Kidney Toxicity of Drugs for the Heart: An Updated Perspective
Abstract
1. Introduction
2. Mechanisms of Drug-Induced Nephrotoxicity
2.1. Changes in Glomerular Hemodynamics
2.2. Tubular Cell Toxicity
2.3. Inflammation
2.4. Crystal Nephropathy
2.5. Rhabdomyolysis
2.6. Thrombotic Microangiopathy
3. Renal Toxicity of Cardiovascular Drugs
3.1. Diuretics
3.2. Angiotensin-Converting Enzyme Inhibitors and Angiotensin Receptor Blockers
3.3. Calcium Channel Blockers
3.4. Beta-Blockers
3.5. Antiplatelet Agents
3.6. Anticoagulants
3.7. Statins
3.8. Proton-Pump Inhibitors
3.9. Contrast Media
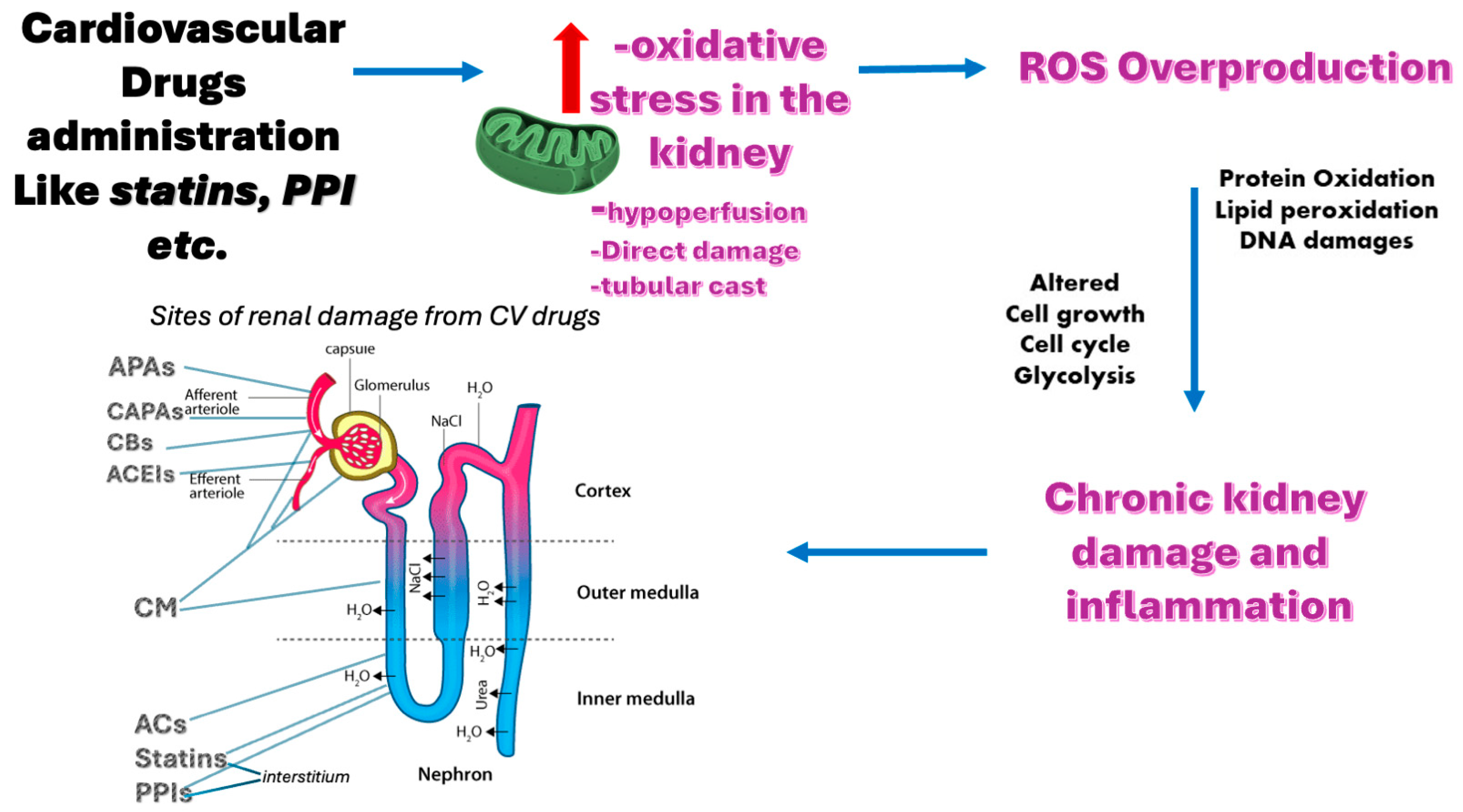
4. Role of Oxidative Stress in Drug-Induced Nephrotoxicity
- developing novel and specific biomarkers of oxidative stress and drug-induced nephrotoxicity that can reflect the degree and location of renal injury, predict the risk and outcome of drug-induced nephrotoxicity, monitor the response to treatment, and guide personalized therapy [10];
- identifying novel and effective antioxidants that can target specific sources or pathways of oxidative stress and drug-induced nephrotoxicity, modulate redox signaling, protect renal cells and tissues from oxidative damage, and preserve or restore renal function [192];
- designing personalized and precise antioxidant therapy based on individual characteristics and needs, such as genetic background, epigenetic modifications, comorbidities, co-administered drugs, environmental factors, and oxidative stress status;
- exploring the potential synergistic or additive effects of combining antioxidant therapy with other therapeutic modalities, such beta-blockers [193];
- assessing the long-term benefits and risks of antioxidant therapy for drug-induced nephrotoxicity on various clinical outcomes, such as renal function preservation, cardiovascular protection, quality of life improvement, and survival extension.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Van Vleet, T.R.; Schnellmann, R.G. Toxic nephropathy: Environmental chemicals. Semin. Nephrol. 2003, 23, 500–508. [Google Scholar] [CrossRef] [PubMed]
- Dalal, R.; Bruss, Z.S.; Sehdev, J.S. Physiology, Renal Blood Flow and Filtration. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK482248/ (accessed on 12 December 2024).
