From Inflammation to Infertility: How Oxidative Stress and Infections Disrupt Male Reproductive Health
Abstract
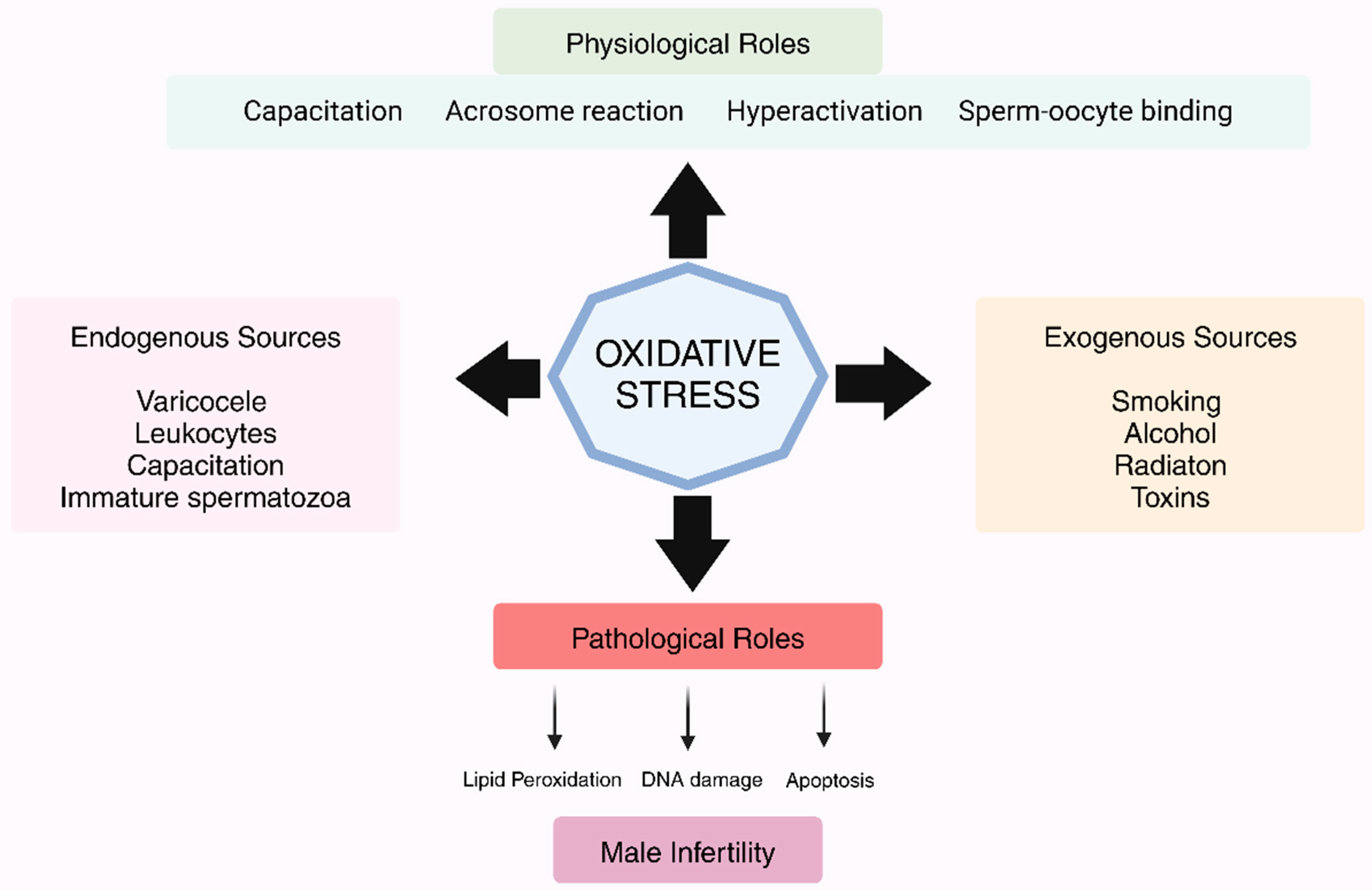
1. Introduction
2. OS and Fertility Pathophysiological Mechanisms and Clinical Implications
2.1. The Role of Infection, Inflammation, and Leukocytes in OS
2.2. Mitochondrial Dysfunction as a Key Mediator of OS
2.3. Lipid Peroxidation and Sperm Function
2.4. OS, Telomere Damage, and Apoptosis in Sperm Health
2.5. How Do Free Radicals Target the Sperm Ion Channels and After Sperm Function?
2.6. Downstream Signaling Mechanisms of Free Radicals in the Regulation of Sperm Function
3. Causes of OS in Male Reproductive Health
Exogenous Parameters and Their Role in Free Radical Generation and Sperm Function
4. Biomarkers and Diagnosis
5. Antioxidants and Their Role in Counteracting OS
5.1. Enzymatic Antioxidant Defense Mechanisms in the Body
5.2. Non-Enzymatic Antioxidant Defense of the Body
5.3. Dietary and Supplementary Antioxidants
6. Infections of the Genital Tract
Neutrophil Extracellular Traps (NETs) and Their Role in Infection and Sperm Function
7. Microbiota on Male Reproductive System and OS
8. The Role of Inflammation and OS
9. Impact of Inflammation and Systemic Factors on Male Fertility
10. Discussion
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, R.; Jia, Z.; Trush, M.A. Defining ROS in Biology and Medicine. React. Oxyg. Species 2016, 1, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Phaniendra, A.; Jestadi, D.B.; Periyasamy, L. Free radicals: Properties, sources, targets, and their implication in various diseases. Indian J. Clin. Biochem. 2015, 30, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Makker, K.; Agarwal, A.; Sharma, R. Oxidative stress & male infertility. Indian J. Med. Res. 2009, 129, 357–367. [Google Scholar]
- Dutta, S.; Majzoub, A.; Agarwal, A. Oxidative stress and sperm function: A systematic review on evaluation and management. Arab. J. Urol. 2019, 17, 87–97. [Google Scholar] [CrossRef]
- Hussain, T.; Kandeel, M.; Metwally, E.; Murtaza, G.; Kalhoro, D.H.; Yin, Y.; Tan, B.; Chughtai, M.I.; Yaseen, A.; Afzal, A.; et al. Unraveling the harmful effect of oxidative stress on male fertility: A mechanistic insight. Front. Endocrinol. 2023, 14, 1070692. [Google Scholar] [CrossRef]
- Shahini, A.; Shahini, A. Role of interleukin-6-mediated inflammation in the pathogenesis of inflammatory bowel disease: Focus on the available therapeutic approaches and gut microbiome. J. Cell Commun. Signal 2023, 17, 55–74. [Google Scholar] [CrossRef]
- Dutta, S.; Sengupta, P.; Slama, P.; Roychoudhury, S. Oxidative Stress, Testicular Inflammatory Pathways, and Male Reproduction. Int. J. Mol. Sci. 2021, 22, 10043. [Google Scholar] [CrossRef]
