FADS1 and FADS2 Gene Polymorphisms Affect Omega-3 and Omega-6 Erythrocyte Fatty Acid Composition and Influence the Association Between Dietary Fatty Acid Intake and Lipid Profile in Brazilian Adults
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. SNP Selection and Genotyping
2.3. Fatty Acid Composition in Erythrocyte Membranes
2.4. Dietary Intake Assessment
2.5. Biochemical Measurements
2.6. Other Covariates
2.7. Statistical Analysis
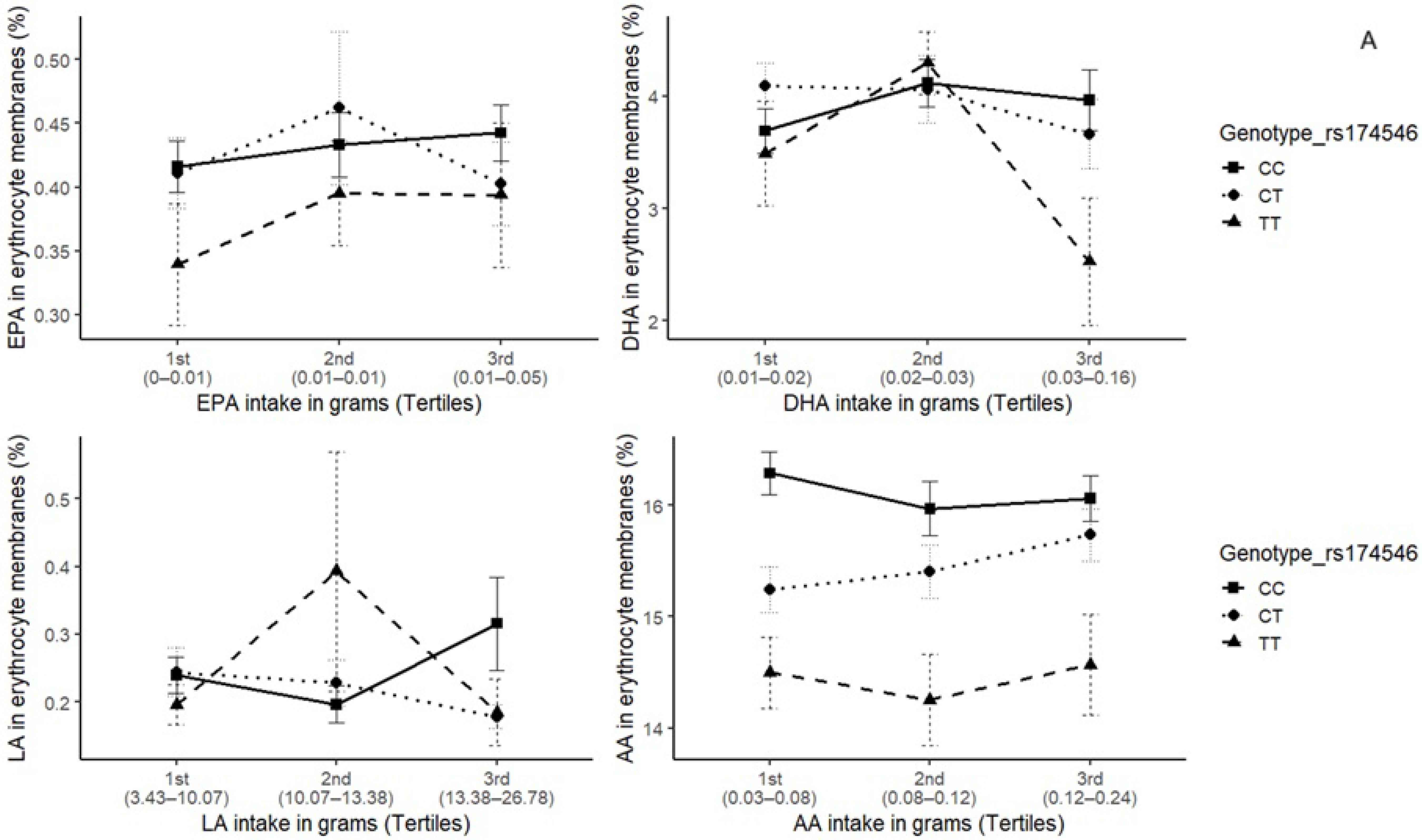
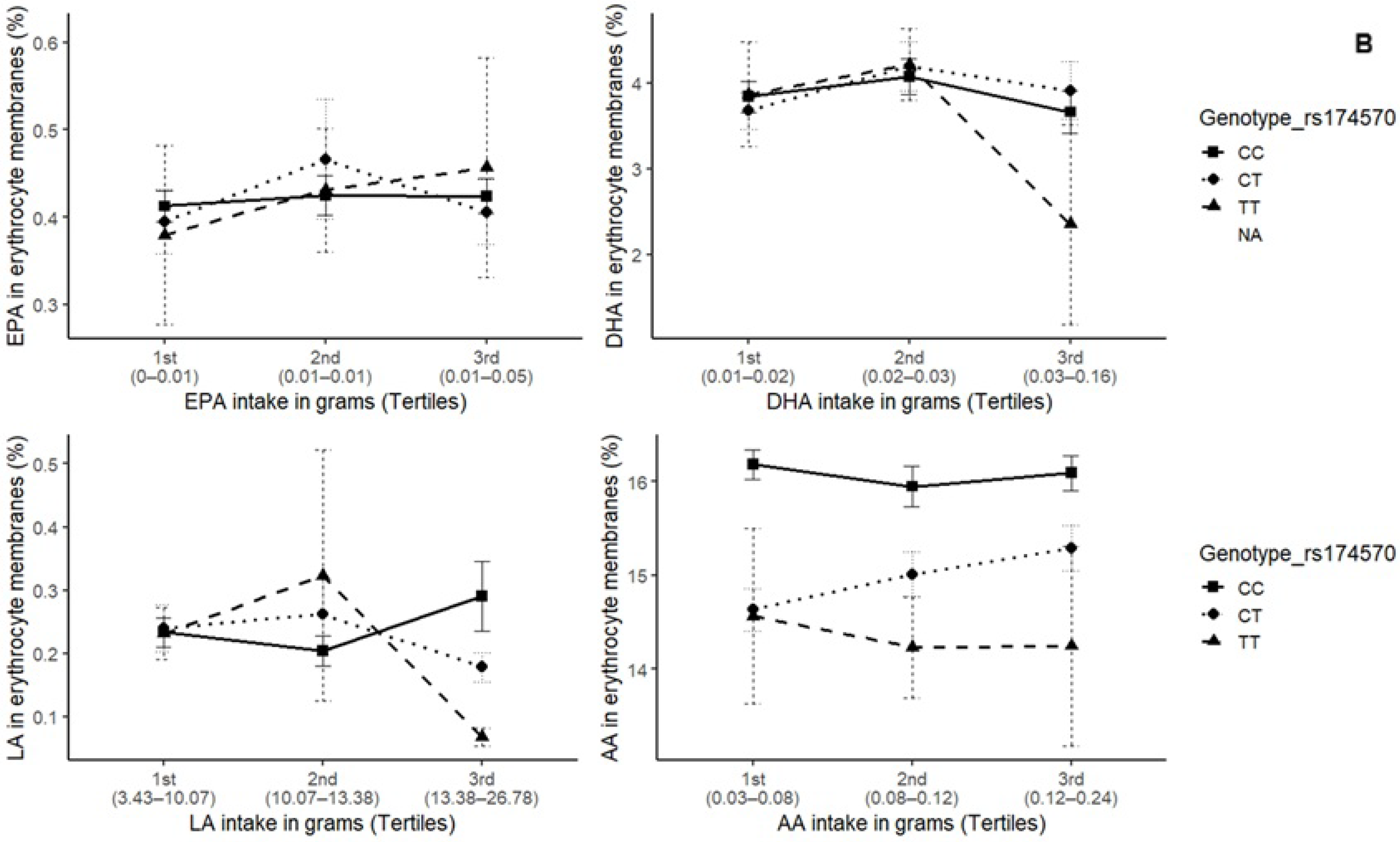
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 24HR | 24-Hour Dietary Recall |
| AA | Arachidonic Acid |
| AFR | African |
| ALA | Alpha-Linolenic Acid |
| AMR | Admixed American |
| BMI | Body Mass Index |
| CVD | Cardiovascular Disease |
| DHA | Docosahexaenoic Acid |
| EPA | Eicosapentaenoic Acid |
| EUR | European |
| FADS1/FADS2 | Fatty Acid Desaturase 1/Fatty Acid Desaturase 2 |
| FFQ | Food Frequency Questionnaire |
| GC | Gas Chromatograph |
| GLM | Generalized Linear Model |
| GWAS | Genome-Wide Association Study |
| HDL-c | High-Density Lipoprotein Cholesterol |
| HUFA/SAT | Highly Unsaturated Fatty Acids-to-Saturated Fatty Acids Ratio |
| HWE | Hardy–Weinberg Equilibrium |
| IPAQ | International Physical Activity Questionnaire |
| IQR | Interquartile Range |
| ISA-Nutrition | Health Survey of São Paulo with a focus on Nutrition |
| LA | Linoleic Acid |
| LD | Linkage Disequilibrium |
| LDL-c | Low-Density Lipoprotein Cholesterol |
| MAF | Minor Allele Frequency |
| MSM | Multiple Source Method |
| NDSR | Nutrition Data System for Research |
| Non-HDL-c | Non-High-Density Lipoprotein Cholesterol |
| O3I | Omega-3 Index |
| PAHO | Pan American Health Organization |
| PAL | Physical Activity Level |
| PUFA | Polyunsaturated Fatty Acid |
| SNP | Single-Nucleotide Polymorphism |
| STROBE | Strengthening the Reporting of Observational Studies in Epidemiology |
| TG | Triglycerides |
| VLDL | Very-Low-Density Lipoprotein |
| WHO | World Health Organization |
| ω-3 | Omega-3 Fatty Acid |
| ω-6 | Omega-6 Fatty Acid |
References
- Hasani, W.S.R.; Muhamad, N.A.; Hanis, T.M.; Maamor, N.H.; Chen, X.W.; Omar, M.A.; Kueh, Y.C.; Karim, Z.A.; Abu Hassan, M.R.; Musa, K.I. The global estimate of premature cardiovascular mortality: A systematic review and meta-analysis of age-standardized mortality rate. BMC Public Health 2023, 23, 1561. [Google Scholar] [CrossRef]
- Willett, W.C. Dietary fats and coronary heart disease. J. Intern. Med. 2012, 272, 13–24. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Zähringer, J.; Beyerbach, J.; Werner, S.S.; Heseker, H.; Koletzko, B.; Meerpohl, J.J. Total Dietary Fat Intake, Fat Quality, and Health Outcomes: A Scoping Review of Systematic Reviews of Prospective Studies. Ann. Nutr. Metab. 2021, 77, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, T.; Yoshida, M.; Arita, M. Omega-3 fatty acid-derived mediators that control inflammation and tissue homeostasis. Int. Immunol. 2019, 31, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Innes, J.K.; Calder, P.C. Omega-6 fatty acids and inflammation. Prostaglandins Leukot. Essent. Fat. Acids 2018, 132, 41–48. [Google Scholar] [CrossRef]
