Effect of Metabolic and Bariatric Surgery Associated with Changes in Weight Loss, Free Leptin Index, and Soluble Leptin Receptor
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Approval
2.2. Study Population
2.3. Study Protocols for Metabolic and Bariatric Surgery
2.4. Sample Preparation and Biochemical Assay
2.5. Assay of Ghrelin, Leptin, Soluble Leptin Receptor Levels, and Free Leptin Index (FLI)
2.6. Statistical Analyses
3. Results
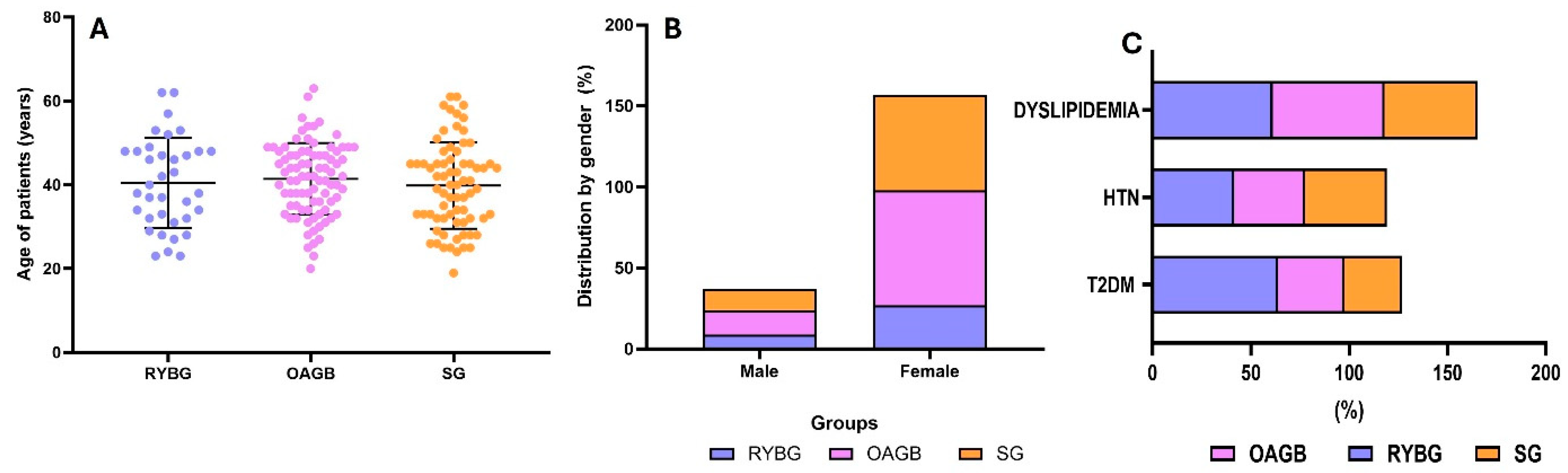
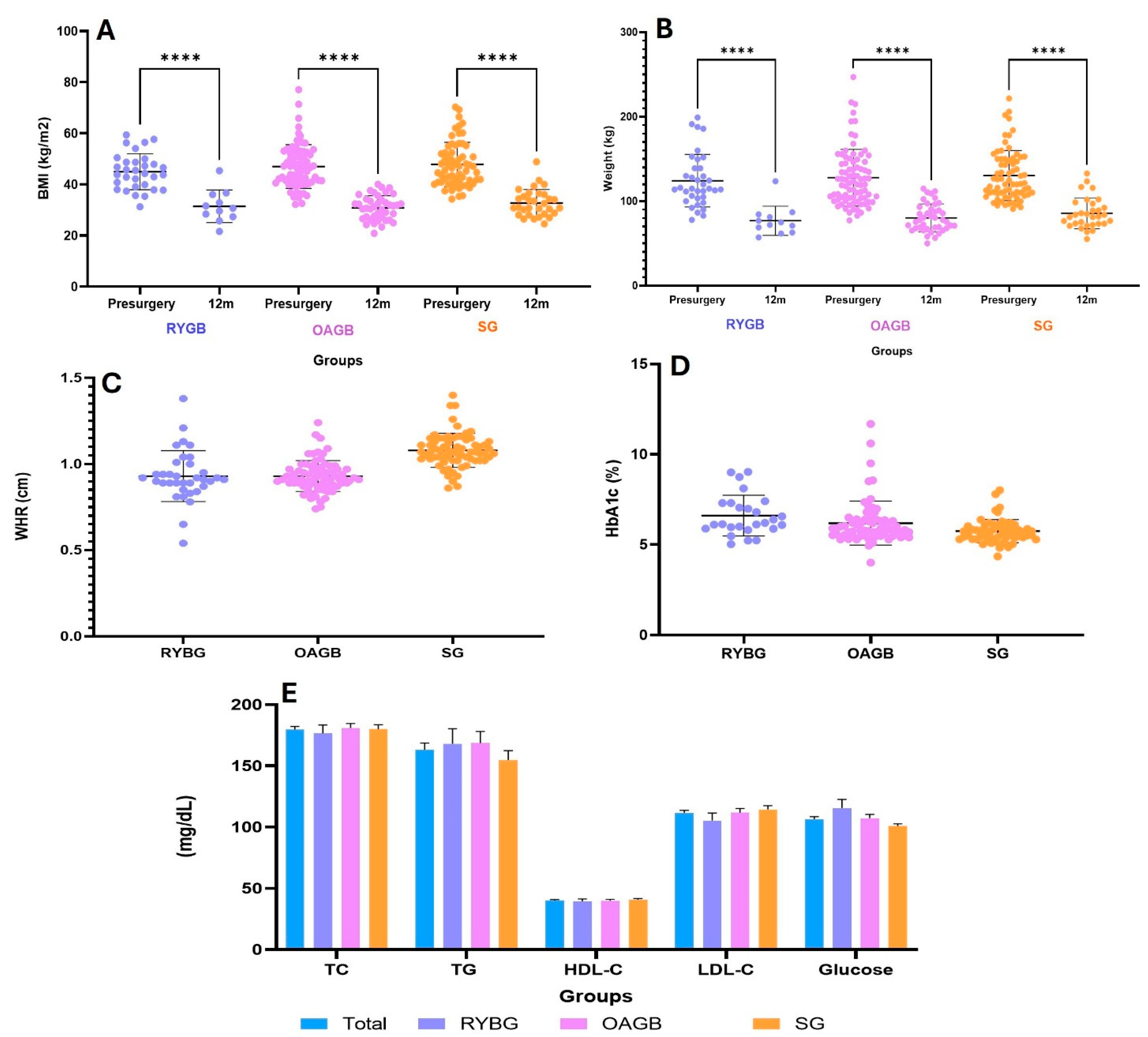
3.1. Clinical Characteristics and Anthropometric of Patients
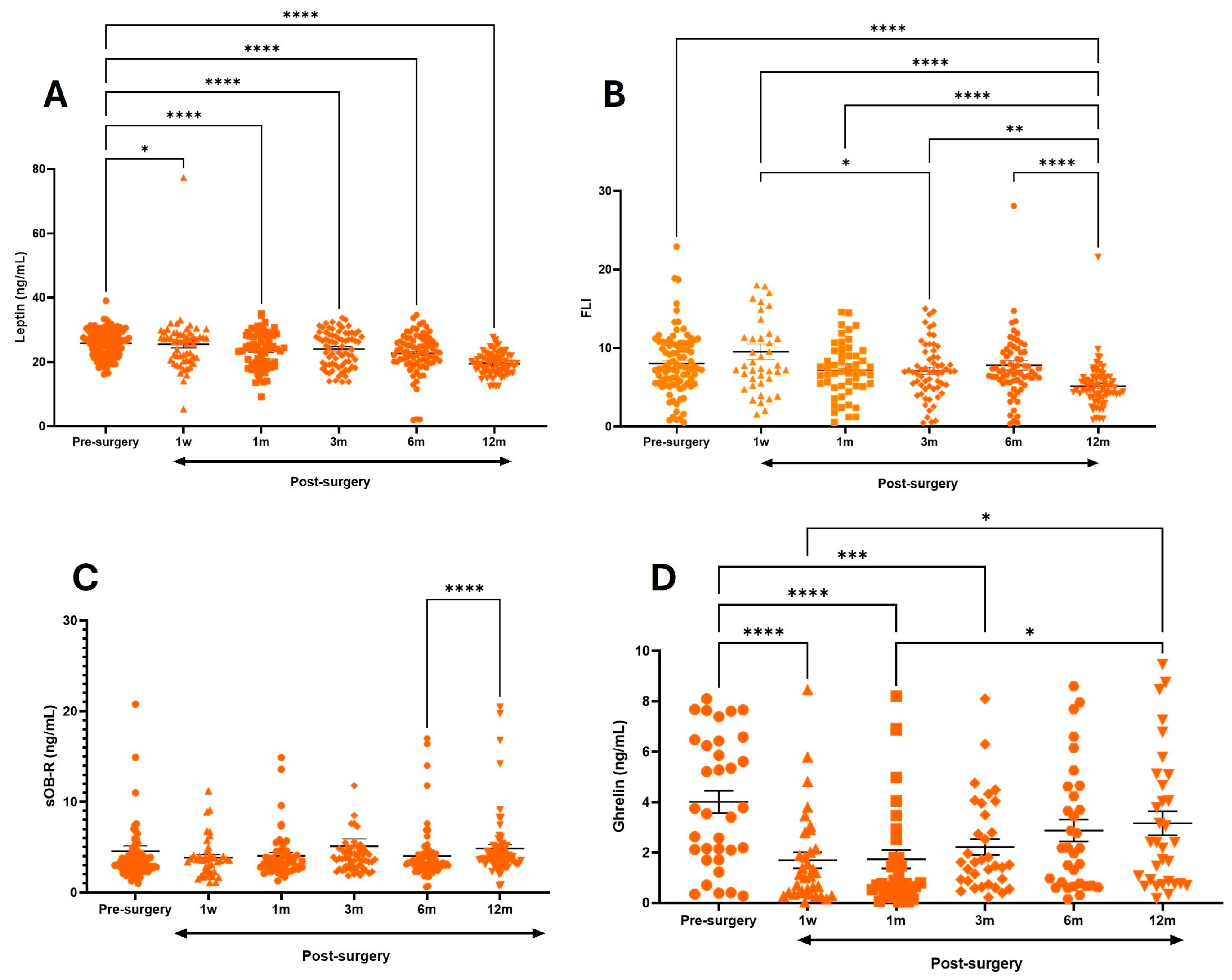
3.2. Effect of Metabolic and Bariatric Surgery on %EWL and Metabolic Markers
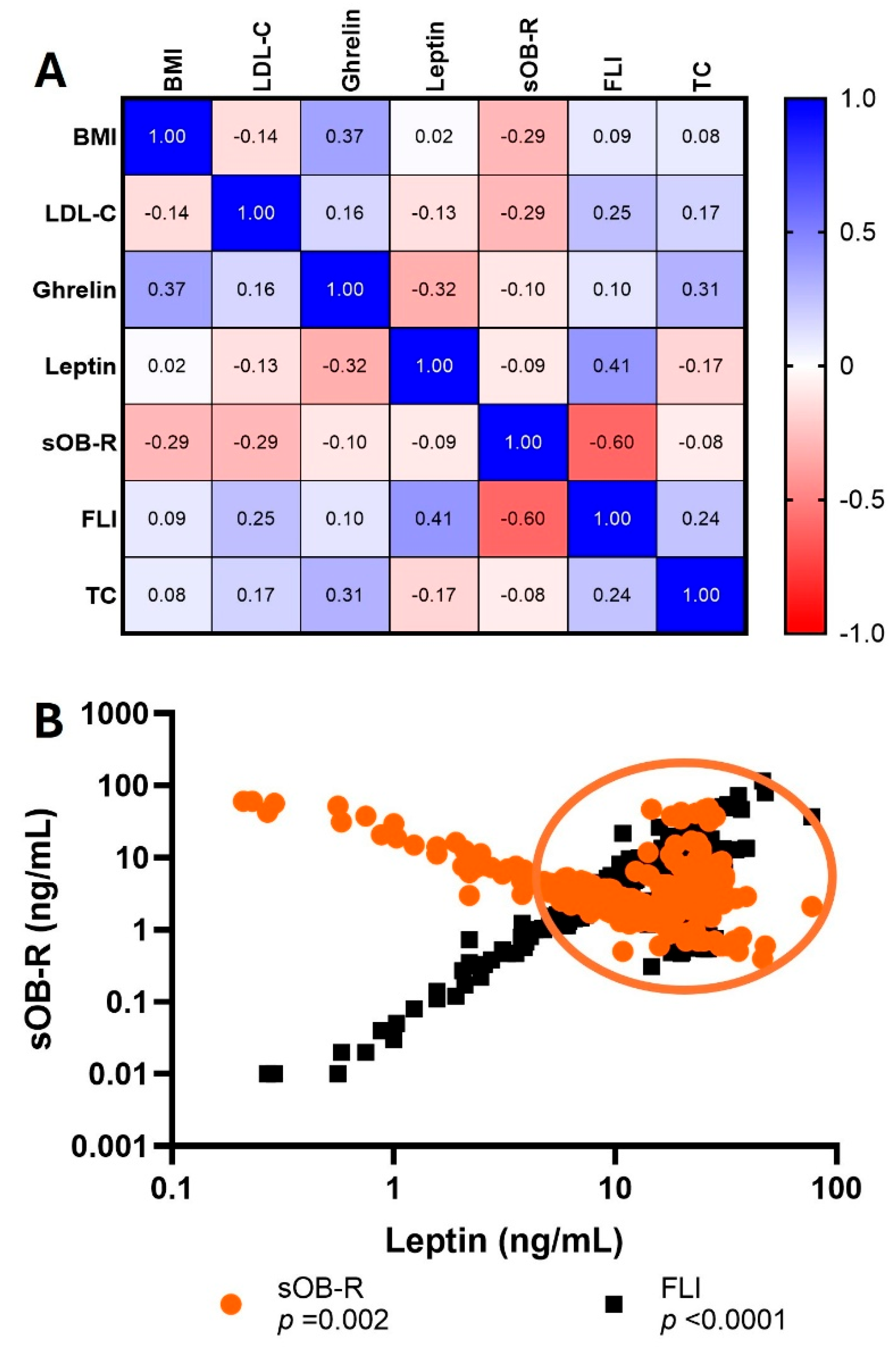
3.3. Association Between Ghrelin, FLI, sOB-R, and Metabolic Variables
4. Discussion
5. Conclusions
6. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BMI | Body mass index |
| BPD-DS | Biliopancreatic diversion with duodenal switch |
| CV | Cardiovascular disease |
| T2DM | Type 2 diabetes |
| ELISA | Enzyme-linked immunosorbent assay |
| ESR | Erythrocyte sedimentation rate |
| EWL | Excess weight loss |
| FLI | Free leptin index |
| HbA1c | Glycated hemoglobin A1c |
| HDL-C | High-density lipoprotein cholesterol |
| IR | Insulin resistance |
| LDL-C | Low-density lipoprotein cholesterol |
| MBS | Metabolic and bariatric surgery |
| OAGB | One anastomosis gastric bypass |
| RYGB | Roux-en-Y gastric bypass surgery |
| ROS | Reactive oxygen species |
| SG | Sleeve gastrectomy |
| sOB-R | Serum soluble leptin receptor |
| SOD | Superoxide dismutase |
| TC | Total cholesterol |
| TG | Triglycerides |
| TNF-α | Tumor necrosis factor alpha |
| VAT | Visceral adipose tissue |
| WAT | White adipose tissue |
| WHR | Waist-to-hip ratio |
| WMA | World Medical Association |
References
- Jaacks, L.M.; Vandevijvere, S.; Pan, A.; McGowan, C.J.; Wallace, C.; Imamura, F.; Mozaffarian, D.; Swinburn, B.; Ezzati, M. The obesity transition: Stages of the global epidemic. Lancet Diabetes Endocrinol. 2019, 7, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Blüher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; López, M.Y.; Rahmouni, K. Cellular and molecular basis of leptin and ghrelin resistance in obesity. Nat. Rev. Endocrinol. 2017, 13, 338–351. [Google Scholar] [CrossRef] [PubMed]
