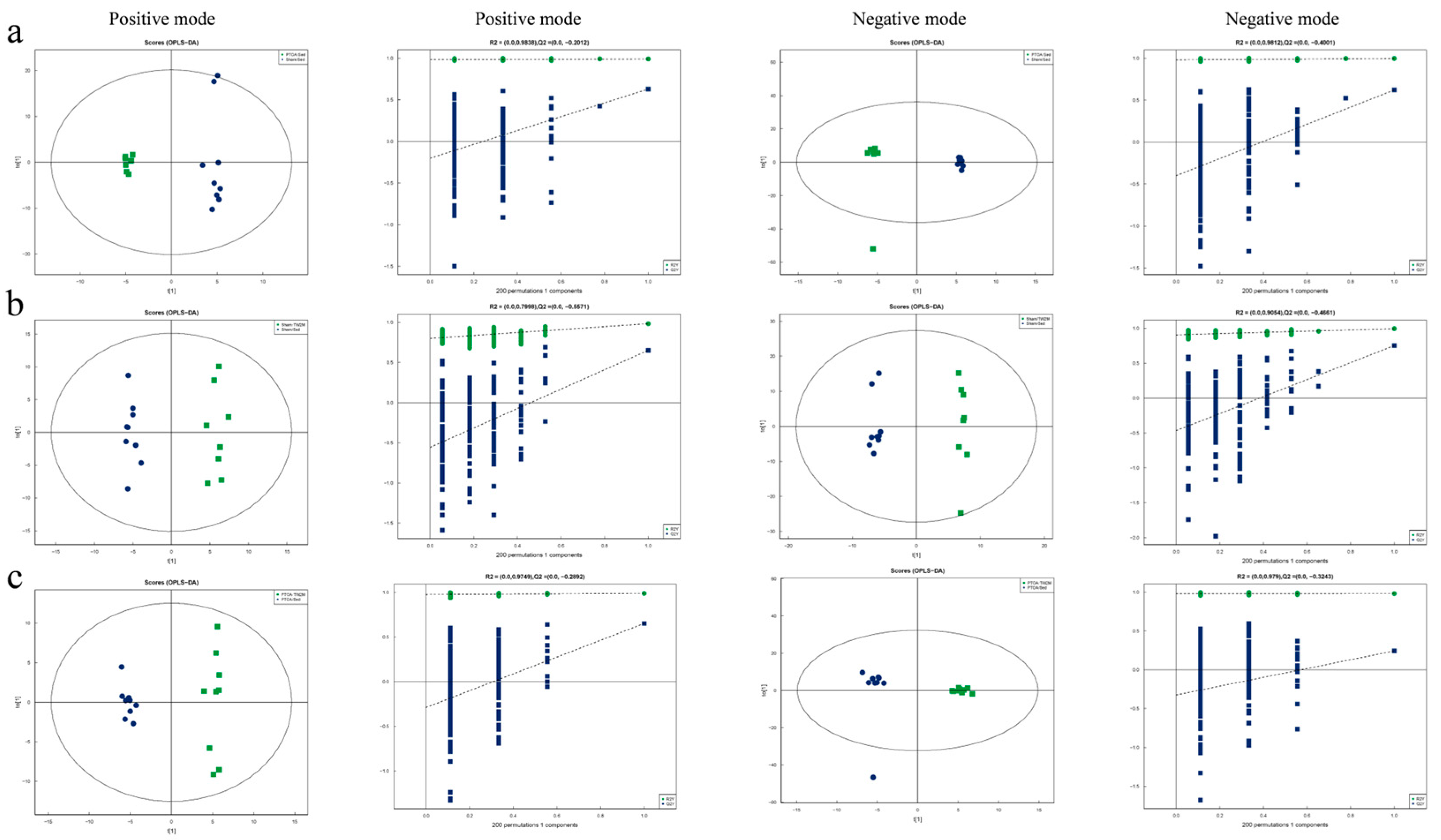
3.3. Exercise Modifies the PTOA-Relevant Serum Metabolic Profiles
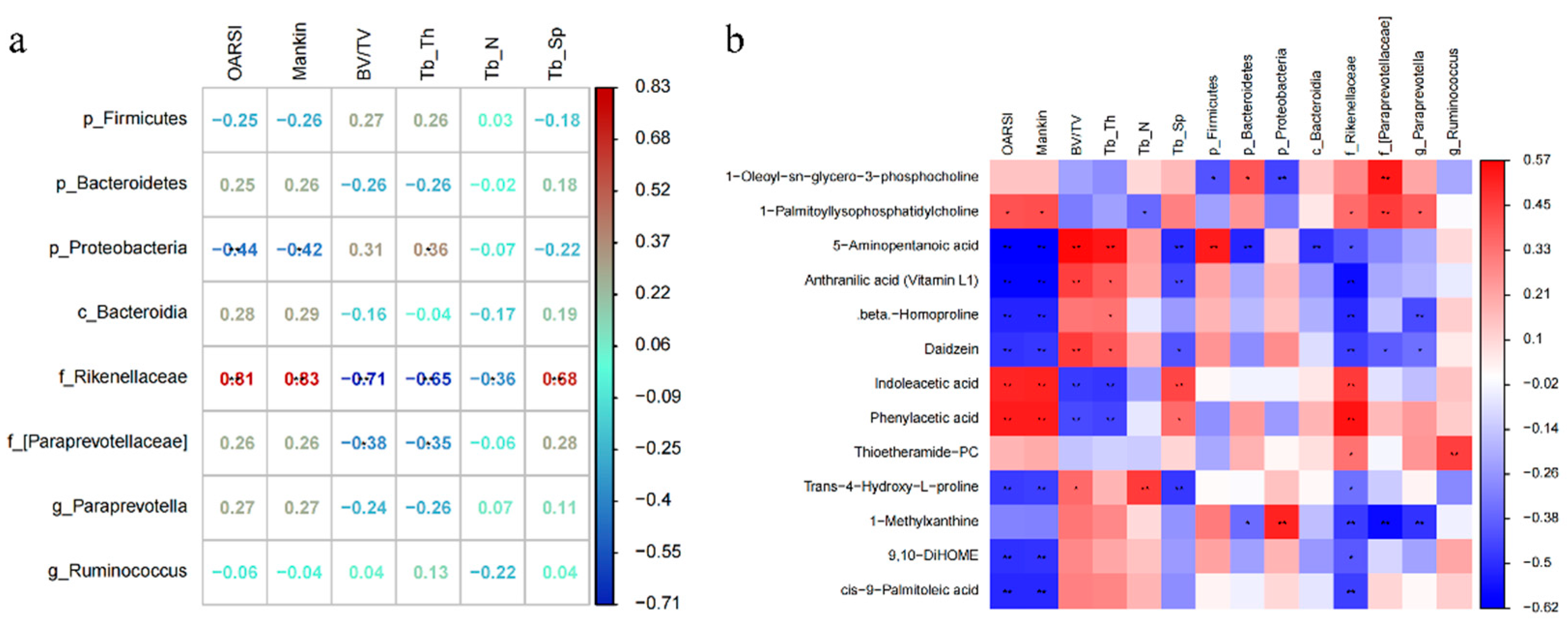
3.4. Exercise-Induced Changes in Serum Metabolism Are Related to the Integrity of Cartilage–Subchondral Bone Unit and Gut Microbiome
4. Discussion
5. Limitations
6. Conclusions and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Kraus, V.B.; Blanco, F.J.; Englund, M.; Karsdal, M.A.; Lohmander, L.S. Call for standardized definitions of osteoarthritis and risk stratification for clinical trials and clinical use. Osteoarthr. Cartil. 2015, 23, 1233–1241. [Google Scholar] [CrossRef] [PubMed]
- Long, H.; Liu, Q.; Yin, H.; Wang, K.; Diao, N.; Zhang, Y.; Lin, J.; Guo, A. Prevalence Trends of Site-Specific Osteoarthritis from 1990 to 2019: Findings from the Global Burden of Disease Study 2019. Arthritis Rheumatol. 2022, 74, 1172–1183. [Google Scholar] [CrossRef] [PubMed]
- Katz, J.N.; Arant, K.R.; Loeser, R.F. Diagnosis and Treatment of Hip and Knee Osteoarthritis: A Review. J. Am. Med. Assoc. 2021, 325, 568–578. [Google Scholar] [CrossRef] [PubMed]
- Robinson, W.H.; Lepus, C.M.; Wang, Q.; Raghu, H.; Mao, R.; Lindstrom, T.M.; Sokolove, J. Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 2016, 12, 580–592. [Google Scholar] [CrossRef]
- Chapman, J.H.; Ghosh, D.; Attari, S.; Ude, C.C.; Laurencin, C.T. Animal Models of Osteoarthritis: Updated Models and Outcome Measures 2016–2023. Regen. Eng. Transl. Med. 2024, 10, 127–146. [Google Scholar] [CrossRef]
- Lieberthal, J.; Sambamurthy, N.; Scanzello, C. Inflammation in joint injury and post-traumatic osteoarthritis. Osteoarthr. Cartil. 2015, 23, 1825–1834. [Google Scholar] [CrossRef]
- Buford, T.W. (Dis)Trust your gut: The gut microbiome in age-related inflammation, health, and disease. Microbiome 2017, 5, 80. [Google Scholar] [CrossRef]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef]
- Vallianou, N.; Stratigou, T.; Christodoulatos, G.S.; Dalamaga, M. Understanding the Role of the Gut Microbiome and Microbial Metabolites in Obesity and Obesity-Associated Metabolic Disorders: Current Evidence and Perspectives. Curr. Obes. Rep. 2019, 8, 317–332. [Google Scholar] [CrossRef]