- Wang, Y.; Fu, X.; Li, H. Mechanisms of oxidative stress-induced sperm dysfunction. Front. Endocrinol. 2025, 16, 1520835. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, K.; Yao, Y.; Li, J.; Deng, S. Bacterial Infections Affect Male Fertility: A Focus on the Oxidative Stress-Autophagy Axis. Front. Cell Dev. Biol. 2021, 9, 727812. [Google Scholar] [CrossRef]
- Li, X.; Ni, M.; Xing, S.; Yu, Y.; Zhou, Y.; Yang, S.; Li, H.; Zhu, R.; Han, M. Reactive Oxygen Species Secreted by Leukocytes in Semen Induce Self-Expression of Interleukin-6 and Affect Sperm Quality. Am. J. Mens. Health 2020, 14, 1557988320970053. [Google Scholar] [CrossRef]
- Costa, J.; Braga, P.C.; Rebelo, I.; Oliveira, P.F.; Alves, M.G. Mitochondria Quality Control and Male Fertility. Biology 2023, 12, 827. [Google Scholar] [CrossRef] [PubMed]
- Ayala, A.; Munoz, M.F.; Arguelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef] [PubMed]
- Gredilla, R.; Bohr, V.A.; Stevnsner, T. Mitochondrial DNA repair and association with aging—An update. Exp. Gerontol. 2010, 45, 478–488. [Google Scholar] [CrossRef] [PubMed]
- Moustakli, E.; Zikopoulos, A.; Skentou, C.; Bouba, I.; Tsirka, G.; Stavros, S.; Vrachnis, D.; Vrachnis, N.; Potiris, A.; Georgiou, I.; et al. Sperm Mitochondrial Content and Mitochondrial DNA to Nuclear DNA Ratio Are Associated with Body Mass Index and Progressive Motility. Biomedicines 2023, 11, 3014. [Google Scholar] [CrossRef]
- Nowicka-Bauer, K.; Nixon, B. Molecular Changes Induced by Oxidative Stress that Impair Human Sperm Motility. Antioxidants 2020, 9, 134. [Google Scholar] [CrossRef]
- Tvrdá, E.; Benko, F.; Ďuračka, M. Oxidative Stress as an Underlying Mechanism of Bacteria-Inflicted Damage to Male Gametes. Oxygen 2022, 2, 547–569. [Google Scholar] [CrossRef]
- Gualtieri, R.; Kalthur, G.; Barbato, V.; Longobardi, S.; Di Rella, F.; Adiga, S.K.; Talevi, R. Sperm Oxidative Stress during In Vitro Manipulation and Its Effects on Sperm Function and Embryo Development. Antioxidants 2021, 10, 1025. [Google Scholar] [CrossRef]
- Gavia-Garcia, G.; Rosado-Perez, J.; Arista-Ugalde, T.L.; Aguiniga-Sanchez, I.; Santiago-Osorio, E.; Mendoza-Nunez, V.M. Telomere Length and Oxidative Stress and Its Relation with Metabolic Syndrome Components in the Aging. Biology 2021, 10, 253. [Google Scholar] [CrossRef]
- Kierszenbaum, A.L. Apoptosis during spermatogenesis: The thrill of being alive. Mol. Reprod. Dev. 2001, 58, 1–3. [Google Scholar] [CrossRef]
- Sharma, P.; Kaushal, N.; Saleth, L.R.; Ghavami, S.; Dhingra, S.; Kaur, P. Oxidative stress-induced apoptosis and autophagy: Balancing the contrary forces in spermatogenesis. Biochim. Biophys. Acta Mol. Basis Dis. 2023, 1869, 166742. [Google Scholar] [CrossRef]
