Integrative Genomic and Metabolomic Analysis Identifies mQTLs Associated with Genetic Selection for Tenderness in Nellore Cattle
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Calf Sampling
2.2. Processing of Blood Serum and Metabolite Extraction
2.3. 1H NMR-Based Metabolomic Profiling
2.4. Genotyping and SNP Quality Control
2.5. mQTL Identification and Enrichment Analysis
3. Results
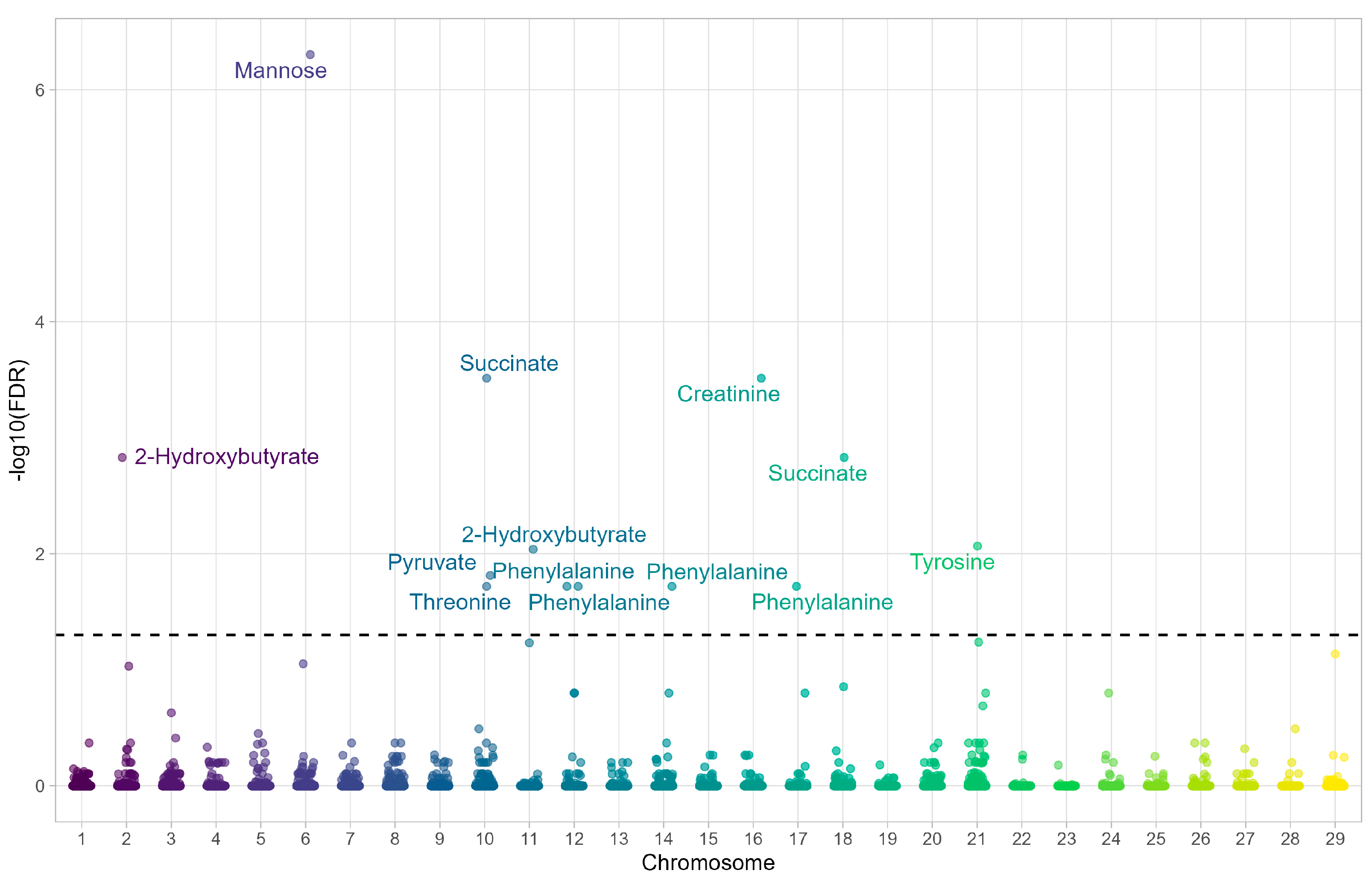
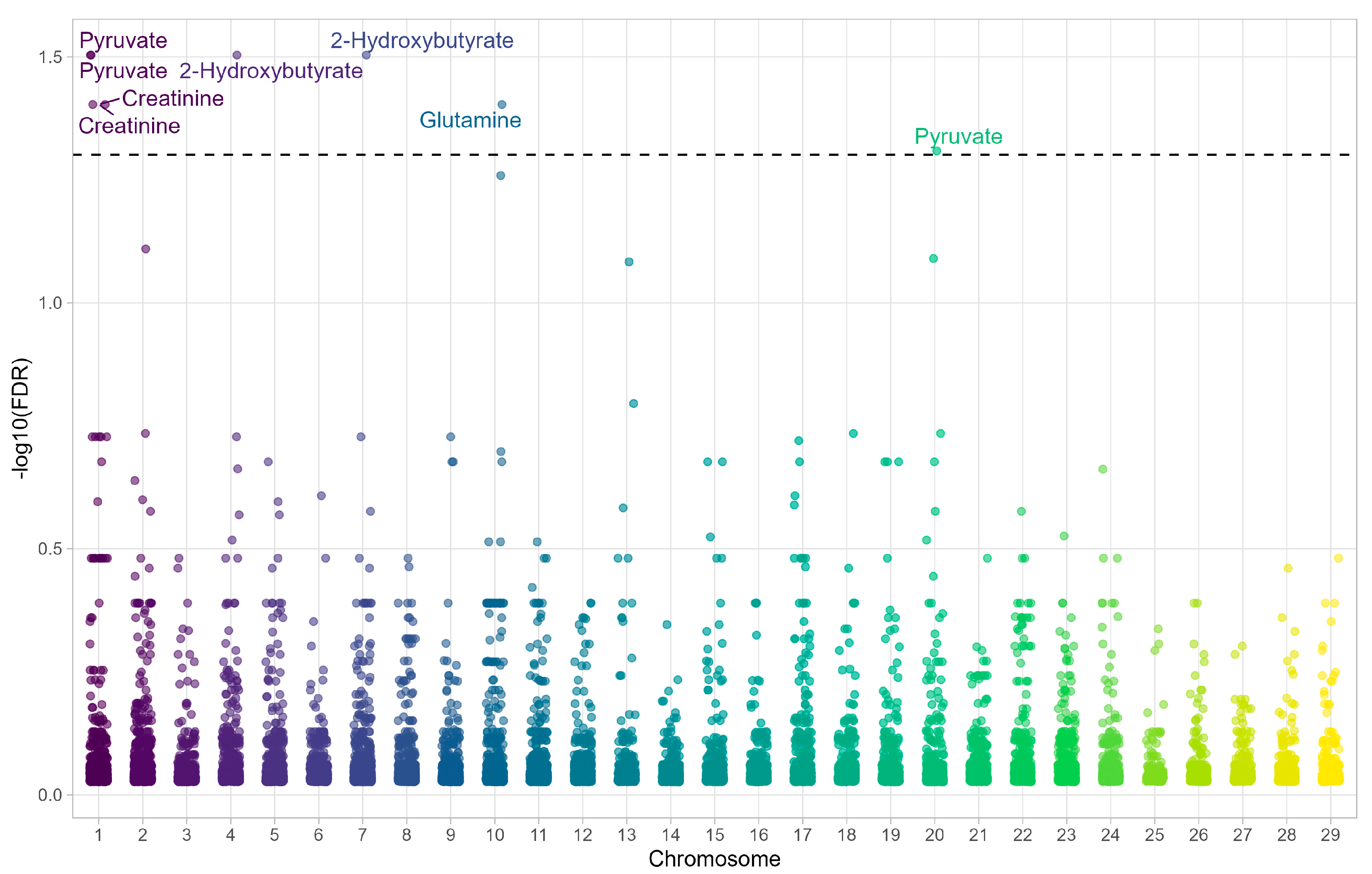
3.1. mQTL Profiles Associated with Favorable and Unfavorable EPD for Meat Tenderness
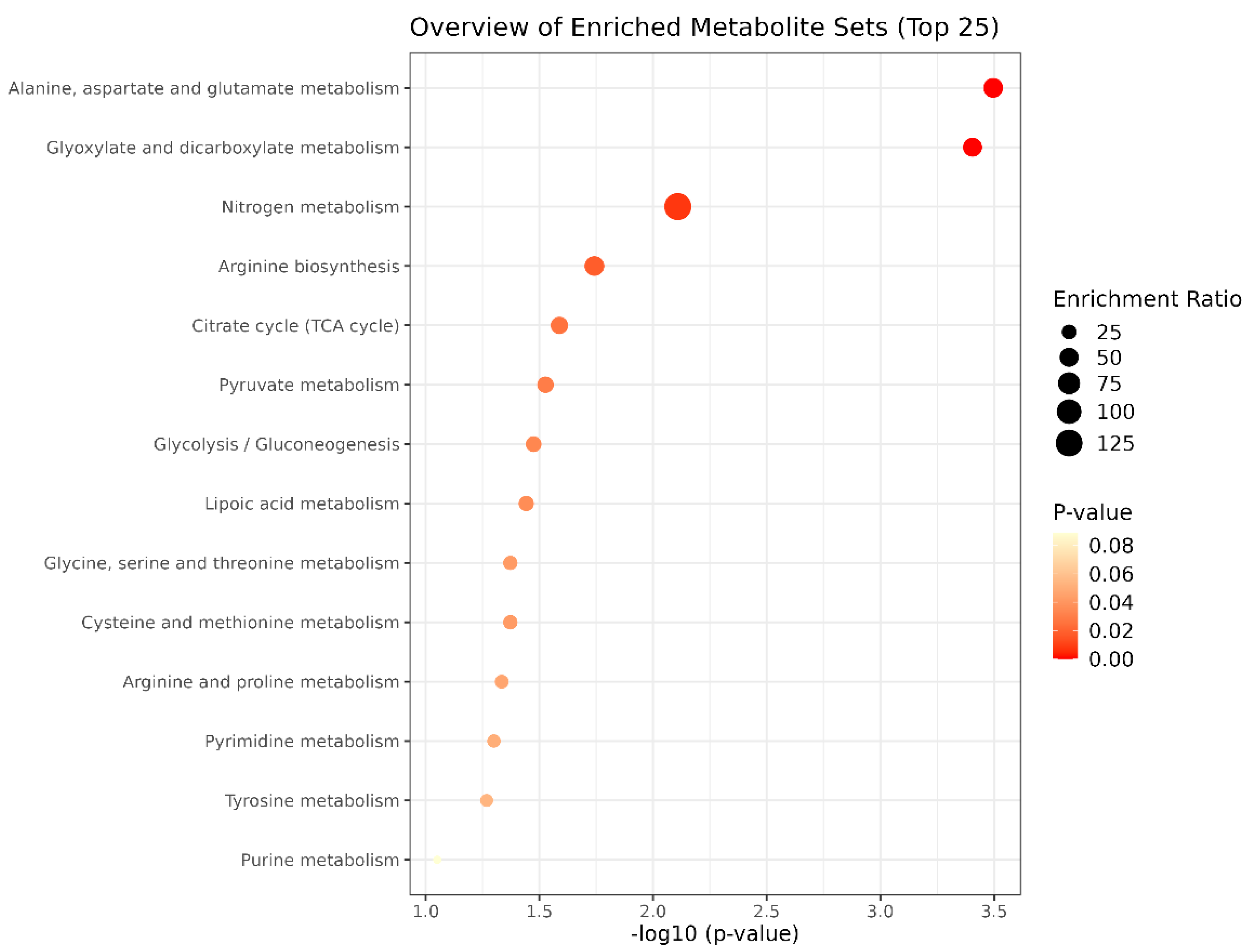
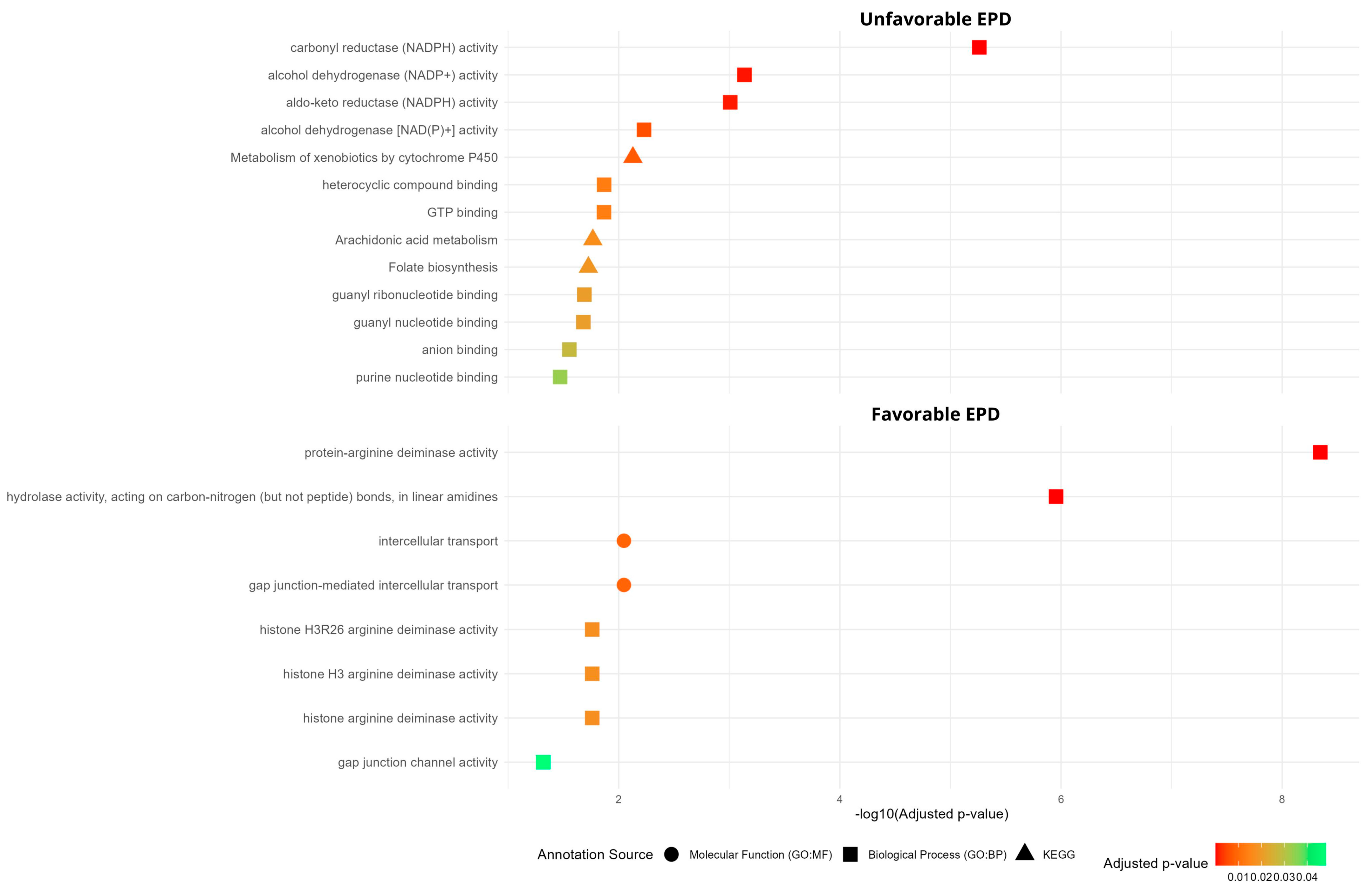
3.2. Pathway Enrichment Associated with Favorable and Unfavorable EPD for Meat Tenderness
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ATP | Adenosine Triphosphate |
| CEUA | Ethics Committee on Animal Use |
| DSS-d6 | 4,4-Dimethyl-4-silapentane-1-sulfonic acid-d6 (internal standard for NMR) |
| EPD | Expected Progeny Difference |
| FA-T | Favorable EPD for Tenderness |
| FDR | False Discovery Rate |
| FTAI | Fixed-Time Artificial Insemination |
| MAF | Minor Allele Frequency |
| mQTL | Metabolic Quantitative Trait Locus |
| NMR | Nuclear Magnetic Resonance |
| ppm | Parts per million |
| SNP | Single Nucleotide Polymorphism |
| UF-T | Unfavorable EPD for Tenderness |
| WBSF | Warner–Bratzler Shear Force |
| 1H NMR | Proton Nuclear Magnetic Resonance |
| TXI | Triple Resonance Inverse detection probe |
References
- Robinson, D.L.; Ferguson, D.M.; Oddy, V.H.; Perry, D.; Thompson, J. Genetic and Environmental Influences on Beef Tenderness. Aust. J. Exp. Agric. 2001, 41, 997. [Google Scholar] [CrossRef]
- Warner, R.D.; Greenwood, P.L.; Pethick, D.W.; Ferguson, D.M. Genetic and Environmental Effects on Meat Quality. Meat Sci. 2010, 86, 171–183. [Google Scholar] [CrossRef]
- Drey, L.N.; Legako, J.F.; Brooks, J.C.; Miller, M.F.; O’quinn, T.G. The Contribution of Tenderness, Juiciness, and Flavor to Overall Consumer Beef Eating Experience. Meat Muscle Biol. 2017, 1, 13. [Google Scholar] [CrossRef]
- Warner, R.D.; Wheeler, T.L.; Ha, M.; Li, X.; Bekhit, A.E.-D.; Morton, J.; Vaskoska, R.; Dunshea, F.R.; Liu, R.; Purslow, P.; et al. Meat Tenderness: Advances in Biology, Biochemistry, Molecular Mechanisms and New Technologies. Meat Sci. 2022, 185, 108657. [Google Scholar] [CrossRef]
- Boleman, S.L.; Boleman, S.J.; Morgan, W.W.; Hale, D.S.; Griffin, D.B.; Savell, J.W.; Ames, R.P.; Smith, M.T.; Tatum, J.D.; Field, T.G.; et al. National Beef Quality Audit-1995: Survey of Producer-Related Defects and Carcass Quality and Quantity Attributes. J. Anim. Sci. 1998, 76, 96. [Google Scholar] [CrossRef] [PubMed]