- Liu, Q.K. Triglyceride-lowering and anti-inflammatory mechanisms of omega-3 polyunsaturated fatty acids for atherosclerotic cardiovascular risk reduction. J. Clin. Lipidol. 2021, 15, 556–568. [Google Scholar] [CrossRef]
- Abdelhamid, A.S.; Brown, T.J.; Brainard, J.S.; Biswas, P.; Thorpe, G.C.; Moore, H.J.; Deane, K.H.; AlAbdulghafoor, F.K.; Summerbell, C.D.; Worthington, H.V.; et al. Omega-3 fatty acids for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2018, 11, CD003177. [Google Scholar]
- Sun, Y.; Xu, H.; Ye, K. GWAS and multi-omics integrative analysis reveal novel loci and their molecular mechanisms for circulating fatty acids. HGG Adv. 2025, 6, 100470. [Google Scholar] [CrossRef]
- De Groot, R.H.M.; Emmett, R.; Meyer, B.J. Non-dietary factors associated with n-3 long-chain PUFA levels in humans—A systematic literature review. Br. J. Nutr. 2019, 121, 793–808. [Google Scholar] [CrossRef]
- Nuotio, P.; Lankinen, M.A.; Meuronen, T.; de Mello, V.D.; Sallinen, T.; Virtanen, K.A.; Pihlajamäki, J.; Laakso, M.; Schwab, U. Dietary n-3 alpha-linolenic and n-6 linoleic acids modestly lower serum lipoprotein(a) concentration but differentially influence other atherogenic lipoprotein traits: A randomized trial. Atherosclerosis 2024, 395, 117562. [Google Scholar] [CrossRef]
- Reyes-Pérez, S.D.; González-Becerra, K.; Barrón-Cabrera, E.; Muñoz-Valle, J.F.; Armendáriz-Borunda, J.; Martínez-López, E. FADS1 Genetic Variant and Omega-3 Supplementation Are Associated with Changes in Fatty Acid Composition in Red Blood Cells of Subjects with Obesity. Nutrients 2024, 16, 3522. [Google Scholar] [CrossRef]
- Gillingham, L.G.; Harding, S.V.; Rideout, T.C.; Yurkova, N.; Cunnane, S.C.; Eck, P.K.; Jones, P.J. Dietary oils and FADS1-FADS2 genetic variants modulate [13C] α-linolenic acid metabolism and plasma fatty acid composition. Am. J. Clin. Nutr. 2013, 97, 195–207. [Google Scholar] [CrossRef]
- Fisberg, R.M.; Sales, C.H.; Fontanelli, M.M.; Pereira, J.L.; Alves, M.C.G.P.; Escuder, M.M.L.; César, C.L.G.; Goldbaum, M. 2015 Health Survey of São Paulo with Focus in Nutrition: Rationale, Design, and Procedures. Nutrients 2018, 10, 169. [Google Scholar] [CrossRef] [PubMed]
- Lachat, C.; Hawwash, D.; Ocké, M.C.; Berg, C.; Forsum, E.; Hörnell, A.; Larsson, C.; Sonestedt, E.; Wirfält, E.; Åkesson, A.; et al. Strengthening the Reporting of Observational Studies in Epidemiology—Nutritional Epidemiology (STROBE-nut): An Extension of the STROBE Statement. PLoS Med. 2016, 13, e1002036. [Google Scholar] [CrossRef] [PubMed]