- Ahima, R.S.; Flier, J.S. Leptin. Annu. Rev. Physiol. 2000, 62, 413–437.2. [Google Scholar] [CrossRef]
- Myers, M.G., Jr. Leptin receptor signaling and the regulation of mammalian physiology. Recent Prog. Horm. Res. 2004, 59, 287–304. [Google Scholar] [CrossRef]
- Friedman, J.M.; Halaas, J.L. Leptin and the regulation of body weight in mammals. Nature 1998, 395, 763–770.6. [Google Scholar] [CrossRef]
- Adamska-Patruno, E.; Ostrowska, L.; Goscik, J.; Pietraszewska, B.; Kretowski, A.; Gorska, M. Relación entre la proporción leptina/grelina y comidas con diversos contenidos de macronutrientes en hombres con diferente estado nutricional: Un estudio cruzado aleatorizado. Nutr. J. 2018, 17, 118. [Google Scholar] [CrossRef]
- Gregor, M.F.; Hotamisligil, G.S. Inflammatory Mechanisms in Obesity. Annu. Rev. Immunol. 2011, 29, 415–445. [Google Scholar] [CrossRef]
- Godoy-Matos, A.F.; Silva Júnior, W.S.; Valerio, C.M. NAFLD as a Continuum: From Obesity to Metabolic Syndrome and Diabetes. Diabetol. Metab. Syndr. 2020, 12, 60. [Google Scholar] [CrossRef]
- Boutari, C.; Tziomalos, K.; Athyros, V.G. The Adipokines in the Pathogenesis and Treatment of Nonalcoholic Fatty Liver Disease. Hippokratia 2016, 20, 259. [Google Scholar]
- Lammert, A.; Kiess, W.; Bottner, A.; Glasowab, A.; Kratzsch, J. Soluble leptin receptor represents the main leptin binding activity in human blood. Biochem. Biophys Res. Commun. 2001, 283, 982–988. [Google Scholar] [CrossRef]
- Ogier, V.; Ziegler, O.; Mejean, L.; Nicolas, J.; Stricker-Krongrad, A. Obesity is associated with decreasing levels of the circulating soluble leptin receptor in humans. Int. J. Obes. Relat. Metab. Disord. 2002, 26, 496–503.16. [Google Scholar] [CrossRef] [PubMed]
- Ingelsson, E.; Larson, M.G.; Yin, X.; Wang, T.J.; Meigs, J.B.; Lipinska, I.; Benjamin, E.J.; Keaney, J.F.; Vasan, R.S. Circulating ghrelin, leptin, and soluble leptin receptor concentrations and cardiometabolic risk factors in a community-based sample. J. Clin. Endocrinol. Metab 2008, 93, 3149–3157. [Google Scholar] [CrossRef] [PubMed]