- McKenna, D.R.; Roebert, D.L.; Bates, P.K.; Schmidt, T.B.; Hale, D.S.; Griffin, D.B.; Savell, J.W.; Brooks, J.C.; Morgan, J.B.; Montgomery, T.H.; et al. National Beef Quality Audit-2000: Survey of Targeted Cattle and Carcass Characteristics Related to Quality, Quantity, and Value of Fed Steers and Heifers. J. Anim. Sci. 2002, 80, 1212–1222. [Google Scholar] [CrossRef]
- O’Quinn, T.G.; Legako, J.F.; Brooks, J.C.; Miller, M.F. Evaluation of the Contribution of Tenderness, Juiciness, and Flavor to the Overall Consumer Beef Eating Experience1. Transl. Anim. Sci. 2018, 2, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Koohmaraie, M.; Wheeler, T.L.; Shackelford, S.D. Beef Tenderness: Regulation and Prediction; USDA ARS: Washington, DC, USA, 1995.
- Garrick, D. Trends and Developments in Genetic Evaluation of Beef Cattle in the United States. In Proceedings of the 9th World Angus Forum Technical Meeting: Angus in the Global Market, Cape Town, South Africa, 19 March 2005; American Angus Association: Kansas City, MO, USA, 2005; pp. 24–31. [Google Scholar]
- Bolsen, J.W.; Bormann, J.M.; Moser, D.W.; Marston, T.T. Relationship between Sire Tenderness EPD and Progeny Carcass Performance. J. Anim. Sci. 2006, 82, 4. [Google Scholar]
- Carvalho, M.E.; Baldi, F.S.; Santana, M.H.A.; Ventura, R.V.; Oliveira, G.A.; Bueno, R.S.; Bonin, M.N.; Rezende, F.M.; Coutinho, L.L.; Eler, J.P.; et al. Identification of Genomic Regions Related to Tenderness in Nellore Beef Cattle. Adv. Anim. Biosci. 2017, 8, s42–s44. [Google Scholar] [CrossRef]
- Regatieri, I.C.; Boligon, A.A.; Baldi, F.; Albuquerque, L.G. Genetic Correlations between Mature Cow Weight and Productive and Reproductive Traits in Nellore Cattle. Genet. Mol. Res. 2012, 11, 2979–2986. [Google Scholar] [CrossRef]
- Van Eenennaam, A.L.; Li, J.; Thallman, R.M.; Quaas, R.L.; Dikeman, M.E.; Gill, C.A.; Franke, D.E.; Thomas, M.G. Validation of Commercial DNA Tests for Quantitative Beef Quality Traits1,2. J. Anim. Sci. 2007, 85, 891–900. [Google Scholar] [CrossRef]
- Wiggans, G.R.; Cole, J.B.; Hubbard, S.M.; Sonstegard, T.S. Genomic Selection in Dairy Cattle: The USDA Experience. Annu. Rev. Anim. Biosci. 2017, 5, 309–327. [Google Scholar] [CrossRef]
- Hayes, B.J.; Lewin, H.A.; Goddard, M.E. The Future of Livestock Breeding: Genomic Selection for Efficiency, Reduced Emissions Intensity, and Adaptation. Trends Genet. 2013, 29, 206–214. [Google Scholar] [CrossRef]
- Smith, K.V.; DeLong, K.L.; Griffith, A.P.; Boyer, C.N.; Martinez, C.; Jensen, K.L. Cow-Calf Producer Preferences for Bull Genomic Enhanced Expected Progeny Differences. J. Agric. Resour. Econ. 2023, 48, 520–539. [Google Scholar]
- Castro, L.M.d.; Magnabosco, C.U.; Sainz, R.D.; Faria, C.U.d.; Lopes, F.B. Quantitative Genetic Analysis for Meat Tenderness Trait in Polled Nellore Cattle. Rev. Ciência Agronômica 2014, 45, 393–402. [Google Scholar] [CrossRef]
