Effects of Glucose Oxidase and Macleaya cordata Extract on Immune Function, Antioxidant Capacity, and Gut Microbiota in British Shorthair Cats
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals Housing and Breeding
2.2. Diets and Feeding
2.3. Experimental Design
2.4. Blood Sample Collection and Analysis
2.5. Fecal Sample Collection and Analysis
2.6. Analysis of Short-Chain and Branched-Chain Fatty Acids
2.7. Fecal and Serum Untargeted Metabolomics Analysis
2.7.1. Serum Untargeted Metabolomics Analysis
2.7.2. Fecal Untargeted Metabolomics Analysis
2.8. 16S rRNA Sequencing
2.9. Statistical Analysis
3. Results
3.1. Effects of GOx and MCE on Body Weight and Apparent Digestibility
3.2. Effects of GOx and MCE on SCFAs and BCFAs
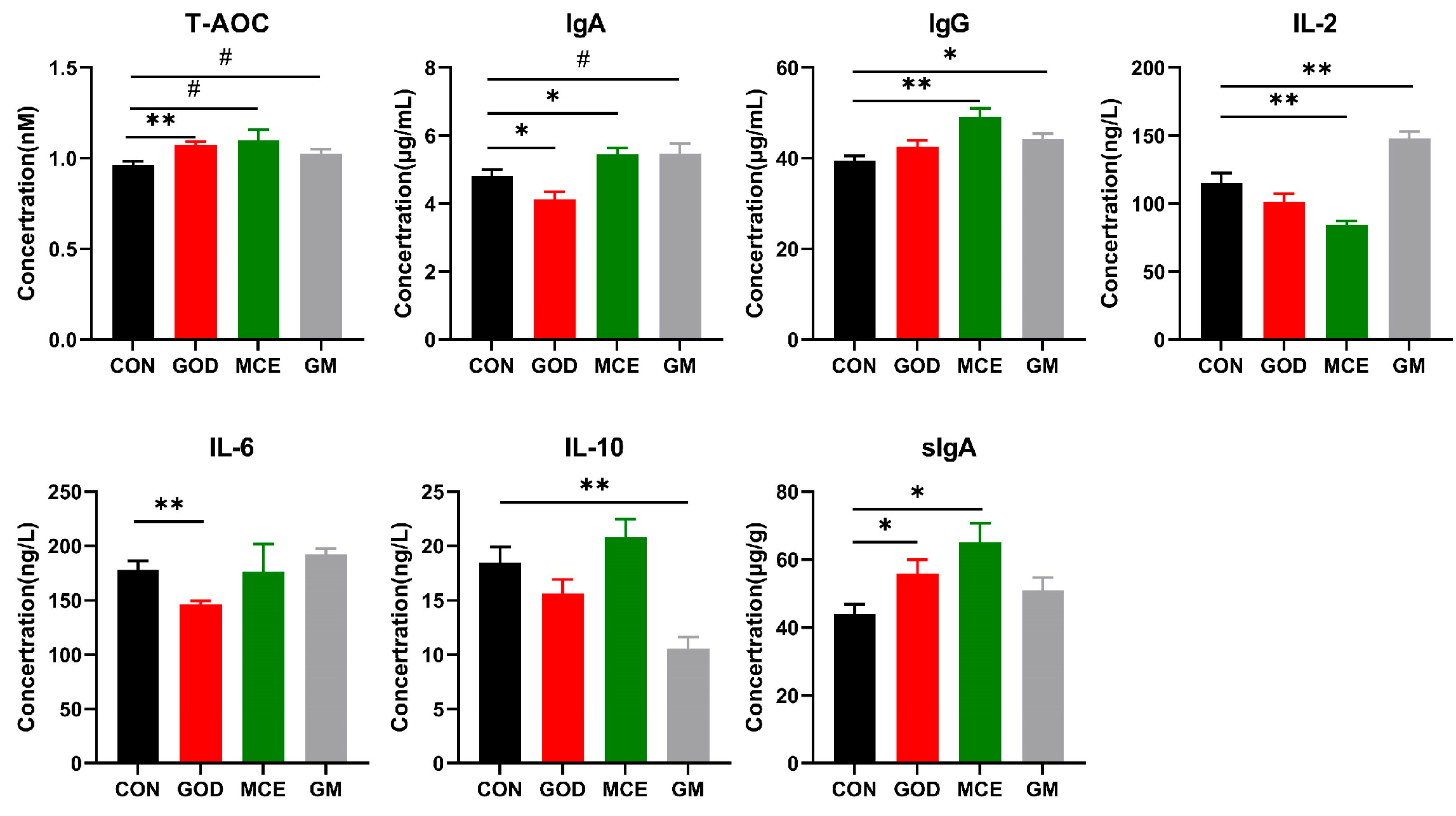
3.3. Effects of GOx and MCE on Immunity, Antioxidant Capacity, and Inflammatory Factors
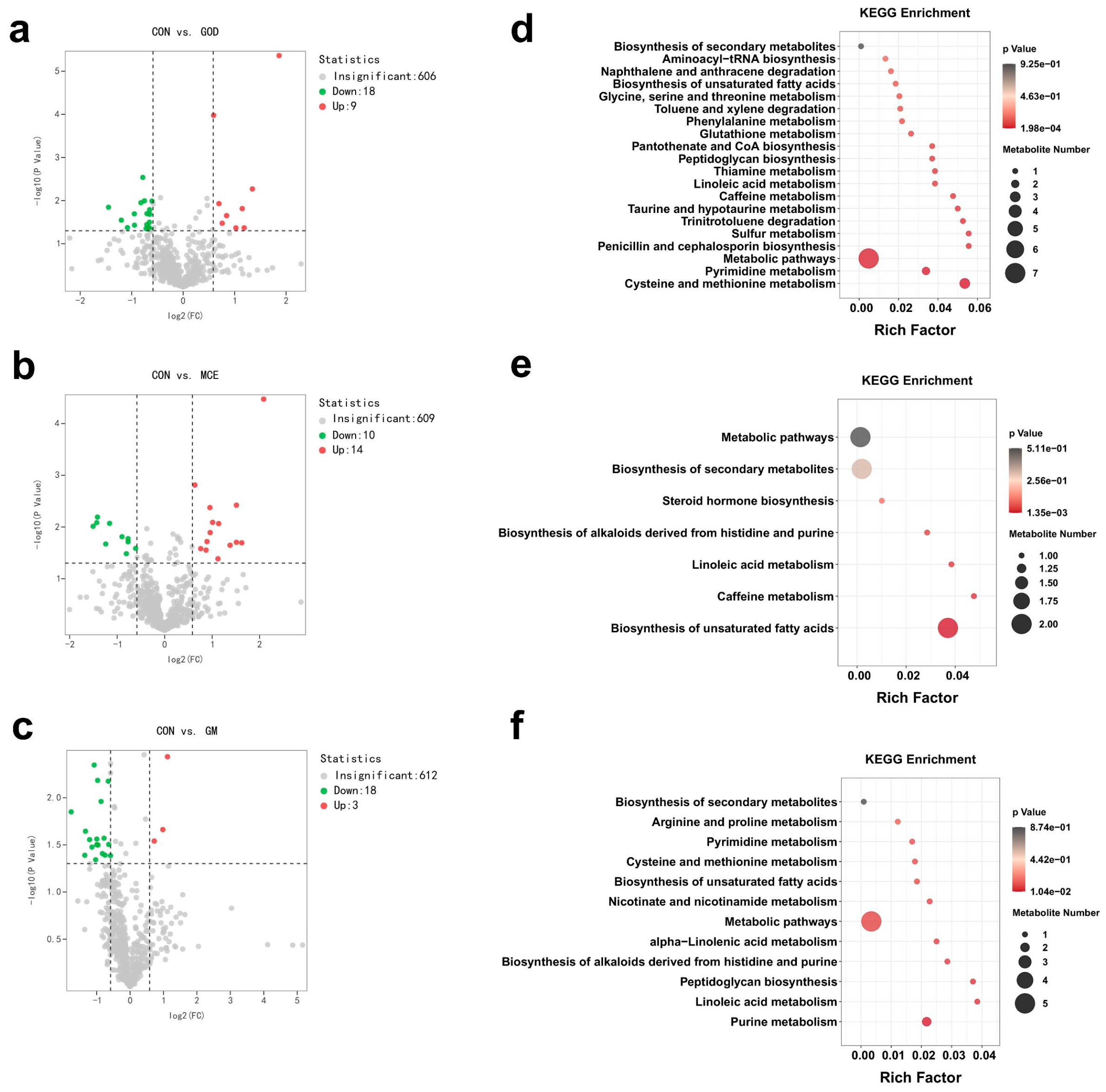
3.4. Effects of GOx and MCE on Serum Metabolome
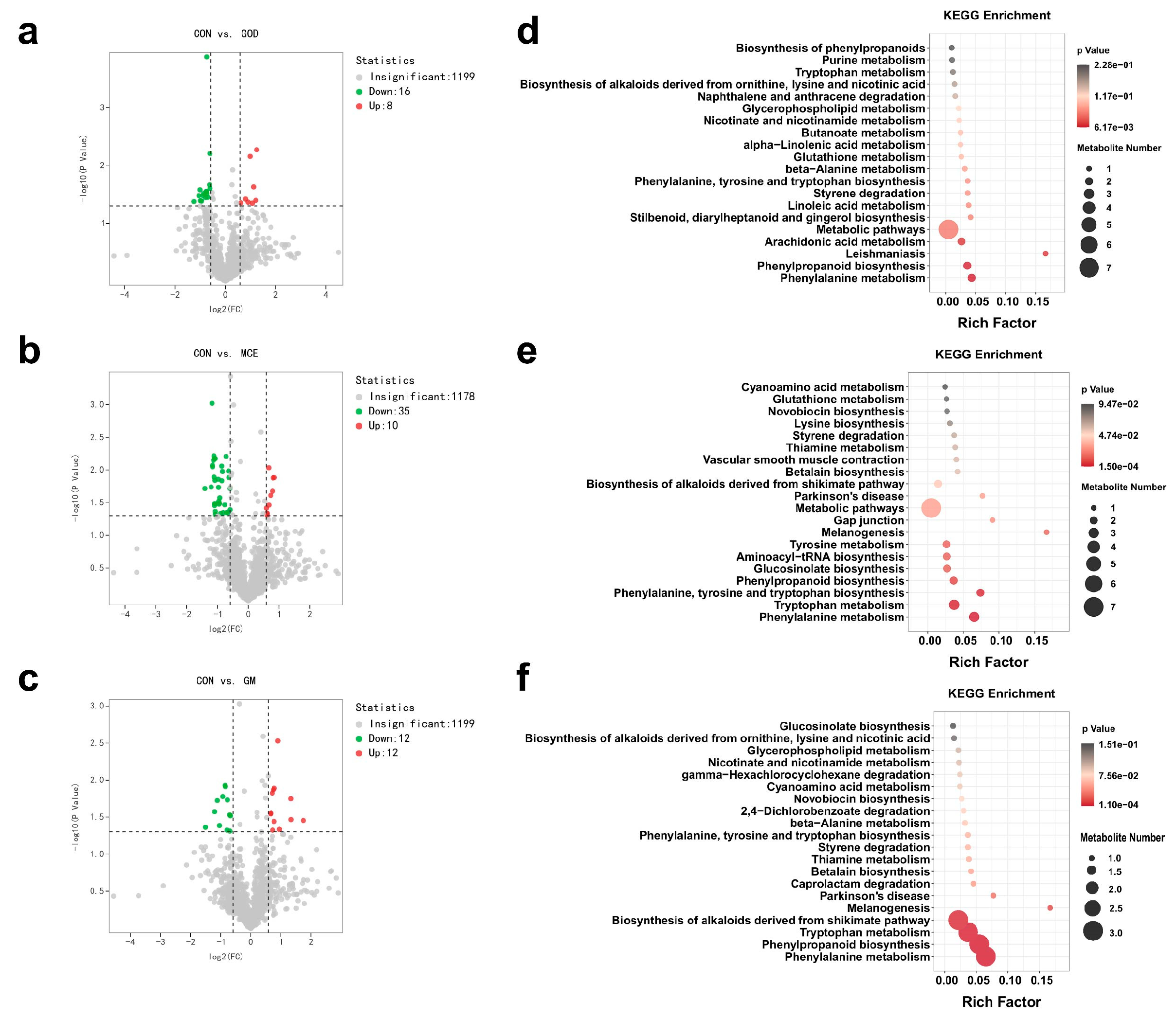
3.5. Effects of GOx and MCE on Fecal Metabolome
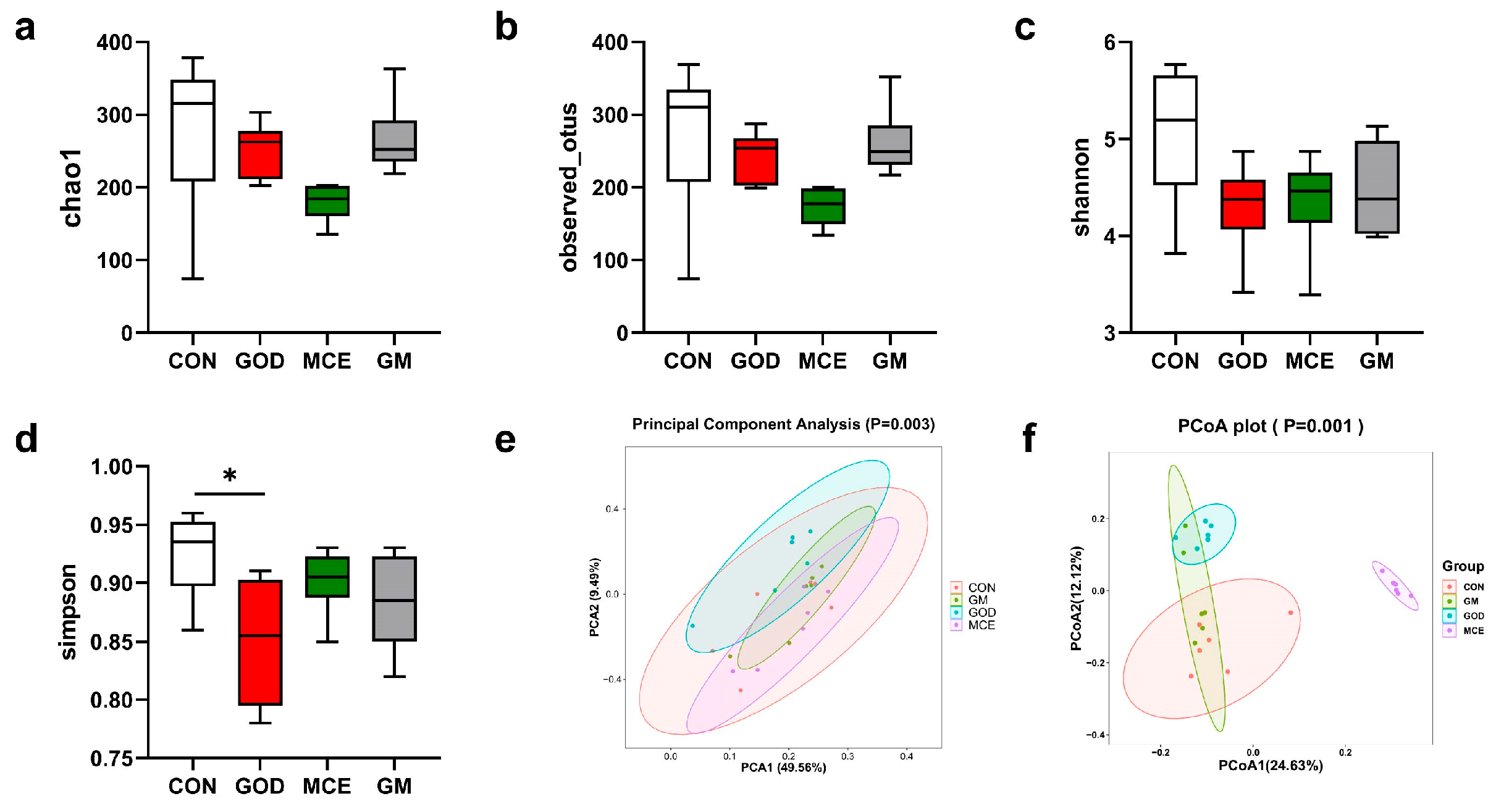
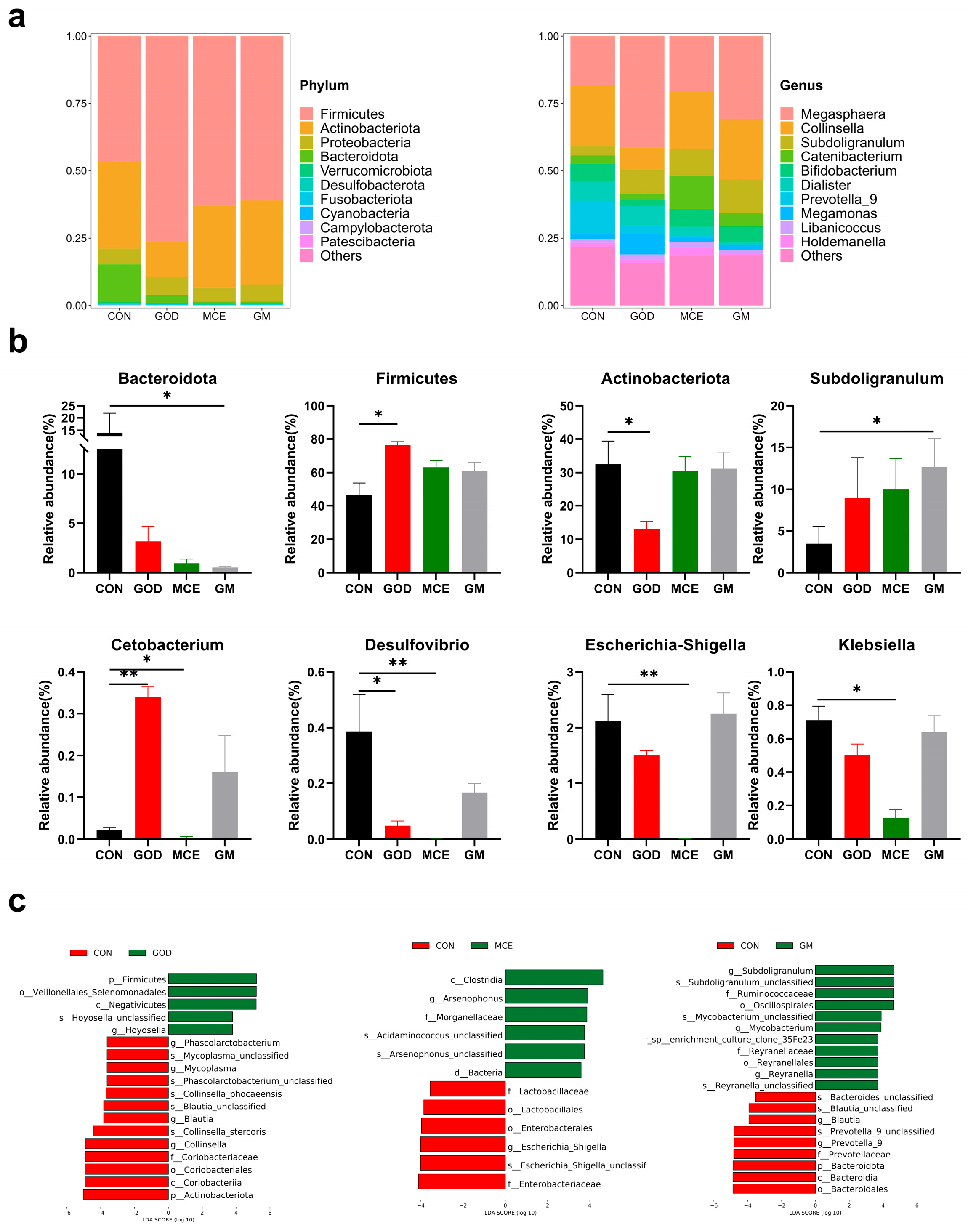
3.6. Effects of GOx and MCE on Fecal Microbiome
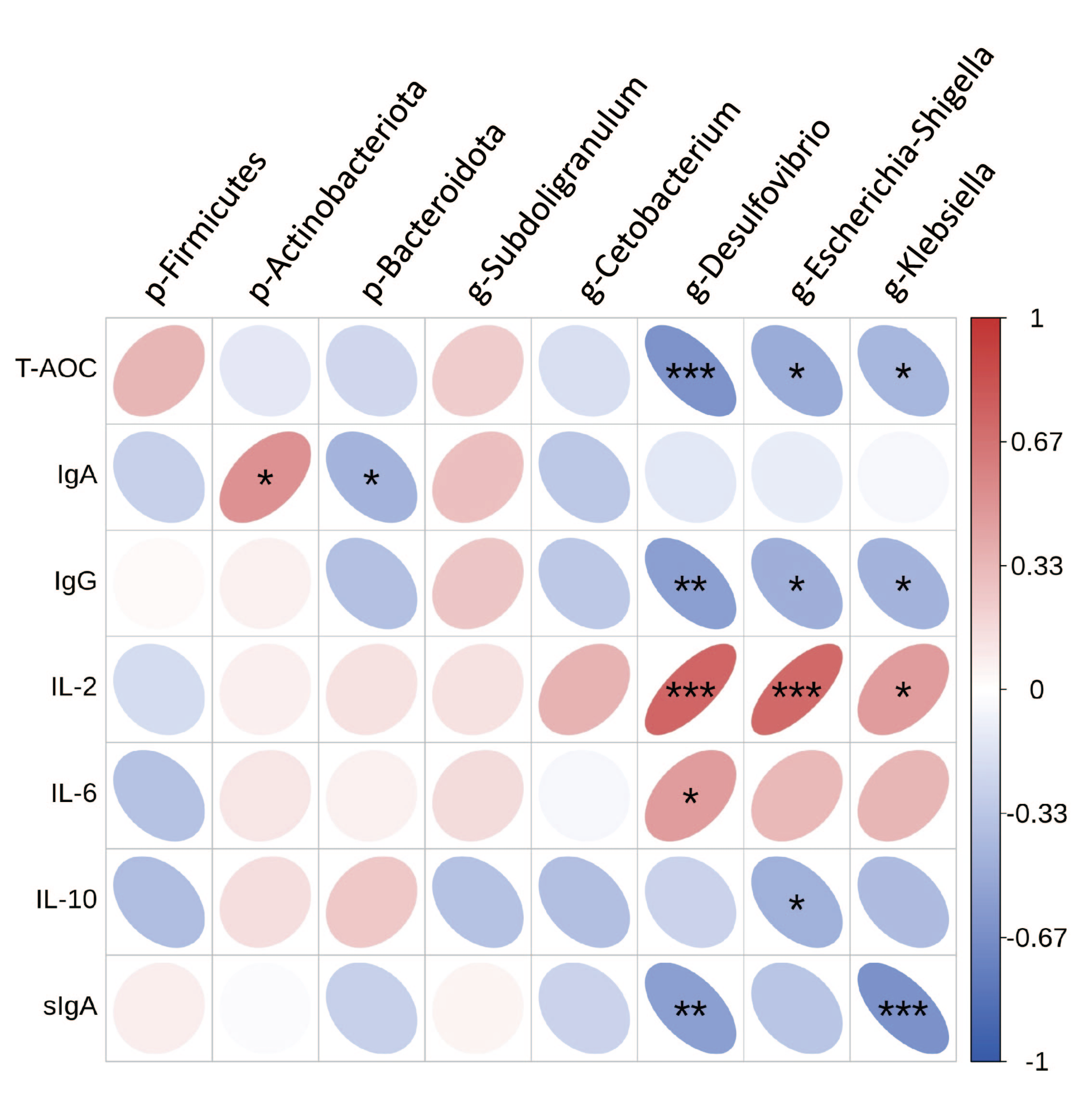
3.7. Correlation of Gut Microbes with Inflammatory Factor and Immune Markers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, K.; Zhang, L.; Liao, P.; Xiao, Z.; Zhang, F.; Sindaye, D.; Xin, Z.; Tan, C.; Deng, J.; Yin, Y.; et al. Impact of Gallic Acid on Gut Health: Focus on the Gut Microbiome, Immune Response, and Mechanisms of Action. Front. Immunol. 2020, 11, 580208. [Google Scholar] [CrossRef]
- Craig, J.M. Additives in pet food: Are they safe? J. Small Anim. Pr. 2021, 62, 624–635. [Google Scholar] [CrossRef]
- Guo, D.; Zhang, L.; Zhang, L.; Han, S.; Yang, K.; Lin, X.; Wen, C.; Tong, A.; Zhang, M.; Yin, Y.; et al. Effect of Dietary Methylsulfonylmethane Supplementation on Growth Performance, Hair Quality, Fecal Microbiota, and Metabolome in Ragdoll Kittens. Front. Microbiol. 2022, 13, 838164. [Google Scholar] [CrossRef]
- Rombach, M.; Dean, D.L. Just Love Me, Feed Me, Never Leave Me: Understanding Pet Food Anxiety, Feeding and Shopping Behavior of US Pet Owners in Covidian Times. Animals 2021, 11, 3101. [Google Scholar] [CrossRef]
- Alem, W.T. Effect of herbal extracts in animal nutrition as feed additives. Heliyon 2024, 10, e24973. [Google Scholar] [CrossRef]
- Dillon, G.P.; Gaffney, M.A.; Curran, C.M.; Moran, C.A. Dietary safety of a dual-enzyme preparation for animal feed: Acute and subchronic oral toxicity and genotoxicity studies. Regul. Toxicol. Pharm. 2017, 88, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Yao, B.; Gao, X.; Yang, P.; Wang, Z.; Zhang, G. Effects of glucose oxidase on the growth performance, serum parameters and faecal microflora of piglets. S. Afr. J. Anim. Sci. 2016, 46, 14–20. [Google Scholar] [CrossRef]
- Wu, S.; Li, T.; Niu, H.; Zhu, Y.; Liu, Y.; Duan, Y.; Sun, Q.; Yang, X. Effects of glucose oxidase on growth performance, gut function, and cecal microbiota of broiler chickens. Poult. Sci. 2019, 98, 828–841. [Google Scholar] [CrossRef]