- Medici, V.; Ali, M.R.; Seo, S.; Aoki, C.A.; Rossaro, L.; Kim, K.; Fuller, W.D.; Vidovszky, T.J.; Smith, W.; Jiang, J.X. Increased soluble leptin receptor levels in morbidly obese patients with insulin resistance and nonalcoholic fatty liver disease. Obesity 2010, 18, 2268–2273. [Google Scholar] [CrossRef] [PubMed]
- Schaab, M.; Kausch, H.; Klammt, J.; Nowicki, M.; Anderegg, U.; Gebhardt, R.; Rose-John, S.; Scheller, J.; Thiery, J.; Kratzsch, J. Novel regulatory mechanisms for generation of the soluble leptin receptor: Implications for leptin action. PLoS ONE 2012, 7, e34787. [Google Scholar] [CrossRef]
- Kishimoto, I.; Tokudome, T.; Hosoda, H.; Miyazato, M.; Kangawa, K. Ghrelin and cardiovascular diseases. J. Cardiol. 2012, 59, 8–13. [Google Scholar] [CrossRef]
- Terra, X.; Auguet, T.; Guiu-Jurado, E.; Berlanga, A.; Orellana-Gavaldà, J.M.; Hernández, M.; Sabench, F.; Porras, J.A.; Llutart, J.; Martinez, S.; et al. Long-term changes in leptin, chemerin and ghrelin levels following different bariatric surgery procedures: Roux-en-Y gastric bypass and sleeve gastrectomy. Obes Surg. 2013, 23, 1790–1798. [Google Scholar] [CrossRef]
- Skoracka, K.; Hryhorowicz, S.; Schulz, P.; Zawada, A.; Ratajczak-Pawłowska, A.E.; Rychter, A.M.; Słomski, R.; Dobrowolska, A.; Krela-Kaźmierczak, I. The role of leptin and ghrelin in the regulation of appetite in obesity. Peptides 2025, 186, 171367. [Google Scholar] [CrossRef]
- Abizaid, A. Stress and obesity: The ghrelin connection. J. Neuroendocrinol. 2019, 31, e12693. [Google Scholar] [CrossRef]
- NORMA Oficial Mexicana NOM-008-SSA3-2017, DIARIO OFICIAL. Estados Unidos Mexicanos.- Secretaría de Salud. Available online: https://www.gob.mx/cms/uploads/attachment/file/512100/NOM-008-SSA3-2017.pdf (accessed on 19 November 2025).
- Deitel, M.; Gawdat, K.; Melissas, J. Reporting weight loss. Obes Surg. 2007, 17, 565–568, Erratum in Obes. Surg. 2007, 17, 996. [Google Scholar] [CrossRef]
- Yang, G.Q.; Ge, H.F.; Boucher, A.; Yu, X.X.; Li, C. Modulation of direct leptin signaling by soluble leptin receptor. Mol. Endocrinol. 2004, 18, 1354–1362. [Google Scholar] [CrossRef]
