A Data-Driven Approach to Link GC-MS and LC-MS with Sensory Attributes of Chicken Bouillon with Added Yeast-Derived Flavor Products in a Combined Prediction Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Food Materials and Bouillon Preparation
2.2. Sensory Evaluation
2.3. Untargeted Volatile Analysis
2.4. Untargeted and Targeted Non-Volatile Analysis
2.5. Variable Selection and Predictive Models
3. Results
3.1. Discriminative Performance of the Sensory Panel
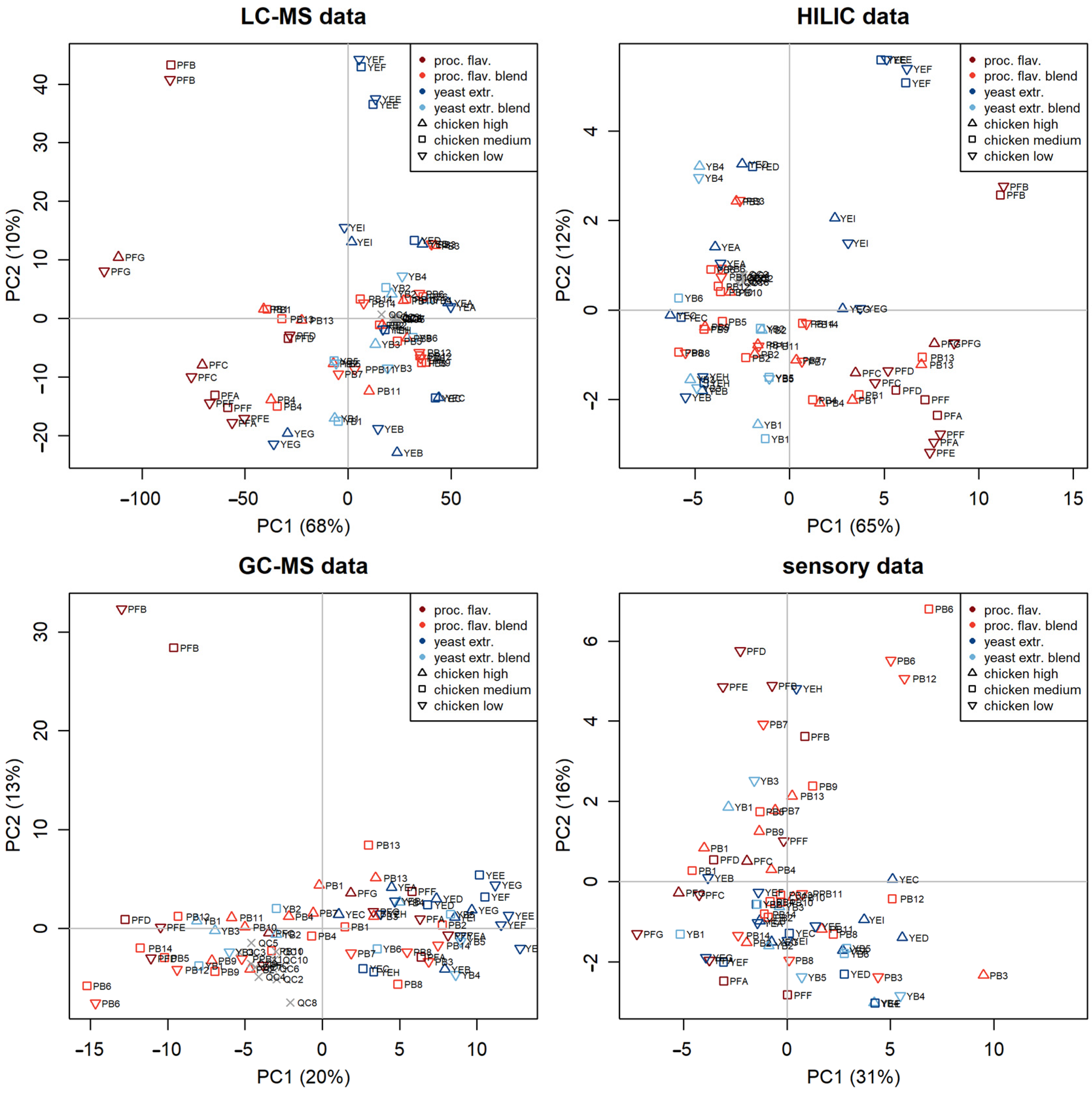
3.2. Principal Component Analysis Quality Assurance
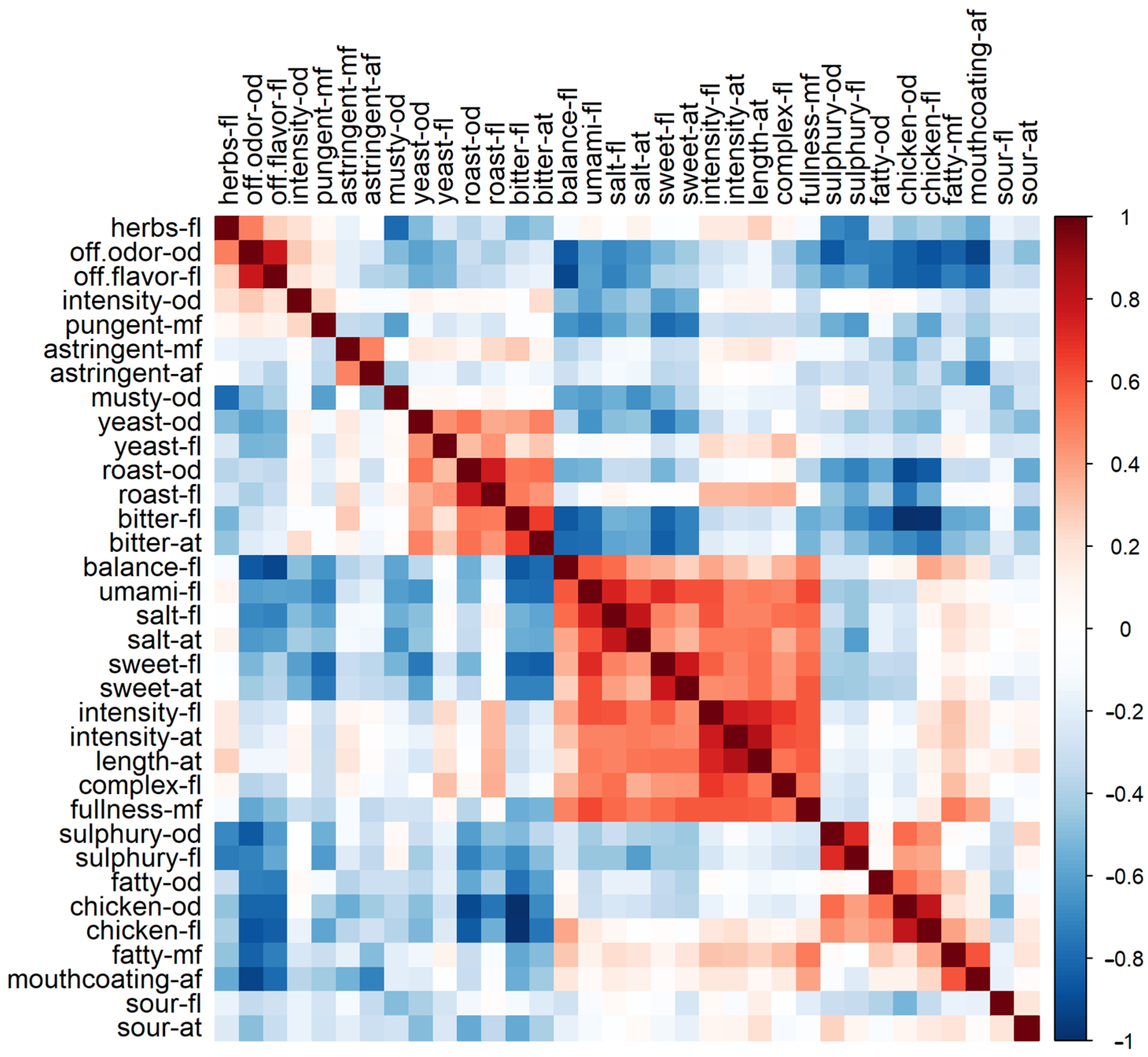
3.3. Pearson Correlation of Taste Attributes
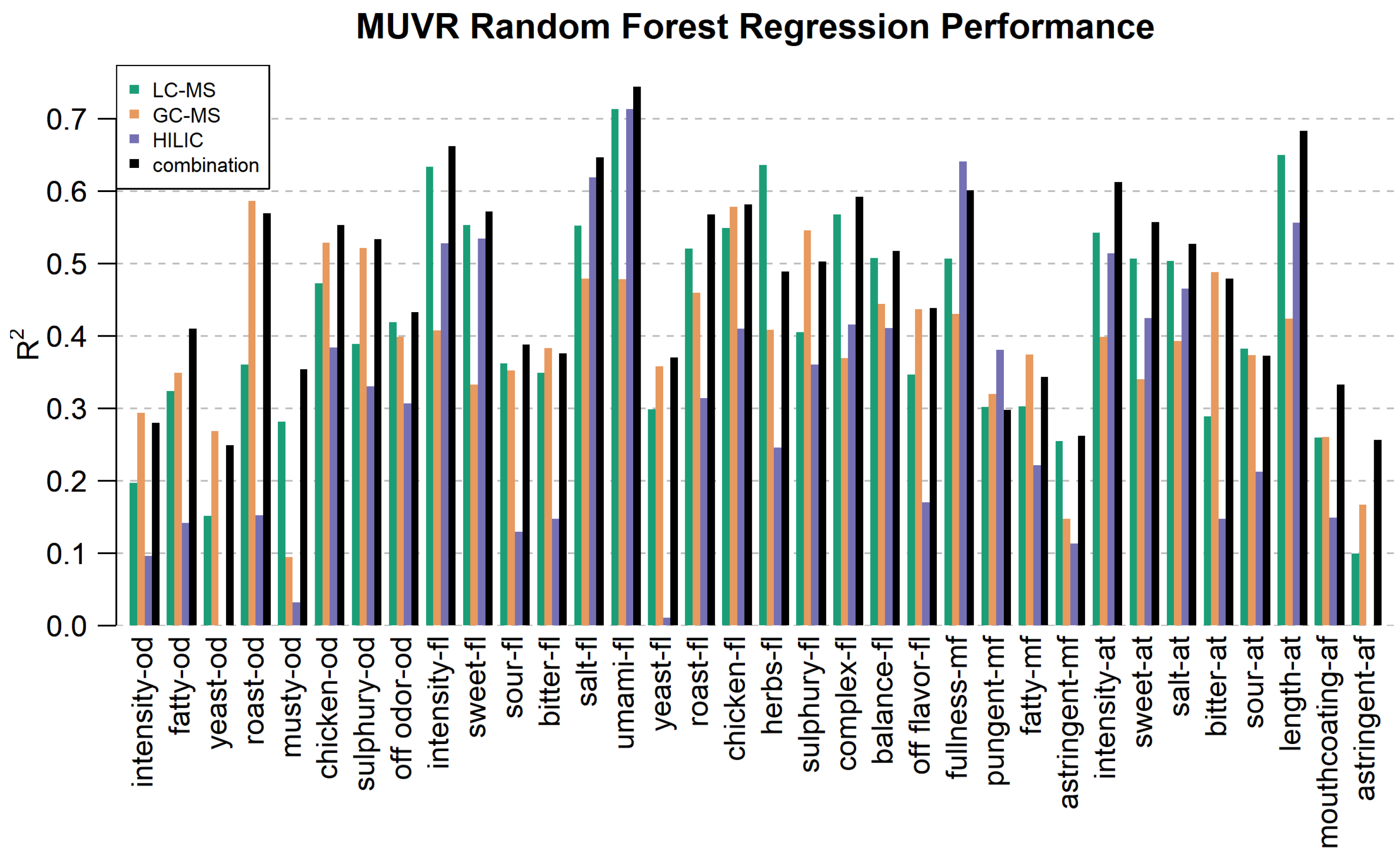
3.4. Random Forest Regression Performance—Modeling the Link Between Sensory and Chemical Profiles
3.5. Importance of Volatile and Non-Volatile Variables per Sensory Attribute
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jaeger, H.; Janositz, A.; Knorr, D. The Maillard Reaction and Its Control during Food Processing. The Potential of Emerging Technologies. Pathol. Biol. 2010, 58, 207–213. [Google Scholar] [CrossRef]
