1. Introduction
Metabolic-associated fatty liver disease (MAFLD) refers to a fatty liver disease accompanied by one or more cardiovascular and metabolic risk factors, in the absence of excessive alcohol consumption, affecting approximately 25% of the general population globally and over half of patients with metabolic disorders [
1]. MAFLD has become one of the most significant causes of liver diseases worldwide and may soon emerge as a primary driver of liver diseases progressing to end-stage liver diseases [
2]. Its pathogenesis is driven by metabolic inflammation (meta-inflammation), a chronic low-grade inflammatory state originating from adipose tissue dysfunction and hepatocyte lipotoxicity, ultimately leading to progressive liver injury [
3].
Cytochrome P450 (CYP) enzymes are a superfamily of heme-dependent monooxygenases widely distributed in organisms, with the highest expression in the liver. In humans, 57 putatively functional genes have been identified, approximately 12 of which are crucial for the metabolism of most clinically used drugs [
4]. The expression and activity of these enzymes exhibit significant variabilities both between and within individuals. Key determinants of interindividual differences include genetic polymorphisms, sex, age, disease status, and environmental factors [
5]. As the core enzyme system of drug metabolism, CYP is involved in approximately 75% of clinical drug oxidative metabolism [
6]. Recent studies have revealed that CYP activities are closely associated with the pathogenesis of metabolic diseases. The dysregulation of hepatic lipid metabolism can significantly alter CYP expression and function: on one hand, subtypes such as CYP2E1 and CYP4A are overactivated to promote lipid peroxidation and reactive oxygen species (ROS) production, thereby exacerbating liver injuries [
7]; on the other hand, the activity of drug-metabolizing enzymes like CYP3A4 and CYP2C19 is inhibited, leading to reduced drug clearance and increased medication risks for patients with MAFLD [
8].
Schisandra chinensis (Turcz.) Baill. is a dried mature fruit of the Magnoliaceae plant. As a traditional Chinese medicine, its medicinal history covers thousands of years [
9].
Schisandra chinensis is commonly used in clinical practice to treat chemical and viral liver injuries. The chemical composition of
Schisandra chinensis is relatively complex, with the main active ingredients including lignans and polysaccharides. Studies have shown that lignans in
Schisandra chinensis can significantly delay the progression of MAFLD [
10,
11,
12].
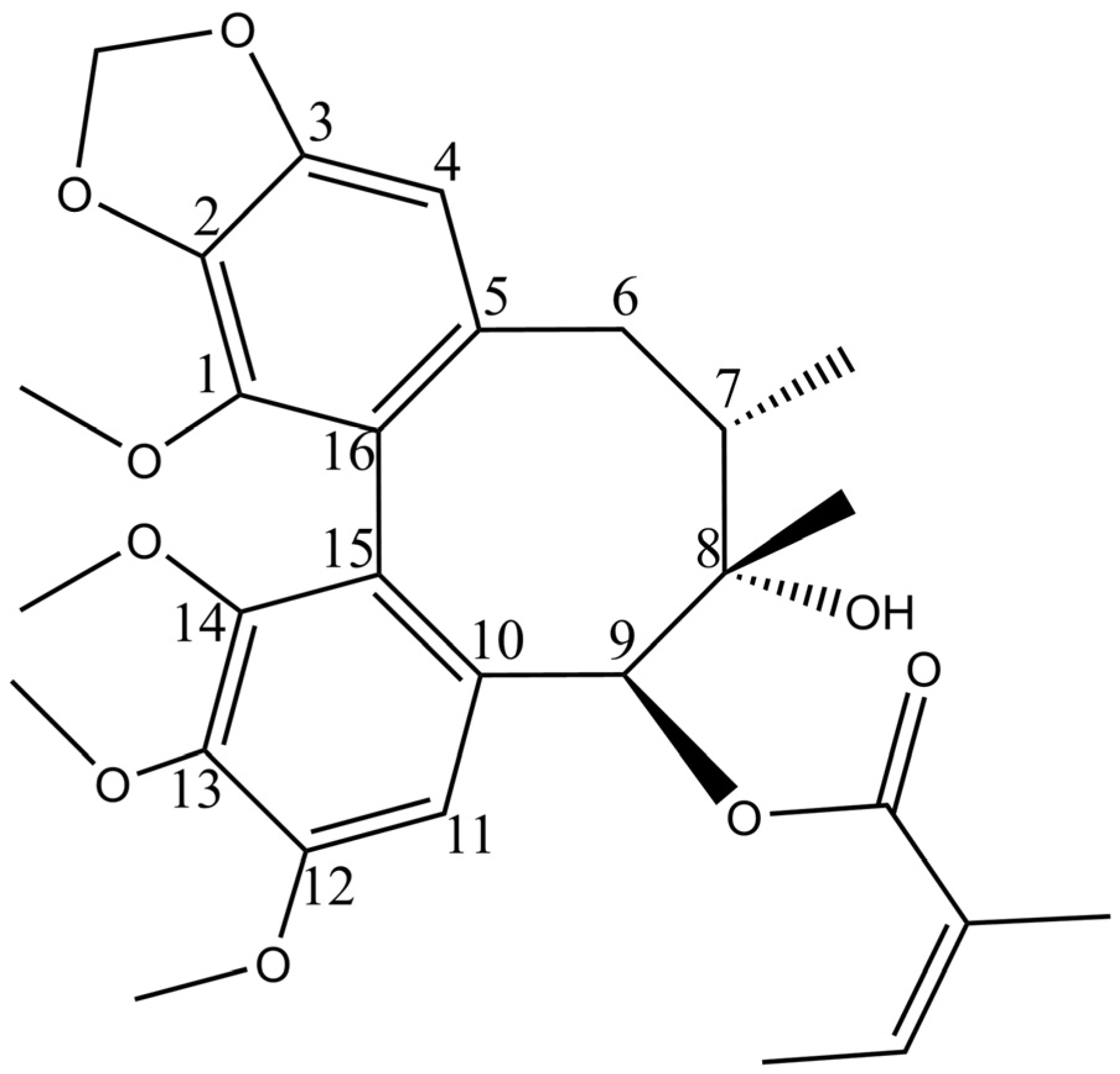
Our previous studies showed that schisantherin B (STB,
Figure 1) could regulate various indicators in the serum of hyperlipidemic mice, including reducing the levels of total cholesterol (TC), triglycerides (TG), and low-density lipoprotein cholesterol (LDL-C) and increasing the levels of high-density lipoprotein cholesterol (HDL-C). STB can correct blood lipid disorders and alleviate liver steatosis, indicating that it is one of the effective ingredients in
Schisandra chinensis for the treatment of MAFLD, with a good research value [
13].