- Kunešová, M.; Sedláčková, B.; Bradnová, O.; Tvrzická, E.; Staňková, B.; Šrámková, P.; DoleŽalová, K.; Kalousková, P.; Hlavatý, P.; Hill, M.; et al. Fatty acid composition of adipose tissue triglycerides in obese diabetic women after bariatric surgery: A 2-year follow up. Physiol. Res. 2015, 64 (Suppl. 2), S155–S166. [Google Scholar] [CrossRef] [PubMed]
- Schigt, A.; Gerdes, V.E.; Cense, H.A.; Berends, F.J.; van Dielen, F.M.; Janssen, I.; van der Laar, A.; van Wagensveld, B.A.; Romijn, J.A.; Serlie, M.J. Bariatric surgery is an effective treatment for morbid obesity. Neth J. Med. 2013, 71, 4–9. [Google Scholar] [PubMed]
- Sjostrom, L.; Peltonen, M.; Jacobson, P.; Sjostrom, C.D.; Karason, K.; Wedel, H.; Ahlin, S.; Anveden, A.; Bengtsson, C.; Bergmark, G.; et al. Bariatric surgery and long-term cardiovascular events. JAMA 2012, 307, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Ortega, F.J.; Fernandez-Real, J.M. Inflammation in adipose tissue fatty acid anabolism: When enough is enough! Horm. Metab. Res 2013, 45, 1009–1019. [Google Scholar] [CrossRef]
- Witasp, A.; Nordfors, L.; Schalling, M.; Nygren, J.; Ljungqvist, O.; Thorell, A. Expression of inflammatory and insulin signaling genes in adipose tissue in response to elective surgery. J. Clin. Endocrinol. Metab. 2010, 95, 3460–3469. [Google Scholar] [CrossRef]
- Pouwels, S.; Sakran, N.; Graham, Y.; Leal, A.; Pintar, T.; Yang, W.; Kassir, R.; Singhal, R.; Mahawar, K.; Ramnarain, D. Non Alcoholic Fatty Liver Disease (NAFLD): A Review of Pathophysiology, Clinical Management and Effects of Weight Loss. BMC Endocr. Disord. 2022, 22, 63. [Google Scholar] [CrossRef]
- Askarpour, M.; Alizadeh, S.; Hadi, A.; Symonds, M.E.; Miraghajani, M.; Sheikhi, A.; Ghaedi, E. Effect of Bariatric Surgery on Serum Inflammatory Factors of Obese Patients: A Systematic Review and Meta-Analysis. Obes. Surg. 2019, 29, 2631–2647. [Google Scholar] [CrossRef]
- Sat-Muñoz, D.; Martínez-Herrera, B.-E.; Quiroga-Morales, L.-A.; Trujillo-Hernández, B.; González-Rodríguez, J.-A.; Gutiérrez-Rodríguez, L.-X.; Leal-Cortés, C.-A.; Portilla-De-Buen, E.; Rubio-Jurado, B.; Salazar-Páramo, M.; et al. Adipocytokines and Insulin Resistance: Their Role as Benign Breast Disease and Breast Cancer Risk Factors in a High-Prevalence Overweight-Obesity Group of Womenover 40 Years Old. Int. J. Environ. Res. Public Health 2022, 19, 6093. [Google Scholar] [CrossRef]