- Kesari, K.K.; Agarwal, A.; Henkel, R. Radiations and male fertility. Reprod. Biol. Endocrinol. 2018, 16, 118. [Google Scholar] [CrossRef] [PubMed]
- Asadi, A.; Ghahremani, R.; Abdolmaleki, A.; Rajaei, F. Role of sperm apoptosis and oxidative stress in male infertility: A narrative review. Int. J. Reprod. Biomed. 2021, 19, 493–504. [Google Scholar] [CrossRef] [PubMed]
- Tvrda, E.; Benko, F.; Slanina, T.; du Plessis, S.S. The Role of Selected Natural Biomolecules in Sperm Production and Functionality. Molecules 2021, 26, 5196. [Google Scholar] [CrossRef]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal 2014, 20, 1126–1167. [Google Scholar] [CrossRef]
- Xu, Y.R.; Dong, H.S.; Yang, W.X. Regulators in the apoptotic pathway during spermatogenesis: Killers or guards? Gene 2016, 582, 97–111. [Google Scholar] [CrossRef]
- Antinozzi, C.; Di Luigi, L.; Sireno, L.; Caporossi, D.; Dimauro, I.; Sgrò, P. Protective Role of Physical Activity and Antioxidant Systems During Spermatogenesis. Biomolecules 2025, 15, 478. [Google Scholar] [CrossRef]
- Juan, C.A.; Pérez De La Lastra, J.M.; Plou, F.J.; Pérez-Lebeña, E. The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef]
- Anwar, S.; Alrumaihi, F.; Sarwar, T.; Babiker, A.Y.; Khan, A.A.; Prabhu, S.V.; Rahmani, A.H. Exploring Therapeutic Potential of Catalase: Strategies in Disease Prevention and Management. Biomolecules 2024, 14, 697. [Google Scholar] [CrossRef]
- Kothari, S.; Thompson, A.; Agarwal, A.; du Plessis, S.S. Free radicals: Their beneficial and detrimental effects on sperm function. Indian J. Exp. Biol. 2010, 48, 425–435. [Google Scholar]
- Son, Y.; Cheong, Y.K.; Kim, N.H.; Chung, H.T.; Kang, D.G.; Pae, H.O. Mitogen-Activated Protein Kinases and Reactive Oxygen Species: How Can ROS Activate MAPK Pathways? J. Signal Transduct. 2011, 2011, 792639. [Google Scholar] [CrossRef]
- Visconti, P.E. Understanding the molecular basis of sperm capacitation through kinase design. Proc. Natl. Acad. Sci. USA 2009, 106, 667–668. [Google Scholar] [CrossRef] [PubMed]
- Chandimali, N.; Bak, S.G.; Park, E.H.; Lim, H.-J.; Won, Y.-S.; Kim, E.-K.; Park, S.-I.; Lee, S.J. Free radicals and their impact on health and antioxidant defenses: A review. Cell Death Discov. 2025, 11, 19. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Henkel, R.; Sengupta, P.; Agarwal, A. Physiological Role of ROS in Sperm Function. In Male Infertility; Parekattil, S.J., Esteves, S.C., Agarwal, A., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 337–345. Available online: http://link.springer.com/10.1007/978-3-030-32300-4_27 (accessed on 31 March 2025).