- Braz, C.U.; Taylor, J.F.; Bresolin, T.; Espigolan, R.; Feitosa, F.L.B.; Carvalheiro, R.; Baldi, F.; de Albuquerque, L.G.; de Oliveira, H.N. Sliding Window Haplotype Approaches Overcome Single SNP Analysis Limitations in Identifying Genes for Meat Tenderness in Nelore Cattle. BMC Genet. 2019, 20, 8. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, M.E.; Eler, J.P.; Bonin, M.N.; Rezende, F.M.; Biase, F.H.; Meirelles, F.V.; Regitano, L.C.A.; Coutinho, L.L.; Balieiro, J.C.C.; Ferraz, J.B.S. Genotypic and Allelic Frequencies of Gene Polymorphisms Associated with Meat Tenderness in Nellore Beef Cattle. Genet. Mol. Res. 2017, 16, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, T.M.; de Almeida Regitano, L.C.; Koltes, J.E.; Cesar, A.S.M.; da Silva Andrade, S.C.; Mourão, G.B.; Gasparin, G.; Moreira, G.C.M.; Fritz-Waters, E.; Reecy, J.M.; et al. Gene Co-Expression Analysis Indicates Potential Pathways and Regulators of Beef Tenderness in Nellore Cattle. Front. Genet. 2018, 9, 441. [Google Scholar] [CrossRef]
- McClure, M.C.; Ramey, H.R.; Rolf, M.M.; McKay, S.D.; Decker, J.E.; Chapple, R.H.; Kim, J.W.; Taxis, T.M.; Weaber, R.L.; Schnabel, R.D.; et al. Genome-wide Association Analysis for Quantitative Trait Loci Influencing Warner–Bratzler Shear Force in Five Taurine Cattle Breeds. Anim. Genet. 2012, 43, 662–673. [Google Scholar] [CrossRef]
- Pinto, L.F.B.; Ferraz, J.B.S.; Pedrosa, V.B.; Eler, J.P.; Meirelles, F.V.; Bonin, M.N.; Rezende, F.M.; Carvalho, M.E.; Cucco, D.C.; Silva, R.C.G. Single Nucleotide Polymorphisms in CAPN and Leptin Genes Associated with Meat Color and Tenderness in Nellore Cattle. Genet. Mol. Res. 2011, 10, 2057–2064. [Google Scholar] [CrossRef]
- Pinto, L.F.B.; Ferraz, J.B.S.; Meirelles, F.V.; Eler, J.P.; Rezende, F.M.; Carvalho, M.E.; Almeida, H.B.; Silva, R.C.G. Association of SNPs on CAPN1 and CAST Genes with Tenderness in Nellore Cattle. Genet. Mol. Res. 2010, 9, 1431–1442. [Google Scholar] [CrossRef]
- Fontanesi, L. Metabolomics and Livestock Genomics: Insights into a Phenotyping Frontier and Its Applications in Animal Breeding. Anim. Front. 2016, 6, 73–79. [Google Scholar] [CrossRef]
- Goldansaz, S.A.; Guo, A.C.; Sajed, T.; Steele, M.A.; Plastow, G.S.; Wishart, D.S. Livestock Metabolomics and the Livestock Metabolome: A Systematic Review. PLoS ONE 2017, 12, e0177675. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandro, A.; Marrocco, C.; Rinalducci, S.; Mirasole, C.; Failla, S.; Zolla, L. Chianina Beef Tenderness Investigated through Integrated Omics. J. Proteom. 2012, 75, 4381–4398. [Google Scholar] [CrossRef] [PubMed]
- Magan, J.B.; O’Callaghan, T.F.; Zheng, J.; Zhang, L.; Mandal, R.; Hennessy, D.; Fenelon, M.A.; Wishart, D.S.; Kelly, A.L.; McCarthy, N.A. Impact of Bovine Diet on Metabolomic Profile of Skim Milk and Whey Protein Ingredients. Metabolites 2019, 9, 305. [Google Scholar] [CrossRef]