- Diez-Simon, C.; Mumm, R.; Hall, R.D. Mass Spectrometry-Based Metabolomics of Volatiles as a New Tool for Understanding Aroma and Flavour Chemistry in Processed Food Products. Metabolomics 2019, 15, 41. [Google Scholar] [CrossRef]
- Chambers IV, E.; Koppel, K. Associations of Volatile Compounds with Sensory Aroma and Flavor: The Complex Nature of Flavor. Molecules 2013, 18, 4887–4905. [Google Scholar] [CrossRef] [PubMed]
- McKay, M.; Bauer, F.F.; Panzeri, V.; Mokwena, L.; Buica, A. Profiling Potentially Smoke Tainted Red Wines: Volatile Phenols and Aroma Attributes. S. Afr. J. Enol. Vitic. 2019, 40, 221–236. [Google Scholar] [CrossRef]
- Biospringer Benefits of Yeast Extract. Available online: https://biospringer.com/en/benefits-of-yeast-extract/ (accessed on 1 July 2024).
- Jayasena, D.D.; Ahn, D.U.; Nam, K.C.; Jo, C. Flavour Chemistry of Chicken Meat: A Review. Asian-Australas. J. Anim. Sci. 2013, 26, 732–742. [Google Scholar] [CrossRef] [PubMed]
- Aliani, M.; Farmer, L.J. Precursors of Chicken Flavor. II. Identification of Key Flavor Precursors Using Sensory Methods. J. Agric. Food Chem. 2005, 53, 6455–6462. [Google Scholar] [CrossRef]