- Sanchez-Alamo, B.; Cases-Corona, C.; Fernandez-Juarez, G. Facing the Challenge of Drug-Induced Acute Interstitial Nephritis. Nephron 2023, 147, 78–90. [Google Scholar] [CrossRef]
- Rennke, H.G.; Bradley, M.D. Renal Pathophysiology: The Essentials, 4th ed.; Wolters Kluwer-Lippincot Williams & Wilkins: Baltimore, MD, USA, 2014. [Google Scholar]
- Petejova, N.; Martinek, A.; Zadrazil, J.; Teplan, V. Acute toxic kidney injury. Ren. Fail. 2019, 41, 576–594. [Google Scholar] [CrossRef] [PubMed]
- Ghane Shahrbaf, F.; Assadi, F. Drug-induced renal disorders. J. Ren. Inj. Prev. 2015, 4, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Pazhayattil, G.S.; Shirali, A.C. Drug-induced impairment of renal function. Int. J. Nephrol. Renov. Dis. 2014, 7, 457–468. [Google Scholar] [CrossRef]
- Hales, C.M.; Servais, J.; Martin, C.B.; Kohen, D. Prescription Drug Use Among Adults Aged 40–79 in the United States and Canada. NCHS Data Brief 2019, 347, 1–8. [Google Scholar]
- Dobrek, L. A Synopsis of Current Theories on Drug-Induced Nephrotoxicity. Life 2023, 13, 325. [Google Scholar] [CrossRef]
- Kim, S.Y.; Moon, A. Drug-induced nephrotoxicity and its biomarkers. Biomol. Ther. 2012, 20, 268–272. [Google Scholar] [CrossRef]
- Kaufman, D.P.; Basit, H.; Knohl, S.J. Physiology, Glomerular Filtration Rate. In StatPearls; StatPearls Publishing LLC: Treasure Island, FL, USA, 2023. [Google Scholar]
- Hostetter, T.H.; Rosenberg, M.E. Hemodynamic effects of glomerular permselectivity. Am. J. Nephrol. 1990, 10 (Suppl. S1), 24–27. [Google Scholar] [CrossRef]
- Horl, W.H. Nonsteroidal Anti-Inflammatory Drugs and the Kidney. Pharmaceuticals 2010, 3, 2291–2321. [Google Scholar] [CrossRef]
- Brown, N.J.; Vaughan, D.E. Angiotensin-Converting Enzyme Inhibitors. Circulation 1998, 97, 1411–1420. [Google Scholar] [CrossRef] [PubMed]
- Hall, A.M.; Trepiccione, F.; Unwin, R.J. Drug toxicity in the proximal tubule: New models, methods and mechanisms. Pediatr. Nephrol. 2022, 37, 973–982. [Google Scholar] [CrossRef]
- Gai, Z.; Gui, T.; Kullak-Ublick, G.A.; Li, Y.; Visentin, M. The Role of Mitochondria in Drug-Induced Kidney Injury. Front. Physiol. 2020, 11, 1079. [Google Scholar] [CrossRef]
- Krause, K.M.; Serio, A.W.; Kane, T.R.; Connolly, L.E. Aminoglycosides: An Overview. Cold Spring Harb. Perspect. Med. 2016, 6, a027029. [Google Scholar] [CrossRef] [PubMed]
- Yano, T.; Itoh, Y.; Kawamura, E.; Maeda, A.; Egashira, N.; Nishida, M.; Kurose, H.; Oishi, R. Amphotericin B-induced renal tubular cell injury is mediated by Na+ Influx through ion-permeable pores and subsequent activation of mitogen-activated protein kinases and elevation of intracellular Ca2+ concentration. Antimicrob. Agents Chemother. 2009, 53, 1420–1426. [Google Scholar] [CrossRef] [PubMed]
- Varlam, D.E.; Siddiq, M.M.; Parton, L.A.; Rüssmann, H. Apoptosis contributes to amphotericin B-induced nephrotoxicity. Antimicrob. Agents Chemother. 2001, 45, 679–685. [Google Scholar] [CrossRef] [PubMed]
- Tanji, N.; Tanji, K.; Kambham, N.; Markowitz, G.S.; Bell, A.; D’Agati, V.D. Adefovir nephrotoxicity: Possible role of mitochondrial DNA depletion. Hum. Pathol. 2001, 32, 734–740. [Google Scholar] [CrossRef] [PubMed]
- Deray, G.; Martinez, F.; Katlama, C.; Levaltier, B.; Beaufils, H.; Danis, M.; Rozenheim, M.; Baumelou, A.; Dohin, E.; Gentilini, M.; et al. Foscarnet nephrotoxicity: Mechanism, incidence and prevention. Am. J. Nephrol. 1989, 9, 316–321. [Google Scholar] [CrossRef]
- Caiazza, A.; Russo, L.; Sabbatini, M.; Russo, D. Hemodynamic and tubular changes induced by contrast media. BioMed Res. Int. 2014, 2014, 578974. [Google Scholar] [CrossRef]
- Imig, J.D.; Ryan, M.J. Immune and inflammatory role in renal disease. Compr. Physiol. 2013, 3, 957–976. [Google Scholar] [CrossRef]
- Barreto, E.F.; Rule, A.D. Management of Drug-Associated Acute Interstitial Nephritis. Kidney360 2020, 1, 62–64. [Google Scholar] [CrossRef] [PubMed]
- Lucas, G.N.C.; Leitão, A.C.C.; Alencar, R.L.; Xavier, R.M.F.; Daher, E.F.; Silva Junior, G.B.D. Pathophysiological aspects of nephropathy caused by non-steroidal anti-inflammatory drugs. J. Bras. Nefrol. 2019, 41, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Farouk, S.S.; Rein, J.L. The Many Faces of Calcineurin Inhibitor Toxicity-What the FK? Adv. Chronic Kidney Dis. 2020, 27, 56–66. [Google Scholar] [CrossRef]
- Gong, R.; Wang, P.; Dworkin, L. What we need to know about the effect of lithium on the kidney. Am. J. Physiology. Ren. Physiol. 2016, 311, F1168–F1171. [Google Scholar] [CrossRef]
- Santos, M.L.C.; de Brito, B.B.; da Silva, F.A.F.; Botelho, A.; de Melo, F.F. Nephrotoxicity in cancer treatment: An overview. World J. Clin. Oncol. 2020, 11, 190–204. [Google Scholar] [CrossRef]
- Keen, M.U.; Aeddula, N.R. Analgesic Nephropathy. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Perazella, M.A.; Herlitz, L.C. The Crystalline Nephropathies. Kidney Int. Rep. 2021, 6, 2942–2957. [Google Scholar] [CrossRef] [PubMed]
- Mulay, S.R.; Shi, C.; Ma, X.; Anders, H.J. Novel Insights into Crystal-Induced Kidney Injury. Kidney Dis. 2018, 4, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Genc, G.; Ozkaya, O.; Acikgöz, Y.; Yapici, O.; Bek, K.; Gülnar Sensoy, S.; Ozyürek, E. Acute renal failure with acyclovir treatment in a child with leukemia. Drug Chem. Toxicol. 2010, 33, 217–219. [Google Scholar] [CrossRef] [PubMed]
- Izzedine, H.; Launay-Vacher, V.; Deray, G. Antiviral drug-induced nephrotoxicity. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2005, 45, 804–817. [Google Scholar] [CrossRef]
- Derebail, V.K.; McGregor, J.G.; Colindres, R.E.; Singh, H.K.; Kshirsagar, A.V. The Case: Acute kidney injury in a patient with P. carinii pneumonia. Kidney Int. 2009, 75, 865–866. [Google Scholar] [CrossRef] [PubMed]
- Perazella, M.A. Crystal-induced acute renal failure. Am. J. Med. 1999, 106, 459–465. [Google Scholar] [CrossRef]