- Ramírez-Zamudio, G.D.; Silva, L.H.P.; Vieira, N.M.; Vilela, R.S.R.; Assis, D.E.F.; Assis, G.J.F.; Estrada, M.M.; Rodrigues, R.T.S.; Duarte, M.S.; Chizzotti, M.L. Effect of Short-Term Dietary Protein Restriction before Slaughter on Meat Quality and Skeletal Muscle Metabolomic Profile in Culled Ewes. Livest. Sci. 2022, 261, 104956. [Google Scholar] [CrossRef]
- Polizel, G.H.G.; Cançado, F.A.C.Q.; Dias, E.F.F.; Fernandes, A.C.; Cracco, R.C.; Carmona, B.T.; Castellar, H.H.; Poleti, M.D.; Santana, M.H.d.A. Effects of Different Prenatal Nutrition Strategies on the Liver Metabolome of Bulls and Its Correlation with Body and Liver Weight. Metabolites 2022, 12, 441. [Google Scholar] [CrossRef]
- Antonelo, D.S.; Cônsolo, N.R.B.; Gómez, J.F.M.; Beline, M.; Goulart, R.S.; Corte, R.R.P.S.; Colnago, L.A.; Schilling, M.W.; Gerrard, D.E.; Silva, S.L. Metabolite Profile and Consumer Sensory Acceptability of Meat from Lean Nellore and Angus × Nellore Crossbreed Cattle Fed Soybean Oil. Food Res. Int. 2020, 132, 109056. [Google Scholar] [CrossRef]
- Antonelo, D.; Gómez, J.F.M.; Cônsolo, N.R.B.; Beline, M.; Colnago, L.A.; Schilling, W.; Zhang, X.; Suman, S.P.; Gerrard, D.E.; Balieiro, J.C.C.; et al. Metabolites and Metabolic Pathways Correlated With Beef Tenderness. Meat Muscle Biol. 2020, 4, 1. [Google Scholar] [CrossRef]
- Cônsolo, N.R.B.; Rosa, A.F.; Barbosa, L.C.G.S.; Maclean, P.H.; Higuera-Padilla, A.; Colnago, L.A.; Titto, E.A.L. Preliminary Study on the Characterization of Longissimus Lumborum Dark Cutting Meat in Angus × Nellore Crossbreed Cattle Using NMR-Based Metabolomics. Meat Sci. 2021, 172, 108350. [Google Scholar] [CrossRef]
- Bovo, S.; Ribani, A.; Fanelli, F.; Galimberti, G.; Martelli, P.L.; Trevisi, P.; Bertolini, F.; Bolner, M.; Casadio, R.; Dall’Olio, S.; et al. Merging Metabolomics and Genomics Provides a Catalog of Genetic Factors That Influence Molecular Phenotypes in Pigs Linking Relevant Metabolic Pathways. Genet. Sel. Evol. 2025, 57, 11. [Google Scholar] [CrossRef]
- Dumas, M.-E.; Wilder, S.P.; Bihoreau, M.-T.; Barton, R.H.; Fearnside, J.F.; Argoud, K.; D’Amato, L.; Wallis, R.H.; Blancher, C.; Keun, H.C.; et al. Direct Quantitative Trait Locus Mapping of Mammalian Metabolic Phenotypes in Diabetic and Normoglycemic Rat Models. Nat. Genet. 2007, 39, 666–672. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, G.; Rantalainen, M.; Li, J.V.; Maher, A.D.; Malmodin, D.; Ahmadi, K.R.; Faber, J.H.; Barrett, A.; Min, J.L.; Rayner, N.W.; et al. A Genome-Wide Metabolic QTL Analysis in Europeans Implicates Two Loci Shaped by Recent Positive Selection. PLoS Genet. 2011, 7, e1002270. [Google Scholar] [CrossRef]