- Gromski, P.S.; Muhamadali, H.; Ellis, D.I.; Xu, Y.; Correa, E.; Turner, M.L.; Goodacre, R. A Tutorial Review: Metabolomics and Partial Least Squares-Discriminant Analysis—A Marriage of Convenience or a Shotgun Wedding. Anal. Chim. Acta 2015, 879, 10–23. [Google Scholar] [CrossRef]
- Shi, L.; Westerhuis, J.A.; Rosén, J.; Landberg, R.; Brunius, C. Variable Selection and Validation in Multivariate Modelling. Bioinformatics 2019, 35, 972–980. [Google Scholar] [CrossRef]
- Leygeber, S.; Grossmann, J.L.; Diez-Simon, C.; Karu, N.; Dubbelman, A.-C.; Harms, A.C.; Westerhuis, J.A.; Jacobs, D.M.; Lindenburg, P.W.; Hendriks, M.M.W.B.; et al. Flavor Profiling Using Comprehensive Mass Spectrometry Analysis of Metabolites in Tomato Soups. Metabolites 2022, 12, 1194. [Google Scholar] [CrossRef]
- ISO 6658:2017; Sensory Analysis—Methodology—General Guidance. International Organization for Standardization: Geneva, Switzerland, 2017. Available online: https://www.iso.org/standard/65519.html (accessed on 7 April 2025).