- Kalinowski, P.; Paluszkiewicz, R.; Wróblewski, T.; Remiszewski, P.; Grodzicki, M.; Bartoszewicz, Z.; Krawczyk, M. Ghrelin, leptin, and glycemic control after sleeve gastrectomy versus Roux-en-Y gastric bypass-results of a randomized clinical trial. Surg. Obes. Relat. Dis. 2017, 13, 181–188. [Google Scholar] [CrossRef]
- Liou, J.M.; Lin, J.T.; Lee, W.J.; Wang, H.P.; Lee, Y.C.; Chiu, H.M.; Wu, M.S. The serial changes of ghrelin and leptin levels and their relations to weight loss after laparoscopic minigastric bypass surgery. Obes. Surg. 2008, 18, 84–89. [Google Scholar] [CrossRef]
- Giovambattista, A.; Gaillard, R.C.; Spinedi, E. Ghrelin gene-related peptides modulate rat white adiposity. Vitam. Horm. 2007, 77, 171–205. [Google Scholar] [CrossRef]
- Perez-Tilve, D.; Heppner, K.; Kirchner, H.; Lockie, S.H.; Woods, S.C.; Smiley, D.L.; Tschöp, M.; Pfluger, P. Ghrelin-induced adiposity is independent of orexigenic effects. FASEB J. 2011, 25, 2814–2822. [Google Scholar] [CrossRef]
- Abu-Farha, M.; Dehbi, M.; Noronha, F.; Tiss, A.; Alarouj, M.; Behbehani, K.; Bennakhi, A.; Elkum, N. Gender differences in ghrelin association with cardiometabolic risk factors in arab population. Int. J. Endocrinol. 2014, 2014, 8. [Google Scholar] [CrossRef] [PubMed]
- Geary, N.; Asarian, L. Modulation of appetite by gonadal steroid hormones. Philos. Trans. R. Soc. B Biol. Sci. 2006, 361, 1251–1263. [Google Scholar] [CrossRef]
- Cummings, D.E.; Weigle, D.S.; Scott, R.; Patrica, A.; Breen, M.K. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. Nutr. Clin. Métabolisme 2002, 16, 219–220. [Google Scholar] [CrossRef] [PubMed]
- Rouault, A.A.J.; Rosselli-Murai, L.K.; Hernandez, C.C.; Gimenez, L.E.; Tall, G.G.; Sebag, J.A. The GPCR accessory protein MRAP2 regulates both biased signaling and constitutive activity of the ghrelin receptor GHSR1a. Sci. Signal. 2020, 13, eaax4569. [Google Scholar] [CrossRef]
- Al Massadi, O.; López, M.; Tschöp, M.; Diéguez, C.; Nogueiras, R. Current understanding of the hypothalamic ghrelin pathways inducing appetite and adiposity. Trends Neurosci. 2017, 40, 167–180. [Google Scholar] [CrossRef]