- Lobionda, S.; Sittipo, P.; Kwon, H.Y.; Lee, Y.K. The Role of Gut Microbiota in Intestinal Inflammation with Respect to Diet and Extrinsic Stressors. Microorganisms 2019, 7, 271. [Google Scholar] [CrossRef]
- Liu, S.; Li, G.; Xu, H.; Wang, Q.; Wei, Y.; Yang, Q.; Xiong, A.; Yu, F.; Weng, J.; Zeng, H. “Cross-talk” between gut microbiome dysbiosis and osteoarthritis progression: A systematic review. Front. Immunol. 2023, 14, 1150572. [Google Scholar]
- Ulici, V.; Kelley, K.; Azcarate-Peril, M.; Cleveland, R.; Sartor, R.; Schwartz, T.; Loeser, R. Osteoarthritis induced by destabilization of the medial meniscus is reduced in germ-free mice. Osteoarthr. Cartil. 2018, 26, 1098–1109. [Google Scholar] [CrossRef] [PubMed]
- Guan, Z.; Jia, J.; Zhang, C.; Sun, T.; Zhang, W.; Yuan, W.; Leng, H.; Song, C. Gut microbiome dysbiosis alleviates the progression of osteoarthritis in mice. Clin. Sci. 2020, 134, 3159–3174. [Google Scholar] [CrossRef] [PubMed]
- Boer, C.G.; Radjabzadeh, D.; Medina-Gomez, C.; Garmaeva, S.; Schiphof, D.; Arp, P.; Koet, T.; Kurilshikov, A.; Fu, J.; Ikram, M.A.; et al. Intestinal microbiome composition and its relation to joint pain and inflammation. Nat. Commun. 2019, 10, 4881. [Google Scholar] [CrossRef]
- Liu, Y.; Ding, W.; Wang, H.; Dai, L.; Zong, W.; Wang, Y.; Bi, J.; Han, W.; Dong, G. Gut microbiota and obesity-associated osteoarthritis. Osteoarthr. Cartil. 2019, 27, 1257–1265. [Google Scholar] [CrossRef]
- Hao, X.; Shang, X.; Liu, J.; Chi, R.; Zhang, J.; Xu, T. The gut microbiota in osteoarthritis: Where do we stand and what can we do? Arthritis Res. Ther. 2021, 23, 42. [Google Scholar] [CrossRef]
- Fu, L.; Duan, H.; Cai, Y.; Chen, X.; Zou, B.; Yuan, L.; Liu, G. Moxibustion ameliorates osteoarthritis by regulating gut microbiota via impacting cAMP-related signaling pathway. Biomed. Pharmacother 2024, 170, 116031. [Google Scholar] [CrossRef]
- Rushing, B.; McRitchie, S.; Arbeeva, L.; Nelson, A.; Azcarate-Peril, M.; Li, Y.-Y.; Qian, Y.; Pathmasiri, W.; Sumner, S.; Loeser, R. Fecal metabolomics reveals products of dysregulated proteolysis and altered microbial metabolism in obesity-related osteoarthritis. Osteoarthr. Cartil. 2022, 30, 81–91. [Google Scholar] [CrossRef]
