Impact of Natural Fermentation on Mineral Composition, Resistant and Non-Resistant Starches, Microbial Diversity, and Global Metabolite Profiles in Commercial Poi from Hawai‘i
Abstract
1. Introduction
2. Materials and Methods
2.1. Commercial Poi
2.2. Poi Fermentation
2.3. Measurement of Resistant Starch (RS) and Non-Resistant Starch (NRS)
2.4. Mineral and Moisture Analysis
2.5. DNA Extraction
2.6. Library Preparation, 16S Sequencing, and Microbial Data Processing
- 5′-TCGTCGGCAGCGTCAGATGTGTATAAGAGACAGCCTACGGGNGGCWGCAG-3′
- 5′-TCTCGTGGGCTCGGAGATGTGTATAAGAGACAGGACTACHVGGGTATCTAATCC-3′
2.7. Global Metabolomic Analysis
2.8. Statistical Data Analysis
3. Results
3.1. Nutrition Composition of Poi
3.2. Fermentation Had No Effect on Mineral Contents of Poi
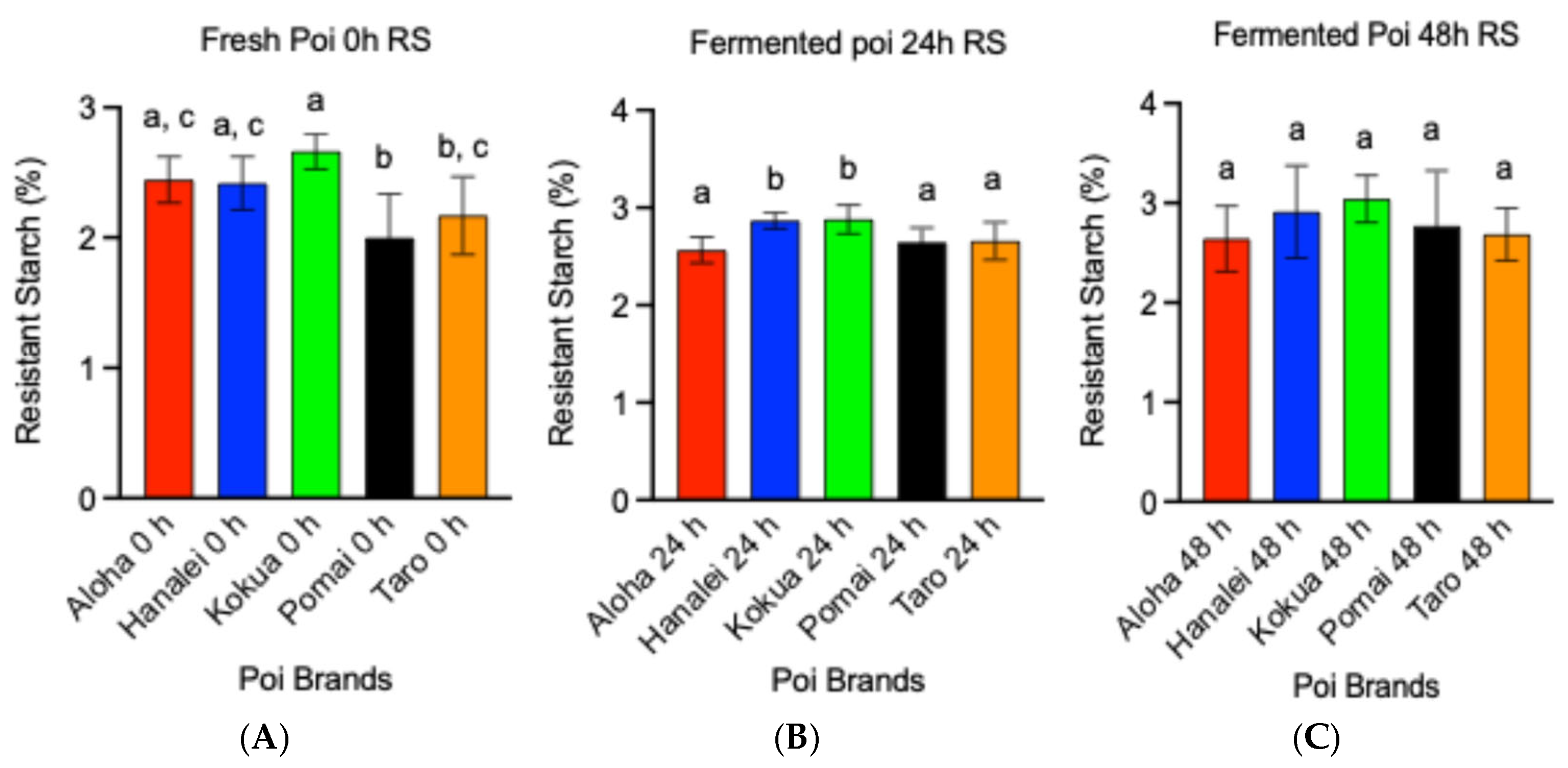
3.3. Fermentation Increases Resistant Starch (RS) Contents in Poi