- Hamed, K.M.; Dighriri, I.M.; Baomar, A.F.; Alharthy, B.T.; Alenazi, F.E.; Alali, G.H.; Alenazy, R.H.; Alhumaidi, N.T.; Alhulayfi, D.H.; Alotaibi, Y.B.; et al. Overview of Methotrexate Toxicity: A Comprehensive Literature Review. Cureus 2022, 14, e29518. [Google Scholar] [CrossRef] [PubMed]
- Yarlagadda, S.G.; Perazella, M.A. Drug-induced crystal nephropathy: An update. Expert Opin. Drug Saf. 2008, 7, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Hebert, J.F.; Burfeind, K.G.; Malinoski, D.; Hutchens, M.P. Molecular Mechanisms of Rhabdomyolysis-Induced Kidney Injury: From Bench to Bedside. Kidney Int. Rep. 2023, 8, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Marcoff, L.; Thompson, P.D. The Role of Coenzyme Q10 in Statin-Associated Myopathy: A Systematic Review. J. Am. Coll. Cardiol. 2007, 49, 2231–2237. [Google Scholar] [CrossRef]
- Mollazadeh, H.; Tavana, E.; Fanni, G.; Bo, S.; Banach, M.; Pirro, M.; von Haehling, S.; Jamialahmadi, T.; Sahebkar, A. Effects of statins on mitochondrial pathways. J. Cachexia Sarcopenia Muscle 2021, 12, 237–251. [Google Scholar] [CrossRef] [PubMed]
- Daras, M.; Kakkouras, L.; Tuchman, A.J.; Koppel, B.S. Rhabdomyolysis and hyperthermia after cocaine abuse: A variant of the neuroleptic malignant syndrome? Acta Neurol. Scand. 1995, 92, 161–165. [Google Scholar] [CrossRef]
- Fernández-Cuadros, M.E.; Goizueta-San-Martin, G.; Varas-de-Dios, B.; Casique-Bocanegra, L.O.; Manrique-de-Lara-Cadiñanos, P.; Albaladejo-Florin, M.J.; Algarra-López, R.; Pérez-Moro, O.S. Colchicine-Induced Rhabdomyolysis: Clinical, Biochemical, and Neurophysiological Features and Review of the Literature. Clin. Med. Insights. Arthritis Musculoskelet. Disord. 2019, 12, 1179544119849883. [Google Scholar] [CrossRef] [PubMed]
- Packard, K.; Price, P.; Hanson, A. Antipsychotic use and the risk of rhabdomyolysis. J. Pharm. Pract. 2014, 27, 501–512. [Google Scholar] [CrossRef]
- Khosla, U.; Ruel, K.S.; Hunt, D.P. Antihistamine-induced rhabdomyolysis. South. Med. J. 2003, 96, 1023–1026. [Google Scholar] [CrossRef] [PubMed]
- Comelli, I.; Lippi, G.; Magnacavallo, A.; Cervellin, G. Mefloquine-associated rhabdomyolysis. Am. J. Emerg. Med. 2016, 34, 2250.e2255–2250.e2256. [Google Scholar] [CrossRef]
- Blain, P.G.; Lane, R.J.; Bateman, D.N.; Rawlins, M.D. Opiate-induced rhabdomyolysis. Hum. Toxicol. 1985, 4, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Arnold, D.M.; Patriquin, C.J.; Nazy, I. Thrombotic microangiopathies: A general approach to diagnosis and management. CMAJ Can. Med. Assoc. J. 2017, 189, E153–E159. [Google Scholar] [CrossRef] [PubMed]
- Mazzierli, T.; Allegretta, F.; Maffini, E.; Allinovi, M. Drug-induced thrombotic microangiopathy: An updated review of causative drugs, pathophysiology, and management. Front. Pharmacol. 2022, 13, 1088031. [Google Scholar] [CrossRef]
- Zakarija, A.; Kwaan, H.C.; Moake, J.L.; Bandarenko, N.; Pandey, D.K.; McKoy, J.M.; Yarnold, P.R.; Raisch, D.W.; Winters, J.L.; Raife, T.J.; et al. Ticlopidine- and clopidogrel-associated thrombotic thrombocytopenic purpura (TTP): Review of clinical, laboratory, epidemiological, and pharmacovigilance findings (1989–2008). Kidney Int. Suppl. 2009, 75, S20–S24. [Google Scholar] [CrossRef] [PubMed]
- Zakarija, A.; Bennett, C. Drug-induced thrombotic microangiopathy. Semin. Thromb. Hemost. 2005, 31, 681–690. [Google Scholar] [CrossRef] [PubMed]
- Page, E.E.; Little, D.J.; Vesely, S.K.; George, J.N. Quinine-Induced Thrombotic Microangiopathy: A Report of 19 Patients. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2017, 70, 686–695. [Google Scholar] [CrossRef] [PubMed]
- Daviet, F.; Rouby, F.; Poullin, P.; Moussi-Francès, J.; Sallée, M.; Burtey, S.; Mancini, J.; Duffaud, F.; Sabatier, R.; Pourroy, B.; et al. Thrombotic microangiopathy associated with gemcitabine use: Presentation and outcome in a national French retrospective cohort. Br. J. Clin. Pharmacol. 2019, 85, 403–412. [Google Scholar] [CrossRef]
- Hilburg, R.; Geara, A.S.; Qiu, M.K.; Palmer, M.B.; Chiang, E.Y.; Burger, R.A.; Hogan, J.J. Bevacizumab-associated thrombotic microangiopathy treated with eculizumab: A case series and systematic review of the literature. Clin. Nephrol. 2021, 96, 51–59. [Google Scholar] [CrossRef]
- Noronha, V.; Punatar, S.; Joshi, A.; Desphande, R.V.; Prabhash, K. Sunitinib-induced thrombotic microangiopathy. J. Cancer Res. Ther. 2016, 12, 6–11. [Google Scholar] [CrossRef]
- Arumugham, V.B.; Shahin, M.H. Therapeutic Uses of Diuretic Agents. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Oh, S.W.; Han, S.Y. Loop Diuretics in Clinical Practice. Electrolyte Blood Press 2015, 13, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Ho, K.M.; Power, B.M. Benefits and risks of furosemide in acute kidney injury. Anaesthesia 2010, 65, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Hegde, A. Diuretics in Acute Kidney Injury. Indian J. Crit. Care Med. 2020, 24 (Suppl. S3), S98–S99. [Google Scholar] [CrossRef] [PubMed]
- Miltiadous, G.; Mikhailidis, D.P.; Elisaf, M. Acid-base and electrolyte abnormalities observed in patients receiving cardiovascular drugs. J. Cardiovasc. Pharmacol. Ther. 2003, 8, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.; Liu, H.; Qi, W.; Jiang, H.; Li, Y.; Wu, X.; Sun, H.; Gross, K.; Salvi, R. Ototoxic effects and mechanisms of loop diuretics. J. Otol. 2016, 11, 145–156. [Google Scholar] [CrossRef]
- Kondo, C.S.; Macchionne, M.; Nakagawa, N.K.; de Carvalho, C.R.; King, M.; Saldiva, P.H.; Lorenzi-Filho, G. Effects of intravenous furosemide on mucociliary transport and rheological properties of patients under mechanical ventilation. Crit. Care 2002, 6, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Mullens, W.; Abrahams, Z.; Francis, G.S.; Sokos, G.; Taylor, D.O.; Starling, R.C.; Young, J.B.; Tang, W.H.W. Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J. Am. Coll. Cardiol. 2009, 53, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Damman, K.; van Deursen, V.M.; Navis, G.; Voors, A.A.; van Veldhuisen, D.J.; Hillege, H.L. Increased central venous pressure is associated with impaired renal function and mortality in a broad spectrum of patients with cardiovascular disease. J. Am. Coll. Cardiol. 2009, 53, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Mullens, W.; Abrahams, Z.; Skouri, H.N.; Francis, G.S.; Taylor, D.O.; Starling, R.C.; Paganini, E.; Tang, W.H. Elevated intra-abdominal pressure in acute decompensated heart failure: A potential contributor to worsening renal function? J. Am. Coll. Cardiol. 2008, 51, 300–306. [Google Scholar] [CrossRef]