- Schott, E.M.; Farnsworth, C.W.; Grier, A.; Lillis, J.A.; Soniwala, S.; Dadourian, G.H.; Bell, R.D.; Doolittle, M.L.; Villani, D.A.; Awad, H.; et al. Targeting the gut microbiome to treat the osteoarthritis of obesity. J. Clin. Investig. 2018, 3, e95997. [Google Scholar] [CrossRef]
- Huang, Z.; Chen, J.; Li, B.; Zeng, B.; Chou, C.-H.; Zheng, X.; Xie, J.; Li, H.; Hao, Y.; Chen, G.; et al. Faecal microbiota transplantation from metabolically compromised human donors accelerates osteoarthritis in mice. Ann. Rheum. Dis. 2020, 79, 646–656. [Google Scholar] [CrossRef]
- Hao, X.; Zhang, J.; Shang, X.; Sun, K.; Zhou, J.; Liu, J.; Chi, R.; Xu, T. Exercise modifies the disease-relevant gut microbial shifts in post-traumatic osteoarthritis rats. Bone Jt. Res. 2022, 11, 214–225. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Xu, H.; Hao, X.; Liu, J.; Shang, X.; Xu, T. Effect of moderate exercise on osteoarthritis. EFORT Open Rev. 2023, 8, 148–161. [Google Scholar] [CrossRef] [PubMed]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020, 18, e3000410. [Google Scholar]
- Hao, X.; Wang, S.; Zhang, J.; Xu, T. Effects of body weight-supported treadmill training on cartilage-subchondral bone unit in the rat model of posttraumatic osteoarthritis. J. Orthop. Res. 2020, 39, 1227–1235. [Google Scholar] [CrossRef]
- Shang, X.; Hao, X.; Hou, W.; Liu, J.; Chi, R.; Deng, X.; Pan, C.; Xu, T. Exercise-induced modulation of myokine irisin on muscle-bone unit in the rat model of post-traumatic osteoarthritis. J. Orthop. Surg. Res. 2024, 19, 49. [Google Scholar] [CrossRef]
- Glasson, S.S.; Chambers, M.G.; Van Den Berg, W.B.; Little, C.B. The OARSI histopathology initiative—Recommendations for histological assessments of osteoarthritis in the mouse. Osteoarthr. Cartil. 2010, 18 (Suppl. 3), S17–S23. [Google Scholar] [CrossRef]
- Mankin, H.J.; Dorfman, H.; Lippiello, L.; Zarins, A. Biochemical and Metabolic Abnormalities in Articular Cartilage from Osteo-Arthritic Human Hips: II. Correlation of Morphology with Biochemical and Metabolic Data. J. Bone Jt. Surg. 1971, 53, 523–537. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, J.; Lv, Q.; Tan, Y.; Dong, X.; Liu, H.; Zhao, N.; He, Z.; Kou, Y.; Tan, Y.; et al. Establishment and resilience of transplanted gut microbiota in aged mice. iScience 2022, 25, 103654. [Google Scholar] [CrossRef]