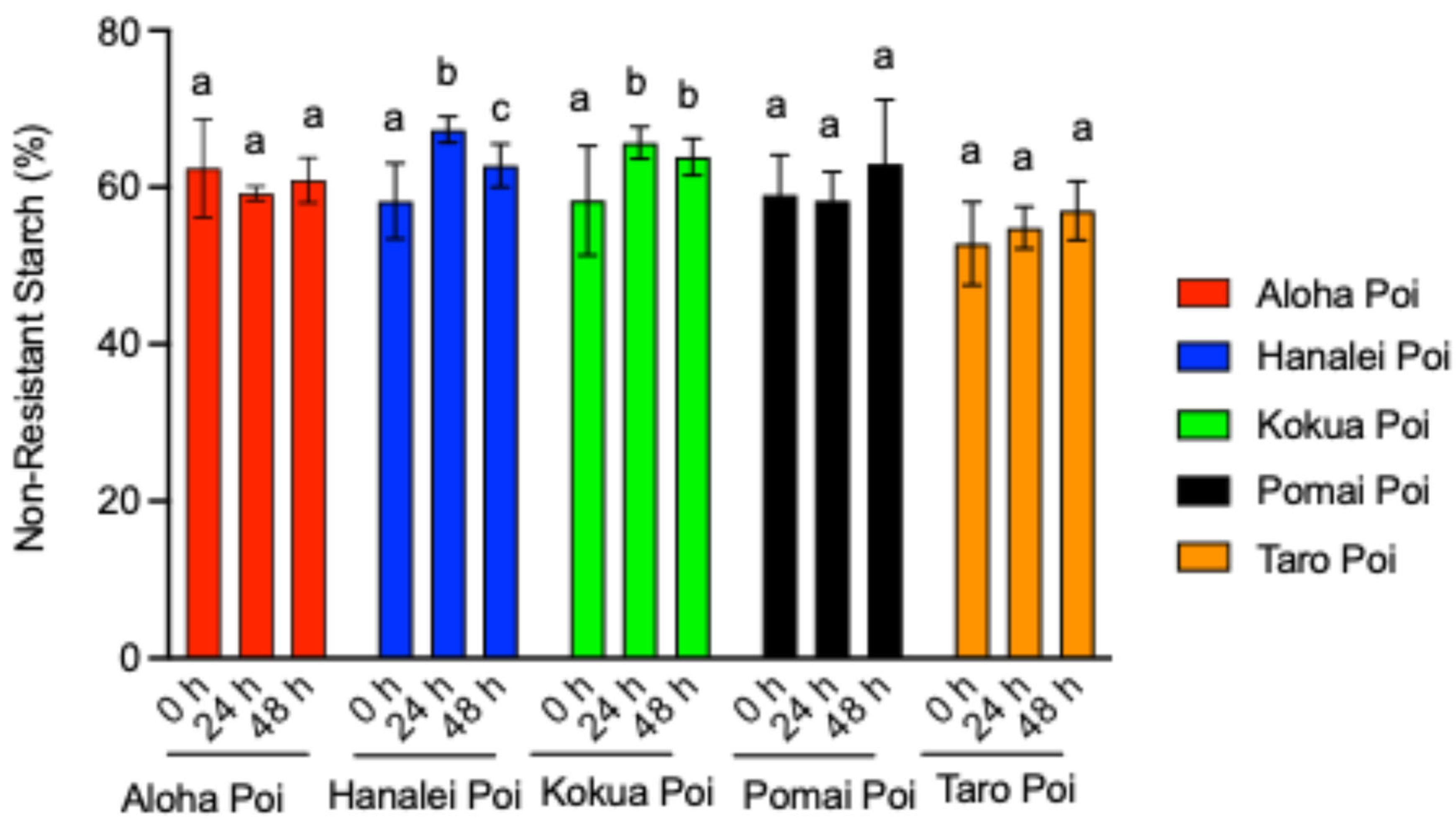
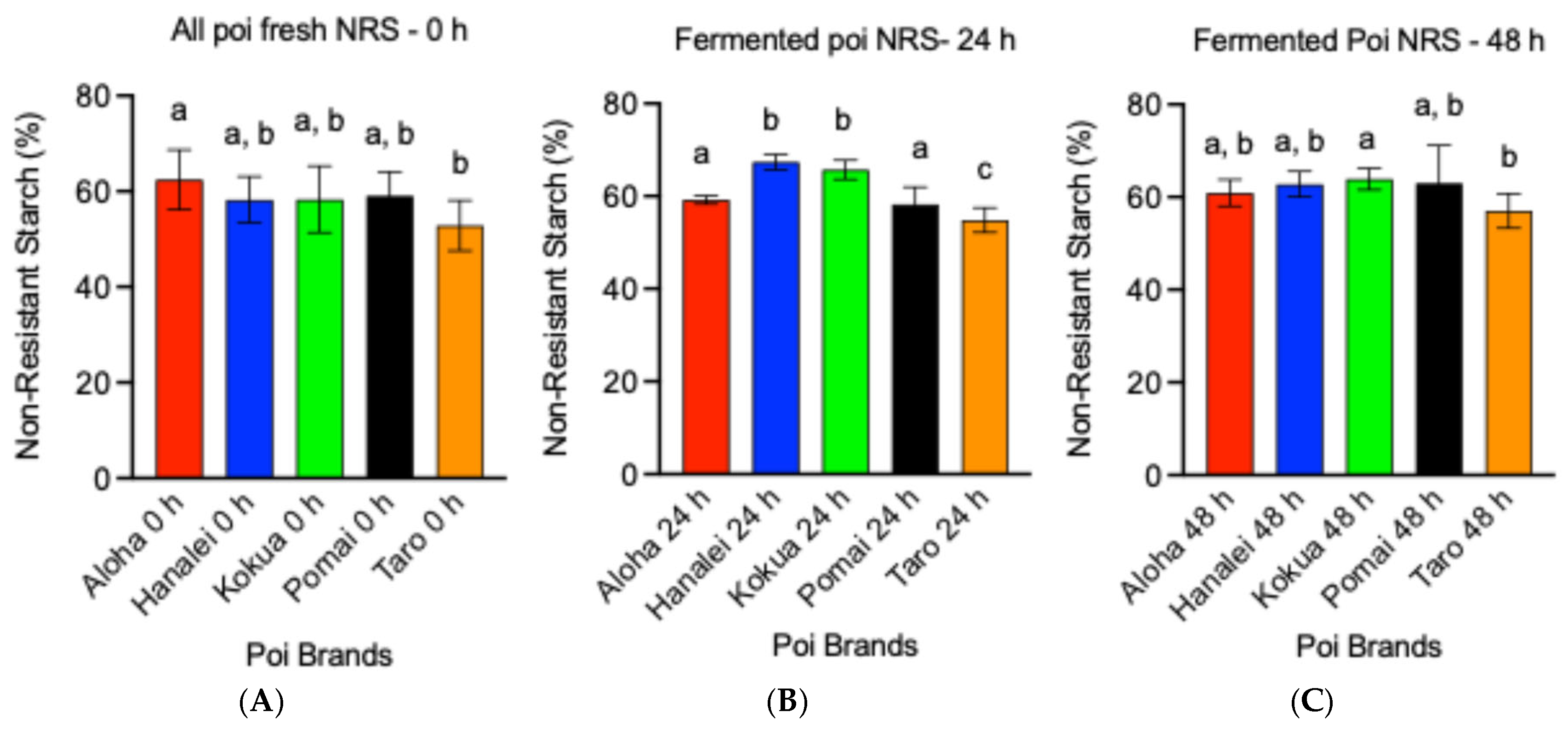
3.4. Fermentation Increases Non-Resistant Starch (NRS) Contents of Poi
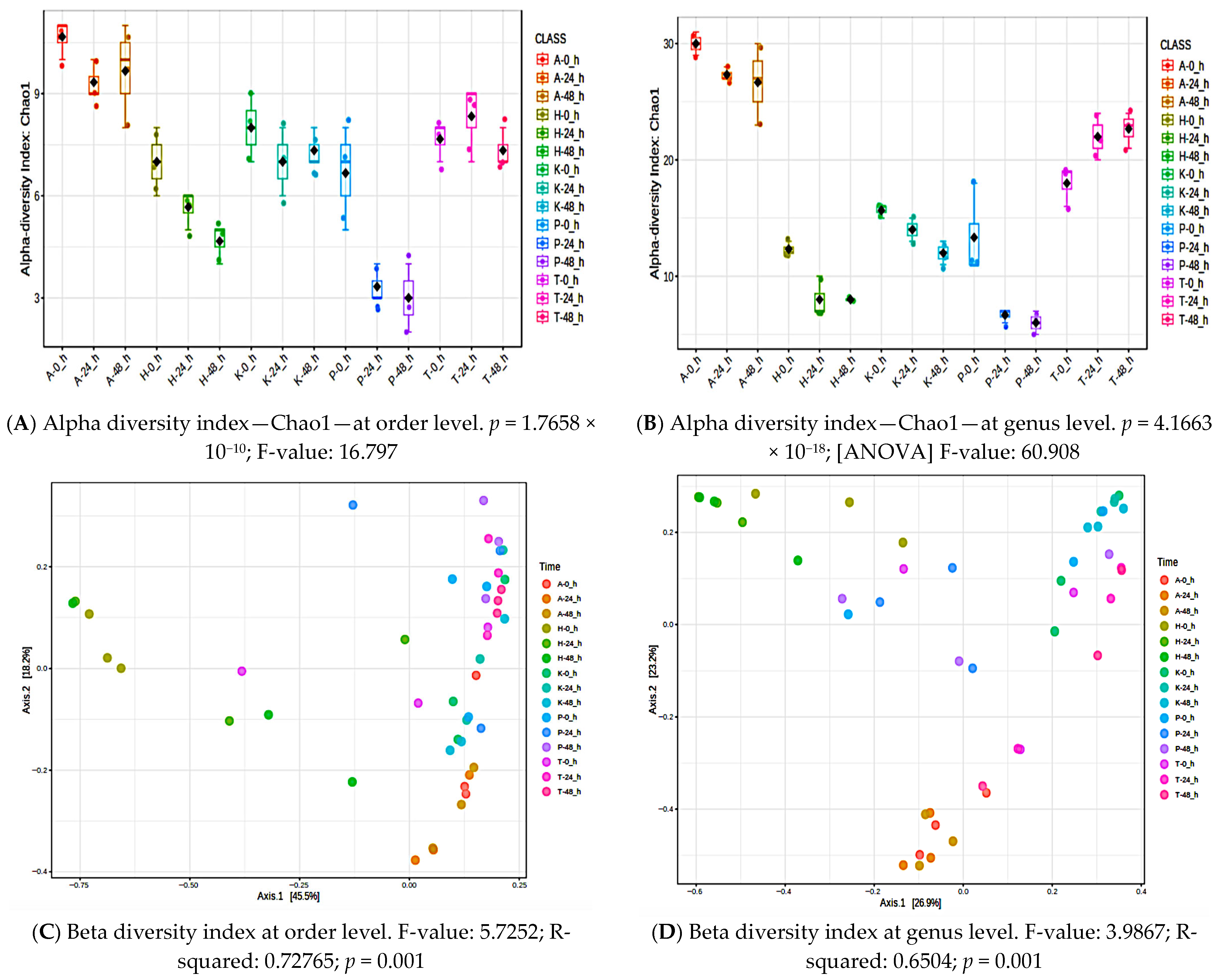