- Ponsuksili, S.; Trakooljul, N.; Hadlich, F.; Methling, K.; Lalk, M.; Murani, E.; Wimmers, K. Genetic Regulation of Liver Metabolites and Transcripts Linking to Biochemical-Clinical Parameters. Front. Genet. 2019, 10, 348. [Google Scholar] [CrossRef] [PubMed]
- Matias, I.F.B.; Santos, E.S.P.; Valim, J.M.B.d.C.; Castro, A.; Ferreira, A.G.; Barbosa, L.C.; Ribeiro, G.H.; Colnago, L.A.; Asnchau, D.G.; Souza, Y.G. da S.; et al. Preparation of Ruminal Fluid and Serum Samples from Beef Cattle for Nuclear Magnetic Resonance Based–Metabolomics. N. Z. J. Agric. Res. 2025, 68, 1401–1414. [Google Scholar] [CrossRef]
- Shabalin, A.A. Matrix EQTL: Ultra Fast EQTL Analysis via Large Matrix Operations. Bioinformatics 2012, 28, 1353–1358. [Google Scholar] [CrossRef]
- Fonseca, P.A.S.; Suárez-Vega, A.; Marras, G.; Cánovas, Á. GALLO: An R Package for Genomic Annotation and Integration of Multiple Data Sources in Livestock for Positional Candidate Loci. Gigascience 2020, 9, giaa149. [Google Scholar] [CrossRef]
- Zimin, A.V.; Delcher, A.L.; Florea, L.; Kelley, D.R.; Schatz, M.C.; Puiu, D.; Hanrahan, F.; Pertea, G.; Van Tassell, C.P.; Sonstegard, T.S.; et al. A Whole-Genome Assembly of the Domestic Cow, Bos Taurus. Genome Biol. 2009, 10, R42. [Google Scholar] [CrossRef] [PubMed]
- King, D.A.; Shackelford, S.D.; Broeckling, C.D.; Prenni, J.E.; Belk, K.E.; Wheeler, T.L. Metabolomic Investigation of Tenderness and Aging Response in Beef Longissimus Steaks. Meat Muscle Biol. 2019, 3, 76–89. [Google Scholar] [CrossRef]
- Garcia-Concejo, A.; Larhammar, D. Protein Kinase C Family Evolution in Jawed Vertebrates. Dev. Biol. 2021, 479, 77–90. [Google Scholar] [CrossRef]
- Seki, T.; Matsubayashi, H.; Amano, T.; Shirai, Y.; Saito, N.; Sakai, N. Phosphorylation of PKC Activation Loop Plays an Important Role in Receptor-mediated Translocation of PKC. Genes Cells 2005, 10, 225–239. [Google Scholar] [CrossRef]
- Fernández, J.C.; Pérez, J.E.; Herrera, N.; Martínez, R.; Bejarano, D.; Rocha, J.F. Research Article Genomic Association Study for Age at First Calving and Calving Interval in Romosinuano and Costeño Con Cuernos Cattle. Genet. Mol. Res. 2019, 18, 1–13. [Google Scholar] [CrossRef]
- Ouali, A.; Gagaoua, M.; Boudida, Y.; Becila, S.; Boudjellal, A.; Herrera-Mendez, C.H.; Sentandreu, M.A. Biomarkers of Meat Tenderness: Present Knowledge and Perspectives in Regards to Our Current Understanding of the Mechanisms Involved. Meat Sci. 2013, 95, 854–870. [Google Scholar] [CrossRef]
- Ren, C.; Hou, C.; Zhang, D.; Li, X.; Xiao, X.; Bai, Y. ATP Regulates the Phosphorylation and Degradation of Myofibrillar Proteins in Ground Ovine Muscle. J. Integr. Agric. 2021, 20, 311–318. [Google Scholar] [CrossRef]