- Stone, H.; Sidel, J.L. Sensory Evaluation Practices, 3rd ed.; Academic Press: Cambridge, MA, USA, 2004; ISBN 978-0-08-047435-9. [Google Scholar]
- Romano, R.; Brockhoff, P.B.; Hersleth, M.; Tomic, O.; Næs, T. Correcting for Different Use of the Scale and the Need for Further Analysis of Individual Differences in Sensory Analysis. Food Qual. Prefer. 2008, 19, 197–209. [Google Scholar] [CrossRef]
- Großmann, J.L.; Westerhuis, J.A.; Næs, T.; Smilde, A.K. Critical Evaluation of Assessor Difference Correction Approaches in Sensory Analysis. Food Qual. Prefer. 2023, 106, 104792. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using Lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. lmerTest Package: Tests in Linear Mixed Effects Models. J. Stat. Softw. 2017, 82, 1–26. [Google Scholar] [CrossRef]
- Diez-Simon, C.; Ammerlaan, B.; van den Berg, M.; van Duynhoven, J.; Jacobs, D.; Mumm, R.; Hall, R.D. Comparison of Volatile Trapping Techniques for the Comprehensive Analysis of Food Flavourings by Gas Chromatography-Mass Spectrometry. J. Chromatogr. A 2020, 1624, 461191. [Google Scholar] [CrossRef]
- Sumner, L.W.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Fan, T.W.M.; Fiehn, O.; Goodacre, R.; Griffin, J.L.; et al. Proposed Minimum Reporting Standards for Chemical Analysis: Chemical Analysis Working Group (CAWG) Metabolomics Standards Initiative (MSI). Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef]
- Chambers, M.C.; Maclean, B.; Burke, R.; Amodei, D.; Ruderman, D.L.; Neumann, S.; Gatto, L.; Fischer, B.; Pratt, B.; Egertson, J.; et al. A Cross-Platform Toolkit for Mass Spectrometry and Proteomics. Nat. Biotechnol. 2012, 30, 918–920. [Google Scholar] [CrossRef] [PubMed]
- Gatto, L.; Gibb, S.; Rainer, J. MSnbase, Efficient and Elegant R-Based Processing and Visualization of Raw Mass Spectrometry Data. J. Proteome Res. 2021, 20, 1063–1069. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; Available online: https://www.R-project.org/ (accessed on 26 July 2023).
- Smith, C.A.; Want, E.J.; O’Maille, G.; Abagyan, R.; Siuzdak, G. XCMS: Processing Mass Spectrometry Data for Metabolite Profiling Using Nonlinear Peak Alignment, Matching, and Identification. Anal. Chem. 2006, 78, 779–787. [Google Scholar] [CrossRef]
- Kuhl, C.; Tautenhahn, R.; Böttcher, C.; Larson, T.R.; Neumann, S. CAMERA: An Integrated Strategy for Compound Spectra Extraction and Annotation of Liquid Chromatography/Mass Spectrometry Data Sets. Anal. Chem. 2012, 84, 283–289. [Google Scholar] [CrossRef]
- Filzmoser, P.; Liebmann, B.; Varmuza, K. Repeated Double Cross Validation. J. Chemom. 2009, 23, 160–171. [Google Scholar] [CrossRef]