- Hahn, S.; Haselhorst, U.; Quadbeck, B.; Tan, S.; Kimmig, R.; Mann, K.; Janssen, O.E. Decreased soluble leptin receptor levels in women with polycystic ovary syndrome. Eur. J. Endocrinol. 2006, 154, 287–294. [Google Scholar] [CrossRef]
- Rojano-Rodriguez, M.E.; Beristain-Hernandez, J.L.; Zavaleta-Villa, B.; Maravilla, P.; Romero-Valdovinos, M.; Olivo-Diaz, A. Leptin receptor gene polymorphisms and morbid obesity in Mexican patients. Hereditas 2016, 153, 2. [Google Scholar] [CrossRef]
- Alnageeb, H.; Abdelgadir, E.; Khalifa, A.; Suliman, M.; Gautam, S.C.; Layani, L.; Subramaniam, S.; Bashier, A. Efficacy of bariatric surgery in improving metabolic outcomes in patients with diabetes. A 24-month follow-up study from a single center in the UAE. Diabetes Metab. Syndr. Obes. 2018, 11, 459–467. [Google Scholar] [CrossRef]
- Morante, J.J.H.; Soler, I.D.; Muñoz, J.S.G.; Sánchez, H.P.; Del Carmen Barberá Ortega, M.; Martínez, C.M.; Ruiz, J.M.M. Moderate weight loss modifies leptin and ghrelin synthesis rhythms but not the subjective sensations of appetite in obesity patients. Nutrients 2020, 12, 916. [Google Scholar] [CrossRef]
- Zhang, Y.; Chua, S., Jr. Leptin Function and Regulation. Compr. Physiol. 2017, 8, 351–369. [Google Scholar] [CrossRef]
- Sahebkar, A.; Giua, R.; Pedone, C. Impact of statin therapy on plasma leptin concentrations: A systematic review and meta-analysis of randomized placebo-controlled trials. Br J. Clin. Pharmacol. 2016, 82, 1674–1684. [Google Scholar] [CrossRef] [PubMed]

| TOTAL (n = 194) | RYBG (n = 36) | OAGB (n = 86) | SG (n = 72) | |
|---|---|---|---|---|
| Pre-Surgery | Pre-Surgery | Pre-Surgery | ||
| anthropometry | ||||
| Age (years) | 40.6 ± 9.6 | 40.4 ± 10.7 | 41.4 ± 8.5 | 39.8 ± 10.2 |
| Gender (M/F) | 37/157 | 9/27 | 15/71 | 13/59 |
| Weight Pre-Surgery (Kg) | 114.9 ± 24.4 | 110.8 ± 26.8 | 117.2 ± 26.4 | 114.3 ± 20.8 |
| BMI | 43.1 ± 8.01 | 41.6 ± 6.9 | 44.7 ± 7.8 | 42.3 ± 8.9 |
| WHR | 0.97 ± 0.58 | 1.15 ± 1.29 | 0.9 ± 0.08 | 1 ± 0.1 |
| Lean body mass (kg) | 57.9 ± 11.4 | 58.2 ± 12.7 | 58.8 ± 11.1 | 56.7 ± 11.2 |
| Body fat (kg) | 55.6 ± 15.4 | 52.4 ± 17.3 | 57.4 ± 16.3 | 55 ± 13.5 |
| Muscle mass (kg) | 55.1 ± 10.5 | 56.2 ± 11.1 | 55.5 ± 10.3 | 54.3 ± 10.6 |
| comorbidities | ||||