Liver enzymes play a crucial role in drug metabolism in the body. Liver disease patients often have pathological changes in the liver, and the pharmacokinetics of drugs may also change accordingly [
14]. It has been shown that the activity of drug-metabolizing enzymes may change in chronic liver diseases, which may be due to their affecting the clearance of drugs through liver metabolism or bile excretion, thereby affecting drug metabolism and excretion processes [
15]; so, it is crucial to jointly study the pharmacokinetics of drugs in the body and the changes in liver enzyme activity. Our research group also studied the pharmacokinetic characteristics of
Schisandra chinensis lignans in vivo and their distribution in tissues [
16,
17]. So far, there have been no studies on the pharmacokinetic differences of STB in normal and MAFLD models in vivo.
Therefore, in this study, HPLC-ESI-MS/MS was used to determine the drug concentrations in the blood and liver of normal and MAFLD mice after oral administration of STB, and the pharmacokinetic characteristics were compared and analyzed. The cocktail method was also used to determine the activity of various subtypes of CYP450 enzymes in MAFLD liver cells. Simultaneously, metabolomics methods were used to conduct in vivo metabolic analysis on blood and liver samples, in order to provide references for the clinical application of Schisandra chinensis.
4. Discussion
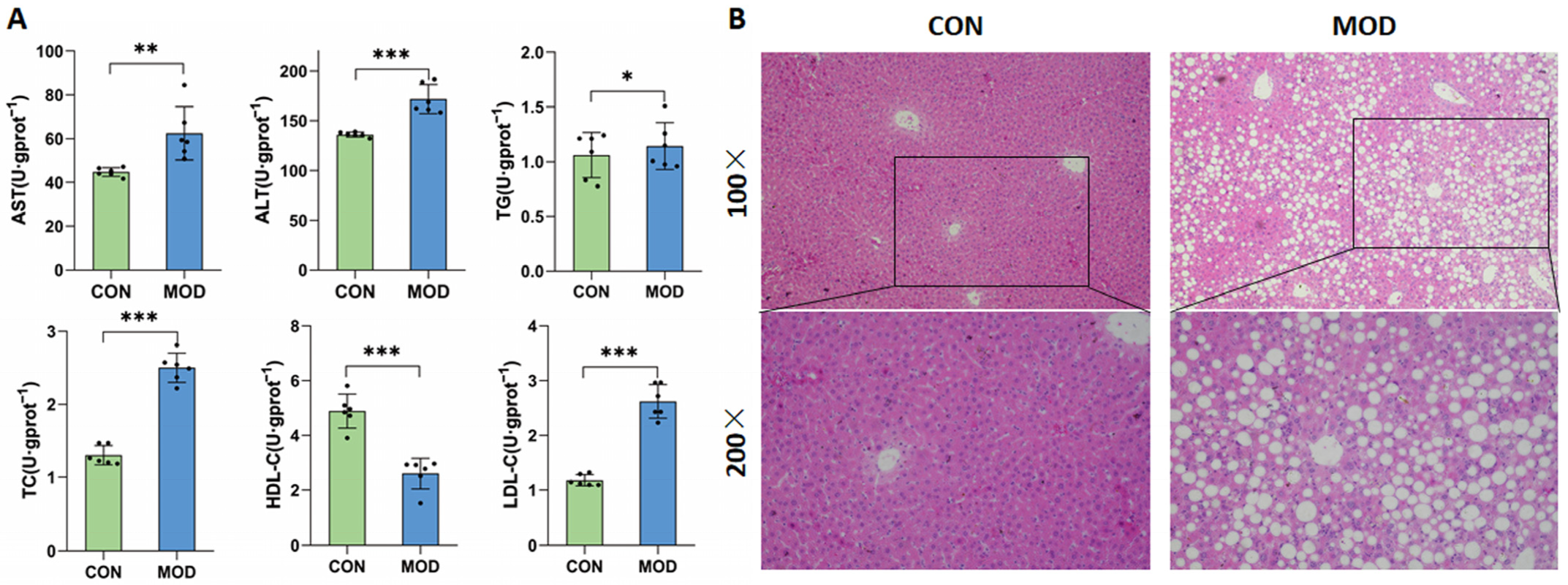
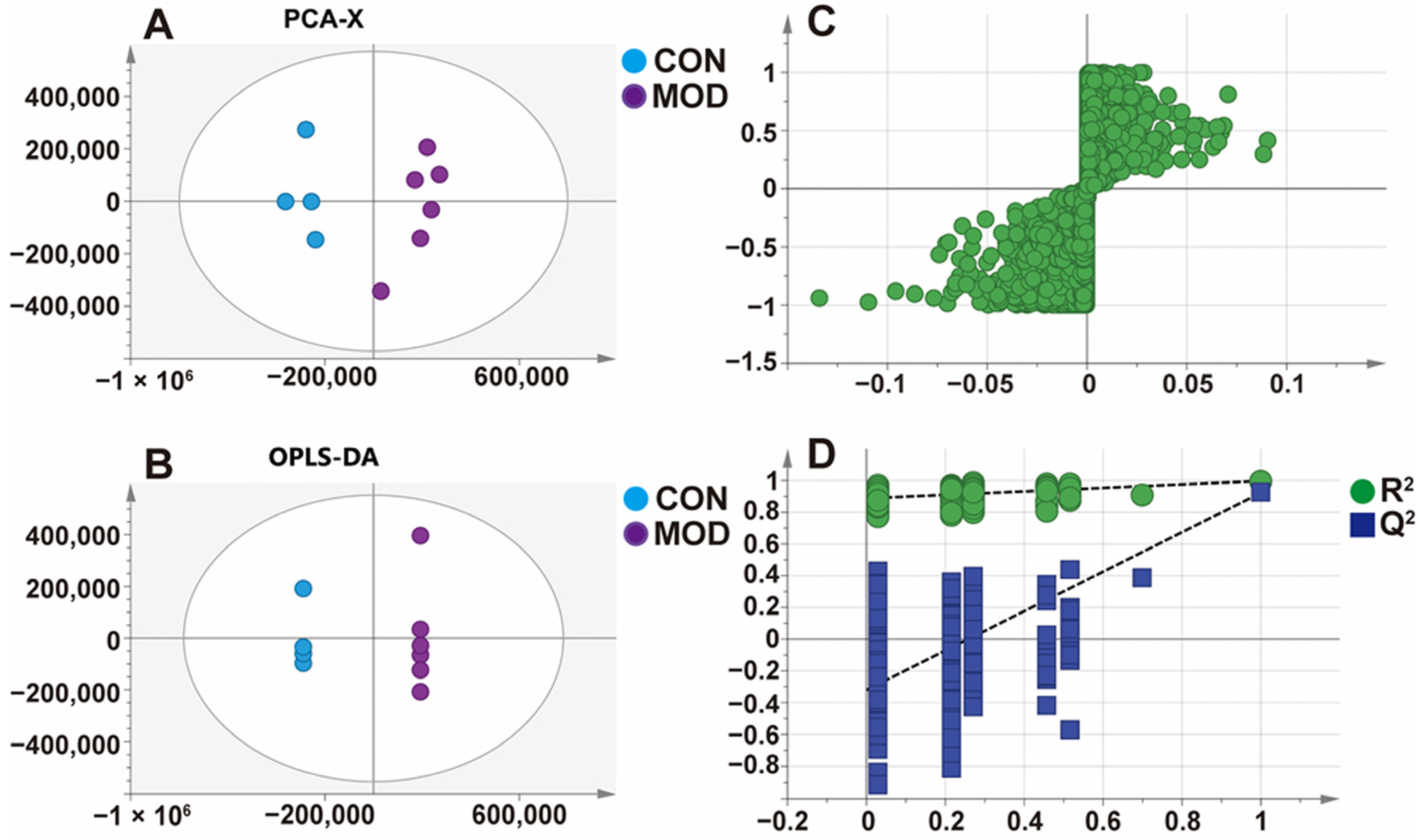
In this study, a MAFLD model was successfully established by feeding mice with a high-fat diet for induction. The model mice exhibited lipid metabolism disorders, with significantly increased levels of the serum AST, ALT, TC, TG, and LDL-C, significantly decreased levels of the serum HDL-C, and a significant hepatic steatosis. AST and ALT are often used as sensitive indicators of liver injury in clinical practice [
24]. Fatty liver disease is closely related to lipid metabolism. Due to the reduced ability of the liver to metabolize fat, free fatty acids enter the body and cannot be metabolized normally, leading to an increase in circulating levels of TC, TG, and LDL-C and a decrease in HDL-C.