- Pereira, J.L.; de Souza, C.A.; Neyra, J.E.M.; Leite, J.M.R.S.; Cerqueira, A.; Mingroni-Netto, R.C.; Soler, J.M.P.; Rogero, M.M.; Sarti, F.M.; Fisberg, R.M. Genetic Ancestry and Self-Reported “Skin Color/Race” in the Urban Admixed Population of São Paulo City, Brazil. Genes 2024, 15, 917. [Google Scholar] [CrossRef]
- Tanaka, T.; Shen, J.; Abecasis, G.R.; Kisialiou, A.; Ordovas, J.M.; Guralnik, J.M.; Singleton, A.; Bandinelli, S.; Cherubini, A.; Arnett, D.; et al. Genome-wide association study of plasma polyunsaturated fatty acids in the InCHIANTI Study. PLoS Genet. 2009, 5, e1000338. [Google Scholar] [CrossRef]
- Schaeffer, L.; Gohlke, H.; Müller, M.; Heid, I.M.; Palmer, L.J.; Kompauer, I.; Demmelmair, H.; Illig, T.; Koletzko, B.; Heinrich, J. Common genetic variants of the FADS1 FADS2 gene cluster and their reconstructed haplotypes are associated with the fatty acid composition in phospholipids. Hum. Mol. Genet. 2006, 15, 1745–1756. [Google Scholar] [CrossRef]
- Batista, L.D.; Tucker, K.L.; Bigornia, S.; Noel, S.E.; Harris, W.S.; Braga, R.A.M.; Neto, J.V.; Damasceno, N.R.T.; Rogero, M.M.; Sarti, F.M.; et al. Association of two cutoff points for the omega-3 index with cardiometabolic risk factors in Brazilian and Puerto Rican middle-aged adults. Br. J. Nutr. 2025, 134, 401–412. [Google Scholar] [CrossRef]
- Pottala, J.V.; Espeland, M.A.; Polreis, J.; Robinson, J.; Harris, W.S. Correcting the effects of −20 °C storage and aliquot size on erythrocyte fatty acid content in the Women’s Health Initiative. Lipids 2012, 47, 835–846. [Google Scholar] [CrossRef]
- Harris, W.S.; Von Schacky, C. The Omega-3 Index: A new risk factor for death from coronary heart disease? Prev. Med. 2004, 39, 212–220. [Google Scholar] [CrossRef]
- Blanton, C.A.; Moshfegh, A.J.; Baer, D.J.; Kretsch, M.J. The USDA Automated Multiple-Pass Method accurately estimates group total energy and nutrient intake. J. Nutr. 2006, 136, 2594–2599. [Google Scholar] [CrossRef] [PubMed]
- Moshfegh, A.J.; Rhodes, D.G.; Baer, D.J.; Murayi, T.; Clemens, J.C.; Rumpler, W.V.; Paul, D.R.; Sebastian, R.S.; Kuczynski, K.J.; Ingwersen, L.A.; et al. The US Department of Agriculture Automated Multiple-Pass Method reduces bias in the collection of energy intakes. Am. J. Clin. Nutr. 2008, 88, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Tabela Brasileira de Composição de Alimentos (TBCA). Available online: http://www.fcf.usp.br/tbca (accessed on 12 December 2024).
- Willet, W. Nutritional Epidemiology, 3rd ed.; Oxford University Press: Oxford, UK, 2013. [Google Scholar]
- Greupner, T.; Kutzner, L.; Pagenkopf, S.; Kohrs, H.; Hahn, A.; Schebb, N.H.; Schuchardt, J.P. Effects of a low and a high dietary LA/ALA ratio on long-chain PUFA concentrations in red blood cells. Food Funct. 2018, 9, 4742–4754. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, H.; Jin, Q.; Wang, X. Effects of Dietary Plant-Derived Low-Ratio Linoleic Acid/Alpha-Linolenic Acid on Blood Lipid Profiles: A Systematic Review and Meta-Analysis. Foods 2023, 12, 3005. [Google Scholar] [CrossRef]