- Wang, W.; Xie, R.; Cao, Q.; Ye, H.; Zhang, C.; Dong, Z.; Feng, D.; Zuo, J. Effects of glucose oxidase on growth performance, clinical symptoms, serum parameters, and intestinal health in piglets challenged by enterotoxigenic Escherichia coli. Front. Microbiol. 2022, 13, 994151. [Google Scholar] [CrossRef] [PubMed]
- Qu, W.; Liu, J. Effects of Glucose Oxidase Supplementation on the Growth Performance, Antioxidative and Inflammatory Status, Gut Function, and Microbiota Composition of Broilers Fed Moldy Corn. Front. Physiol. 2021, 12, 646393. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Xu, H.; Mei, X.; Gong, L.; Wang, B.; Li, W.; Jiang, S. Direct-fed glucose oxidase and its combination with B. amyloliquefaciens SC06 on growth performance, meat quality, intestinal barrier, antioxidative status, and immunity of yellow-feathered broilers. Poult. Sci. 2018, 97, 3540–3549. [Google Scholar] [CrossRef]
- Hoque, M.R.; Chen, N.; Liu, Y.; Kim, I.H. Possibility of using glucose oxidase in the diet to improve selected indicators of blood antioxidant defense, digestibility and growth performance of broiler chicken. Ital. J. Anim. Sci. 2022, 21, 455–462. [Google Scholar] [CrossRef]
- Sun, X.; Piao, L.; Jin, H.; Nogoy, K.; Zhang, J.; Sun, B.; Jin, Y.; Lee, D.H.; Choi, S.; Li, X. Dietary glucose oxidase and/or catalase supplementation alleviates intestinal oxidative stress induced by diquat in weaned piglets. Anim. Sci. J. 2021, 92, e13634. [Google Scholar] [CrossRef]
- Han, D.; Yang, H.; Li, J.; Zhang, C.; Ye, L.; Dong, J.; Zhang, C.; Guo, R.; Xin, J. Macleaya cordata extract improves growth performance, immune responses and anti-inflammatory capacity in neonatal piglets. Vet. Microbiol. 2024, 293, 110090. [Google Scholar] [CrossRef]
- de Medeiros, F.J.; Mello, C.A.; Bastos, F.L.; Pereira, R.A.; Duarte, M.A.; Araujo, J.A.; Da, S.A.; de Oliveira, G.A. Effects of Macleaya cordata extract supplementation on digestive parameters of ponies. Arch. Anim. Nutr. 2023, 77, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Xue, G.D.; Wu, S.B.; Choct, M.; Pastor, A.; Steiner, T.; Swick, R.A. Impact of a Macleaya cordata—Derived alkaloid extract on necrotic enteritis in broilers. Poult. Sci. 2017, 96, 3581–3585. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, J.; Zhao, W.; Wang, J.; Pan, X.; Lai, M.; Chimeze, V.W.N.; Pamete, E. Optimizing the Production of Antioxidant and Antibacterial Alkaloids from Macleaya cordata by Subcritical Water Extraction Technology. Chem. Biodivers. 2023, 20, e202300048. [Google Scholar] [CrossRef]
- Liu, G.; Guan, G.; Fang, J.; Martinez, Y.; Chen, S.; Bin, P.; Duraipandiyan, V.; Gong, T.; Tossou, M.C.B.; Al-Dhabi, N.A.; et al. Macleaya cordata Extract Decreased Diarrhea Score and Enhanced Intestinal Barrier Function in Growing Piglets. Biomed Res. Int. 2016, 2016, 1069585. [Google Scholar]
- Godowski, K.C. Antimicrobial action of sanguinarine. J. Clin. Dent. 1989, 1, 96–101. [Google Scholar] [PubMed]