- DeSantis, T.Z.; Hugenholtz, P.; Larsen, N.; Rojas, M.; Brodie, E.L.; Keller, K.; Huber, T.; Dalevi, D.; Hu, P.; Andersen, G.L. Greengenes, a Chimera-Checked 16S rRNA Gene Database and Workbench Compatible with ARB. Appl. Env. Microbiol. 2006, 72, 5069–5072. [Google Scholar] [CrossRef]
- Ma, N.; Yang, Y.; Liu, X.; Li, S.; Qin, Z.; Li, J. Plasma metabonomics and proteomics studies on the anti-thrombosis mechanism of aspirin eugenol ester in rat tail thrombosis model. J. Proteom. 2020, 215, 103631. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zheng, Y.; Liu, Z.; Su, M.; Wu, Z.; Zhang, H.; Zhang, C.; Xu, X. Insights into characteristic metabolites and potential bioactive peptides profiles of fresh cheese fermented with three novel probiotics based metabolomics and peptidomics. Food Chem. X 2024, 21, 101147. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Liu, Z.; Gong, L. Systematic evaluation of sample preparation strategy for GC-MS-based plasma metabolomics and its application in osteoarthritis. Anal. Biochem. 2021, 621, 114153. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.-M.; Zeng, S.-L.; Huang, T.-Q.; Lin, Y.; Wang, F.-F.; Gao, X.-J.; Li, J.; Li, P.; Liu, E.-H. Sinomenine ameliorates rheumatoid arthritis by modulating tryptophan metabolism and activating aryl hydrocarbon receptor via gut microbiota regulation. Sci. Bull. 2023, 68, 1540–1555. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kwon, Y.M.; Kim, I.S.; Kim, J.A.; Yu, D.Y.; Adhikari, B.; Lee, S.S.; Choi, I.S.; Cho, K.K. Effects of the Brown Seaweed Laminaria japonica Supplementation on Serum Concentrations of IgG, Triglycerides, and Cholesterol, and Intestinal Microbiota Composition in Rats. Front. Nutr. 2018, 5, 23. [Google Scholar] [CrossRef]
- Ozaki, D.; Kubota, R.; Maeno, T.; Abdelhakim, M.; Hitosugi, N. Association between gut microbiota, bone metabolism, and fracture risk in postmenopausal Japanese women. Osteoporos. Int. 2021, 32, 145–156. [Google Scholar] [CrossRef]