- Zhao, C.C.; Scott, M.; Eisenberg, M.L. Male Fertility as a Proxy for Health. J. Clin. Med. 2024, 13, 5559. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Wang, Z.; Kong, Y.; Jin, M.; Ma, L. Global, regional and national burden of male infertility in 204 countries and territories between 1990 and 2019: An analysis of global burden of disease study. BMC Public Health 2023, 23, 2195. [Google Scholar] [CrossRef]
- Pasqualotto, F.F.; Sharma, R.K.; Kobayashi, H.; Nelson, D.R.; Thomas, A.J., Jr.; Agarwal, A. Oxidative stress in normospermic men undergoing infertility evaluation. J. Androl. 2001, 22, 316–322. [Google Scholar] [CrossRef]
- Agarwal, A.; Virk, G.; Ong, C.; du Plessis, S.S. Effect of oxidative stress on male reproduction. World J. Men Health 2014, 32, 1–17. [Google Scholar] [CrossRef]
- Huang, R.; Chen, J.; Guo, B.; Jiang, C.; Sun, W. Diabetes-induced male infertility: Potential mechanisms and treatment options. Mol. Med. 2024, 30, 11. [Google Scholar] [CrossRef]
- Koklesova, L.; Mazurakova, A.; Samec, M.; Biringer, K.; Samuel, S.M.; Busselberg, D.; Kubatka, P.; Golubnitschaja, O. Homocysteine metabolism as the target for predictive medical approach, disease prevention, prognosis, and treatments tailored to the person. EPMA J. 2021, 12, 477–505. [Google Scholar] [CrossRef]
- Tyagi, N.; Sedoris, K.C.; Steed, M.; Ovechkin, A.V.; Moshal, K.S.; Tyagi, S.C. Mechanisms of homocysteine-induced oxidative stress. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, H2649–H2656. [Google Scholar] [CrossRef]
- Vahedi Raad, M.; Firouzabadi, A.M.; Tofighi Niaki, M.; Henkel, R.; Fesahat, F. The impact of mitochondrial impairments on sperm function and male fertility: A systematic review. Reprod. Biol. Endocrinol. 2024, 22, 83. [Google Scholar] [CrossRef]
- Stavros, S.; Potiris, A.; Molopodi, E.; Mavrogianni, D.; Zikopoulos, A.; Louis, K.; Karampitsakos, T.; Nazou, E.; Sioutis, D.; Christodoulaki, C.; et al. Sperm DNA Fragmentation: Unraveling Its Imperative Impact on Male Infertility Based on Recent Evidence. Int. J. Mol. Sci. 2024, 25, 10167. [Google Scholar] [CrossRef] [PubMed]
- Pizzol, D.; Foresta, C.; Garolla, A.; Demurtas, J.; Trott, M.; Bertoldo, A.; Smith, L. Pollutants and sperm quality: A systematic review and meta-analysis. Environ. Sci. Pollut. Res. Int. 2021, 28, 4095–4103. [Google Scholar] [CrossRef] [PubMed]
- Chung, E.; Brock, G.B. Cryptorchidism and its impact on male fertility: A state of art review of current literature. Can. Urol. Assoc. J. 2011, 5, 210–214. [Google Scholar] [CrossRef] [PubMed]
- Hasan, H.; Bhushan, S.; Fijak, M.; Meinhardt, A. Mechanism of Inflammatory Associated Impairment of Sperm Function, Spermatogenesis and Steroidogenesis. Front. Endocrinol. 2022, 13, 897029. [Google Scholar] [CrossRef]
- Walke, G.; Gaurkar, S.S.; Prasad, R.; Lohakare, T.; Wanjari, M. The Impact of Oxidative Stress on Male Reproductive Function: Exploring the Role of Antioxidant Supplementation. Cureus 2023, 15, e42583. [Google Scholar] [CrossRef]
- Ayad, B.; Omolaoye, T.S.; Louw, N.; Ramsunder, Y.; Skosana, B.T.; Oyeipo, P.I.; Du Plessis, S.S. Oxidative Stress and Male Infertility: Evidence From a Research Perspective. Front. Reprod. Health 2022, 4, 822257. [Google Scholar] [CrossRef]
- Zhou, Q.; Chen, J.; Zhang, J.; Zhou, F.; Zhao, J.; Wei, X.; Zheng, K.; Wu, J.; Li, B.; Pan, B. Toxicity and endocrine-disrupting potential of PM2.5: Association with particulate polycyclic aromatic hydrocarbons, phthalate esters, and heavy metals. Environ. Pollut. 2022, 292, 118349. [Google Scholar] [CrossRef]
- Nsonwu-Anyanwu, A.C.; Ekong, E.R.; Offor, S.J.; Awusha, O.F.; Orji, O.C.; Umoh, E.I.; Owhorji, J.A.; Emetonjor, F.R.; Usoro, C.A. Heavy metals, biomarkers of oxidative stress and changes in sperm function: A case-control study. Int. J. Reprod. Biomed. 2019, 17, 163. [Google Scholar] [CrossRef]
- Wang, J.-J.; Wang, S.-X.; Tehmina; Feng, Y.; Zhang, R.-F.; Li, X.-Y.; Sun, Q.; Ding, J. Age-Related Decline of Male Fertility: Mitochondrial Dysfunction and the Antioxidant Interventions. Pharmaceuticals 2022, 15, 519. [Google Scholar] [CrossRef]