- Jessup, M.; Costanzo, M.R. The cardiorenal syndrome: Do we need a change of strategy or a change of tactics? J. Am. Coll. Cardiol. 2009, 53, 597–599. [Google Scholar] [CrossRef] [PubMed]
- Palazzuoli, A.; Ruocco, G.; Ronco, C.; McCullough, P.A. Loop diuretics in acute heart failure: Beyond the decongestive relief for the kidney. Crit. Care 2015, 19, 296. [Google Scholar] [CrossRef]
- Hasselblad, V.; Gattis Stough, W.; Shah, M.R.; Lokhnygina, Y.; O’Connor, C.M.; Califf, R.M.; Adams, K.F., Jr. Relation between dose of loop diuretics and outcomes in a heart failure population: Results of the ESCAPE trial. Eur. J. Heart Fail. 2007, 9, 1064–1069. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, W.; Ren, H.; Chen, X.; Xie, J.; Chen, N. Diuretics associated acute kidney injury: Clinical and pathological analysis. Ren. Fail. 2014, 36, 1051–1055. [Google Scholar] [CrossRef] [PubMed]
- Nasr, S.H.; Milliner, D.S.; Wooldridge, T.D.; Sethi, S. Triamterene crystalline nephropathy. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2014, 63, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; He, D.; Zhang, W.; Xing, Y.; Guo, Y.; Wang, F.; Jia, J.; Yan, T.; Liu, Y.; Lin, S. ACE Inhibitor Benefit to Kidney and Cardiovascular Outcomes for Patients with Non-Dialysis Chronic Kidney Disease Stages 3–5: A Network Meta-Analysis of Randomised Clinical Trials. Drugs 2020, 80, 797–811. [Google Scholar] [CrossRef] [PubMed]
- Baltatzi, M.; Savopoulos, C.; Hatzitolios, A. Role of angiotensin converting enzyme inhibitors and angiotensin receptor blockers in hypertension of chronic kidney disease and renoprotection. Study results. Hippokratia 2011, 15, 27–32. [Google Scholar]
- Schoolwerth, A.C.; Sica, D.A.; Ballermann, B.J.; Wilcox, C.S. Renal Considerations in Angiotensin Converting Enzyme Inhibitor Therapy. Circulation 2001, 104, 1985–1991. [Google Scholar] [CrossRef] [PubMed]
- Knight, E.L.; Glynn, R.J.; McIntyre, K.M.; Mogun, H.; Avorn, J. Predictors of decreased renal function in patients with heart failure during angiotensin-converting enzyme inhibitor therapy: Results from the Studies of Left Ventricular Dysfunction (SOLVD). Am. Heart J. 1999, 138, 849–855. [Google Scholar] [CrossRef]
- Weinfeld, M.S.; Chertow, G.M.; Stevenson, L.W. Aggravated renal dysfunction during intensive therapy for advanced chronic heart failure. Am. Heart J. 1999, 138, 285–290. [Google Scholar] [CrossRef]
- Muslih, A.I. Reduction of mean arterial pressure and proteinuria by the effect of ACEIs (Lisinopril) in Kurdish hypertensive patients in Hawler City. Glob. J. Health Sci. 2012, 4, 14–19. [Google Scholar] [CrossRef]
- Swartz, S.L. The role of prostaglandins in mediating the effects of angiotensin converting enzyme inhibitors and other antihypertensive drugs. Cardiovasc. Drugs Ther. 1987, 1, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Banas, J.S., Jr. Effects of inhibitors of angiotensin-converting enzyme on regional hemodynamics. Am. J. Cardiol. 1992, 69, 40c–45c. [Google Scholar] [CrossRef] [PubMed]
- Lant, A.F. Evolution of diuretics and ACE inhibitors, their renal and antihypertensive actions–parallels and contrasts. Br. J. Clin. Pharmacol. 1987, 23 (Suppl. S1), 27s–41s. [Google Scholar] [CrossRef]
- Mandal, A.K.; Markert, R.J.; Saklayen, M.G.; Mankus, R.A.; Yokokawa, K. Diuretics potentiate angiotensin converting enzyme inhibitor-induced acute renal failure. Clin. Nephrol. 1994, 42, 170–174. [Google Scholar] [PubMed]
- Packer, M.; Lee, W.H.; Medina, N.; Yushak, M.; Kessler, P.D. Functional Renal Insufficiency During Long-Term Therapy with Captopril and Enalapril in Severe Chronic Heart Failure. Ann. Intern. Med. 1987, 106, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Wynckel, A.; Ebikili, B.; Melin, J.-P.; Randoux, C.; Lavaud, S.; Chanard, J. Long-term follow-up of acute renal failure caused by angiotensin converting enzyme inhibitors. Am. J. Hypertens. 1998, 11, 1080–1086. [Google Scholar] [CrossRef]
- Main, J. Atherosclerotic renal artery stenosis, ACE inhibitors, and avoiding cardiovascular death. Heart 2005, 91, 548–552. [Google Scholar] [CrossRef]
- Sica, D.A. Angiotensin-Converting Enzyme Inhibitors’ Side Effects—Physiologic and Non-Physiologic Considerations. J. Clin. Hypertens. 2007, 7 (Suppl. S8), 17–23. [Google Scholar] [CrossRef]
- Ponticelli, C.; Cucchiari, D. Renin-angiotensin system inhibitors in kidney transplantation: A benefit-risk assessment. J. Nephrol. 2017, 30, 155–157. [Google Scholar] [CrossRef]
- Calvo Barbado, D.M.; Saiz Fernández, L.C.; Leache Alegría, L.; Celaya Lecea, M.C.; Gutiérrez-Valencia, M. Acute Kidney Injury associated with “Triple whammy” combination: A protocol for a systematic review. F1000Research 2022, 11, 496. [Google Scholar] [CrossRef]
- Leete, J.; Wang, C.; López-Hernández, F.J.; Layton, A.T. Determining risk factors for triple whammy acute kidney injury. Math. Biosci. 2022, 347, 108809. [Google Scholar] [CrossRef] [PubMed]
- Loboz, K.K.; Shenfield, G.M. Drug combinations and impaired renal function—The ‘triple whammy’. Br. J. Clin. Pharmacol. 2005, 59, 239–243. [Google Scholar] [CrossRef]
- Patil, V.P.; Salunke, B.G. Fluid Overload and Acute Kidney Injury. Indian J. Crit. Care Med. 2020, 24, S94–S97. [Google Scholar] [CrossRef]
- Busse, L.W.; Ostermann, M. Vasopressor Therapy and Blood Pressure Management in the Setting of Acute Kidney Injury. Semin. Nephrol. 2019, 39, 462–472. [Google Scholar] [CrossRef] [PubMed]