- Pittari, E.; Moio, L.; Piombino, P. Interactions between Polyphenols and Volatile Compounds in Wine: A Literature Review on Physicochemical and Sensory Insights. Appl. Sci. 2021, 11, 1157. [Google Scholar] [CrossRef]
- Choi, S.M.; Lee, D.J.; Kim, J.Y.; Lim, S.T. Volatile Composition and Sensory Characteristics of Onion Powders Prepared by Convective Drying. Food Chem. 2017, 231, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Kamani, M.H.; Meera, M.S.; Bhaskar, N.; Modi, V.K. Partial and Total Replacement of Meat by Plant-Based Proteins in Chicken Sausage: Evaluation of Mechanical, Physico-Chemical and Sensory Characteristics. J. Food Sci. Technol. 2019, 56, 2660–2669. [Google Scholar] [CrossRef]
- De Toffoli, A.; Monteleone, E.; Bucalossi, G.; Veneziani, G.; Fia, G.; Servili, M.; Zanoni, B.; Pagliarini, E.; Gallina Toschi, T.; Dinnella, C. Sensory and Chemical Profile of a Phenolic Extract from Olive Mill Waste Waters in Plant-Base Food with Varied Macro-Composition. Food Res. Int. 2019, 119, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Seisonen, S.; Vene, K.; Koppel, K. The Current Practice in the Application of Chemometrics for Correlation of Sensory and Gas Chromatographic Data. Food Chem. 2016, 210, 530–540. [Google Scholar] [CrossRef]
- Pattee, H.E.; Isleib, T.G.; Giesbrecht, F.G.; McFeeters, R.F. Relationships of Sweet, Bitter, and Roasted Peanut Sensory Attributes with Carbohydrate Components in Peanuts. J. Agric. Food Chem. 2000, 48, 757–763. [Google Scholar] [CrossRef]
- Pattee, H.E.; Isleib, T.G.; Giesbrecht, F.G. Variation in Intensity of Sweet and Bitter Sensory Attributes Across Peanut Genotypes1. Peanut Sci. 1998, 25, 63–69. [Google Scholar] [CrossRef]
- Onuma, T.; Maruyama, H.; Sakai, N. Enhancement of Saltiness Perception by Monosodium Glutamate Taste and Soy Sauce Odor: A near-Infrared Spectroscopy Study. Chem. Senses 2018, 43, 151–167. [Google Scholar] [CrossRef]
- Li, X.; Staszewski, L.; Xu, H.; Durick, K.; Zoller, M.; Adler, E. Human Receptors for Sweet and Umami Taste. Proc. Natl. Acad. Sci. USA 2002, 99, 4692–4696. [Google Scholar] [CrossRef]
- Shim, J.; Son, H.J.; Kim, Y.; Kim, K.H.; Kim, J.T.; Moon, H.; Kim, M.J.; Misaka, T.; Rhyu, M.R. Modulation of Sweet Taste by Umami Compounds via Sweet Taste Receptor. PLoS ONE 2015, 10, e0124030. [Google Scholar] [CrossRef]
- Maruyama, Y.; Yasuda, R.; Kuroda, M.; Eto, Y. Kokumi Substances, Enhancers of Basic Tastes, Induce Responses in Calcium-Sensing Receptor Expressing Taste Cells. PLoS ONE 2012, 7, e34489. [Google Scholar] [CrossRef] [PubMed]