- Daniel, H.; Gholami, A.M.; Berry, D.; Desmarchelier, C.; Hahne, H.; Loh, G.; Mondot, S.; Lepage, P.; Rothballer, M.; Walker, A.; et al. High-fat diet alters gut microbiota physiology in mice. ISME J. 2013, 8, 295–308. [Google Scholar] [CrossRef]
- Prinz, E.; Schlupp, L.; Dyson, G.; Barrett, M.; Szymczak, A.; Velasco, C.; Izda, V.; Dunn, C.M.; A Jeffries, M. OA susceptibility in mice is partially mediated by the gut microbiome, is transferrable via microbiome transplantation and is associated with immunophenotype changes. Ann. Rheum. Dis. 2024, 83, 382–393. [Google Scholar] [CrossRef]
- Tang, J.; Song, X.; Zhao, M.; Chen, H.; Wang, Y.; Zhao, B.; Yu, S.; Ma, T.; Gao, L. Oral administration of live combined Bacillus subtilis and Enterococcus faecium alleviates colonic oxidative stress and inflammation in osteoarthritic rats by improving fecal microbiome metabolism and enhancing the colonic barrier. Front. Microbiol. 2022, 13, 1005842. [Google Scholar] [CrossRef]
- Şenol, H.; Çağman, Z.; Katmerlikaya, T.G.; Tokalı, F.S. New Anthranilic Acid Hydrazones as Fenamate Isosteres: Synthesis, Characterization, Molecular Docking, Dynamics & in Silico ADME, in Vitro Anti-Inflammatory and Anticancer Activity Studies. Chem. Biodivers. 2023, 20, e202300773. [Google Scholar]
- Rosser, E.C.; Piper, C.J.; Matei, D.E.; Blair, P.A.; Rendeiro, A.F.; Orford, M.; Alber, D.G.; Krausgruber, T.; Catalan, D.; Klein, N.; et al. Microbiota-Derived Metabolites Suppress Arthritis by Amplifying Aryl-Hydrocarbon Receptor Activation in Regulatory B Cells. Cell Metab. 2020, 31, 837–851. [Google Scholar] [CrossRef]
- Binvignat, M.; Emond, P.; Mifsud, F.; Miao, B.; Courties, A.; Lefèvre, A.; Maheu, E.; Crema, M.D.; Klatzmann, D.; Kloppenburg, M.; et al. Serum tryptophan metabolites are associated with erosive hand osteoarthritis and pain: Results from the DIGICOD cohort. Osteoarthr. Cartil. 2023, 31, 1132–1143. [Google Scholar] [CrossRef] [PubMed]
- Kassab, R.B.; Elhenawy, A.A.; Theyab, A.; Hawsawi, Y.M.; Al-Amer, O.M.; Oyouni, A.A.A.; Habotta, O.A.; Althagafi, H.A.; Alharthi, F.; Lokman, M.S.; et al. Modulation of inflammatory, oxidative, and apoptotic stresses mediates the renoprotective effect of daidzein against glycerol-induced acute kidney injury in rats. Environ. Sci. Pollut. Res. 2023, 30, 119016–119033. [Google Scholar] [CrossRef] [PubMed]