- Mushiyakh, Y.; Dangaria, H.; Qavi, S.; Ali, N.; Pannone, J.; Tompkins, D. Treatment and pathogenesis of acute hyperkalemia. J. Community Hosp. Intern. Med. Perspect. 2011, 1, 7372. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, S.; Aucella, F.; De Nicola, L.; Genovesi, S.; Paoletti, E.; Regolisti, G. Management of hyperkalemia in patients with kidney disease: A position paper endorsed by the Italian Society of Nephrology. J. Nephrol. 2019, 32, 499–516. [Google Scholar] [CrossRef]
- Griffin, K.A.; Bidani, A.K. Potential risks of calcium channel blockers in chronic kidney disease. Curr. Cardiol. Rep. 2008, 10, 448–455. [Google Scholar] [CrossRef]
- Caiati, C.; Argentiero, A.; Favale, S.; Lepera, M.E. Cardiorenal Syndrome Triggered by Slowly Progressive Drugs Toxicity-Induced Renal Failure along with Minimal Mitral Disease: A Case Report. Endocr. Metab. Immune Disord. Drug Targets 2022, 22, 970–977. [Google Scholar] [CrossRef]
- Griffin, K.A.; Abu-Amarah, I.; Picken, M.; Bidani, A.K. Renoprotection by ACE Inhibition or Aldosterone Blockade Is Blood Pressure–Dependent. Hypertension 2003, 41, 201–206. [Google Scholar] [CrossRef]
- Mishima, E.; Maruyama, K.; Nakazawa, T.; Abe, T.; Ito, S. Acute Kidney Injury from Excessive Potentiation of Calcium-channel Blocker via Synergistic CYP3A4 Inhibition by Clarithromycin Plus Voriconazole. Intern. Med. 2017, 56, 1687–1690. [Google Scholar] [CrossRef]
- Gandhi, S.; Fleet, J.L.; Bailey, D.G.; McArthur, E.; Wald, R.; Rehman, F.; Garg, A.X. Calcium-channel blocker-clarithromycin drug interactions and acute kidney injury. JAMA 2013, 310, 2544–2553. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, R. Beta-blockers and renal function. Drugs 1982, 23, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Zech, P.; Pozet, N.; Labeeuw, M.; Laville, M.; Hadj-Aissa, A.; Arkouche, W.; Poncet, J.F. Acute renal effects of beta-blockers. Am. J. Nephrol. 1986, 6 (Suppl. S2), 15–19. [Google Scholar] [CrossRef]
- Sullivan, J.M.; Adams, D.F.; Hollenberg, N.K. beta-adrenergic blockade in essential hypertension: Reduced renin release despite renal vasoconstriction. Circ. Res. 1976, 39, 532–536. [Google Scholar] [CrossRef] [PubMed]
- Epstein, M.; Oster, J.R. Beta blockers and renal function: A reappraisal. J. Clin. Hypertens. 1985, 1, 85–99. [Google Scholar]
- Bakris, G.L.; Hart, P.; Ritz, E. Beta blockers in the management of chronic kidney disease. Kidney Int. 2006, 70, 1905–1913. [Google Scholar] [CrossRef]
- Kalaitzidis, R.; Bakris, G. Should nephrologists use beta-blockers? A perspective. Nephrol. Dial. Transplant. 2009, 24, 701–702. [Google Scholar] [CrossRef][Green Version]
- Hall, M.E.; Rocco, M.V.; Morgan, T.M.; Hamilton, C.A.; Jordan, J.H.; Edwards, M.S.; Hall, J.E.; Hundley, W.G. Beta-Blocker Use Is Associated with Higher Renal Tissue Oxygenation in Hypertensive Patients Suspected of Renal Artery Stenosis. Cardiorenal Med. 2016, 6, 261–268. [Google Scholar] [CrossRef]
- Borchard, U. Pharmacokinetics of beta-adrenoceptor blocking agents: Clinical significance of hepatic and/or renal clearance. Clin. Physiol. Biochem. 1990, 8 (Suppl. S2), 28–34. [Google Scholar]
- Capodanno, D.; Angiolillo, D.J. Antithrombotic Therapy in Patients With Chronic Kidney Disease. Circulation 2012, 125, 2649–2661. [Google Scholar] [CrossRef]
- Tsai, M.H.; Liou, H.H.; Huang, Y.C.; Lee, T.S.; Chen, M.; Fang, Y.W. Hazardous Effect of Low-Dose Aspirin in Patients with Predialysis Advanced Chronic Kidney Disease Assessed by Machine Learning Method Feature Selection. Healthcare 2021, 9, 1484. [Google Scholar] [CrossRef]
- Ricciotti, E.; FitzGerald, G.A. Prostaglandins and inflammation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 986–1000. [Google Scholar] [CrossRef]
- Crofford, L.J. COX-1 and COX-2 tissue expression: Implications and predictions. J. Rheumatol. Suppl. 1997, 49, 15–19. [Google Scholar] [PubMed]
- Shibata, K.; Akagi, Y.; Nozawa, N.; Shimomura, H.; Aoyama, T. Influence of nonsteroidal anti-inflammatory drugs on aspirin’s antiplatelet effects and suggestion of the most suitable time for administration of both agents without resulting in interaction. J. Pharm. Health Care Sci. 2017, 3, 9. [Google Scholar] [CrossRef]
- Bjorkman, D.J. The effect of aspirin and nonsteroidal anti-inflammatory drugs on prostaglandins. Am. J. Med. 1998, 105, 8S–12S. [Google Scholar] [CrossRef] [PubMed]
- Packer, M. Interaction of prostaglandins and angiotensin II in the modulation of renal function in congestive heart failure. Circulation 1988, 77, I64–I73. [Google Scholar] [CrossRef]
- Kramer, H.J.; Stinnesbeck, B.; Klautke, G.; Kipnowski, J.; Klingmueller, D.; Glaenzer, K.; Duesing, R. Interaction of renal prostaglandins with the renin-angiotensin and renal adrenergic nervous systems in healthy subjects during dietary changes in sodium intake. Clin. Sci. 1985, 68, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.H. Renal effects of prostaglandins and cyclooxygenase-2 inhibitors. Electrolyte Blood Press 2008, 6, 35–41. [Google Scholar] [CrossRef]
- Culpepper, R.M.; Andreoli, T.E. Interactions among prostaglandin E2, antidiuretic hormone, and cyclic adenosine monophosphate in modulating Cl- absorption in single mouse medullary thick ascending limbs of Henle. J. Clin. Investig. 1983, 71, 1588–1601. [Google Scholar] [CrossRef]
- Ejaz, P.; Bhojani, K.; Joshi, V.R. NSAIDs and kidney. J. Assoc. Physicians India 2004, 52, 632–640. [Google Scholar]
- Burukoglu, D.; Baycu, C.; Taplamacioglu, F.; Sahin, E.; Bektur, E. Effects of nonsteroidal anti-inflammatory meloxicam on stomach, kidney, and liver of rats. Toxicol. Ind. Health 2016, 32, 980–986. [Google Scholar] [CrossRef]
- Drożdżal, S.; Lechowicz, K.; Szostak, B.; Rosik, J.; Kotfis, K.; Machoy-Mokrzyńska, A.; Białecka, M.; Ciechanowski, K.; Gawrońska-Szklarz, B. Kidney damage from nonsteroidal anti-inflammatory drugs-Myth or truth? Review of selected literature. Pharmacol. Res. Perspect. 2021, 9, e00817. [Google Scholar] [CrossRef]