- Mebazaa, R.; Mahmoudi, A.; Fouchet, M.; Dos Santos, M.; Kamissoko, F.; Nafti, A.; Ben Cheikh, R.; Rega, B.; Camel, V. Characterisation of Volatile Compounds in Tunisian Fenugreek Seeds. Food Chem. 2009, 115, 1326–1336. [Google Scholar] [CrossRef]
- Shahidi, F.; Rubin, L.J.; D’Souza, L.A. Meat Flavor Volatiles: A Review of the Composition, Techniques of Analysis, and Sensory Evaluation. C R C Crit. Rev. Food Sci. Nutr. 1986, 24, 141–243. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Na Jom, K.; Ge, Y. Influence of Roasting Condition on Flavor Profile of Sunflower Seeds: A Flavoromics Approach. Sci. Rep. 2019, 9, 11295. [Google Scholar] [CrossRef]
- Cuicui, L.; Lixia, H. Review on Volatile Flavor Components of Roasted Oilseeds and Their Products. Grain Oil Sci. Technol. 2018, 1, 151–156. [Google Scholar] [CrossRef]
- Yu, A.-N.; Tan, Z.-W.; Shi, B.-A. Influence of the pH on the Formation of Pyrazine Compounds by the Maillard Reaction of L-Ascorbic Acid with Acidic, Basic and Neutral Amino Acids. Asia-Pac. J. Chem. Eng. 2011, 7, 455–462. [Google Scholar] [CrossRef]
- Nishimura, T.; Goto, S.; Miura, K.; Takakura, Y.; Egusa, A.S.; Wakabayashi, H. Umami Compounds Enhance the Intensity of Retronasal Sensation of Aromas from Model Chicken Soups. Food Chem. 2016, 196, 577–583. [Google Scholar] [CrossRef]
- Sun, A.; Wu, W.; Soladoye, O.P.; Aluko, R.E.; Bak, K.H.; Fu, Y.; Zhang, Y. Maillard Reaction of Food-Derived Peptides as a Potential Route to Generate Meat Flavor Compounds: A Review. Food Res. Int. 2022, 151, 110823. [Google Scholar] [CrossRef]
- Chua, L.S.; Abdullah, F.I.; Lim, S.H. Physiochemical Changes and Nutritional Content of Black Garlic during Fermentation. Appl. Food Res. 2022, 2, 100216. [Google Scholar] [CrossRef]
- Yamamoto, T.; Inui-Yamamoto, C. The Flavor-Enhancing Action of Glutamate and Its Mechanism Involving the Notion of Kokumi. NPJ Sci. Food 2023, 7, 3. [Google Scholar] [CrossRef]
- Diepeveen, J.; Moerdijk-Poortvliet, T.C.W.; van der Leij, F.R. Molecular Insights into Human Taste Perception and Umami Tastants: A Review. J. Food Sci. 2022, 87, 1449–1465. [Google Scholar] [CrossRef] [PubMed]
- Rolls, E.T.; Critchley, H.D.; Browning, A.; Hernadi, I. The Neurophysiology of Taste and Olfaction in Primates, and Umami Flavor. Ann. N. Y. Acad. Sci. 1998, 855, 426–437. [Google Scholar] [CrossRef] [PubMed]
- Shibata, M.; Hirotsuka, M.; Mizutani, Y.; Takahashi, H.; Kawada, T.; Matsumiya, K.; Hayashi, Y.; Matsumura, Y. Isolation and Characterization of Key Contributors to the “Kokumi” Taste in Soybean Seeds. Biosci. Biotechnol. Biochem. 2017, 81, 2168–2177. [Google Scholar] [CrossRef] [PubMed]
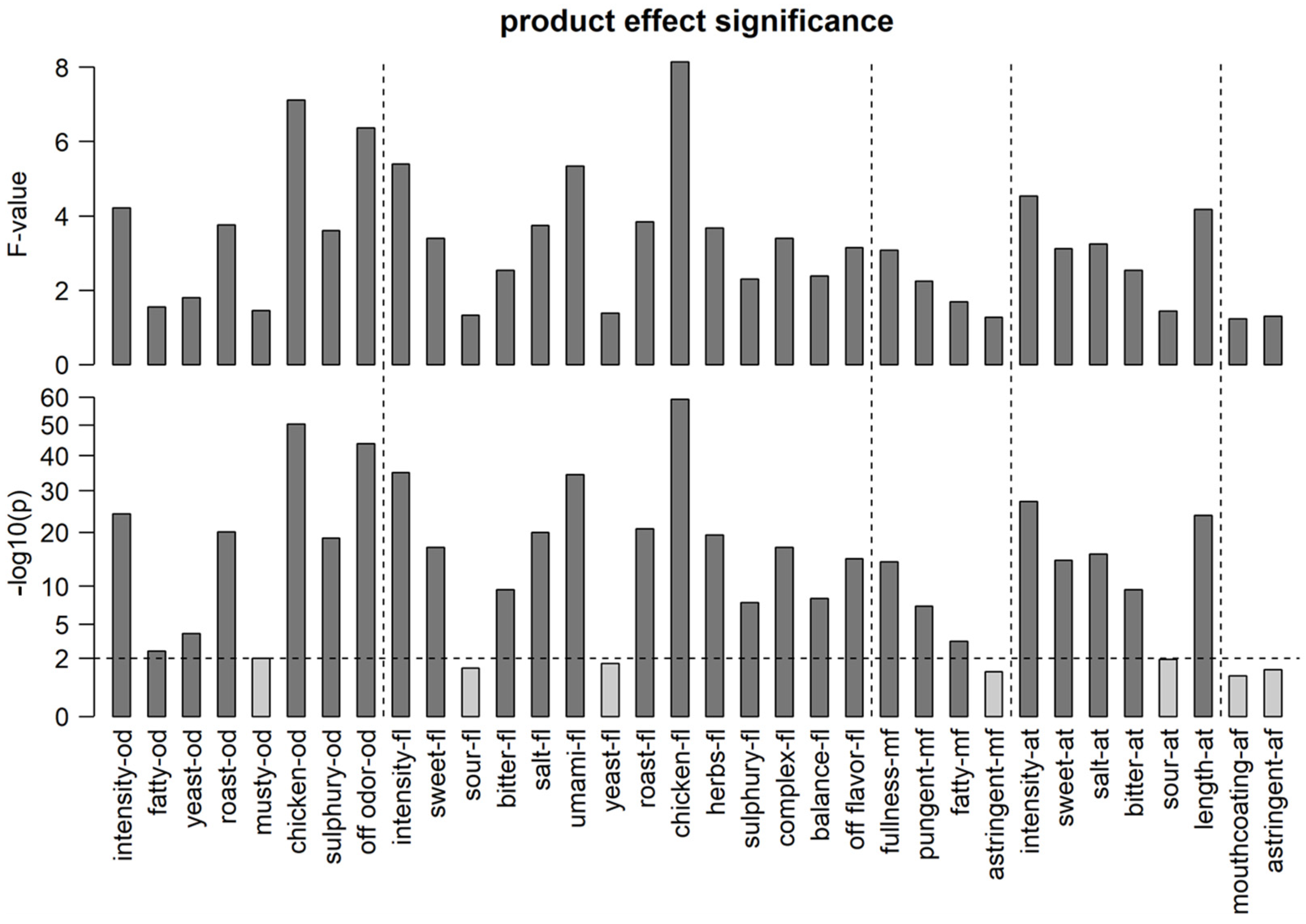
 = low,
= low,  = mid,
= mid,  = high).
= high).
 = low,
= low,  = mid,
= mid,  = high).
= high).