- Carr, S.S.; Hooper, A.J.; Sullivan, D.R.; Burnett, J.R. Non-HDL-cholesterol and apolipoprotein B compared with LDL-cholesterol in atherosclerotic cardiovascular disease risk assessment. Pathology 2019, 51, 148–154. [Google Scholar] [CrossRef]
- Craig, C.L.; Marshall, A.L.; Sjöström, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.L.; Yngve, A.; Sallis, J.F.; et al. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef]
- Matsudo, S.; Matsudo, V.; Andrade, D.; Andrade, E.; Oliveira, L.C.; Braggion, G. Questionário Internacional de Atividade Física (IPAQ): Estudo de Validade e Reprodutibilidade no Brasil. Rev. Bras. Ativ. Fís. Saúde 2012, 6, 5–18. [Google Scholar]
- World Health Organization. Global Recommendations on Physical Activity for Health; World Health Organization: Geneva, Switzerland, 2010. [Google Scholar]
- World Health Organization. Obesity: Preventing and Managing the Global Epidemic: Report of a WHO Consultation on Obesity, Geneva, Switzerland, 3–5 June 1997; World Health Organization: Geneva, Switzerland, 2000. [Google Scholar]
- Pan-American Health Organization. XXXVI Reunión del Comitê Asesor de Ivestigaciones en Salud—Encuestra Multicêntrica—Salud Beinestar y Envejecimeiento (SABE) en América Latina e el Caribe—Informe Preliminar; PAHO: Washington, DC, USA, 2001. [Google Scholar]
- World Health Organization. Waist circumference and waist–hip ratio: Report of a WHO Expert Consultation, Geneva, 8–11 December 2008; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Koletzko, B.; Reischl, E.; Tanjung, C.; Gonzalez-Casanova, I.; Ramakrishnan, U.; Meldrum, S.; Simmer, K.; Heinrich, J.; Demmelmair, H. FADS1 and FADS2 Polymorphisms Modulate Fatty Acid Metabolism and Dietary Impact on Health. Annu. Rev. Nutr. 2019, 39, 21–44. [Google Scholar] [CrossRef]
- Metelcová, T.; Vaňková, M.; Zamrazilová, H.; Hovhannisyan, M.; Staňková, B.; Tvrzická, E.; Hill, M.; Hainer, V.; Včelák, J.; Kunešová, M. FADS1 gene polymorphism(s) and fatty acid composition of serum lipids in adolescents. Lipids 2021, 56, 499–508. [Google Scholar] [CrossRef]
- Lankinen, M.; Uusitupa, M.; Schwab, U. Genes and Dietary Fatty Acids in Regulation of Fatty Acid Composition of Plasma and Erythrocyte Membranes. Nutrients 2018, 10, 1785. [Google Scholar] [CrossRef]
- Van der Meij, B.S.; Wijnhoven, H.A.H.; Lee, J.S.; Houston, D.K.; Hue, T.; Harris, T.B.; Kritchevsky, S.B.; Newman, A.B.; Visser, M. Poor Appetite and Dietary Intake in Community-Dwelling Older Adults. J. Am. Geriatr. Soc. 2017, 65, 2190–2197. [Google Scholar] [CrossRef]