- Pavuluri, H.; Bakhtiary, Z.; Selvam, M.K.P.; Hellstrom, W.J.G. Oxidative Stress-Associated Male Infertility: Current Diagnostic and Therapeutic Approaches. Medicina 2024, 60, 1008. [Google Scholar] [CrossRef]
- Atig, F.; Raffa, M.; Ali, H.B.; Abdelhamid, K.; Saad, A.; Ajina, M. Altered antioxidant status and increased lipid per-oxidation in seminal plasma of tunisian infertile men. Int. J. Biol. Sci. 2012, 8, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Zini, A.; Al-Hathal, N. Antioxidant therapy in male infertility: Fact or fiction? Asian J. Androl. 2011, 13, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Moustakli, E.; Zikopoulos, A.; Sakaloglou, P.; Bouba, I.; Sofikitis, N.; Georgiou, I. Functional association between telomeres, oxidation and mitochondria. Front. Reprod. Health 2023, 5, 1107215. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Sengupta, P.; Roychoudhury, S.; Chakravarthi, S.; Wang, C.W.; Slama, P. Antioxidant Paradox in Male Infertility: ‘A Blind Eye’ on Inflammation. Antioxidants 2022, 11, 167. [Google Scholar] [CrossRef]
- Jomova, K.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Several lines of antioxidant defense against oxidative stress: Antioxidant enzymes, nanomaterials with multiple enzyme-mimicking activities, and low-molecular-weight antioxidants. Arch. Toxicol. 2024, 98, 1323–1367. [Google Scholar] [CrossRef]
- Wang, Y.; Branicky, R.; Noe, A.; Hekimi, S. Superoxide dismutases: Dual roles in controlling ROS damage and regulating ROS signaling. J. Cell Biol. 2018, 217, 1915–1928. [Google Scholar] [CrossRef]
- Yan, L.; Liu, J.; Wu, S.; Zhang, S.; Ji, G.; Gu, A. Seminal superoxide dismutase activity and its relationship with semen quality and SOD gene polymorphism. J. Assist. Reprod. Genet. 2014, 31, 549–554. [Google Scholar] [CrossRef]
- Walton, P.A.; Pizzitelli, M. Effects of peroxisomal catalase inhibition on mitochondrial function. Front. Physiol. 2012, 3, 108. [Google Scholar] [CrossRef]
- Rubio-Riquelme, N.; Huerta-Retamal, N.; Gomez-Torres, M.J.; Martinez-Espinosa, R.M. Catalase as a Molecular Target for Male Infertility Diagnosis and Monitoring: An Overview. Antioxidants 2020, 9, 78. [Google Scholar] [CrossRef]
- Pei, J.; Pan, X.; Wei, G.; Hua, Y. Research progress of glutathione peroxidase family (GPX) in redoxidation. Front. Pharmacol. 2023, 14, 1147414. [Google Scholar] [CrossRef]
- Lubos, E.; Loscalzo, J.; Handy, D.E. Glutathione peroxidase-1 in health and disease: From molecular mechanisms to therapeutic opportunities. Antioxid. Redox Signal 2011, 15, 1957–1997. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Boiti, A.; Vallone, D.; Foulkes, N.S. Reactive Oxygen Species Signaling and Oxidative Stress: Transcriptional Regulation and Evolution. Antioxidants 2024, 13, 312. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Aponte-Mellado, A.; Premkumar, B.J.; Shaman, A.; Gupta, S. The effects of oxidative stress on female reproduction: A review. Reprod. Biol. Endocrinol. 2012, 10, 49. [Google Scholar] [CrossRef] [PubMed]
- Walczak-Jedrzejowska, R.; Wolski, J.K.; Slowikowska-Hilczer, J. The role of oxidative stress and antioxidants in male fertility. Cent. Eur. J. Urol. 2013, 66, 60–67. [Google Scholar] [CrossRef]
- Zhao, F.; Whiting, S.; Lambourne, S.; Aitken, R.J.; Sun, Y.P. Melatonin alleviates heat stress-induced oxidative stress and apoptosis in human spermatozoa. Free Radic. Biol. Med. 2021, 164, 410–416. [Google Scholar] [CrossRef]
- Leandri, R.; Power, K.; Buonocore, S.; De Vico, G. Preliminary Evidence of the Possible Roles of the Ferritinophagy-Iron Uptake Axis in Canine Testicular Cancer. Animals 2024, 14, 2619. [Google Scholar] [CrossRef]
- Palani, A.F. Effect of serum antioxidant levels on sperm function in infertile male. Middle East Fertil. Soc. J. 2018, 23, 19–22. [Google Scholar] [CrossRef]
- Traber, M.G.; Stevens, J.F. Vitamins C and E: Beneficial effects from a mechanistic perspective. Free Radic. Biol. Med. 2011, 51, 1000–1013. [Google Scholar] [CrossRef]