| T2DM (%) | 37.6 | 63.8 | 33.7 | 29.1 |
| HTN (%) | 39.17 | 41.6 | 36 | 41.6 |
| Dyslipidemia (%) | 54.1 | 61.1 | 56.9 | 47.2 |
| laboratory tests | ||||
| Leukocytes (×103/mL) | 8.1 ± 1.9 | 7.9 ± 1.8 | 8.5 ± 2.2 | 7.9 ± 1.7 |
| Lymphocytes | 3.2 ±5.5 | 3.9 ± 7.8 | 3.8 ± 7 | 2.2 ± 0.5 |
| Hemoglobin (g/dL) | 14.7 ±1.5 | 14.9 ± 0.9 | 14.8 ± 1.5 | 14.4 ± 1.7 |
| Albumin (g/dL) | 4.4 ± 5 | 6.7 ± 12.2 | 3.9 ± 0.3 | 3.9 ± 0.3 |
| Hematocrit (%) | 44.4 ± 6.3 | 45.2 ± 3 | 43.9 ± 8.3 | 44.5 ± 5.2 |
| Platelets | 275.1 ± 63.5 | 274.6 ± 48.6 | 273 ± 66.1 | 276.6 ± 67.5 |
| Glucose (mg/dL) | 106.5 ± 28.5 | 115.6 ± 42 | 107.3 ± 28.8 | 100.9 ± 15.9 * |
| HbA1c (%) | 6.07 ± 1 | 6.7 ± 1.1 | 6.2 ± 1.3 * | 5.6 ± 0.5 *** |
| Creatinine (mg/dL) | 0.7 ± 0.17 | 0.7 ± 0.1 | 0.7 ± 0.1 | 0.7 ± 0.2 |
| TC (mg/dL) | 179.6 ± 34.5 | 176.5 ± 40.9 | 180.9 ± 34 | 179.8 ± 31.8 |
| TG (mg/dL) | 163.1 ± 77.2 | 168 ± 73.5 | 168.5 ± 88.7 | 154.7 ± 64.9 |
| HDL-C (mg/dL) | 40.2 ± 10 | 39.6 ± 10.4 | 40 ± 10 | 40.6 ± 9.8 |
| LDL-C (mg/dL) | 111.6 ± 30.2 | 105.4 ± 36.7 | 111.8 ± 30.6 | 114.4 ± 26.1 |
| TOTAL (n = 194) | RYBG (n = 36) | OAGB (n = 86) | SG (n = 72) | |||||
|---|---|---|---|---|---|---|---|---|
| Kg | %EWL | Kg | %EWL | Kg | %EWL | Kg | %EWL | |
| Pre-Surgery | 114.9 ± 24.4 | -- | 110.8 ± 26.8 | -- | 117.2 ± 26.4 | -- | 114.3 ± 20.8 | -- |
| Post-surgery times | ||||||||
| 1 w | 107.8 ± 24.3 | 27 ± 9.4 | 105 ± 27.2 | 27.2 ± 9.6 | 108.2 ± 24 | 27.4 ± 9.1 | 108.2 ± 23.5 | 26.6 ± 9.7 |
| 1 m | 101.8 ± 25.8 | 35 ± 10.6 | 99 ± 27.6 | 37.7 ± 11.3 | 104.2 ± 24.2 | 33.6 ± 9.2 | 102 ± 24.8 | 35.4 ± 11.4 |
| 3 m | 95.6 ± 22.2 | 47 ± 11.5 | 97.1 ± 29.9 | 50 ± 12.5 | 95.3 ± 21.7 | 47.7 ± 12.1 | 95.5 ± 19.5 | 45.2 ± 10.4 |
| 6 m | 95.8 ± 72.3 | 56.9 ± 13.4 | 90.7 ± 24.9 | 56.5 ± 13.2 | 86.7 ± 19.5 | 59 ± 14.6 | 106 ± 107 | 55.3 ± 12.5 |
| 12 m | 78 ± 17.9 | 65.2 ± 16 | 74.8 ± 8.6 | 47.1 ± 16.9 | 74.1 ± 19.4 | 65.6 ± 12.2 | 83.9 ± 16.8 | 64.8 ± 19.4 |
| Leptin ANOVA | FLI ANOVA | sOB-R ANOVA | Ghrelin ANOVA | |||||
|---|---|---|---|---|---|---|---|---|
| ng/mL | p-Value | ng/mL | p-Value | ng/mL | p-Value | ng/mL | p-Value | |
| Pre-Surgery | 25.9 ± 4 | -- | 8.0 ± 3.9 | -- | 4.6 ± 5 | -- | 4.0 ± 2.5 | -- |