- Dreischulte, T.; Morales, D.R.; Bell, S.; Guthrie, B. Combined use of nonsteroidal anti-inflammatory drugs with diuretics and/or renin-angiotensin system inhibitors in the community increases the risk of acute kidney injury. Kidney Int. 2015, 88, 396–403. [Google Scholar] [CrossRef] [PubMed]
- McLeish, K.R.; Senitzer, D.; Gohara, A.F. Acute interstitial nephritis in a patient with aspirin hypersensitivity. Clin. Immunol. Immunopathol. 1979, 14, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Dixit, M.P.; Nguyen, C.; Carson, T.; Guedes, B.; Dixit, N.M.; Bell, J.M.; Wang, Y. Non-steroidal anti-inflammatory drugs-associated acute interstitial nephritis with granular tubular basement membrane deposits. Pediatr. Nephrol. 2008, 23, 145–148. [Google Scholar] [CrossRef]
- Ravnskov, U. Glomerular, tubular and interstitial nephritis associated with non-steroidal antiinflammatory drugs. Evidence of a common mechanism. Br. J. Clin. Pharmacol. 1999, 47, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Harirforoosh, S.; Asghar, W.; Jamali, F. Adverse effects of nonsteroidal antiinflammatory drugs: An update of gastrointestinal, cardiovascular and renal complications. J. Pharm. Pharm. Sci. 2013, 16, 821–847. [Google Scholar] [CrossRef]
- Leung, S.J.; Cisu, T.; Grob, B.M. Bilateral Ureteral Obstruction Secondary to Papillary Necrosis From Non-Steroidal Anti-Inflammatory Drug Use in an Adult Patient. Cureus 2021, 13, e16926. [Google Scholar] [CrossRef]
- Samuel, G.; Atanda, A.C.; Onyemeh, A.; Awan, A.; Ajiboye, O. A Unique Case of Drug Interaction between Ticagrelor and Statin Leading to Acute Renal Failure. Cureus 2017, 9, e1633. [Google Scholar] [CrossRef]
- Park, I.S.; Lee, S.B.; Song, S.H.; Seong, E.Y.; Kim, I.Y.; Rhee, H.; Kim, M.J.; Lee, D.W. Ticagrelor-induced acute kidney injury can increase serum concentration of statin and lead to concurrence of rhabdomyolysis. Anatol. J. Cardiol. 2018, 19, 225–226. [Google Scholar] [CrossRef]
- Tada, K.; Ito, K.; Hamauchi, A.; Takahashi, K.; Watanabe, R.; Uchida, A.; Abe, Y.; Yasuno, T.; Miyake, K.; Sasatomi, Y.; et al. Clopidogrel-induced Thrombotic Microangiopathy in a Patient with Hypocomplementemia. Intern. Med. 2016, 55, 969–973. [Google Scholar] [CrossRef][Green Version]
- Etta, P.K.; Gowrishankar, S. Clopidogrel Induced Thrombotic Microangiopathy Successfully Treated with Conservative Approach. Indian J. Nephrol. 2020, 30, 209–210. [Google Scholar] [CrossRef]
- Afiari, A.; Drekolias, D.; Jacob, J. Anticoagulant-Related Nephropathy: A Common, Under-Diagnosed Clinical Entity. Cureus 2022, 14, e22038. [Google Scholar] [CrossRef]
- Zakrocka, I.; Załuska, W. Anticoagulant-related nephropathy: Focus on novel agents. A review. Adv. Clin. Exp. Med. Off. Organ Wroc. Med. Univ. 2022, 31, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Brodsky, S.; Eikelboom, J.; Hebert, L.A. Anticoagulant-Related Nephropathy. J. Am. Soc. Nephrol. 2018, 29, 2787–2793. [Google Scholar] [CrossRef] [PubMed]
- Oliver, T.; Salman, L.A.; Ciaudelli, B.; Cohen, D.A. Anticoagulation-Related Nephropathy: The Most Common Diagnosis You’ve Never Heard Of. Am. J. Med. 2019, 132, e631–e633. [Google Scholar] [CrossRef] [PubMed]
- Brodsky, S.V.; Satoskar, A.; Chen, J.; Nadasdy, G.; Eagen, J.W.; Hamirani, M.; Hebert, L.; Calomeni, E.; Nadasdy, T. Acute kidney injury during warfarin therapy associated with obstructive tubular red blood cell casts: A report of 9 cases. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2009, 54, 1121–1126. [Google Scholar] [CrossRef]
- Brodsky, S.V.; Mhaskar, N.S.; Thiruveedi, S.; Dhingra, R.; Reuben, S.C.; Calomeni, E.; Ivanov, I.; Satoskar, A.; Hemminger, J.; Nadasdy, G.; et al. Acute kidney injury aggravated by treatment initiation with apixaban: Another twist of anticoagulant-related nephropathy. Kidney Res. Clin. Pract. 2017, 36, 387–392. [Google Scholar] [CrossRef]
- Mezue, K.; Ram, P.; Egbuche, O.; Menezes, R.G.; Lerma, E.; Rangaswami, J. Anticoagulation-related nephropathy for the internist: A concise review. Am. J. Cardiovasc. Dis. 2020, 10, 301–305. [Google Scholar]
- Liao, J.K.; Laufs, U. Pleiotropic effects of statins. Annu. Rev. Pharmacol. Toxicol. 2005, 45, 89–118. [Google Scholar] [CrossRef]
- Mach, F.; Ray, K.K.; Wiklund, O.; Corsini, A.; Catapano, A.L.; Bruckert, E.; De Backer, G.; Hegele, R.A.; Hovingh, G.K.; Jacobson, T.A.; et al. Adverse effects of statin therapy: Perception vs. the evidence—Focus on glucose homeostasis, cognitive, renal and hepatic function, haemorrhagic stroke and cataract. Eur. Heart J. 2018, 39, 2526–2539. [Google Scholar] [CrossRef] [PubMed]
- Ward, N.C.; Watts, G.F.; Eckel, R.H. Statin Toxicity. Circ. Res. 2019, 124, 328–350. [Google Scholar] [CrossRef]
- Wolfe, S.M. Dangers of rosuvastatin identified before and after FDA approval. Lancet 2004, 363, 2189–2190. [Google Scholar] [CrossRef]
- van Zyl-Smit, R.; Firth, J.C.; Duffield, M.; Marais, A.D. Renal tubular toxicity of HMG-CoA reductase inhibitors. Nephrol. Dial. Transplant. 2004, 19, 3176–3179. [Google Scholar] [CrossRef] [PubMed]
- Londrino, F.; Zattera, T.; Falqui, V.; Corbani, V.; Cavallini, M.; Stefanini, T.; Chiappini, N.; Ardini, M.; Martina, V.; Rombolà, G. Rosuvastatin-induced acute interstitial nephritis. Case Rep. Nephrol. Urol. 2013, 3, 87–90. [Google Scholar] [CrossRef] [PubMed]
- Annigeri, R.A.; Mani, R.M. Acute interstitial nephritis due to statin and its class effect. Indian J. Nephrol. 2015, 25, 54–56. [Google Scholar] [CrossRef]
- Ezad, S.; Cheema, H.; Collins, N. Statin-induced rhabdomyolysis: A complication of a commonly overlooked drug interaction. Oxf. Med. Case Rep. 2018, 2018, omx104. [Google Scholar] [CrossRef]
- Botshekan, S.; Yalameha, B. Are statins toxic or safe for kidney diseases? An updated mini-review study. J. Nephropathol. 2020, 9, e38. [Google Scholar] [CrossRef]
- Liu, A.; Wu, Q.; Guo, J.; Ares, I.; Rodríguez, J.-L.; Martínez-Larrañaga, M.-R.; Yuan, Z.; Anadón, A.; Wang, X.; Martínez, M.-A. Statins: Adverse reactions, oxidative stress and metabolic interactions. Pharmacol. Ther. 2019, 195, 54–84. [Google Scholar] [CrossRef]