- Koutsidis, G.; Elmore, J.S.; Oruna-Concha, M.J.; Campo, M.M.; Wood, J.D.; Mottram, D.S. Water-Soluble Precursors of Beef Flavour: I. Effect of Diet and Breed. Meat Sci. 2008, 79, 124–130. [Google Scholar] [CrossRef]
- Giovanini de Oliveira Sartori, A.; Silva Antonelo, D.; Ribeiro, G.H.; Colnago, L.A.; de Carvalho Balieiro, J.C.; Francisquine Delgado, E.; Contreras Castillo, C.J. Lipidome and Metabolome Profiling of Longissimus Lumborum Beef with Different Ultimate PH and Postmortem Aging. Meat Sci. 2024, 217, 109621. [Google Scholar] [CrossRef]
- Lu, G.; Li, Y.; Mao, K.; Zang, Y.; Zhao, X.; Qiu, Q.; Qu, M.; Ouyang, K. Effects of Rumen-Protected Creatine Pyruvate on Meat Quality, Hepatic Gluconeogenesis, and Muscle Energy Metabolism of Long-Distance Transported Beef Cattle. Front. Anim. Sci. 2022, 3, 904503. [Google Scholar] [CrossRef]
- Lomiwes, D.; Farouk, M.M.; Wu, G.; Young, O.A. The Development of Meat Tenderness is Likely to Be Compartmentalised by Ultimate PH. Meat Sci. 2014, 96, 646–651. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.K.F.; Rowe, H.C.; Hansen, B.G.; Kliebenstein, D.J. The Complex Genetic Architecture of the Metabolome. PLoS Genet. 2010, 6, e1001198. [Google Scholar] [CrossRef]
- Liao, Y.; Hu, R.; Wang, Z.; Peng, Q.; Dong, X.; Zhang, X.; Zou, H.; Pu, Q.; Xue, B.; Wang, L. Metabolomics Profiling of Serum and Urine in Three Beef Cattle Breeds Revealed Different Levels of Tolerance to Heat Stress. J. Agric. Food Chem. 2018, 66, 6926–6935. [Google Scholar] [CrossRef]
- Serrano-Contreras, J.I.; García-Pérez, I.; Meléndez-Camargo, M.E.; Zepeda, L.G. NMR-Based Metabonomic Analysis of Physiological Responses to Starvation and Refeeding in the Rat. J. Proteome Res. 2016, 15, 3241–3254. [Google Scholar] [CrossRef]
- Li, J.L.; Guo, Z.Y.; Li, Y.J.; Zhang, L.; Gao, F.; Zhou, G.H. Effect of Creatine Monohydrate Supplementation on Carcass Traits, Meat Quality and Postmortem Energy Metabolism of Finishing Pigs. Anim. Prod. Sci. 2016, 56, 48. [Google Scholar] [CrossRef]
- Wicks, J.; Beline, M.; Gomez, J.F.M.; Luzardo, S.; Silva, S.L.; Gerrard, D. Muscle Energy Metabolism, Growth, and Meat Quality in Beef Cattle. Agriculture 2019, 9, 195. [Google Scholar] [CrossRef]
- Bendall, J.R. The Shortening of Rabbit Muscles during Rigor Mortis: Its Relation to the Breakdown of Adenosine Triphosphate and Creatine Phosphate and to Muscular Contraction. J. Physiol. 1951, 114, 71–88. [Google Scholar] [CrossRef] [PubMed]
- Bate-Smith, E.C.; Bendall, J.R. Factors Determining the Time Course of Rigor Mortis. J. Physiol. 1949, 110, 47–65. [Google Scholar] [CrossRef] [PubMed]