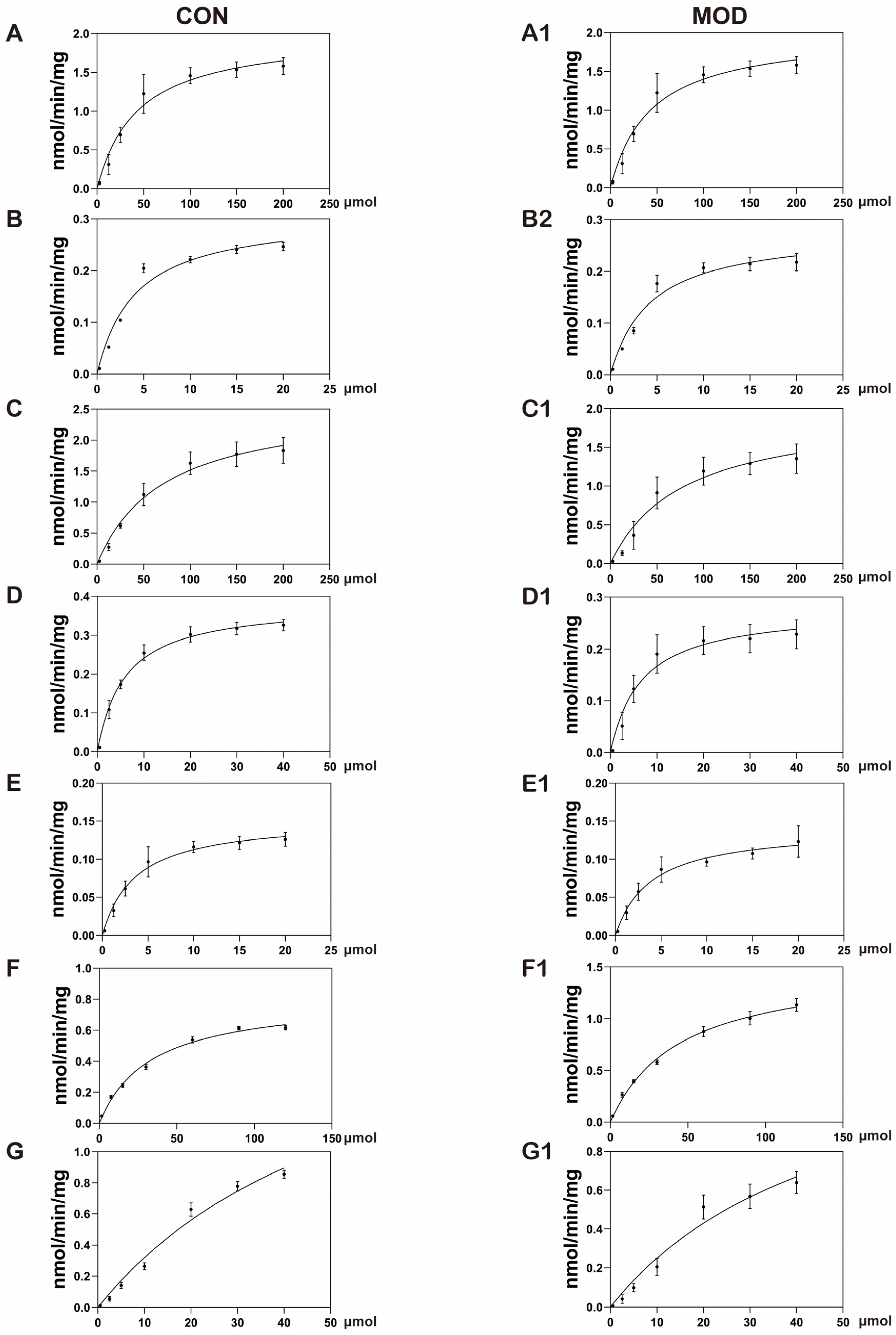
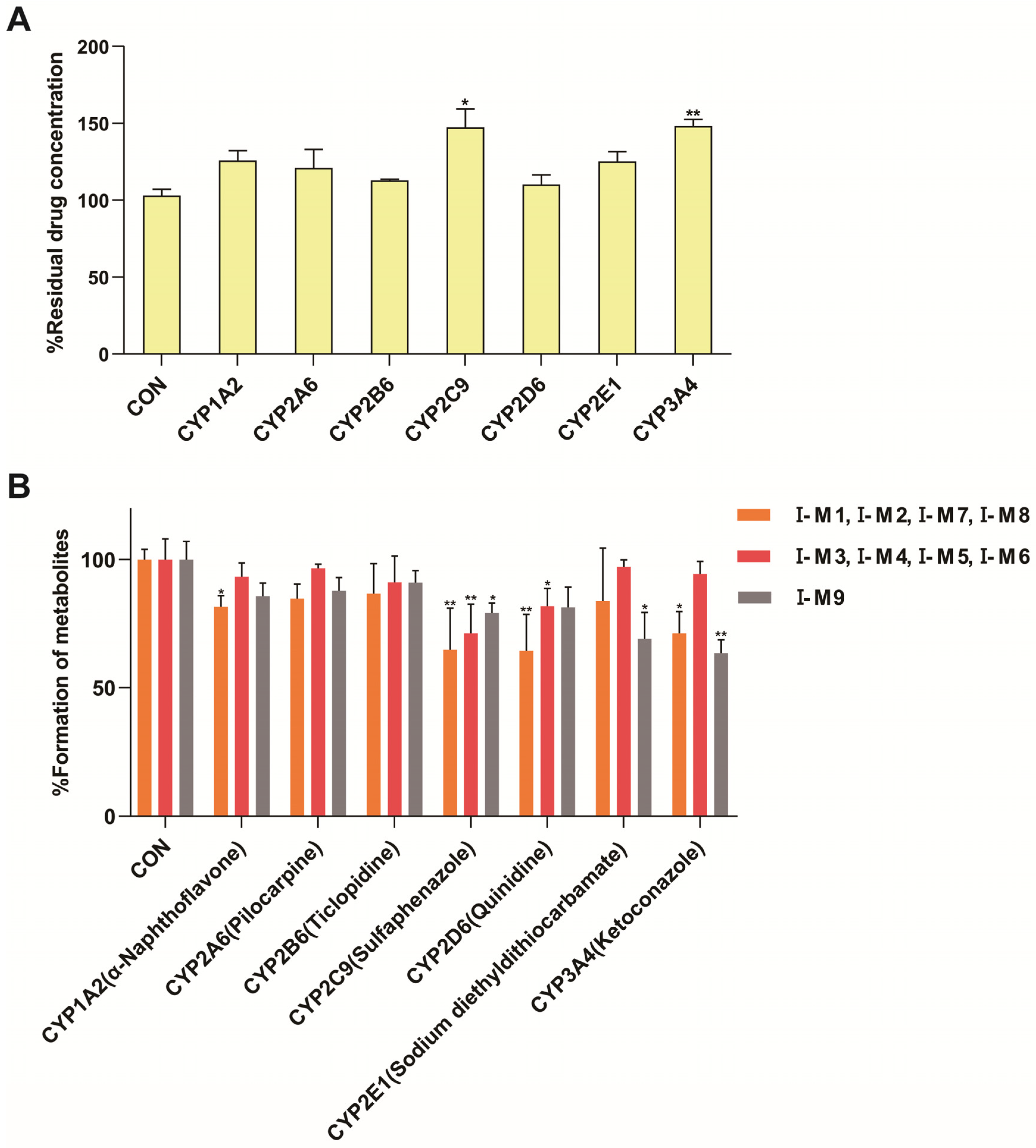
The in vitro incubation of AML-12 cells showed that multiple changes in cytochrome P450 enzymes could be observed in MAFLD model mice, including a 23% decrease in CYP1A2 activity, a 24% decrease in CYP2B6 activity, a 26% decrease in CYP2C9 activity, and a 33% decrease in CYP3A4 activity. There was no significant change in the activity of CYP2A6 and CYP2D6, while CYP2E1 activity increased nearly two-fold. A study analyzing liver biopsy samples from MAFLD patients showed a 30% decrease in CYP3A4 activity during the simple fatty lesion stage and a 50% decrease during the NASH stage, while the CYP2E1 activity increased by 2.5 times during the NASH stage [
25]. Another study on the free fatty acid-induced MAFLD model of human liver cells also showed similar results, in which a 30–50% decrease in the activity of CYP1A2 and CYP2C9 was found [
26]. The reasons for the changes in the activity of cytochrome P450 enzymes in the MAFLD model are shown in
Table 8.