- Greco, E.; Iacobelli, M.; Rienzi, L.; Ubaldi, F.; Ferrero, S.; Tesarik, J. Reduction of the incidence of sperm DNA fragmentation by oral antioxidant treatment. J. Androl. 2005, 26, 349–353. [Google Scholar] [CrossRef]
- Stradaioli, G.; Sylla, L.; Zelli, R.; Chiodi, P.; Monaci, M. Effect of L-carnitine administration on the seminal characteristics of oligoasthenospermic stallions. Theriogenology 2004, 62, 761–777. [Google Scholar] [CrossRef]
- Kooshesh, L.; Nateghian, Z.; Aliabadi, E. Evaluation of L-Carnitine Potential in Improvement of Male Fertility. J. Reprod. Infertil. 2023, 24, 69–84. [Google Scholar] [CrossRef] [PubMed]
- Torres-Arce, E.; Vizmanos, B.; Babio, N.; Marquez-Sandoval, F.; Salas-Huetos, A. Dietary Antioxidants in the Treatment of Male Infertility: Counteracting Oxidative Stress. Biology 2021, 10, 241. [Google Scholar] [CrossRef] [PubMed]
- Balercia, G.; Buldreghini, E.; Vignini, A.; Tiano, L.; Paggi, F.; Amoroso, S.; Ricciardo-Lamonica, G.; Boscaro, M.; Lenzi, A.; Littarru, G. Coenzyme Q10 treatment in infertile men with idiopathic asthenozoospermia: A placebo-controlled, double-blind randomized trial. Fertil. Steril. 2009, 91, 1785–1792. [Google Scholar] [CrossRef] [PubMed]
- Alahmar, A.T. Coenzyme Q10 improves sperm motility and antioxidant status in infertile men with idiopathic oligoasthenospermia. Clin. Exp. Reprod. Med. 2022, 49, 277–284. [Google Scholar] [CrossRef]
- Lafuente, R.; Gonzalez-Comadran, M.; Sola, I.; Lopez, G.; Brassesco, M.; Carreras, R.; Checa, M.A. Coenzyme Q10 and male infertility: A meta-analysis. J. Assist. Reprod. Genet. 2013, 30, 1147–1156. [Google Scholar] [CrossRef]
- Allouche-Fitoussi, D.; Breitbart, H. The Role of Zinc in Male Fertility. Int. J. Mol. Sci. 2020, 21, 7796. [Google Scholar] [CrossRef]
- Fallah, A.; Mohammad-Hasani, A.; Colagar, A.H. Zinc is an Essential Element for Male Fertility: A Review of Zn Roles in Men’s Health, Germination, Sperm Quality, and Fertilization. J. Reprod. Infertil. 2018, 19, 69–81. [Google Scholar]
- Yuan, S.; Zhang, Y.; Dong, P.Y.; Chen Yan, Y.M.; Liu, J.; Zhang, B.Q.; Chen, M.M.; Zhang, S.E.; Zhang, X.F. A comprehensive review on potential role of selenium, selenoproteins and selenium nanoparticles in male fertility. Heliyon 2024, 10, e34975. [Google Scholar] [CrossRef]
- Jannatifar, R.; Parivar, K.; Roodbari, N.H.; Nasr-Esfahani, M.H. Effects of N-acetyl-cysteine supplementation on sperm quality, chromatin integrity and level of oxidative stress in infertile men. Reprod. Biol. Endocrinol. 2019, 17, 24. [Google Scholar] [CrossRef]
- Safarinejad, M.R.; Safarinejad, S. Efficacy of selenium and/or N-acetyl-cysteine for improving semen parameters in infertile men: A double-blind, placebo controlled, randomized study. J. Urol. 2009, 181, 741–751. [Google Scholar] [CrossRef]
- Khalafalla, K.; El Ansari, W.; Sengupta, P.; Majzoub, A.; Elbardisi, H.; Canguven, O.; El-Ansari, K.; Arafa, M. Are sexually transmitted infections associated with male infertility? A systematic review and in-depth evaluation of the evidence and mechanisms of action of 11 pathogens. Arab. J. Urol. 2023, 21, 216–232. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.L.; Apicella, M.A. The molecular mechanisms used by Neisseria gonorrhoeae to initiate infection differ between men and women. Clin. Microbiol. Rev. 2004, 17, 965–981. [Google Scholar] [CrossRef] [PubMed]
- Henkel, R.; Offor, U.; Fisher, D. The role of infections and leukocytes in male infertility. Andrologia 2021, 53, e13743. [Google Scholar] [CrossRef]
- Gimenes, F.; Souza, R.P.; Bento, J.C.; Teixeira, J.J.; Maria-Engler, S.S.; Bonini, M.G.; Consolaro, M.E. Male infertility: A public health issue caused by sexually transmitted pathogens. Nat. Rev. Urol. 2014, 11, 672–687. [Google Scholar] [CrossRef]
- Sengupta, P.; Pinggera, G.M.; Calogero, A.E.; Agarwal, A. Oxidative stress affects sperm health and fertility-Time to apply facts learned at the bench to help the patient: Lessons for busy clinicians. Reprod. Med. Biol. 2024, 23, e12598. [Google Scholar] [CrossRef]