- Gundogdu, G.; Miloglu, F.D.; Gundogdu, K.; Tasci, S.Y.; Albayrak, M.; Demirci, T.; Cetin, M. Investigation of the efficacy of daidzein in experimental knee osteoarthritis-induced with monosodium iodoacetate in rats. Clin. Rheumatol. 2020, 39, 2399–2408. [Google Scholar] [CrossRef]
- Kang, K.Y.; Lee, S.H.; Jung, S.M.; Park, S.-H.; Jung, B.-H.; Ju, J.H. Downregulation of Tryptophan-related Metabolomic Profile in Rheumatoid Arthritis Synovial Fluid. J. Rheumatol. 2015, 42, 2003–2011. [Google Scholar] [CrossRef]
- Ekeuku, S.O.; Tan, J.-K.; Al-Saadi, H.M.; Ahmad, F.; Suhana, M.R.E.; Arlamsyah, A.M.; Sidik, F.Z.J.; Hamid, J.A.; Ima-Nirwana, S.; Chin, K.-Y. Serum Metabolomic Alteration in Rats with Osteoarthritis Treated with Palm Tocotrienol-Rich Fraction Alone or in Combination with Glucosamine Sulphate. Life 2023, 13, 2343. [Google Scholar] [CrossRef]
- Zhai, G.; Sun, X.; Randell, E.W.; Liu, M.; Wang, N.; Tolstykh, I.; Rahman, P.; Torner, J.; Lewis, C.E.; Nevitt, M.C.; et al. Phenylalanine Is a Novel Marker for Radiographic Knee Osteoarthritis Progression: The MOST Study. J. Rheumatol. 2021, 48, 123–128. [Google Scholar] [CrossRef]
- Riederer, M.; Lechleitner, M.; Hrzenjak, A.; Koefeler, H.; Desoye, G.; Heinemann, A.; Frank, S. Endothelial lipase (EL) and EL-generated lysophosphatidylcholines promote IL-8 expression in endothelial cells. Atherosclerosis 2011, 214, 338–344. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, H.; Xie, Z.; Huang, Y.; Sun, G.; Qi, D.; Furey, A.; Randell, E.W.; Rahman, P.; Zhai, G. Glutathione, polyamine, and lysophosphatidylcholine synthesis pathways are associated with circulating pro-inflammatory cytokines. Metabolomics 2022, 18, 76. [Google Scholar] [CrossRef]
- Zhang, W.; Sun, G.; Aitken, D.; Likhodii, S.; Liu, M.; Martin, G.; Furey, A.; Randell, E.; Rahman, P.; Jones, G.; et al. Lysophosphatidylcholines to phosphatidylcholines ratio predicts advanced knee osteoarthritis. Rheumatology 2016, 55, 1566–1574. [Google Scholar] [CrossRef]
- Nguyen, T.L.; Vieira-Silva, S.; Liston, A.; Raes, J. How informative is the mouse for human gut microbiota research? Dis. Model Mech. 2015, 8, 1–16. [Google Scholar] [CrossRef]