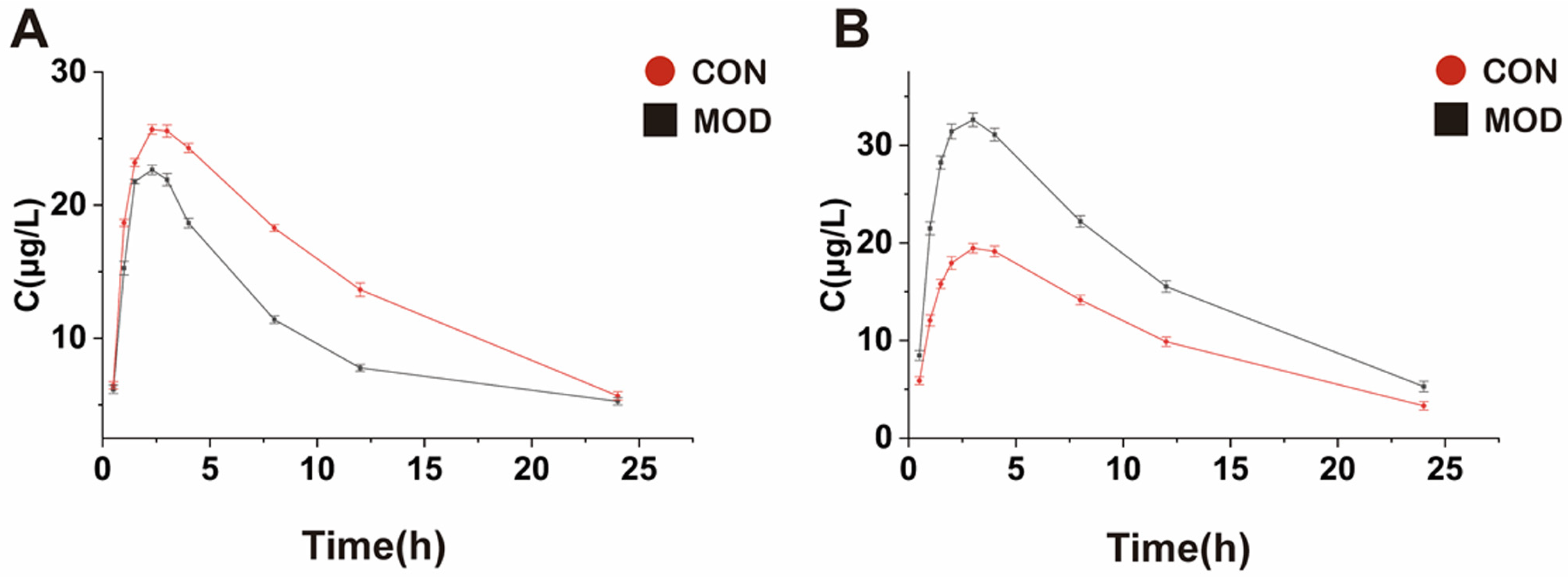
The study on pharmacokinetic characteristics revealed significant differences in the pharmacokinetic characteristics of STB between normal and MAFLD mice. Based on the bioequivalence criteria, the significant difference in drug exposure in MAFLD patients is defined as a 20% increase or decrease in the mean
AUC in the model group compared to the control group [
33]. In this study, the
AUC of STB in the liver tissue of MAFLD mice was significantly reduced (the mean
AUC was decreased by 40.2%),
Cmax was significantly decreased,
t1/2 was prolonged,
Tmax and
MRT were shortened, and
CL was increased. The results showed that the total concentration of STB in the liver of MAFLD mice decreased, which is likely due to the occurrence of fatty lesions in the liver, leading to a decrease in microvascular blood flow and total effective liver cells, further resulting in a relative decrease in effective blood flow to the liver, ultimately reducing the exposure of STB in the liver of MAFLD mice. MAFLD is often accompanied by increased intestinal permeability and gastrointestinal motility abnormalities, which may lead to a reduced drug absorption. The increase in
CL and the prolongation of
t1/2 may be due to the effect of changes in metabolic enzyme activity and hepatic blood flow on the metabolism and excretion of STB.
Contrary to the results in the liver, the
AUC of STB in the blood of MAFLD mice significantly increased (the mean
AUC increased by 42.58%), the
Cmax increased,
t1/2,
Tmax, and
MRT were shortened, and
CL decreased, which may be likely due to the decreased activity of liver microsomal enzymes in MAFLD mice, leading to a decrease in STB metabolism and an increase in its content. An increase in
Cmax and a decrease in
Tmax indicate an accelerated absorption of STB in the intestine or a weakened first pass effect. A significant increase in
AUC and a decrease in
CL may indicate impaired liver metabolism or bile excretion disorders. Xie et al. also successfully established a MAFLD model by administering a high-fat diet to rats and studied the pharmacokinetic behavior of lovastatin in MAFLD rats. The experimental results showed that the exposure of lovastatin in the plasma of MAFLD rats increased. After analyzing the reasons, the researchers believe that the main metabolic enzyme of lovastatin is CYP3A2. The pathological state of liver injury may lead to a decrease in the expression of this enzyme, thereby slowing down the metabolism of lovastatin, and the results are consistent with those reported in the related studies [
34,
35].