- Mackraj, I.; Govender, T.; Gathiram, P. Sanguinarine. Cardiovasc. Ther. 2008, 26, 75–83. [Google Scholar] [CrossRef]
- Guan, G.; Ding, S.; Yin, Y.; Duraipandiyan, V.; Al-Dhabi, N.A.; Liu, G. Macleaya cordata extract alleviated oxidative stress and altered innate immune response in mice challenged with enterotoxigenic Escherichia coli. Sci. China Life Sci. 2019, 62, 1019–1027. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Wang, X.L.; Ou, S.Q.; Hou, D.X.; He, J.H. Sanguinarine modulate gut microbiome and intestinal morphology to enhance growth performance in broilers. PLoS ONE 2020, 15, e0234920. [Google Scholar] [CrossRef]
- Wang, F.; Yin, Y.; Yang, M.; Chen, J.; Fu, C.; Huang, K. Effects of Combined Supplementation of Macleaya cordata Extract and Benzoic Acid on the Growth Performance, Immune Responses, Antioxidant Capacity, Intestinal Morphology, and Microbial Composition in Weaned Piglets. Front. Vet. Sci. 2021, 8, 708597. [Google Scholar] [CrossRef]
- Song, B.; He, J.; Pan, X.; Kong, L.; Xiao, C.; Keerqin, C.; Song, Z. Dietary Macleaya cordata extract supplementation improves the growth performance and gut health of broiler chickens with necrotic enteritis. J. Anim. Sci. Biotechno. 2023, 14, 113. [Google Scholar] [CrossRef]
- Chen, F.; Wang, Y.; Wang, K.; Chen, J.; Jin, K.; Peng, K.; Chen, X.; Liu, Z.; Ouyang, J.; Wang, Y.; et al. Effects of Litsea cubeba essential oil on growth performance, blood antioxidation, immune function, apparent digestibility of nutrients, and fecal microflora of pigs. Front. Pharmacol. 2023, 14, 1166022. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Jian, S.; Zhang, L.; Deng, B. Effect of compound polysaccharide on immunity, antioxidant capacity, gut microbiota, and serum metabolome in kittens. Front. Microbiol. 2025, 16, 1500961. [Google Scholar] [CrossRef]
- Xin, Z.; Ma, S.; Ren, D.; Liu, W.; Han, B.; Zhang, Y.; Xiao, J.; Yi, L.; Deng, B. UPLC-Orbitrap-MS/MS combined with chemometrics establishes variations in chemical components in green tea from Yunnan and Hunan origins. Food Chem. 2018, 266, 534–544. [Google Scholar] [CrossRef] [PubMed]
- Bavarsadi, M.; Mahdavi, A.H.; Ansari-Mahyari, S.; Jahanian, E. Effects of different levels of sanguinarine on antioxidant indices, immunological responses, ileal microbial counts and jejunal morphology of laying hens fed diets with different levels of crude protein. J. Anim. Physiol. Anim. Nutr. 2017, 101, 936–948. [Google Scholar] [CrossRef]
- Chen, J.; Kang, B.; Yao, K.; Fu, C.; Zhao, Y. Effects of dietary Macleaya cordata extract on growth performance, immune responses, antioxidant capacity, and intestinal development in weaned piglets. J. Appl. Anim. Res. 2019, 47, 349–356. [Google Scholar] [CrossRef]
- Khatami, S.H.; Vakili, O.; Ahmadi, N.; Fard, E.S.; Mousavi, P.; Khalvati, B.; Maleksabet, A.; Savardashtaki, A.; Taheri-Anganeh, M.; Movahedpour, A. Glucose oxidase: Applications, sources, and recombinant production. Biotechnol. Appl. Biochem. 2022, 69, 939–950. [Google Scholar] [CrossRef]