- O’Keeffe, M.; Kelly, M.; O’Herlihy, E.; O’Toole, P.W.; Kearney, P.M.; Timmons, S.; O’Shea, E.; Stanton, C.; Hickson, M.; Rolland, Y.; et al. Potentially modifiable determinants of malnutrition in older adults: A systematic review. Clin. Nutr. 2019, 38, 2477–2498. [Google Scholar] [CrossRef]
- Batista, L.D.; de França, N.A.G.; Fontanelli, M.M.; Martinez-Arroyo, A.G.; Fisberg, R.M. Misreporting of dietary energy intake obtained by 24 h recalls in older adults: A comparison of five previous methods using doubly labeled water. Eur. J. Clin. Nutr. 2022, 76, 535–543. [Google Scholar] [CrossRef]
- Wien, M.; Rajaram, S.; Oda, K.; Sabaté, J. Decreasing the linoleic acid to alpha-linolenic acid diet ratio increases eicosapentaenoic acid in erythrocytes in adults. Lipids 2010, 45, 683–692. [Google Scholar] [CrossRef]
- Martinelli, N.; Girelli, D.; Malerba, G.; Guarini, P.; Illig, T.; Trabetti, E.; Sandri, M.; Friso, S.; Pizzolo, F.; Schaeffer, L.; et al. FADS genotypes and desaturase activity estimated by the ratio of arachidonic acid to linoleic acid are associated with inflammation and coronary artery disease. Am. J. Clin. Nutr. 2008, 88, 941–949. [Google Scholar] [CrossRef]
- Kim, S.; Won, C.W. Sex-different changes of body composition in aging: A systemic review. Arch. Gerontol. Geriatr. 2022, 102, 104711. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Patel, K.V.; Lavie, C.J. Obesity, Central Adiposity, and Fitness: Understanding the Obesity Paradox in the Context of Other Cardiometabolic Parameters. Mayo Clin. Proc. 2018, 93, 676–678. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhu, G.; Chen, G.; Luo, M.; Liu, X.; Chen, Z.; Qian, J. Distribution of lipid levels and prevalence of hyperlipidemia: Data from the NHANES 2007–2018. Lipids Health Dis. 2022, 21, 111. [Google Scholar] [CrossRef]
- Sergeant, S.; Keith, B.A.; Seeds, M.C.; Legins, J.A.; Young, C.B.; Vitolins, M.Z.; Chilton, F.H. Impact of FADS gene variation and dietary fatty acid exposure on biochemical and anthropomorphic phenotypes in a Hispanic/Latino cohort. Front. Nutr. 2023, 10, 1111624. [Google Scholar] [CrossRef]
- Zietemann, V.; Kröger, J.; Enzenbach, C.; Jansen, E.; Fritsche, A.; Weikert, C.; Boeing, H.; Schulze, M.B. Genetic variation of the FADS1 FADS2 gene cluster and n-6 PUFA composition in erythrocyte membranes in the European Prospective Investigation into Cancer and Nutrition-Potsdam study. Br. J. Nutr. 2010, 104, 1748–1759. [Google Scholar] [CrossRef]
- Verly, E.; Fisberg, R.M.; Marchioni, D.M. Is the food frequency consumption essential as covariate to estimate usual intake of episodically consumed foods? Eur. J. Clin. Nutr. 2012, 66, 1254–1258. [Google Scholar] [CrossRef] [PubMed]

| Total Population (n = 294) | Age Groups | |||
|---|---|---|---|---|
| 20–59 Years (n = 131) | 60+ Years (n = 163) | |||
| Median (IQR) | Median (IQR) | Median (IQR) | p | |