| post-surgery times | ||||||||
| 1 w | 25.5 ± 4.1 | 0.0250 | 9.5 ± 6.2 | 0.8644 | 3.8 ± 2 | 0.9975 | 1.6 ± 1.8 & | <0.0001 |
| 1 m | 23.7 ± 5.5 | <0.0001 | 7.2 ± 3.3 | 0.9412 | 4.1 ± 2.5 | 0.9999 | 1.7 ± 2 & | <0.0001 |
| 3 m | 24.0 ± 5.5 | <0.0001 | 7.1 ± 3.4 | 0.1694 | 5.1 ± 2.6 | 0.9997 | 2.2 ± 1.5 | 0.0009 |
| 6 m | 22.7 ± 6.2 | <0.0001 | 7.8 ± 4.9 | 0.9895 | 4.0 ± 2.9 | 0.9994 | 2.8 ± 2 | 0.0821 |
| 12 m | 19.45 ± 3.4 | <0.0001 | 5.1 ± 2.8 | <0.0001 | 4.8 ± 3 &&& | 0.9867 | 3.1 ± 2.4 | 0.3211 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basurto, L.; Basurto-Acevedo, N.; Oviedo, N.; Santa Cruz-Galicia, E.; Rodríguez-Martínez, A.I.; Tesoro-Cruz, E.; Martínez-Murillo, C.; García-Estrada, A.G.; Nájera Meneses, A.C.; Manuel-Apolinar, L. Effect of Metabolic and Bariatric Surgery Associated with Changes in Weight Loss, Free Leptin Index, and Soluble Leptin Receptor. Metabolites 2025, 15, 764. https://doi.org/10.3390/metabo15120764
Basurto L, Basurto-Acevedo N, Oviedo N, Santa Cruz-Galicia E, Rodríguez-Martínez AI, Tesoro-Cruz E, Martínez-Murillo C, García-Estrada AG, Nájera Meneses AC, Manuel-Apolinar L. Effect of Metabolic and Bariatric Surgery Associated with Changes in Weight Loss, Free Leptin Index, and Soluble Leptin Receptor. Metabolites. 2025; 15(12):764. https://doi.org/10.3390/metabo15120764
Chicago/Turabian StyleBasurto, Lourdes, Norma Basurto-Acevedo, Norma Oviedo, Erika Santa Cruz-Galicia, Ana Isabel Rodríguez-Martínez, Emiliano Tesoro-Cruz, Carlos Martínez-Murillo, Ariana Grisel García-Estrada, Andrea Cristina Nájera Meneses, and Leticia Manuel-Apolinar. 2025. "Effect of Metabolic and Bariatric Surgery Associated with Changes in Weight Loss, Free Leptin Index, and Soluble Leptin Receptor" Metabolites 15, no. 12: 764. https://doi.org/10.3390/metabo15120764
APA StyleBasurto, L., Basurto-Acevedo, N., Oviedo, N., Santa Cruz-Galicia, E., Rodríguez-Martínez, A. I., Tesoro-Cruz, E., Martínez-Murillo, C., García-Estrada, A. G., Nájera Meneses, A. C., & Manuel-Apolinar, L. (2025). Effect of Metabolic and Bariatric Surgery Associated with Changes in Weight Loss, Free Leptin Index, and Soluble Leptin Receptor. Metabolites, 15(12), 764. https://doi.org/10.3390/metabo15120764