- Chelakkot, C.; Ghim, J.; Ryu, S.H. Mechanisms regulating intestinal barrier integrity and its pathological implications. Exp. Mol. Med. 2018, 50, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Neurath, M.F. Targeting immune cell circuits and trafficking in inflammatory bowel disease. Nat. Immunol. 2019, 20, 970–979. [Google Scholar] [CrossRef]
- Turner, M.D.; Nedjai, B.; Hurst, T.; Pennington, D.J. Cytokines and chemokines: At the crossroads of cell signalling and inflammatory disease. Biochim. Biophys. Acta 2014, 1843, 2563–2582. [Google Scholar] [CrossRef]
- Zhou, P. Emerging mechanisms and applications of low-dose IL-2 therapy in autoimmunity. Cytokine Growth Factor Rev. 2022, 67, 80–88. [Google Scholar] [CrossRef]
- Troia, R.; Mascalzoni, G.; Agnoli, C.; Lalonde-Paul, D.; Giunti, M.; Goggs, R. Cytokine and Chemokine Profiling in Cats with Sepsis and Septic Shock. Front. Vet. Sci. 2020, 7, 305. [Google Scholar] [CrossRef] [PubMed]
- Batfalsky, A.; Lohr, A.; Heussen, N.; Neunhoeffer, F.; Orlikowsky, T.W. Diagnostic value of an interleukin-6 bedside test in term and preterm neonates at the time of clinical suspicion of early- and late-onset bacterial infection. Neonatology 2012, 102, 37–44. [Google Scholar] [CrossRef]
- Beebe, A.M.; Cua, D.J.; de Waal, M.R. The role of interleukin-10 in autoimmune disease: Systemic lupus erythematosus (SLE) and multiple sclerosis (MS). Cytokine Growth Factor Rev. 2002, 13, 403–412. [Google Scholar] [CrossRef]
- Rasquinha, M.T.; Sur, M.; Lasrado, N.; Reddy, J. IL-10 as a Th2 Cytokine: Differences Between Mice and Humans. J. Immunol. 2021, 207, 2205–2215. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.F.; Wu, G.Y.; Liao, Y.P.; Houb, Z.P.; Liu, H.J.; Yin, F.G.; Li, T.J.; Huang, R.L.; Zhang, Y.M.; Deng, D.; et al. Dietary supplementation with Chinese herbal ultra—Fine powder enhances cellular and humoral immunity in early—Weaned piglets. Livest. Sci. 2007, 108, 94–98. [Google Scholar] [CrossRef]
- Kumar, B.S.; Parker, B.W.; Malyutin, A.G.; Haloi, N.; Huey-Tubman, K.E.; Tajkhorshid, E.; Stadtmueller, B.M. The structures of secretory and dimeric immunoglobulin A. Elife 2020, 9, e56098. [Google Scholar] [CrossRef] [PubMed]
- Doron, I.; Kusakabe, T.; Iliev, I.D. Immunoglobulins at the interface of the gut mycobiota and anti-fungal immunity. Semin. Immunol. 2023, 67, 101757. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Wang, K.; Yin, J.; Chen, S.; Liu, G.; Tan, B.; Wu, G.; Bazer, F.W.; Peng, Y.; Yin, Y. Glutamine-induced Secretion of intestinal Secretory immunoglobulin A: A Mechanistic Perspective. Front. Immunol. 2016, 7, 503. [Google Scholar] [CrossRef]
- Liu, C.; Li, Y.; Li, H.; Wang, Y.; Zhao, K. Nano-Selenium and Macleaya cordata Extracts Improved Immune Functions of Intrauterine Growth Retardation Piglets under Maternal Oxidation Stress. Biol. Trace Elem Res. 2022, 200, 3975–3982. [Google Scholar] [CrossRef]
- Reddy, V.P. Oxidative Stress in Health and Disease. Biomedicines 2023, 11, 2925. [Google Scholar] [CrossRef] [PubMed]