- Puerta Suarez, J.; Sanchez, L.R.; Salazar, F.C.; Saka, H.A.; Molina, R.; Tissera, A.; Rivero, V.E.; Cardona Maya, W.D.; Motrich, R.D. Chlamydia trachomatis neither exerts deleterious effects on spermatozoa nor impairs male fertility. Sci. Rep. 2017, 7, 1126. [Google Scholar] [CrossRef]
- Wang, Y.; Du, C.; Zhang, Y.; Zhu, L. Composition and Function of Neutrophil Extracellular Traps. Biomolecules 2024, 14, 416. [Google Scholar] [CrossRef]
- Iturrieta, V.; Schulz, M.; Sánchez, R.; Uribe, P.; Rivera-Concha, R.; Taubert, A.; Hermosilla, C.; Zambrano, F. Inflammatory Pathologies in Reproductive Tract: Role of NETs and METs on Fertilization Disorders. Int. J. Morphol. 2024, 42, 1783–1792. [Google Scholar] [CrossRef]
- Azenabor, A.; Ekun, A.O.; Akinloye, O. Impact of Inflammation on Male Reproductive Tract. J. Reprod. Infertil. 2015, 16, 123–129. [Google Scholar]
- Amulic, B.; Hayes, G. Neutrophil extracellular traps. Curr. Biol. 2011, 21, R297–R298. [Google Scholar] [CrossRef]
- Spooner, R.; Yilmaz, O. The role of reactive-oxygen-species in microbial persistence and inflammation. Int. J. Mol. Sci. 2011, 12, 334–352. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.; Liu, X.; Deng, W.; Zhou, Z.; Li, Y.; Tu, Y.; Chen, L.; Wang, G.; Fu, B. Pharmacological Interventions for Bacterial Prostatitis. Front. Pharmacol. 2020, 11, 504. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Yu, X.; Liu, Y.F.; Song, B.; Sun, Z.; Zhao, S. Immunoregulation and male reproductive function: Impacts and mechanistic insights into inflammation. Andrology 2024. Early View. [Google Scholar] [CrossRef] [PubMed]
- Peris, M.P.; Alonso, H.; Escolar, C.; Tristancho-Baro, A.; Abad, M.P.; Rezusta, A.; Milagro, A. Detection of Chlamydia trachomatis and Neisseria gonorrhoeae (and Its Resistance to Ciprofloxacin): Validation of a Molecular Biology Tool for Rapid Diagnosis and Treatment. Antibiotics 2024, 13, 1011. [Google Scholar] [CrossRef]
- Bonner, M.; Sheele, J.M.; Cantillo-Campos, S.; Elkins, J.M. A Descriptive Analysis of Men Diagnosed With Epididymitis, Orchitis, or Both in the Emergency Department. Cureus 2021, 13, e15800. [Google Scholar] [CrossRef]
- Monageng, E.; Offor, U.; Takalani, N.B.; Mohlala, K.; Opuwari, C.S. A Review on the Impact of Oxidative Stress and Medicinal Plants on Leydig Cells. Antioxidants 2023, 12, 1559. [Google Scholar] [CrossRef]
- Cristofori, F.; Dargenio, V.N.; Dargenio, C.; Miniello, V.L.; Barone, M.; Francavilla, R. Anti-Inflammatory and Immunomodulatory Effects of Probiotics in Gut Inflammation: A Door to the Body. Front. Immunol. 2021, 12, 578386. [Google Scholar] [CrossRef]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef]
- Roh, J.S.; Sohn, D.H. Damage-Associated Molecular Patterns in Inflammatory Diseases. Immune Netw. 2018, 18, e27. [Google Scholar] [CrossRef]
- Stavros, S.; Panagopoulos, P.; Machairiotis, N.; Potiris, A.; Mavrogianni, D.; Sfakianakis, A.; Drakaki, E.; Christodoulaki, C.; Panagiotopoulos, D.; Sioutis, D.; et al. Association between cytokine polymorphisms and recurrent pregnancy loss: A review of current evidence. Int. J. Gynaecol. Obstet. 2024, 167, 45–57. [Google Scholar] [CrossRef]
- Hedger, M.P.; Meinhardt, A. Cytokines and the immune-testicular axis. J. Reprod. Immunol. 2003, 58, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Cannarella, R.; Crafa, A.; Curto, R.; Condorelli, R.A.; La Vignera, S.; Calogero, A.E. Obesity and male fertility disorders. Mol. Aspects Med. 2024, 97, 101273. [Google Scholar] [CrossRef] [PubMed]
- Barbagallo, F.; Condorelli, R.A.; Mongioi, L.M.; Cannarella, R.; Cimino, L.; Magagnini, M.C.; Crafa, A.; La Vignera, S.; Calogero, A.E. Molecular Mechanisms Underlying the Relationship between Obesity and Male Infertility. Metabolites 2021, 11, 840. [Google Scholar] [CrossRef]
- Akhigbe, R.E.; Dutta, S.; Hamed, M.A.; Ajayi, A.F.; Sengupta, P.; Ahmad, G. Viral Infections and Male Infertility: A Comprehensive Review of the Role of Oxidative Stress. Front. Reprod. Health 2022, 4, 782915. [Google Scholar] [CrossRef]