| Total cholesterol (mg/dL) | 187 (57) | 189 (52) | 186 (59) | 0.301 |
| HDL cholesterol (mg/dL) | 43 (19) | 41.5 (20) | 44 (17) | 0.315 |
| LDL cholesterol (mg/dL) | 113 (49) | 118.5 (44) | 112 (53) | 0.320 |
| VLDL cholesterol (mg/dL) | 24 (15) | 24 (16) | 24 (14) | 0.952 |
| Triglycerides (mg/dL) | 120 (73.5) | 121 (77) | 118 (72) | 0.902 |
| non-HDL cholesterol (mg/dL) | 144 (60) | 148.5 (56) | 138 (60) | 0.177 |
| Adjusted usual energy intake (kcal/day) | 1679 (595) | 1772 (621) | 1615 (534) | 0.003 |
| Dietary total fat intake (%) | 30.9 (6.7) | 31.4 (7.0) | 30.7 (6.7) | 0.112 |
| Total polyunsaturated fatty acids intake (%) | 7.3 (2.1) | 7.2 (2.1) | 7.4 (2.1) | 0.717 |
| Total ω-3 fatty acids usual intake (g) | 1.6 (0.7) | 1.7 (0.7) | 1.5 (0.7) | 0.046 |
| Eicosapentaenoic (EPA) usual intake (g) | 0.01 (0.01) | 0.01 (0.01) | 0.01 (0.01) | 0.836 |
| Docosahexaenoic (DHA) usual intake (g) | 0.03 (0.01) | 0.03 (0.02) | 0.03 (0.01) | 0.291 |
| Alpha-Linolenic acid (ALA) usual intake (g) | 1.5 (0.6) | 1.7 (0.6) | 1.5 (0.5) | 0.036 |
| Linoleic acid usual intake (g) | 12.0 (5.3) | 12.6 (5.7) | 11.5 (4.6) | 0.016 |
| Linoleic/ALA ratio | 7.7 (1.1) | 7.8 (1.1) | 7.6 (1.1) | 0.016 |
| Sex—n (%) * | ||||
| Male | 169 (57.5) | 75 (57.2) | 94 (57.7) | 0.943 |
| Female | 125 (42.5) | 56 (42.8) | 69 (42.3) | |
| Nutritional Status—n (%) * | ||||
| Without overweight/obesity | 135 (46.1) | 46 (35.1) | 89 (54.9) | 0.001 |
| Excess weight (overweight or obesity) | 158 (53.9) | 85 (64.9) | 73 (45.1) | |
| Central adiposity—n (%) * | ||||
| <88 cm (women) or <102 cm (men) | 132 (45.5) | 70 (54.7) | 62 (38.3) | 0.005 |
| ≥88 cm (women) or ≥102 cm (men) | 158 (54.5) | 58 (45.3) | 100 (61.7) | |
| Leisure-Time Physical Activity—n (%) * | ||||
| <150 min/week | 241 (83.1) | 109 (83.9) | 132 (82.5) | 0.761 |
| ≥150 min/week | 49 (16.9) | 21 (16.1) | 28 (17.5) | |
| Smoking status—n (%) * | ||||
| Never | 170 (58.0) | 80 (61.1) | 90 (55.6) | 0.448 |
| Current or past | 123 (42.0) | 51 (38.9) | 72 (44.4) | |
| Statins or antilipidemic use—n (%) ** | ||||
| No | 263 (89.5) | 127 (96.9) | 136 (83.4) | <0.001 |
| Yes | 31 (10.5) | 4 (3.1) | 27 (16.6) | |
| SNP | Gene | M/m Alleles | Genotype | MAF (%) | HWE | ||
|---|---|---|---|---|---|---|---|
| CC | CT | TT | |||||
| rs174537 | FADS1 | C/T | 151 (51.5%) | 109 (37.2%) | 33 (11.3%) | 29.9% | 0.069 |
| rs174546 | FADS1 | C/T | 151 (51.4%) | 110 (37.4%) | 33 (11.2%) | 29.9% | 0.070 |
| rs174556 | FADS1 | C/T | 161 (54.8%) | 109 (37.1%) | 24 (8.2%) | 26.7% | 0.372 |
| rs174570 | FADS2 | C/T | 190 (64.9%) | 88 (30.0%) | 15 (5.1%) | 20.1% | 0.275 |
| rs174583 | FADS2 | C/T | 117 (39.8%) | 125 (42.5%) | 52 (17.7%) | 38.9% | 0.067 |
| rs174546—FADS1 | rs174570—FADS2 | |||||||
|---|---|---|---|---|---|---|---|---|
| ω-3 Fatty Acids | CC (n = 151) | CT (n = 110) | TT (n = 33) | p | CC (n = 190) | CT (n = 88) | TT (n = 15) | p |