- Sinha, K.; Das, J.; Pal, P.B.; Sil, P.C. Oxidative stress: The mitochondria-dependent and mitochondria-independent pathways of apoptosis. Arch. Toxicol. 2013, 87, 1157–1180. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, B.; Zhan, X.; Wang, Y.; Li, W. Effects of glucose oxidase and its combination with B. amyloliquefaciens SC06 on intestinal microbiota, immune response and antioxidative capacity in broilers. Animal 2022, 16, 100473. [Google Scholar] [CrossRef]
- Sun, X.; Piao, L.; Jin, H.; Nogoy, K.M.C.; Zhang, J.; Sun, B.; Jin, Y.; Lee, D.H.; Choi, S.; Smith, S.B.; et al. Effects of dietary supplementation of glucose oxidase, catalase, or both on reproductive performance, oxidative stress, fecal microflora and apoptosis in multiparous sows. Anim. Biosci. 2022, 35, 75–86. [Google Scholar] [CrossRef]
- Zhao, W.; Huang, Y.; Cui, N.; Wang, R.; Xiao, Z.; Su, X. Glucose oxidase as an alternative to antibiotic growth promoters improves the immunity function, antioxidative status, and cecal microbiota environment in white-feathered broilers. Front. Microbiol. 2023, 14, 1100465. [Google Scholar] [CrossRef]
- Pilla, R.; Suchodolski, J.S. The Gut Microbiome of Dogs and Cats, and the Influence of Diet. Vet. Clin. N. Am.-Small 2021, 51, 605–621. [Google Scholar] [CrossRef]
- Masuoka, H.; Shimada, K.; Kiyosue-Yasuda, T.; Kiyosue, M.; Oishi, Y.; Kimura, S.; Ohashi, Y.; Fujisawa, T.; Hotta, K.; Yamada, A.; et al. Transition of the intestinal microbiota of cats with age. PLoS ONE 2017, 12, e0181739. [Google Scholar] [CrossRef] [PubMed]
- Suchodolski, J.S. Analysis of the gut microbiome in dogs and cats. Vet. Clin. Pathol. 2022, 50 (Suppl. S1), 6–17. [Google Scholar] [CrossRef]
- Lyu, Y.; Su, C.; Verbrugghe, A.; Van de Wiele, T.; Martos, M.A.; Hesta, M. Past, Present, and Future of Gastrointestinal Microbiota Research in Cats. Front. Microbiol. 2020, 11, 1661. [Google Scholar] [CrossRef]
- Yang, K.; Jian, S.; Guo, D.; Wen, C.; Xin, Z.; Zhang, L.; Kuang, T.; Wen, J.; Yin, Y.; Deng, B. Fecal microbiota and metabolomics revealed the effect of long-term consumption of gallic acid on canine lipid metabolism and gut health. Food Chem. X 2022, 15, 100377. [Google Scholar] [CrossRef] [PubMed]
- Cole, L.P.; Hardas, A.; Priestnall, S.L.; Tinson, E.W. Waterhouse—Friderichsen syndrome in a cat with Klebsiella spp. infection. J. Vet. Emerg. Crit. Care 2021, 31, 531–536. [Google Scholar] [CrossRef]
- Rubio, L.A.; Peinado, M.J.; Ruiz, R.; Suarez-Pereira, E.; Ortiz, M.C.; Garcia, F.J. Correlations between changes in intestinal microbiota composition and performance parameters in broiler chickens. J. Anim. Physiol. Anim. Nutr. 2015, 99, 418–423. [Google Scholar] [CrossRef]
- Zhang, M.; Mo, R.; Li, M.; Qu, Y.; Wang, H.; Liu, T.; Liu, P.; Wu, Y. Comparison of the Effects of Enzymolysis Seaweed Powder and Saccharomyces boulardii on Intestinal Health and Microbiota Composition in Kittens. Metabolites 2023, 13, 637. [Google Scholar] [CrossRef]