| Comparative Group | Metabolites | VIP | Fold Change | p-Value |
|---|---|---|---|---|
| PTOA/Sed versus Sham/Sed | beta-Homoproline | 2.400925861 | 0.576463827 | 0.001 |
| Daidzein | 1.939621477 | 0.32599906 | 0.001 | |
| Phenylacetic acid | 1.691965753 | 1.253189416 | 0.002 | |
| 5-Aminopentanoic acid | 1.515618194 | 0.539227609 | 0.003 | |
| Anthranilic acid (Vitamin L1) | 6.114384325 | 0.579288897 | 0.003 | |
| L-Anserine | 3.448507904 | 0.485224903 | 0.019 | |
| 1-Palmitoyllysophosphatidylcholine | 3.241282054 | 1.150208786 | 0.026 | |
| Trimethylamine N-oxide | 2.238067382 | 0.674405948 | 0.036 | |
| L-Methionine | 1.426522831 | 0.747339917 | 0.036 | |
| 7-Oxocholesterol | 1.109913427 | 0.727777146 | 0.040 | |
| Thioetheramide-PC | 16.14239843 | 1.422210189 | 0.041 | |
| Isobutyrylglycine | 1.429748753 | 1.632478727 | 0.043 | |
| Indoleacetic acid | 1.64116589 | 1.486730841 | 0.044 | |
| Thr-Met | 1.198055767 | 0.367348409 | 0.046 | |
| Sham/TW2M versus Sham/Sed | Caproic acid | 2.322510891 | 3.147297216 | 0.001 |
| Trans-4-Hydroxy-L-proline | 1.722552698 | 8.129422426 | 0.001 | |
| 3-Hydroxyisovaleric acid | 1.1778371 | 0.127366318 | 0.001 | |
| 1-Myristoyl-sn-glycero-3-phosphocholine | 5.221306055 | 0.673725935 | 0.005 | |
| Phe-Tyr | 1.071113318 | 2.505113841 | 0.005 | |
| D-Mannose | 1.552197047 | 1.396176604 | 0.018 | |
| Indoleacetic acid | 1.155273074 | 0.661480492 | 0.027 | |
| beta-Homoproline | 1.780179794 | 0.699808117 | 0.035 | |
| PTOA/TW2M versus PTOA/Sed | Daidzein | 1.567454134 | 2.695336031 | 0.001 |
| L-Carnosine | 1.372057913 | 2.361096252 | 0.003 | |
| L-Anserine | 6.373354859 | 3.939618039 | 0.005 | |
| Isobutyrylglycine | 1.578019604 | 0.501190958 | 0.012 | |
| Sphinganine | 1.018051966 | 1.602814019 | 0.013 | |
| Trimethylamine N-oxide | 1.98858132 | 1.48755549 | 0.015 | |
| Anthranilic acid (Vitamin L1) | 3.914777318 | 1.449357496 | 0.016 | |
| 1-Oleoyl-sn-glycero-3-phosphocholine | 12.9633525 | 0.851623797 | 0.016 | |
| 3-Methylhistidine | 1.58895438 | 0.834093177 | 0.017 | |
| 1-Palmitoyllysophosphatidylcholine | 3.387267701 | 0.852708007 | 0.033 |