- England, E.M.; Matarneh, S.K.; Mitacek, R.M.; Abraham, A.; Ramanathan, R.; Wicks, J.C.; Shi, H.; Scheffler, T.L.; Oliver, E.M.; Helm, E.T.; et al. Presence of Oxygen and Mitochondria in Skeletal Muscle Early Postmortem. Meat Sci. 2018, 139, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Matarneh, S.K.; Yen, C.-N.; Bodmer, J.; El-Kadi, S.W.; Gerrard, D.E. Mitochondria Influence Glycolytic and Tricarboxylic Acid Cycle Metabolism under Postmortem Simulating Conditions. Meat Sci. 2021, 172, 108316. [Google Scholar] [CrossRef]
- Rocha, R.d.F.B.; Garcia, A.O.; Otto, P.I.; dos Santos, M.G.; da Silva, M.V.B.; Martins, M.F.; Machado, M.A.; Panetto, J.C.d.C.; Guimarães, S.E.F. Single-Step Genome-Wide Association Studies and Post-GWAS Analyses for the Number of Oocytes and Embryos in Gir Cattle. Mamm. Genome 2023, 34, 497–508. [Google Scholar] [CrossRef]
- Scheffler, T.L.; Gerrard, D.E. Mechanisms Controlling Pork Quality Development: The Biochemistry Controlling Postmortem Energy Metabolism. Meat Sci. 2007, 77, 7–16. [Google Scholar] [CrossRef]
- Haworth, R.A.; Hunter, D.R. The Ca2+-Induced Membrane Transition in Mitochondria. Arch. Biochem. Biophys. 1979, 195, 460–467. [Google Scholar] [CrossRef]
- Ramos, P.M.; Li, C.; Elzo, M.A.; Wohlgemuth, S.E.; Scheffler, T.L. Mitochondrial Oxygen Consumption in Early Postmortem Permeabilized Skeletal Muscle Fibers Is Influenced by Cattle Breed. J. Anim. Sci. 2020, 98, skaa044. [Google Scholar] [CrossRef] [PubMed]
- Dang, D.S.; Buhler, J.F.; Davis, H.T.; Thornton, K.J.; Scheffler, T.L.; Matarneh, S.K. Inhibition of Mitochondrial Calcium Uniporter Enhances Postmortem Proteolysis and Tenderness in Beef Cattle. Meat Sci. 2020, 162, 108039. [Google Scholar] [CrossRef] [PubMed]
- Buckley, D.M.; Burroughs-Garcia, J.; Kriks, S.; Lewandoski, M.; Waters, S.T. Gbx1 and Gbx2 Are Essential for Normal Patterning and Development of Interneurons and Motor Neurons in the Embryonic Spinal Cord. J. Dev. Biol. 2020, 8, 9. [Google Scholar] [CrossRef]
- Tsoukalas, D.; Fragoulakis, V.; Papakonstantinou, E.; Antonaki, M.; Vozikis, A.; Tsatsakis, A.; Buga, A.M.; Mitroi, M.; Calina, D. Prediction of Autoimmune Diseases by Targeted Metabolomic Assay of Urinary Organic Acids. Metabolites 2020, 10, 502. [Google Scholar] [CrossRef] [PubMed]
- den Hertog-Meischke, M.J.A.; Smulders, F.J.M.; Houben, J.H.; Eikelenboom, G. The Effect of Dietary Vitamin E Supplementation on Drip Loss of Bovine Longissimus Lumborum, Psoas Major and Semitendinosus Muscles. Meat Sci. 1997, 45, 153–160. [Google Scholar] [CrossRef]
- Dou, L.; Jin, Y.; Li, H.; Liu, C.; Yang, Z.; Chen, X.; Sun, L.; Zhao, L.; Su, L. Effect of Feeding System on Muscle Fiber Composition, Antioxidant Capacity, and Nutritional and Organoleptic Traits of Goat Meat. Animals 2023, 13, 172. [Google Scholar] [CrossRef]

| Sire | Progeny, n | Genetic Selection for Tenderness | ||
|---|---|---|---|---|
| Treatment | EPD | Accuracy | ||
| Sire 1 | 15 | FA-T | −0.1217 | 0.79 |