- Sharma, P.; Reichert, M.; Lu, Y.; Markello, T.C.; Adams, D.R.; Steinbach, P.J.; Fuqua, B.K.; Parisi, X.; Kaler, S.G.; Vulpe, C.D.; et al. Biallelic HEPHL1 variants impair ferroxidase activity and cause an abnormal hair phenotype. PloS Genet. 2019, 15, e1008143. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, X.; Zhou, C.; Jiao, J.; Tan, Z. Microbiome-transcriptome analysis reveals that dietary supplementation with macleaya cordata extract alters multiple immune pathways with minimal impact on microbial structure. Front. Cell. Infect. Microbiol. 2023, 13, 1264550. [Google Scholar] [CrossRef]
- Koliada, A.; Syzenko, G.; Moseiko, V.; Budovska, L.; Puchkov, K.; Perederiy, V.; Gavalko, Y.; Dorofeyev, A.; Romanenko, M.; Tkach, S.; et al. Association between body mass index and Firmicutes/Bacteroidetes ratio in an adult Ukrainian population. BMC Microbiol. 2017, 17, 120. [Google Scholar] [CrossRef]
- Rahlwes, K.C.; Dias, B.; Campos, P.C.; Alvarez-Arguedas, S.; Shiloh, M.U. Pathogenicity and virulence of Mycobacterium tuberculosis. Virulence 2023, 14, 2150449. [Google Scholar] [CrossRef] [PubMed]
- O’Halloran, C.; Gunn-Moore, D.; Hope, J. Diagnosis of feline mycobacteriosis: Feline mycobacteriosis. Vet. Rec. 2016, 178, 145. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Yang, K.; Wen, J.; Kuang, T.; Cao, Z.; Zhang, L.; Han, S.; Jian, S.; Chen, X.; Zhang, L.; et al. Antimicrobial Peptides Relieve Transportation Stress in Ragdoll Cats by Regulating the Gut Microbiota. Metabolites 2023, 13, 326. [Google Scholar] [CrossRef]
- Shang, L.; Yu, H.; Liu, H.; Chen, M.; Zeng, X.; Qiao, S. Recombinant antimicrobial peptide microcin J25 alleviates DSS-induced colitis via regulating intestinal barrier function and modifying gut microbiota. Biomed. Pharmacother. 2021, 139, 111127. [Google Scholar] [CrossRef]
- Liu, S.; Chen, Y.; Wang, X.; Wang, S.; Bai, L.; Cheng, X.; Wan, J.; Hu, Y.; Ding, Y.; Zhang, X.; et al. Plasma metabolomics identifies metabolic alterations associated with the growth and development of cat. Anim. Model. Exp. Med. 2023, 6, 306–316. [Google Scholar] [CrossRef]
- Chiu, O.; Tal, M.; Sanmugam, A.; Hesta, M.; Gomez, D.E.; Weese, J.S.; Verbrugghe, A. The effects of ambient temperature exposure on feline fecal metabolome. Front. Vet. Sci. 2023, 10, 1141881. [Google Scholar] [CrossRef]
- Yang, K.; Deng, X.; Jian, S.; Zhang, M.; Wen, C.; Xin, Z.; Zhang, L.; Tong, A.; Ye, S.; Liao, P.; et al. Gallic Acid Alleviates Gut Dysfunction and Boosts Immune and Antioxidant Activities in Puppies Under Environmental Stress Based on Microbiome-Metabolomics Analysis. Front. Immunol. 2021, 12, 813890. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H.; Rinna, A. Glutathione: Overview of its protective roles, measurement, and biosynthesis. Mol. Asp. Med. 2009, 30, 1–12. [Google Scholar] [CrossRef]
- Yang, Y.; Li, L.; Hang, Q.; Fang, Y.; Dong, X.; Cao, P.; Yin, Z.; Luo, L. Gamma-glutamylcysteine exhibits anti-inflammatory effects by increasing cellular glutathione level. Redox Biol. 2019, 20, 157–166. [Google Scholar] [CrossRef]