| Comparative Group | Metabolites | VIP | Fold Change | p-Value |
|---|---|---|---|---|
| PTOA/Sed versus Sham/Sed | 1-Oleoyl-L-.alpha.-lysophosphatidic acid | 2.278466783 | 0.55020154 | 0.001 |
| Coumestrol | 2.704798442 | 0.304959452 | 0.002 | |
| cis-9-Palmitoleic acid | 6.569765019 | 0.535145129 | 0.018 | |
| 9,10-DiHOME | 1.610698643 | 0.28558991 | 0.028 | |
| Sham/TW2M versus Sham/Sed | 3-Methoxy-4-hydroxyphenylglycol sulfate | 1.561900233 | 1.795819177 | 0.004 |
| 1-Oleoyl-L-.alpha.-lysophosphatidic acid | 2.087100203 | 0.585488284 | 0.009 | |
| Alpha-D-glucose | 1.062456392 | 1.580477716 | 0.012 | |
| D-Threitol | 1.560157863 | 1.361687968 | 0.042 | |
| PTOA/TW2M versus PTOA/Sed | Coumestrol | 2.781664503 | 3.25340615 | 0.002 |
| Glyceric acid | 1.515536125 | 1.756441364 | 0.005 | |
| D-Ornithine | 2.626868222 | 1.404355261 | 0.023 | |
| Palmitic acid | 16.40253911 | 1.458633594 | 0.024 | |
| 1-Methylxanthine | 1.558013091 | 3.327696176 | 0.029 | |
| 3-Methoxy-4-hydroxyphenylglycol sulfate | 1.011534776 | 1.446836056 | 0.036 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, X.; Shang, X.; Zhang, Y.; Hou, W.; Chi, R.; Pan, C.; Liu, J.; Deng, X.; Zhang, J.; Xu, T. Effects of Exercise on Gut Microbiome and Serum Metabolomics in Post-Traumatic Osteoarthritis Rats. Metabolites 2025, 15, 341. https://doi.org/10.3390/metabo15050341
Hao X, Shang X, Zhang Y, Hou W, Chi R, Pan C, Liu J, Deng X, Zhang J, Xu T. Effects of Exercise on Gut Microbiome and Serum Metabolomics in Post-Traumatic Osteoarthritis Rats. Metabolites. 2025; 15(5):341. https://doi.org/10.3390/metabo15050341
Chicago/Turabian StyleHao, Xiaoxia, Xingru Shang, Yiwen Zhang, Wenjie Hou, Ruimin Chi, Chunran Pan, Jiawei Liu, Xiaofeng Deng, Jiaming Zhang, and Tao Xu. 2025. "Effects of Exercise on Gut Microbiome and Serum Metabolomics in Post-Traumatic Osteoarthritis Rats" Metabolites 15, no. 5: 341. https://doi.org/10.3390/metabo15050341
APA StyleHao, X., Shang, X., Zhang, Y., Hou, W., Chi, R., Pan, C., Liu, J., Deng, X., Zhang, J., & Xu, T. (2025). Effects of Exercise on Gut Microbiome and Serum Metabolomics in Post-Traumatic Osteoarthritis Rats. Metabolites, 15(5), 341. https://doi.org/10.3390/metabo15050341