- Patronia, M.M.; Potiris, A.; Mavrogianni, D.; Drakaki, E.; Karampitsakos, T.; Machairoudias, P.; Topis, S.; Zikopoulos, A.; Vrachnis, D.; Moustakli, E.; et al. The Expression of microRNAs and Their Involvement in Recurrent Pregnancy Loss. J. Clin. Med. 2024, 13, 3361. [Google Scholar] [CrossRef]
- Chen, J.; Chen, J.; Fang, Y.; Shen, Q.; Zhao, K.; Liu, C.; Zhang, H. Microbiology and immune mechanisms associated with male infertility. Front. Immunol. 2023, 14, 1139450. [Google Scholar] [CrossRef]
- Alahmar, A.T.; Calogero, A.E.; Singh, R.; Cannarella, R.; Sengupta, P.; Dutta, S. Coenzyme Q10, oxidative stress, and male infertility: A review. Clin. Exp. Reprod. Med. 2021, 48, 97–104. [Google Scholar] [CrossRef]
- Alexandru, I.; Nistor, D.; Motofelea, A.C.; Cadar Andone, B.A.; Crintea, A.; Tatu, C.; Pop, G.N.; Csep, A.N. Vitamins, Coenzyme Q10, and Antioxidant Strategies to Improve Oocyte Quality in Women with Gynecological Cancers: A Comprehensive Review. Antioxidants 2024, 13, 1567. [Google Scholar] [CrossRef]
- Chatzokou, D.; Tsarna, E.; Davouti, E.; Siristatidis, C.S.; Christopoulou, S.; Spanakis, N.; Tsakris, A.; Christopoulos, P. Semen Microbiome, Male Infertility, and Reproductive Health. Int. J. Mol. Sci. 2025, 26, 1446. [Google Scholar] [CrossRef]


| Biomarker | Measurement Method | Function | Clinical Relevance |
|---|---|---|---|
| Malondialdehyde (MDA) | Seminal plasma analysis | Lipid peroxidation and membrane damage | Increased MDA indicates oxidative damage to sperm membranes, impairing motility and membrane integrity. |
| Glutathione (GSH) | Seminal plasma or sperm cell analysis | Antioxidant capacity | Low levels of GSH suggest insufficient antioxidant defenses, leading to increased vulnerability to ROS-induced damage. |
| Reactive Oxygen Species (ROS) | Chemiluminescence or fluorescence-based assays | Overall OS in semen | Elevated ROS levels correlate with conditions like asthenozoospermia and teratozoospermia. |
| DNA Fragmentation | Sperm DNA fragmentation assay (TUNEL, SCSA, etc.) | DNA damage in sperm | Higher DNA fragmentation is associated with poor fertility outcomes, including lower fertilization rates and early pregnancy loss. |
| Chromatin Condensation | Chromatin condensation assay (e.g., chromomycin A3) | Sperm nuclear structure | Impaired chromatin condensation indicates potential DNA damage and reduced sperm viability. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Potiris, A.; Moustakli, E.; Trismpioti, E.; Drakaki, E.; Mavrogianni, D.; Matsas, A.; Zikopoulos, A.; Sfakianakis, A.; Tsakiridis, I.; Dagklis, T.; et al. From Inflammation to Infertility: How Oxidative Stress and Infections Disrupt Male Reproductive Health. Metabolites 2025, 15, 267. https://doi.org/10.3390/metabo15040267
Potiris A, Moustakli E, Trismpioti E, Drakaki E, Mavrogianni D, Matsas A, Zikopoulos A, Sfakianakis A, Tsakiridis I, Dagklis T, et al. From Inflammation to Infertility: How Oxidative Stress and Infections Disrupt Male Reproductive Health. Metabolites. 2025; 15(4):267. https://doi.org/10.3390/metabo15040267
Chicago/Turabian StylePotiris, Anastasios, Efthalia Moustakli, Eleni Trismpioti, Eirini Drakaki, Despoina Mavrogianni, Alkis Matsas, Athanasios Zikopoulos, Antonios Sfakianakis, Ioannis Tsakiridis, Themistoklis Dagklis, and et al. 2025. "From Inflammation to Infertility: How Oxidative Stress and Infections Disrupt Male Reproductive Health" Metabolites 15, no. 4: 267. https://doi.org/10.3390/metabo15040267
APA StylePotiris, A., Moustakli, E., Trismpioti, E., Drakaki, E., Mavrogianni, D., Matsas, A., Zikopoulos, A., Sfakianakis, A., Tsakiridis, I., Dagklis, T., Zachariou, A., Christopoulos, P., Domali, E., Drakakis, P., & Stavros, S. (2025). From Inflammation to Infertility: How Oxidative Stress and Infections Disrupt Male Reproductive Health. Metabolites, 15(4), 267. https://doi.org/10.3390/metabo15040267