- Veeravalli, K.; Boyd, D.; Iverson, B.L.; Beckwith, J.; Georgiou, G. Laboratory evolution of glutathione biosynthesis reveals natural compensatory pathways. Nat. Chem. Biol. 2011, 7, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lu, S.; Zhang, X.; Zhou, J.; Yan, X.; Liu, H.; Liang, J.; Luo, L.; Zhou, D.; Yin, Z. Gamma-Glutamylcysteine alleviates ethanol-induced hepatotoxicity via suppressing oxidative stress, apoptosis, and inflammation. J. Food Biochem. 2022, 46, e14318. [Google Scholar] [CrossRef]
- Hestad, K.; Alexander, J.; Rootwelt, H.; Aaseth, J.O. The Role of Tryptophan Dysmetabolism and Quinolinic Acid in Depressive and Neurodegenerative Diseases. Biomolecules 2022, 12, 998. [Google Scholar] [CrossRef] [PubMed]
- Reilly, L.M.; Hu, Y.; von Schaumburg, P.C.; de Oliveira, M.; He, F.; Rodriguez-Zas, S.L.; Southey, B.R.; Parsons, C.M.; Utterback, P.; Lambrakis, L.; et al. Chemical composition of selected insect meals and their effect on apparent total tract digestibility, fecal metabolites, and microbiota of adult cats fed insect-based retorted diets. J. Anim. Sci. 2022, 100, skac024. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Shang, Y.; Wang, Q. Exploration of the Mechanism of Linoleic Acid Metabolism Dysregulation in Metabolic Syndrome. Genet. Res. 2022, 2022, 6793346. [Google Scholar] [CrossRef]
- Alexeev, E.E.; Lanis, J.M.; Kao, D.J.; Campbell, E.L.; Kelly, C.J.; Battista, K.D.; Gerich, M.E.; Jenkins, B.R.; Walk, S.T.; Kominsky, D.J.; et al. Microbiota-Derived Indole Metabolites Promote Human and Murine Intestinal Homeostasis through Regulation of Interleukin-10 Receptor. Am. J. Pathol. 2018, 188, 1183–1194. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.K.; Kwon, B. Immune regulation through tryptophan metabolism. Exp. Mol. Med. 2023, 55, 1371–1379. [Google Scholar] [CrossRef]
- Hayes, K.C. Nutritional problems in cats: Taurine deficiency and vitamin A excess. Can. Vet. J. 1982, 23, 2–5. [Google Scholar]
- Brosnan, J.T.; Brosnan, M.E. The sulfur-containing amino acids: An overview. J. Nutr. 2006, 136 (Suppl. S6), 1636S–1640S. [Google Scholar] [CrossRef]
- MacDonald, M.L.; Rogers, Q.R.; Morris, J.G. Nutrition of the domestic cat, a mammalian carnivore. Annu. Rev. Nutr. 1984, 4, 521–562. [Google Scholar] [CrossRef]
- Song, Z.; Liu, L.; Xu, Y.; Cao, R.; Lan, X.; Pan, C.; Zhang, S.; Zhao, H. Caffeine-Induced Sleep Restriction Alters the Gut Microbiome and Fecal Metabolic Profiles in Mice. Int. J. Mol. Sci. 2022, 23, 14837. [Google Scholar] [CrossRef]
- Zengler, K.; Zaramela, L.S. The social network of microorganisms—How auxotrophies shape complex communities. Nat. Rev. Microbiol. 2018, 16, 383–390. [Google Scholar] [CrossRef]
- Bai, M.; Liu, H.; Yan, Y.; Duan, S.; Szeto, I.M.; He, J.; Hu, J.; Fu, Y.; Xu, K.; Xiong, X. Hydrolyzed protein formula improves the nutritional tolerance by increasing intestinal development and altering cecal microbiota in low-birth-weight piglets. Front. Nutr. 2024, 11, 1439110. [Google Scholar] [CrossRef]
| Items | |||
|---|---|---|---|
| Ingredients (%) | Nutrient levels 2 (%) | ||
| Corn | 27.00 | DM | 92.61 |