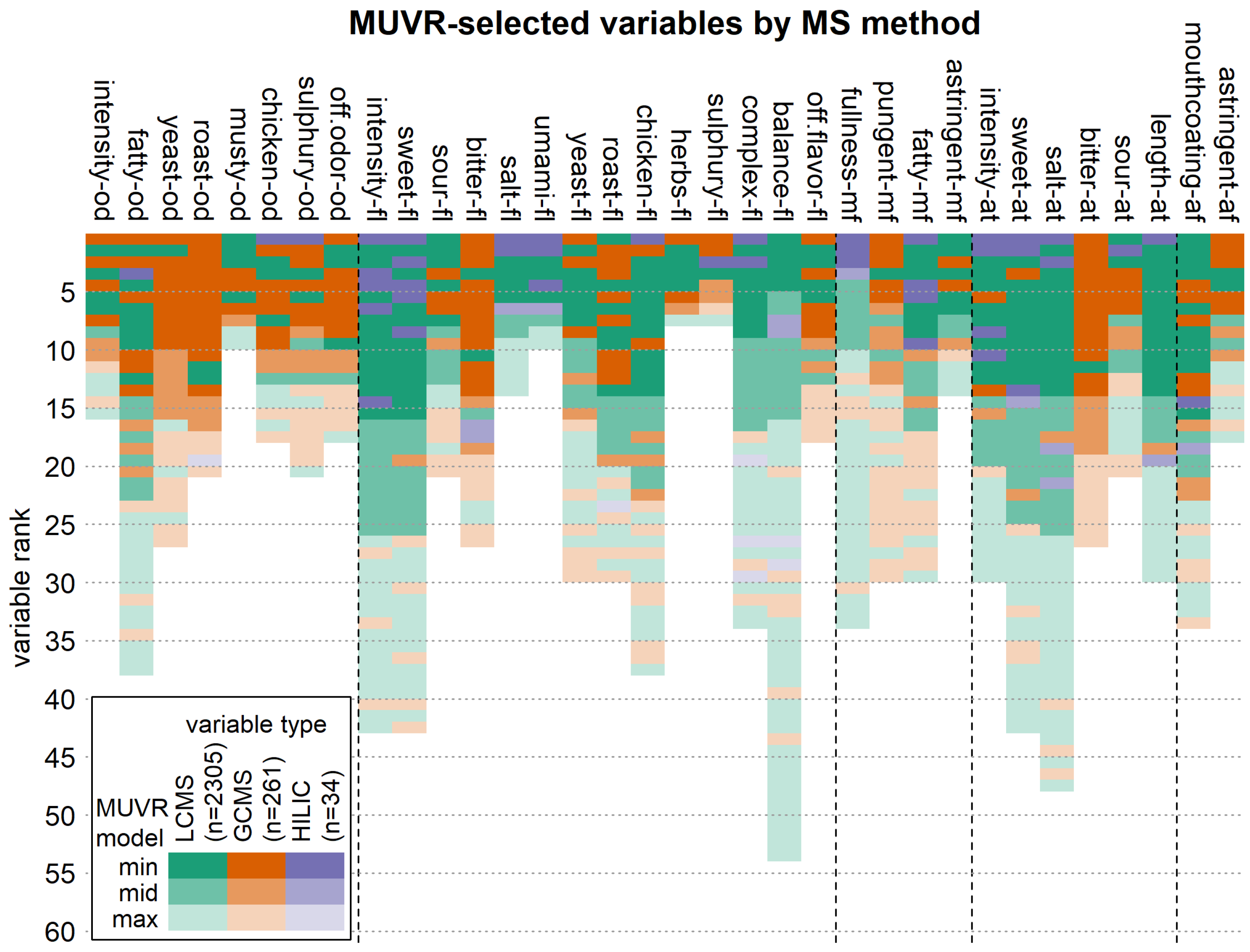
| Sensory Attributes | |||||
|---|---|---|---|---|---|
| Odor (od) | 1 | intensity-od | Mouthfeel (mf) | 23 | fullness-mf |
| 2 | fatty-od | 24 | pungent-mf | ||
| 3 | yeast-od | 25 | fatty-mf | ||
| 4 | roast-od | 26 | astringent-mf | ||
| 5 | musty-od | Aftertaste (at) | 27 | intensity-at | |
| 6 | chicken-od | 28 | sweet-at | ||
| 7 | sulfury-od | 29 | salt-at | ||
| 8 | off-odor-od | 30 | bitter-at | ||
| Flavor (fl) | 9 | intensity-fl | 31 | sour-at | |
| 10 | sweet-fl | 32 | length-at | ||
| 11 | sour-fl | Afterfeel (af) | 33 | mouthcoating-af | |
| 12 | bitter-fl | 34 | astringent-af | ||
| 13 | salt-fl | ||||
| 14 | umami-fl | ||||
| 15 | yeast-fl | ||||
| 16 | roast-fl | ||||
| 17 | chicken-fl | ||||
| 18 | herbs-fl | ||||
| 19 | sulfury-fl | ||||
| 20 | complex-fl | ||||
| 21 | balance-fl | ||||
| 22 | off-flavor-fl | ||||
| Chicken Flavor | Chicken Odor | ||||
| Rank | Measurement | Likely Hit/Formula | Rank | Measurement | Likely Hit/Formula |
| 1 | HILIC | Cystine | 1 | HILIC | Cystine |
| 2 | GC | 1-octen-3-ol | 2 | GC | 2,4-nonadienal |
| 3 | RPLC | C18H7N10NaO | 3 | RPLC | N-Fructosyl glutamylphenylalanine or isomer |
| 4 | RPLC | C19H38N3NaO8S3 | 4 | GC | 2,4-decadienal |
| 5 | RPLC | N-Fructosyl glutamylphenylalanine or isomer | 5 | GC | 1-octen-3-ol |
| 6 | RPLC | Traumatic acid or isomer | |||
| 7 | RPLC | C18H7N10NaO | |||
| 8 | GC | 2,4-decadienal | |||
| Sweet Flavor | Salt | ||||
| Rank | Measurement | Likely Hit/Formula | Flavor | ||
| 1 | HILIC | GMP | Rank | Measurement | Likely Hit/Formula |
| 2 | RPLC | C17H23NNa2O5 | 1 | HILIC | GMP |
| 3 | HILIC | IMP | 2 | HILIC | IMP |
| 4 | HILIC | UMP | 3 | RPLC | N-gamma-L-Glutamyl-L-phenylalanine/Aspartame |
| 5 | HILIC | CMP | 4 | RPLC | C12H22N4O5 |
| 6 | RPLC | C23H41N2O3PS | 5 | RPLC | Aspartylphenylalanine/Phenylalanyl-aspartic acid |
| 7 | RPLC | C16H19N0O5 | 6 | RPLC | C13H25N5NaO2P |
| 8 | HILIC | L.Glutamic.acid | 7 | HILIC | UMP |
| 9 | RPLC | C15H25N3O8 | 8 | RPLC | C16H19N0O5 |
| 10 | RPLC | C15H25N3O8 | Umami Flavor | ||
| 11 | RPLC | C16H19NNa2O3 | Rank | Measurement | Likely Hit/Formula |
| 12 | RPLC | C17H30N6O6 | 1 | HILIC | GMP |
| 13 | GC | homosalate | 2 | HILIC | IMP |
| 14 | RPLC | C19H27N3O4/C14H32N3NaO4S | 3 | RPLC | C18H36N4O4 |
| 15 | RPLC | Methionyl-Leucine or Isomer | 4 | HILIC | CMP |
| 5 | RPLC | Leu-Ala-Ser, Ala-Val-Thr or Thr-Gly-Leu or isomer | |||
| 6 | HILIC | UMP | |||
| 7 | RPLC | C19H27N3O6 | |||
| Roast Flavor | Roast Odor | ||||
| Rank | Measurement | Likely Hit/Formula | Rank | Measurement | Likely Hit/Formula |
| 1 | RPLC | Fructose-isoleucine, Fructose-leucine or isomer | 1 | GC | C14H20O2 |