| Alpha-Linolenic (C18:3 ω3) | 0.15 (0.10) | 0.16 (0.13) | 0.13 (0.10) | 0.285 | 0.15 (0.10) | 0.15 (0.14) | 0.18 (0.15) | 0.854 |
| Eicosapentaenoic (C20:5 ω3) | 0.41 (0.16) | 0.40 (0.16) | 0.35 (0.23) | 0.057 | 0.40 (0.16) | 0.36 (0.14) | 0.37 (0.39) | 0.199 |
| Docosapentaenoic (C22:5 ω3) | 2.24 (0.51) | 2.30 (0.47) | 2.07 (0.40) | 0.010 b,c | 2.27 (0.48) | 2.19 (0.51) | 2.02 (0.35) | 0.081 |
| Docosahexaenoic (C22:6 ω3) | 3.83 (1.58) | 3.93 (1.59) | 3.80 (1.34) | 0.636 | 3.87 (1.59) | 3.86 (1.52) | 3.72 (1.50) | 0.758 |
| ω-3 Index (EPA + DHA) | 4.29 (1.74) | 4.25 (1.59) | 4.09 (1.67) | 0.645 | 4.28 (1.74) | 4.21 (1.54) | 4.03 (1.75) | 0.811 |
| ω-6 Fatty acids | ||||||||
| Linoleic (C18:2 ω6) | 9.42 (1.93) | 9.95 (2.00) | 10.3 (1.67) | 0.006 a,b | 9.40 (1.94) | 10.2 (1.77) | 10.7 (1.96) | 0.001 a |
| Gamma-Linolenic (C18:3 ω6) | 0.18 (0.15) | 0.19 (0.18) | 0.16 (0.17) | 0.715 | 0.18 (0.16) | 0.19 (0.16) | 0.17 (0.22) | 0.604 |
| Eicosadienoic (C20:2 ω6) | 0.23 (0.15) | 0.25 (0.16) | 0.26 (0.09) | 0.300 | 0.23 (0.16) | 0.26 (0.14) | 0.26 (0.20) | 0.208 |
| Dihomo-y-linolenic (C20:3 ω6) | 1.57 (0.44) | 1.84 (0.44) | 2.24 (0.45) | <0.001 a,b,c | 1.64 (0.45) | 1.89 (0.57) | 2.15 (0.54) | <0.001 a,b |
| Arachidonic (C20:4 ω6) | 16.0 (1.88) | 15.3 (1.89) | 14.7 (1.83) | <0.001 a,b,c | 16.0 (1.83) | 14.9 (1.51) | 14.6 (2.38) | <0.001 a,b |
| Docosatetraenoic (C22:4 ω6) | 3.58 (0.77) | 3.41 (0.65) | 3.21 (0.87) | 0.004 b | 3.53 (0.74) | 3.40 (0.59) | 3.34 (1.26) | 0.075 |
| Total PUFA % | 37.9 (3.05) | 37.9 (2.78) | 36.9 (2.63) | 0.201 | 37.9 (2.90) | 37.9 (2.96) | 36.8 (1.40) | 0.220 |
| FADS1 (rs174546) | FADS2 (rs174570) | |||||
|---|---|---|---|---|---|---|
| Total Cholesterol (mg/dL) | β | SE | P | β | SE | P |
| LA/ALA ratio | −0.01 | 0.02 | 0.534 | −0.01 | 0.01 | 0.642 |
| Genotype—CT | 0.31 | 0.19 | 0.103 | 0.49 | 0.18 | 0.008 |
| Genotype—TT | 0.55 | 0.27 | 0.041 | 0.57 | 0.33 | 0.083 |
| Interaction CT vs. LA/ALA ratio | −0.04 | 0.02 | 0.092 | −0.06 | 0.02 | 0.007 |
| Interaction TT vs. LA/ALA ratio | −0.07 | 0.03 | 0.031 | −0.07 | 0.04 | 0.083 |
| DHA_Adjusted for energy | −0.43 | 1.07 | 0.689 | - | ||
| Genotype—CT | 0.06 | 0.07 | 0.397 | |||
| Genotype—TT | 0.17 | 0.10 | 0.081 | |||
| Interaction CT vs. DHA intake | −2.08 | 1.99 | 0.299 | |||
| Interaction TT vs. DHA intake | −6.11 | 2.83 | 0.031 | |||
| VLDL (mg/dL) | ||||||
| LA/ALA ratio | - | −0.04 | 0.04 | 0.273 | ||
| Genotype—CT | 0.27 | 0.52 | 0.603 | |||
| Genotype—TT | 2.01 | 0.86 | 0.020 | |||
| Interaction CT vs. LA/ALA ratio | −0.01 | 0.07 | 0.937 | |||
| Interaction TT vs. LA/ALA ratio | −0.22 | 0.11 | 0.045 | |||
| DHA_Adjusted for energy | −4.64 | 2.79 | 0.097 | −5.80 | 2.5 | 0.022 |
| Genotype—CT | 0.03 | 0.17 | 0.857 | 0.13 | 0.17 | 0.466 |
| Genotype—TT | 0.85 | 0.26 | 0.001 | 0.73 | 0.31 | 0.021 |