Drug-metabolizing enzymes in the body are mainly divided into phase I metabolizing enzymes and phase II metabolizing enzymes. The former mainly comprises oxidases, reducdases, and hydrolases, and the latter mainly comprises conjugated enzymes, of which the key enzyme systems involved in oxidative metabolism mainly include CYP, flavin monooxidase, monoamine oxidase, aldehyde dehydrogenase, ethanol dehydrogenase, aldehyde oxidase, and xanthine oxidase [
36]. The existing research evidence suggests that the expression levels of drug-metabolizing enzymes (especially CYP) in the body are negatively correlated with drug exposure, and when CYP expression is downregulated, the systemic exposure of its metabolites may increase, while when its expression is upregulated, the substrate clearance accelerates, and the exposure decreases [
37], consistent with our experimental results. Therefore, it is necessary to monitor these patients more closely in clinical practice to avoid the risk of toxicity [
38]. In addition, free fatty acids in patients’ bodies can increase in MAFLD, leading to increased oxidative stress, which may result in the overexpression of CYP2E1 and CYP4A mRNA, as well as the increased activity of CYP2E1 [
39,
40]. Existing studies have identified multiple CYP subtypes involved in the metabolism of Schisandra chinensis lignans, mainly including CYP2C6, CYP2C11, CYP2E1, CYP3A, CYP1A2, and CYP2D2 [
23,
41,
42,
43], similar to the results that STB is mainly metabolized by the CYP3A4 and CYP2C9 enzymes.
In recent years, there has been an increasing amount of research on the in vivo metabolic pathways of
Schisandra chinensis lignans.
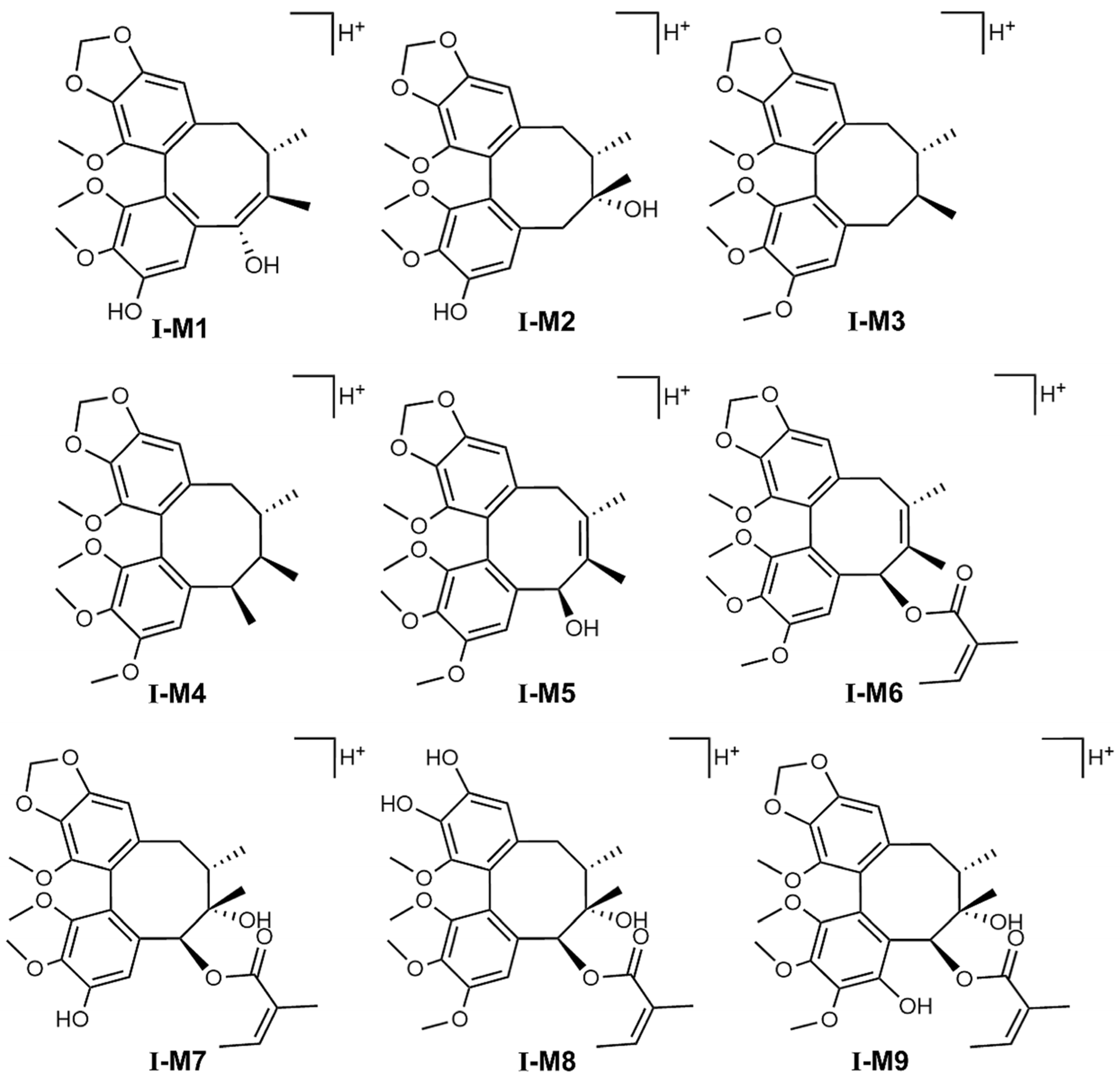
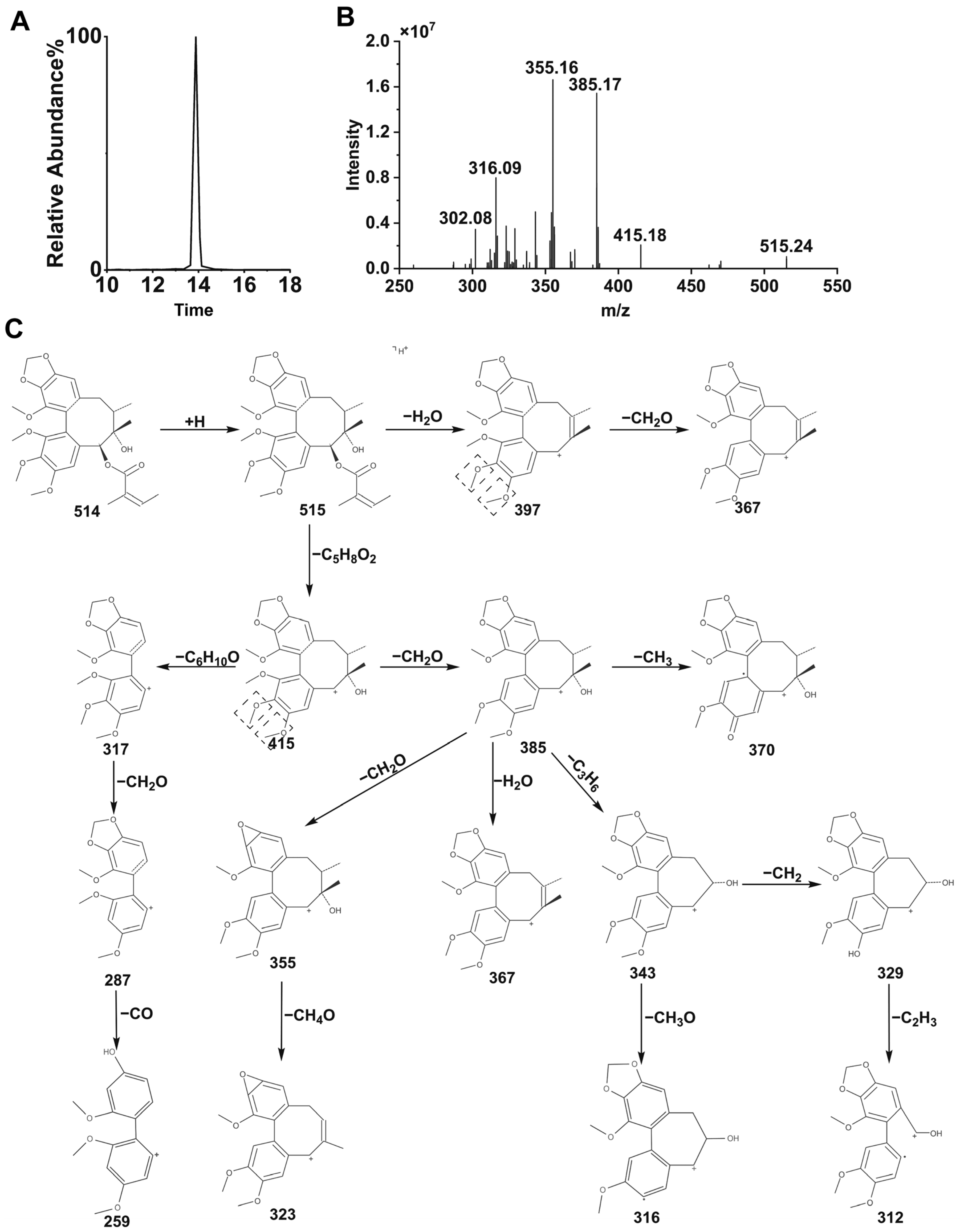
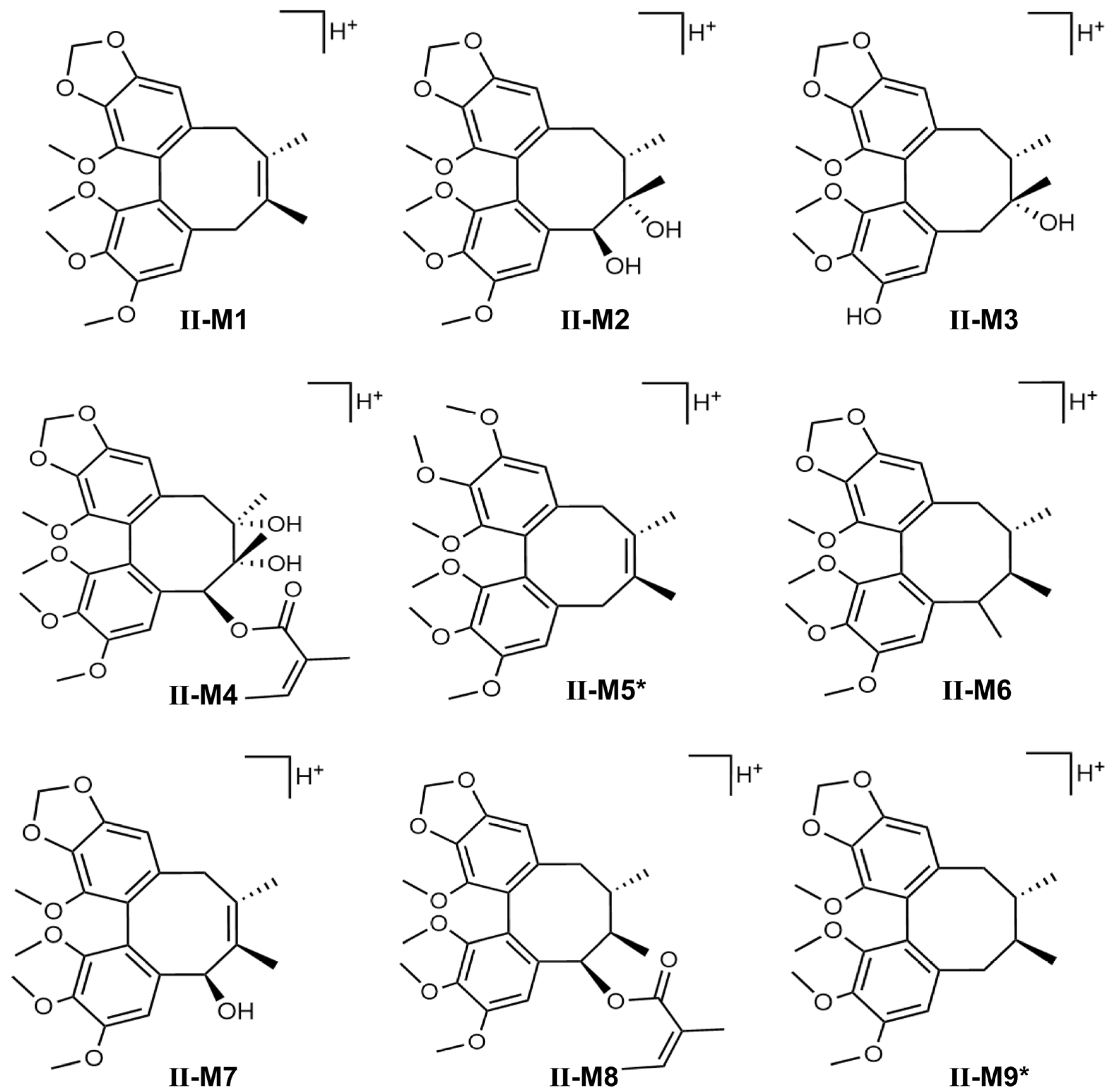
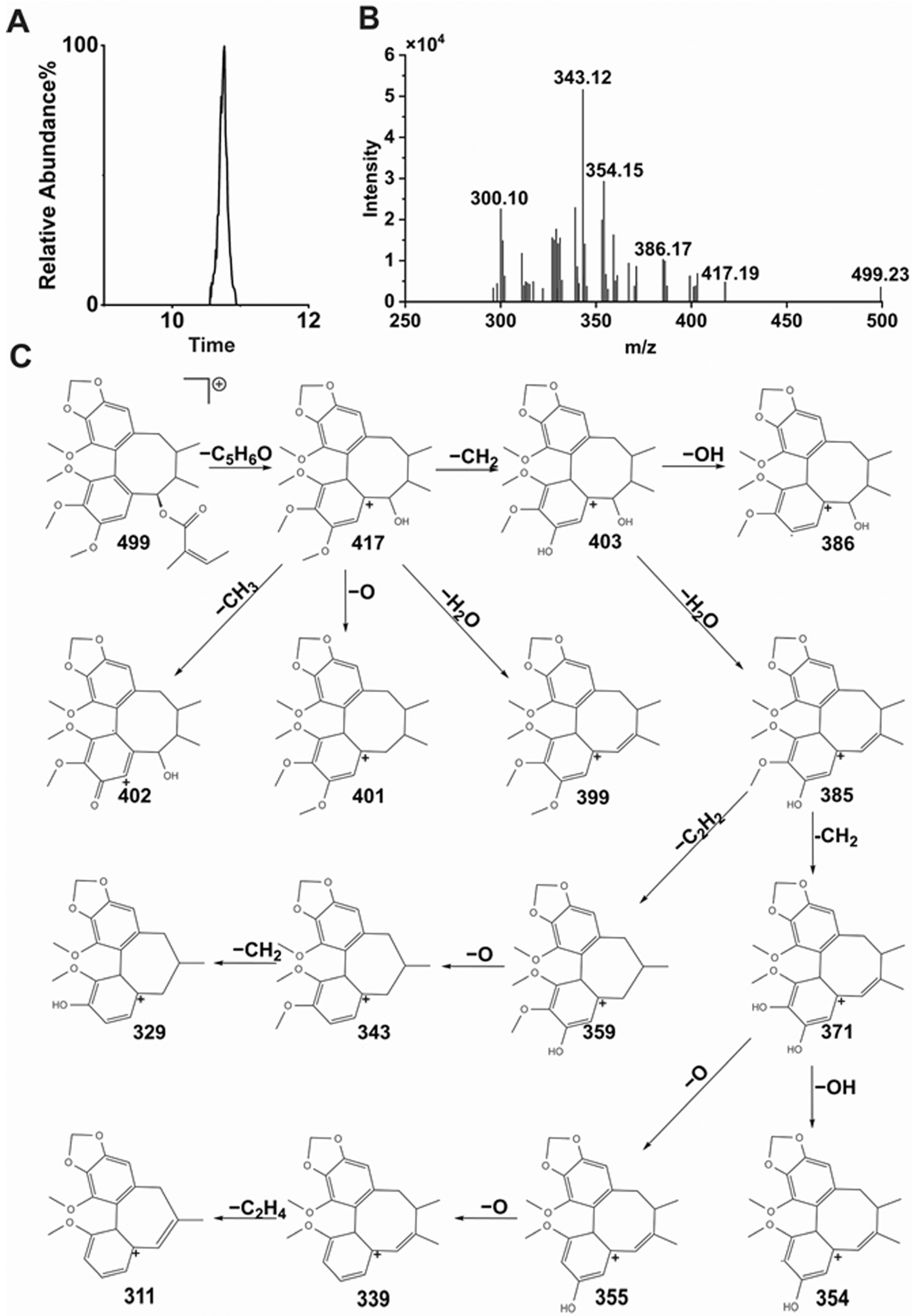
Schisandra chinensis lignans are mostly based on biphenyl cyclooctene as their skeleton structures, with side chains connecting different functional groups, and many studies have shown that substances with similar structures often have the same metabolic patterns. Zhang et al. pointed out that the metabolic pathways of STB in vivo are mainly demethoxylation, demethylation, and oxidation [
23]. The results of this study also confirmed that STB was mainly metabolized in mice through several pathways such as demethoxylation, demethylation, and oxidation, consistent with the reported results.
It can be seen from the in vivo analysis of STB metabolites that its metabolites are mainly concentrated in the liver, with only a portion able to enter the bloodstream, which may reflect specific changes in liver metabolic activity and transport substances in MAFLD, such as the compensatory enhancement of liver metabolic enzyme activity, the obstruction of hepatic metabolite efflux, the accelerated clearance of metabolites in the blood, and differences in tissue distribution. It has been found that Schisandra lignans exhibits significant hepatic intestinal circulation characteristics in the body. Its metabolic process shows that a large amount of prototype drugs and their metabolites can enter the intestine with bile and undergo intestinal reabsorption or be excreted from the intestine. This circulation pattern may lead to a higher distribution of Schisandra lignans metabolites in liver tissue and a lower distribution in the blood. The metabolites of STB could be detected in normal mouse liver, while some of them were not detected in MAFLD mouse liver, which may be due to MAFLD affecting the distribution and metabolism of STB in mouse liver, thereby reducing its metabolites.