- McMurray, J.J.; Dunselman, P.; Wedel, H.; Cleland, J.G.; Lindberg, M.; Hjalmarson, A.; Kjekshus, J.; Waagstein, F.; Apetrei, E.; Barrios, V.; et al. Coenzyme Q10, rosuvastatin, and clinical outcomes in heart failure: A pre-specified substudy of CORONA (controlled rosuvastatin multinational study in heart failure). J. Am. Coll. Cardiol. 2010, 56, 1196–1204. [Google Scholar] [CrossRef]
- Deichmann, R.; Lavie, C.; Andrews, S. Coenzyme q10 and statin-induced mitochondrial dysfunction. Ochsner J. 2010, 10, 16–21. [Google Scholar] [PubMed]
- Sirvent, P.; Mercier, J.; Vassort, G.; Lacampagne, A. Simvastatin triggers mitochondria-induced Ca2+ signaling alteration in skeletal muscle. Biochem. Biophys. Res. Commun. 2005, 329, 1067–1075. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, Y.; Ghorbanihaghjo, A.; Naghi-Zadeh, M.; Yagin, N.L. Oxidative stress as a possible mechanism of statin-induced myopathy. Inflammopharmacology 2018, 26, 667–674. [Google Scholar] [CrossRef]
- Verdoodt, A.; Honore, P.M.; Jacobs, R.; De Waele, E.; Van Gorp, V.; De Regt, J.; Spapen, H.D. Do Statins Induce or Protect from Acute Kidney Injury and Chronic Kidney Disease: An Update Review in 2018. J. Transl. Int. Med. 2018, 6, 21–25. [Google Scholar] [CrossRef]
- Chitralli, D.; Raheja, R.; Br, K. Clinical Rhabdomyolysis with Acute Kidney Injury Secondary to High-Intensity Rosuvastatin Use: A Case Report. Cureus 2020, 12, e10932. [Google Scholar] [CrossRef]
- Morschel, C.F.; Mafra, D.; Eduardo, J.C.C. The relationship between proton pump inhibitors and renal disease. J. Bras. De Nefrol. 2018, 40, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Moledina, D.G.; Perazella, M.A. PPIs and kidney disease: From AIN to CKD. J. Nephrol. 2016, 29, 611–616. [Google Scholar] [CrossRef]
- Van Laecke, S.; Van Biesen, W.; Vanholder, R. Hypomagnesaemia, the kidney and the vessels. Nephrol. Dial. Transpl. 2012, 27, 4003–4010. [Google Scholar] [CrossRef]
- Malavade, P.; Hiremath, S. Proton Pump Inhibitors: More Indigestion than Relief? Indian J. Nephrol. 2017, 27, 249–257. [Google Scholar] [CrossRef]
- Leonard, J.; Marshall, J.K.; Moayyedi, P. Systematic review of the risk of enteric infection in patients taking acid suppression. Am. J. Gastroenterol. 2007, 102, 2047–2056, quiz 2057. [Google Scholar] [CrossRef]
- Arrich, J.; Sodeck, G.H.; Sengolge, G.; Konnaris, C.; Mullner, M.; Laggner, A.N.; Domanovits, H. Clostridium difficile causing acute renal failure: Case presentation and review. World J. Gastroenterol. 2005, 11, 1245–1247. [Google Scholar] [CrossRef] [PubMed]
- Fontecha-Barriuso, M.; Martín-Sanchez, D.; Martinez-Moreno, J.M.; Cardenas-Villacres, D.; Carrasco, S.; Sanchez-Niño, M.D.; Ruiz-Ortega, M.; Ortiz, A.; Sanz, A.B. Molecular pathways driving omeprazole nephrotoxicity. Redox Biol. 2020, 32, 101464. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Gong, X. Research Progress on the Potential Mechanisms of Acute Kidney Injury and Chronic Kidney Disease Induced by Proton Pump Inhibitors. Integr. Med. Nephrol. Androl. 2023, 10, e00027. [Google Scholar] [CrossRef]
- Caiati, C.; Scardapane, A.; Iacovelli, F.; Pollice, P.; Achille, T.I.; Favale, S.; Lepera, M.E. Coronary Flow and Reserve by Enhanced Transthoracic Doppler Trumps Coronary Anatomy by Computed Tomography in Assessing Coronary Artery Stenosis. Diagnostics 2021, 11, 245. [Google Scholar] [CrossRef] [PubMed]
- McCullough, P.A.; Soman, S.S. Contrast-induced nephropathy. Crit. Care Clin. 2005, 21, 261–280. [Google Scholar] [CrossRef]
- Azzalini, L.; Spagnoli, V.; Ly, H.Q. Contrast-Induced Nephropathy: From Pathophysiology to Preventive Strategies. Can. J. Cardiol. 2016, 32, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Morcos, S.K.; Dawson, P.; Pearson, J.D.; Jeremy, J.Y.; Davenport, A.P.; Yates, M.S.; Tirone, P.; Cipolla, P.; de Haën, C.; Muschick, P.; et al. The haemodynamic effects of iodinated water soluble radiographic contrast media: A review. Eur. J. Radiol. 1998, 29, 31–46. [Google Scholar] [CrossRef]
- Tumlin, J.; Stacul, F.; Adam, A.; Becker, C.R.; Davidson, C.; Lameire, N.; McCullough, P.A. Pathophysiology of contrast-induced nephropathy. Am. J. Cardiol. 2006, 98, 14k–20k. [Google Scholar] [CrossRef]
- Persson, P.B.; Hansell, P.; Liss, P. Pathophysiology of contrast medium-induced nephropathy. Kidney Int. 2005, 68, 14–22. [Google Scholar] [CrossRef]
- Li, Y.; Ren, K. The Mechanism of Contrast-Induced Acute Kidney Injury and Its Association with Diabetes Mellitus. Contrast Media Mol. Imaging 2020, 2020, 3295176. [Google Scholar] [CrossRef]
- Seeliger, E.; Sendeski, M.; Rihal, C.S.; Persson, P.B. Contrast-induced kidney injury: Mechanisms, risk factors, and prevention. Eur. Heart J. 2012, 33, 2007–2015. [Google Scholar] [CrossRef]
- McCullough, P.A. Contrast-induced acute kidney injury. J. Am. Coll. Cardiol. 2008, 51, 1419–1428. [Google Scholar] [CrossRef] [PubMed]
- Modi, K.; Padala, S.A.; Gupta, M. Contrast-Induced Nephropathy. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Iftikhar, H.; Saleem, M.; Kaji, A. Metformin-associated Severe Lactic Acidosis in the Setting of Acute Kidney Injury. Cureus 2019, 11, e3897. [Google Scholar] [CrossRef] [PubMed]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxidative Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed]
- Deavall, D.G.; Martin, E.A.; Horner, J.M.; Roberts, R. Drug-induced oxidative stress and toxicity. J. Toxicol. 2012, 2012, 645460. [Google Scholar] [CrossRef]
- Tomsa, A.M.; Alexa, A.L.; Junie, M.L.; Rachisan, A.L.; Ciumarnean, L. Oxidative stress as a potential target in acute kidney injury. PeerJ 2019, 7, e8046. [Google Scholar] [CrossRef]
- Tirichen, H.; Yaigoub, H.; Xu, W.; Wu, C.; Li, R.; Li, Y. Mitochondrial Reactive Oxygen Species and Their Contribution in Chronic Kidney Disease Progression Through Oxidative Stress. Front. Physiol. 2021, 12, 627837. [Google Scholar] [CrossRef]
- Che, R.; Yuan, Y.; Huang, S.; Zhang, A. Mitochondrial dysfunction in the pathophysiology of renal diseases. Am. J. Physiol. Ren. Physiol. 2014, 306, F367–F378. [Google Scholar] [CrossRef]