| 2 | GC | non-identified | 2 | GC | non-identified |
| 3 | GC | 4-methoxy-6-(2-propenyl)-1,3-benzodioxole | 3 | GC | C12H11N |
| 4 | RPLC | C22H15N4NaO5 | 4 | GC | C11H18N2 |
| 5 | GC | 4-terpineol | 5 | GC | non-identified |
| 6 | RPLC | C9H15NO5 | 6 | GC | C10H16N2 |
| 7 | GC | C11H18N2 | 7 | GC | C7H10N2 |
| 8 | GC | non-identified | 8 | GC | non-identified |
| 9 | RPLC | C20H7N3O5P2 | 9 | GC | 2-ethyl-5-methyl pyrazine |
| 10 | GC | terpineol | 10 | RPLC | Fructose-isoleucine, Fructose-leucine or isomer |
| 11 | RPLC | C33H24N2 | 11 | GC | 2,3,5-trimethyl-6-isopentyl pyrazine |
| 12 | RPLC | C11H21N3O2 | 12 | RPLC | non-identified |
| 13 | RPLC | non-identified | |||
| 14 | GC | non-identified | |||
| 15 | RPLC | L,L-Cyclo(leucylprolyl) | |||
| 16 | RPLC | non-identified | |||
| 17 | GC | 2,3,5-trimethyl-6-isopentyl pyrazine | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leygeber, S.; Diez-Simon, C.; Großmann, J.L.; Dubbelman, A.-C.; Harms, A.C.; Westerhuis, J.A.; Jacobs, D.M.; Lindenburg, P.W.; Hendriks, M.M.W.B.; Ammerlaan, B.C.H.; et al. A Data-Driven Approach to Link GC-MS and LC-MS with Sensory Attributes of Chicken Bouillon with Added Yeast-Derived Flavor Products in a Combined Prediction Model. Metabolites 2025, 15, 317. https://doi.org/10.3390/metabo15050317
Leygeber S, Diez-Simon C, Großmann JL, Dubbelman A-C, Harms AC, Westerhuis JA, Jacobs DM, Lindenburg PW, Hendriks MMWB, Ammerlaan BCH, et al. A Data-Driven Approach to Link GC-MS and LC-MS with Sensory Attributes of Chicken Bouillon with Added Yeast-Derived Flavor Products in a Combined Prediction Model. Metabolites. 2025; 15(5):317. https://doi.org/10.3390/metabo15050317
Chicago/Turabian StyleLeygeber, Simon, Carmen Diez-Simon, Justus L. Großmann, Anne-Charlotte Dubbelman, Amy C. Harms, Johan A. Westerhuis, Doris M. Jacobs, Peter W. Lindenburg, Margriet M. W. B. Hendriks, Brenda C. H. Ammerlaan, and et al. 2025. "A Data-Driven Approach to Link GC-MS and LC-MS with Sensory Attributes of Chicken Bouillon with Added Yeast-Derived Flavor Products in a Combined Prediction Model" Metabolites 15, no. 5: 317. https://doi.org/10.3390/metabo15050317
APA StyleLeygeber, S., Diez-Simon, C., Großmann, J. L., Dubbelman, A.-C., Harms, A. C., Westerhuis, J. A., Jacobs, D. M., Lindenburg, P. W., Hendriks, M. M. W. B., Ammerlaan, B. C. H., van den Berg, M. A., van Doorn, R., Mumm, R., Smilde, A. K., Hall, R. D., & Hankemeier, T. (2025). A Data-Driven Approach to Link GC-MS and LC-MS with Sensory Attributes of Chicken Bouillon with Added Yeast-Derived Flavor Products in a Combined Prediction Model. Metabolites, 15(5), 317. https://doi.org/10.3390/metabo15050317