| Interaction CT vs. DHA intake | 3.52 | 4.91 | 0.473 | 3.65 | 4.99 | 0.464 |
| Interaction TT vs. DHA intake | −15.6 | 7.30 | 0.032 | −11.7 | 8.83 | 0.186 |
| LDL cholesterol (mg/dL) | ||||||
| LA/ALA ratio | −0.01 | 0.02 | 0.868 | −0.01 | 0.02 | 0.908 |
| Genotype—CT | 0.62 | 0.27 | 0.021 | 0.82 | 0.26 | 0.002 |
| Genotype—TT | 0.45 | 0.39 | 0.245 | 0.23 | 0.47 | 0.635 |
| Interaction CT vs. LA/ALA ratio | −0.08 | 0.03 | 0.019 | −0.11 | 0.03 | 0.001 |
| Interaction TT vs. LA/ALA ratio | −0.07 | 0.05 | 0.173 | −0.03 | 0.06 | 0.594 |
| Non-HDL cholesterol (mg/dL) | ||||||
| LA/ALA ratio | −0.01 | 0.02 | 0.481 | −0.01 | 0.02 | 0.604 |
| Genotype—CT | 0.49 | 0.26 | 0.064 | 0.74 | 0.25 | 0.004 |
| Genotype—TT | 0.72 | 0.37 | 0.049 | 0.72 | 0.45 | 0.106 |
| Interaction CT vs. LA/ALA ratio | −0.06 | 0.03 | 0.074 | −0.09 | 0.03 | 0.005 |
| Interaction TT vs. LA/ALA ratio | −0.09 | 0.05 | 0.058 | −0.09 | 0.06 | 0.129 |
| DHA_Adjusted for energy | −1.05 | 1.45 | 0.468 | - | ||
| Genotype—CT | 0.07 | 0.09 | 0.454 | |||
| Genotype—TT | 0.32 | 0.13 | 0.016 | |||
| Interaction CT vs. DHA intake | −1.44 | 2.68 | 0.589 | |||
| Interaction TT vs. DHA intake | −9.24 | 3.82 | 0.016 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Batista, L.D.; Rogero, M.M.; Sarti, F.M.; Costa, M.L.; França, J.L.P.; Valentini Neto, J.; Fisberg, R.M. FADS1 and FADS2 Gene Polymorphisms Affect Omega-3 and Omega-6 Erythrocyte Fatty Acid Composition and Influence the Association Between Dietary Fatty Acid Intake and Lipid Profile in Brazilian Adults. Metabolites 2025, 15, 758. https://doi.org/10.3390/metabo15120758
Batista LD, Rogero MM, Sarti FM, Costa ML, França JLP, Valentini Neto J, Fisberg RM. FADS1 and FADS2 Gene Polymorphisms Affect Omega-3 and Omega-6 Erythrocyte Fatty Acid Composition and Influence the Association Between Dietary Fatty Acid Intake and Lipid Profile in Brazilian Adults. Metabolites. 2025; 15(12):758. https://doi.org/10.3390/metabo15120758
Chicago/Turabian StyleBatista, Lais Duarte, Marcelo Macedo Rogero, Flávia Mori Sarti, Marcela Larissa Costa, Jaqueline Lopes Pereira França, João Valentini Neto, and Regina Mara Fisberg. 2025. "FADS1 and FADS2 Gene Polymorphisms Affect Omega-3 and Omega-6 Erythrocyte Fatty Acid Composition and Influence the Association Between Dietary Fatty Acid Intake and Lipid Profile in Brazilian Adults" Metabolites 15, no. 12: 758. https://doi.org/10.3390/metabo15120758
APA StyleBatista, L. D., Rogero, M. M., Sarti, F. M., Costa, M. L., França, J. L. P., Valentini Neto, J., & Fisberg, R. M. (2025). FADS1 and FADS2 Gene Polymorphisms Affect Omega-3 and Omega-6 Erythrocyte Fatty Acid Composition and Influence the Association Between Dietary Fatty Acid Intake and Lipid Profile in Brazilian Adults. Metabolites, 15(12), 758. https://doi.org/10.3390/metabo15120758