- Daenen, K.; Andries, A.; Mekahli, D.; Van Schepdael, A.; Jouret, F.; Bammens, B. Oxidative stress in chronic kidney disease. Pediatr. Nephrol. 2019, 34, 975–991. [Google Scholar] [CrossRef]
- Ho, H.J.; Shirakawa, H. Oxidative Stress and Mitochondrial Dysfunction in Chronic Kidney Disease. Cells 2022, 12, 88. [Google Scholar] [CrossRef]
- Podkowińska, A.; Formanowicz, D. Chronic Kidney Disease as Oxidative Stress- and Inflammatory-Mediated Cardiovascular Disease. Antioxidants 2020, 9, 752. [Google Scholar] [CrossRef]
- Moledina, D.G.; Parikh, C.R. Differentiating Acute Interstitial Nephritis from Acute Tubular Injury: A Challenge for Clinicians. Nephron 2019, 143, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Hosohata, K. Role of Oxidative Stress in Drug-Induced Kidney Injury. Int. J. Mol. Sci. 2016, 17, 1826. [Google Scholar] [CrossRef] [PubMed]
- Arany, I.; Safirstein, R.L. Cisplatin nephrotoxicity. Semin. Nephrol. 2003, 23, 460–464. [Google Scholar] [CrossRef] [PubMed]
- Santos, N.A.; Catão, C.S.; Martins, N.M.; Curti, C.; Bianchi, M.L.; Santos, A.C. Cisplatin-induced nephrotoxicity is associated with oxidative stress, redox state unbalance, impairment of energetic metabolism and apoptosis in rat kidney mitochondria. Arch. Toxicol. 2007, 81, 495–504. [Google Scholar] [CrossRef]
- Lopez-Novoa, J.M.; Quiros, Y.; Vicente, L.; Morales, A.I.; Lopez-Hernandez, F.J. New insights into the mechanism of aminoglycoside nephrotoxicity: An integrative point of view. Kidney Int. 2011, 79, 33–45. [Google Scholar] [CrossRef]
- Kusirisin, P.; Chattipakorn, S.C.; Chattipakorn, N. Contrast-induced nephropathy and oxidative stress: Mechanistic insights for better interventional approaches. J. Transl. Med. 2020, 18, 400. [Google Scholar] [CrossRef] [PubMed]
- Manda, P.; Srinivasa Rao, P.; Bitla, A.R.; Vinapamula, K.S.; Jeyaseelan, L.; Rajasekhar, D.; Vishnubhotla, S. Study of contrast-induced oxidative stress in nondiabetic patients undergoing coronary angiography. Saudi J. Kidney Dis. Transpl. 2019, 30, 45–52. [Google Scholar]
- Hizoh, I.; Haller, C. Radiocontrast-induced renal tubular cell apoptosis: Hypertonic versus oxidative stress. Investig. Radiol. 2002, 37, 428–434. [Google Scholar] [CrossRef]
- Nascimento, M.M.; Bernardo, D.R.D.; de Bragança, A.C.; Massola Shimizu, M.H.; Seguro, A.C.; Volpini, R.A.; Canale, D. Treatment with β-blocker nebivolol ameliorates oxidative stress and endothelial dysfunction in tenofovir-induced nephrotoxicity in rats. Front. Med. 2022, 9, 953749. [Google Scholar] [CrossRef]
- Gokturk, H.; Ulusu, N.N.; Gok, M.; Tuncay, E.; Can, B.; Turan, B. Long-term treatment with a beta-blocker timolol attenuates renal-damage in diabetic rats via enhancing kidney antioxidant-defense system. Mol. Cell. Biochem. 2014, 395, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Coats, A.; Jain, S. Protective effects of nebivolol from oxidative stress to prevent hypertension-related target organ damage. J. Hum. Hypertens. 2017, 31, 376–381. [Google Scholar] [CrossRef]
- Nakamura, K.; Murakami, M.; Miura, D.; Yunoki, K.; Enko, K.; Tanaka, M.; Saito, Y.; Nishii, N.; Miyoshi, T.; Yoshida, M.; et al. Beta-Blockers and Oxidative Stress in Patients with Heart Failure. Pharmaceuticals 2011, 4, 1088–1100. [Google Scholar] [CrossRef] [PubMed]
- Caiati, C.; Stanca, A.; Lepera, M.E. Free Radicals and Obesity-Related Chronic Inflammation Contrasted by Antioxidants: A New Perspective in Coronary Artery Disease. Metabolites 2023, 13, 712. [Google Scholar] [CrossRef] [PubMed]
- Ayza, M.A.; Zewdie, K.A.; Yigzaw, E.F.; Ayele, S.G.; Tesfaye, B.A.; Tafere, G.G.; Abrha, M.G. Potential Protective Effects of Antioxidants against Cyclophosphamide-Induced Nephrotoxicity. Int. J. Nephrol. 2022, 2022, 5096825. [Google Scholar] [CrossRef] [PubMed]
- Ranasinghe, R.; Mathai, M.; Zulli, A. Cytoprotective remedies for ameliorating nephrotoxicity induced by renal oxidative stress. Life Sci. 2023, 318, 121466. [Google Scholar] [CrossRef]
- Tylicki, L.; Rutkowski, B.; Hörl, W.H. Antioxidants: A possible role in kidney protection. Kidney Blood Press Res. 2003, 26, 303–314. [Google Scholar] [CrossRef]
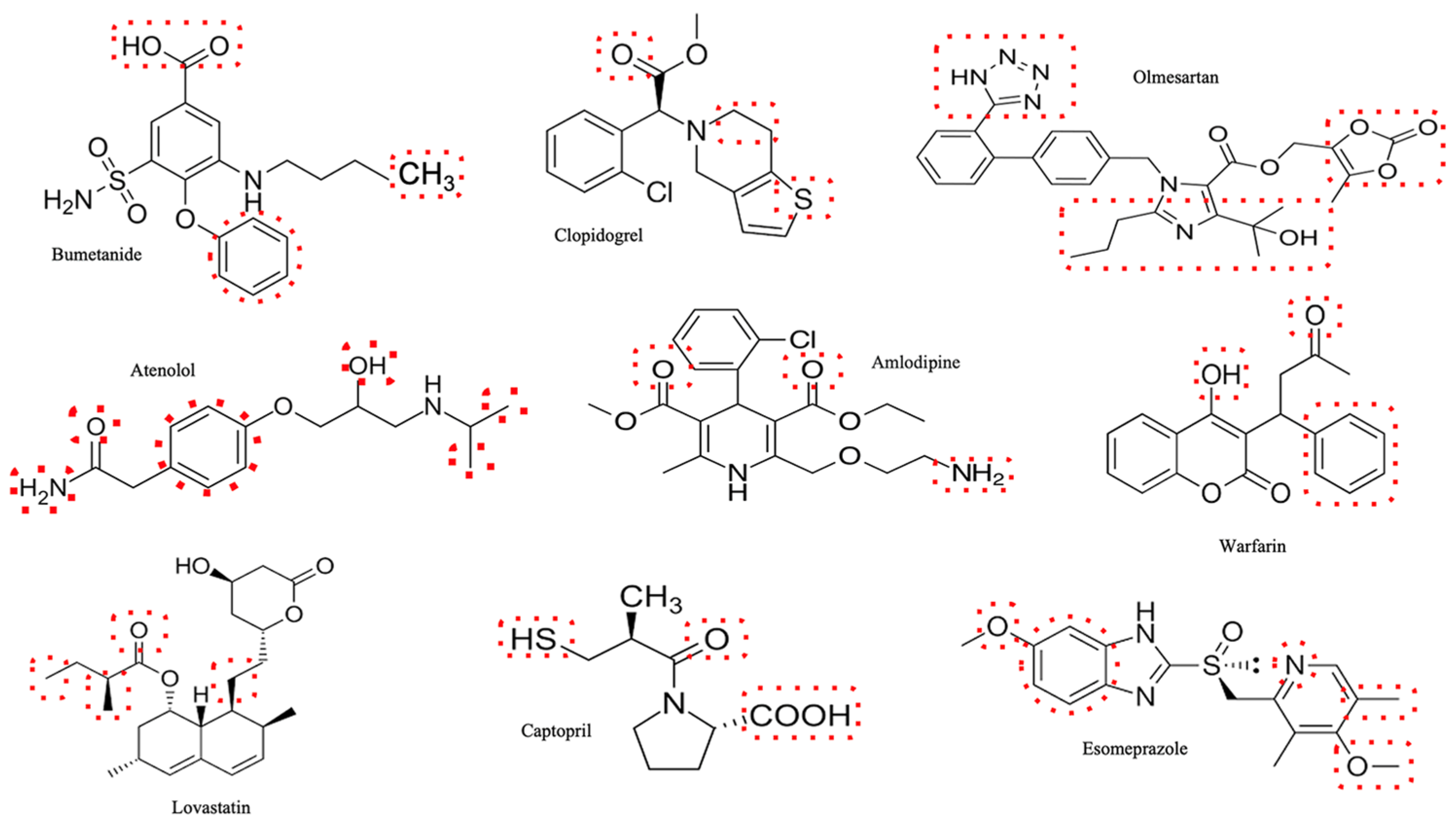
| Glomerulus | Tubules | Other Renal Toxic Effects | Factors That Enhance Toxicity | |
|---|---|---|---|---|
| Diuretics (loop) | Reduction of RBF and GF | Obstruction (Tamm–Horsfall protein formation) | Paradoxical stimulation of RAAS | ACEIs/NSAIDs |
| ACEIs and ARBs | Reduced IG-P (dilation Eart) | - | - | NSAIDs and diuretics (triple whammy) |
| CCBs | Increase IG-P (dilation Aart) (more proteinuria) | - | - | CYP3A4 inhibitors (Clarithromycin) |
| BBs | Decrease in GFR (mild) | - | - | - |
| APAs | Decrease in GFR for intense vasoconstriction (PGEi) | AIN | - | Dehydration, HF, LC, sepsis, diuretics, and ACEIs; statins |
| ACs | Occlusive casts Bowman space | Occlusive cast | - | Dehydration |
| Statins | - | TIN | Suppression Q10 | Rhabdomyolysis |
| PPIs | - | TIN | Hypomagnesemia | Enteric infection |
| CM | Vasospasm | Direct tubular cell damage | - | Hypotension, microembolism, and bleeding; metformin |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caiati, C.; Arrigoni, R.; Stanca, A.; Lepera, M.E. Kidney Toxicity of Drugs for the Heart: An Updated Perspective. Metabolites 2025, 15, 191. https://doi.org/10.3390/metabo15030191
Caiati C, Arrigoni R, Stanca A, Lepera ME. Kidney Toxicity of Drugs for the Heart: An Updated Perspective. Metabolites. 2025; 15(3):191. https://doi.org/10.3390/metabo15030191
Chicago/Turabian StyleCaiati, Carlo, Roberto Arrigoni, Alessandro Stanca, and Mario Erminio Lepera. 2025. "Kidney Toxicity of Drugs for the Heart: An Updated Perspective" Metabolites 15, no. 3: 191. https://doi.org/10.3390/metabo15030191
APA StyleCaiati, C., Arrigoni, R., Stanca, A., & Lepera, M. E. (2025). Kidney Toxicity of Drugs for the Heart: An Updated Perspective. Metabolites, 15(3), 191. https://doi.org/10.3390/metabo15030191