| Sire 2 | 13 | FA-T | −0.0974 | 0.63 |
| Sire 3 | 17 | FA-T | −0.08 | 0.66 |
| Total | 45 | Mean | −0.0997 | 0.69 |
| Sire 4 | 24 | UF-T | 0.0751 | 0.65 |
| Sire 5 | 13 | UF-T | 0.0773 | 0.6 |
| Sire 6 | 9 | UF-T | 0.0531 | 0.59 |
| Total | 46 | Mean | 0.0685 | 0.61 |
| SNP | Metabolite | Gene | Chromosome | FDR |
|---|---|---|---|---|
| ARS-BFGL-NGS-68102 | Threonine | PRKCH | 10 | 0.019 |
| ARS-BFGL-NGS-32801 | Phenylalanine | RNF17 | 12 | 0.019 |
| Hapmap51939-BTA-21630 | Phenylalanine | PARP4 | 12 | 0.019 |
| SNP | Metabolite | Gene | Chromosome | FDR |
|---|---|---|---|---|
| Hapmap57645-rs29027916 | Pyruvate | CHAF1B | 1 | 0.031 |
| BovineHD010 0043594 | Pyruvate | MORC3 | 1 | 0.031 |
| BovineHD040 0033104 | 2-hydroxybutyrate | GBX1 | 4 | 0.031 |
| BovineHD070 0014924 | 2-hydroxybutyrate | CDC25C | 7 | 0.031 |
| BovineHD010 0043594 | Creatinine | MORC3 | 1 | 0.039 |
| Hapmap57645-rs29027916– | Creatinine | CHAF1B | 1 | 0.039 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valim, J.M.B.d.C.; Herreira, V.L.S.; Gôngora, A.L.d.S.M.; Beltrão, L.C.F.; Santos, E.S.P.d.; Oliveira, B.S.d.; Pugliesi, G.; Santana, M.H.d.A.; Polizel, G.H.G.; Colnago, L.A.; et al. Integrative Genomic and Metabolomic Analysis Identifies mQTLs Associated with Genetic Selection for Tenderness in Nellore Cattle. Metabolites 2025, 15, 760. https://doi.org/10.3390/metabo15120760
Valim JMBdC, Herreira VLS, Gôngora ALdSM, Beltrão LCF, Santos ESPd, Oliveira BSd, Pugliesi G, Santana MHdA, Polizel GHG, Colnago LA, et al. Integrative Genomic and Metabolomic Analysis Identifies mQTLs Associated with Genetic Selection for Tenderness in Nellore Cattle. Metabolites. 2025; 15(12):760. https://doi.org/10.3390/metabo15120760
Chicago/Turabian StyleValim, Joao Marcos Bovetto de Campos, Vinicius Laerte Silva Herreira, Ana Laura dos Santos Munhoz Gôngora, Lauro César Ferreira Beltrão, Eduardo Solano Pina dos Santos, Brenda Santos de Oliveira, Guilherme Pugliesi, Miguel Henrique de Almeida Santana, Guilherme Henrique Gebim Polizel, Luiz Alberto Colnago, and et al. 2025. "Integrative Genomic and Metabolomic Analysis Identifies mQTLs Associated with Genetic Selection for Tenderness in Nellore Cattle" Metabolites 15, no. 12: 760. https://doi.org/10.3390/metabo15120760
APA StyleValim, J. M. B. d. C., Herreira, V. L. S., Gôngora, A. L. d. S. M., Beltrão, L. C. F., Santos, E. S. P. d., Oliveira, B. S. d., Pugliesi, G., Santana, M. H. d. A., Polizel, G. H. G., Colnago, L. A., Ocampos, F. M. M., Ramírez-Zamudio, G. D., Silva, S. L., & Cônsolo, N. R. B. (2025). Integrative Genomic and Metabolomic Analysis Identifies mQTLs Associated with Genetic Selection for Tenderness in Nellore Cattle. Metabolites, 15(12), 760. https://doi.org/10.3390/metabo15120760