| Wheat | 10.00 | CP | 31.21 |
| Flours | 8.00 | CF | 10.93 |
| Corn gluten meal | 8.00 | OM | 91.35 |
| Soybean meal | 8.00 | ||
| Beef bone meal | 5.00 | ||
| Chicken meat | 15.00 | ||
| Sugar beet pulp | 3.00 | ||
| Calcium hydrogen Phosphate | 1.00 | ||
| Zeolite powder | 1.00 | ||
| Premix 1 | 2.00 | ||
| Mixed oils | 7.00 | ||
| Composite seasoning | 5.00 | ||
| Total | 100 | ||
| CON | GOD | MCE | GM | |
|---|---|---|---|---|
| Body weight change (kg) | −0.07 ± 0.11 | 0.01 ± 0.21 | 0.03 ± 0.14 | −0.17 ± 0.17 |
| Food intake (g) | 54.10 ± 5.28 | 63.54 ± 5.26 | 57.31 ± 4.67 | 58.22 ± 6.22 |
| Fecal score | 2.47 | 2.49 | 2.50 | 2.50 |
| Digestibility | ||||
| Dry matter (%) | 81.74 ± 1.29 | 79.77 ± 1.30 | 81.30 ± 2.25 | 78.94 ± 0.99 |
| Crude protein (%) | 85.49 ± 0.90 | 84.14 ± 0.75 | 85.43 ± 1.76 | 82.22 ± 0.99 * |
| Crude fat (%) | 94.91 ± 0.97 | 92.74 ± 1.48 | 96.37 ± 0.71 | 93.46 ± 1.11 |
| Organic material (%) | 85.68 ± 0.91 | 84.23 ± 0.92 | 85.27 ± 1.66 | 83.50 ± 0.78 |
| Crude fiber (%) | 37.99 ± 6.85 | 29.45 ± 11.20 | 44.03 ± 7.96 | 18.07 ± 10.73 |
| Items (ng/g) | CON | GOD | MCE | GM |
|---|---|---|---|---|
| Acetic acid | 1282.02 ± 93.09 | 1350.31 ± 49.16 | 1434.59 ± 43.79 | 1439.03 ± 43.12 |
| Propanoic acid | 781.02 ± 84.43 | 769.12 ± 128.15 | 856.10 ± 120.20 | 799.17 ± 115.22 |
| Isobutyric acid | 26.71 ± 4.47 | 29.88 ± 9.18 | 20.77 ± 5.16 | 29.55 ± 8.38 |
| Butyric acid | 428.16 ± 57.42 | 383.46 ± 73.24 | 402.8 ± 42.55 | 464.21 ± 25.51 |
| Isovaleric acid | 54.44 ± 7.64 | 62.88 ± 17.00 | 44.16 ± 10.24 | 60.54 ± 16.41 |
| Valeric acid | 242.95 ± 70.53 | 266.65 ± 57.56 | 252.8 ± 48.67 | 292.00 ± 47.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; He, X.; Kuang, T.; Chen, Z.; Guo, Y.; Huang, Z.; Jian, S.; Jiang, Z.; Zhang, L.; Deng, B.; et al. Effects of Glucose Oxidase and Macleaya cordata Extract on Immune Function, Antioxidant Capacity, and Gut Microbiota in British Shorthair Cats. Metabolites 2025, 15, 759. https://doi.org/10.3390/metabo15120759
Li L, He X, Kuang T, Chen Z, Guo Y, Huang Z, Jian S, Jiang Z, Zhang L, Deng B, et al. Effects of Glucose Oxidase and Macleaya cordata Extract on Immune Function, Antioxidant Capacity, and Gut Microbiota in British Shorthair Cats. Metabolites. 2025; 15(12):759. https://doi.org/10.3390/metabo15120759
Chicago/Turabian StyleLi, Lizhen, Xuanzhen He, Tao Kuang, Zhuoting Chen, Yan Guo, Zhiyi Huang, Shiyan Jian, Zipeng Jiang, Limeng Zhang, Baichuan Deng, and et al. 2025. "Effects of Glucose Oxidase and Macleaya cordata Extract on Immune Function, Antioxidant Capacity, and Gut Microbiota in British Shorthair Cats" Metabolites 15, no. 12: 759. https://doi.org/10.3390/metabo15120759
APA StyleLi, L., He, X., Kuang, T., Chen, Z., Guo, Y., Huang, Z., Jian, S., Jiang, Z., Zhang, L., Deng, B., & Liu, Q. (2025). Effects of Glucose Oxidase and Macleaya cordata Extract on Immune Function, Antioxidant Capacity, and Gut Microbiota in British Shorthair Cats. Metabolites, 15(12), 759. https://doi.org/10.3390/metabo15120759