3.5. Alpha and Beta Diversity for Individual Poi
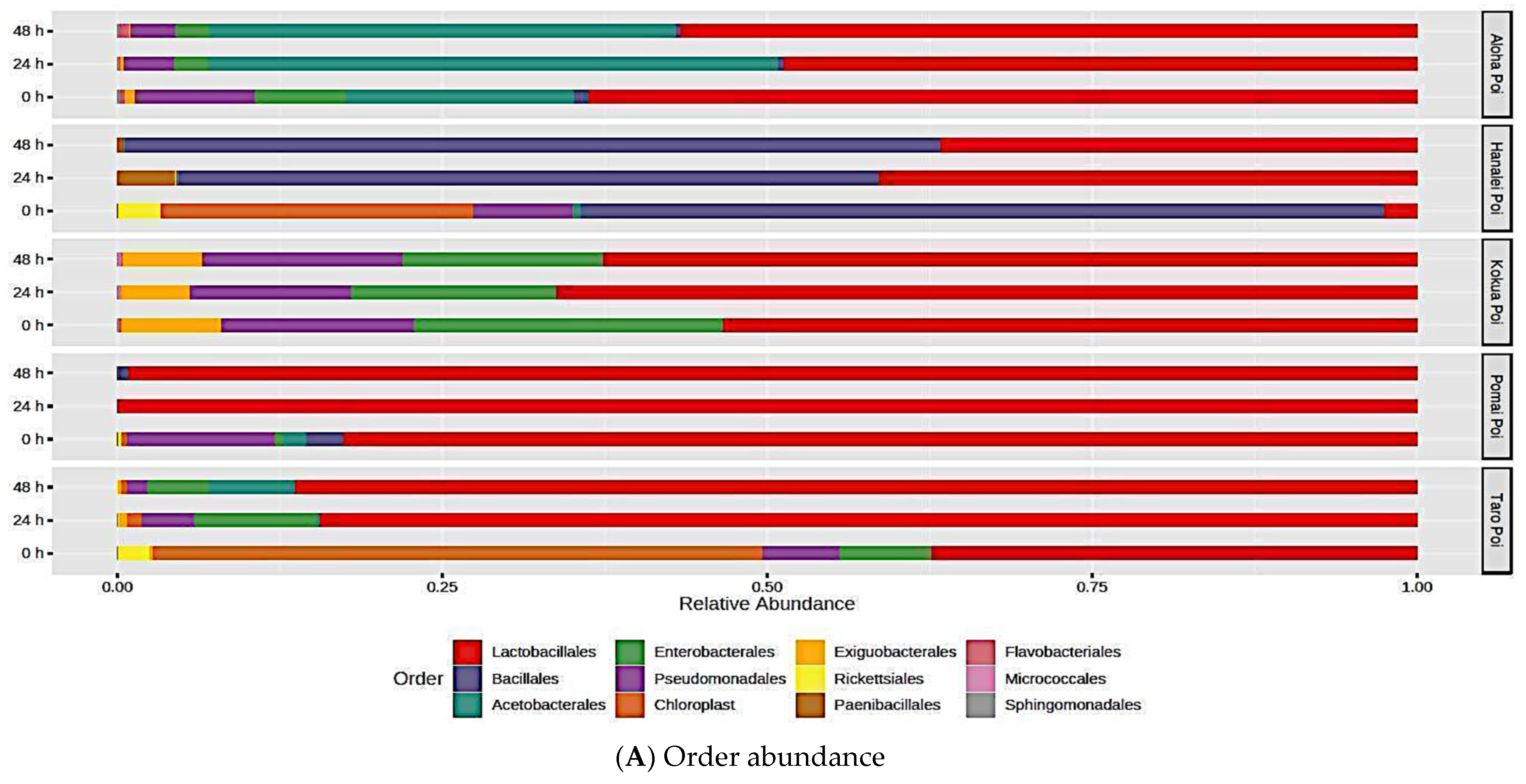
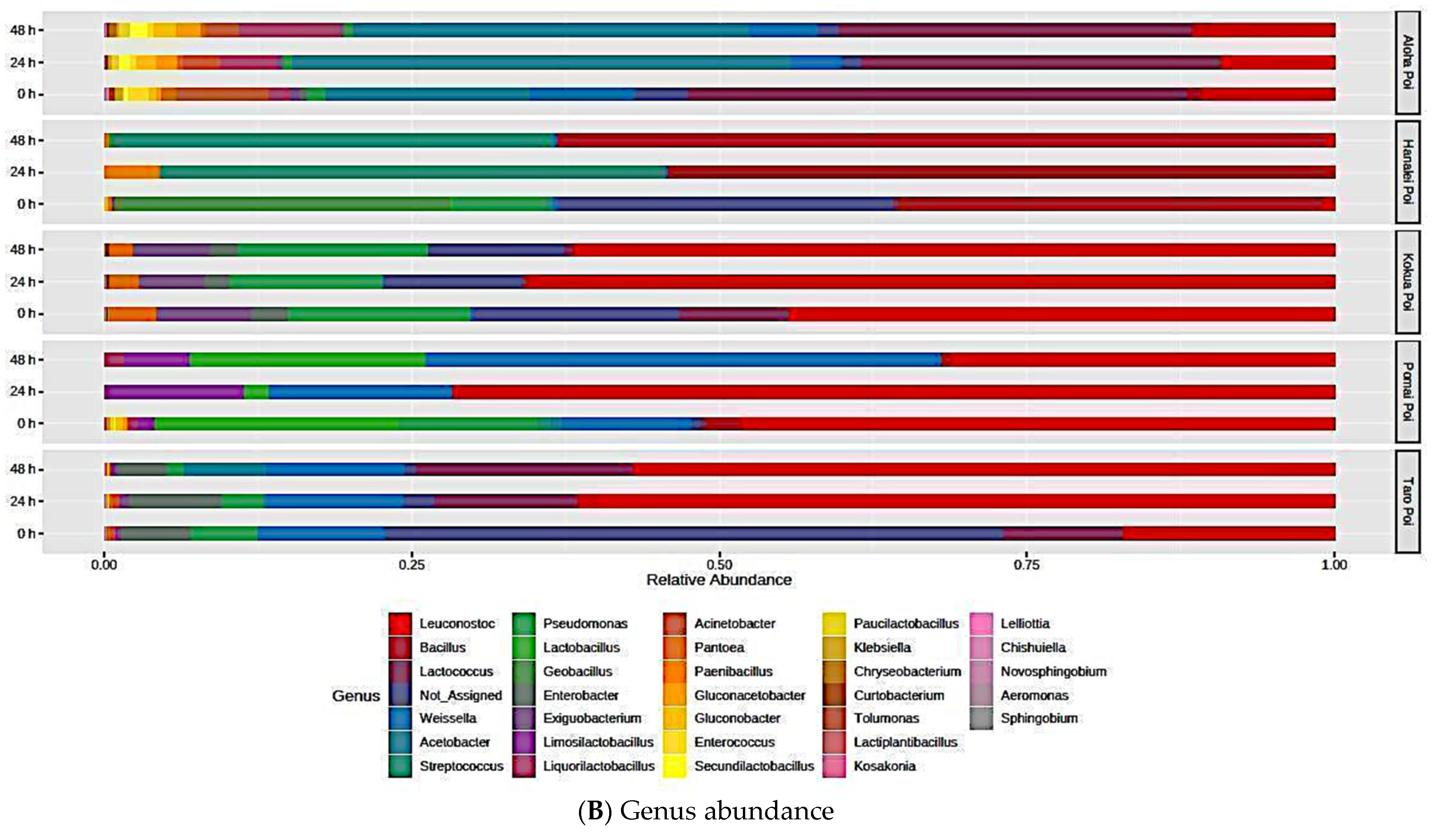
3.6. Fermentation Redistributes the Relative Abundance and Microbial Diversity of Poi
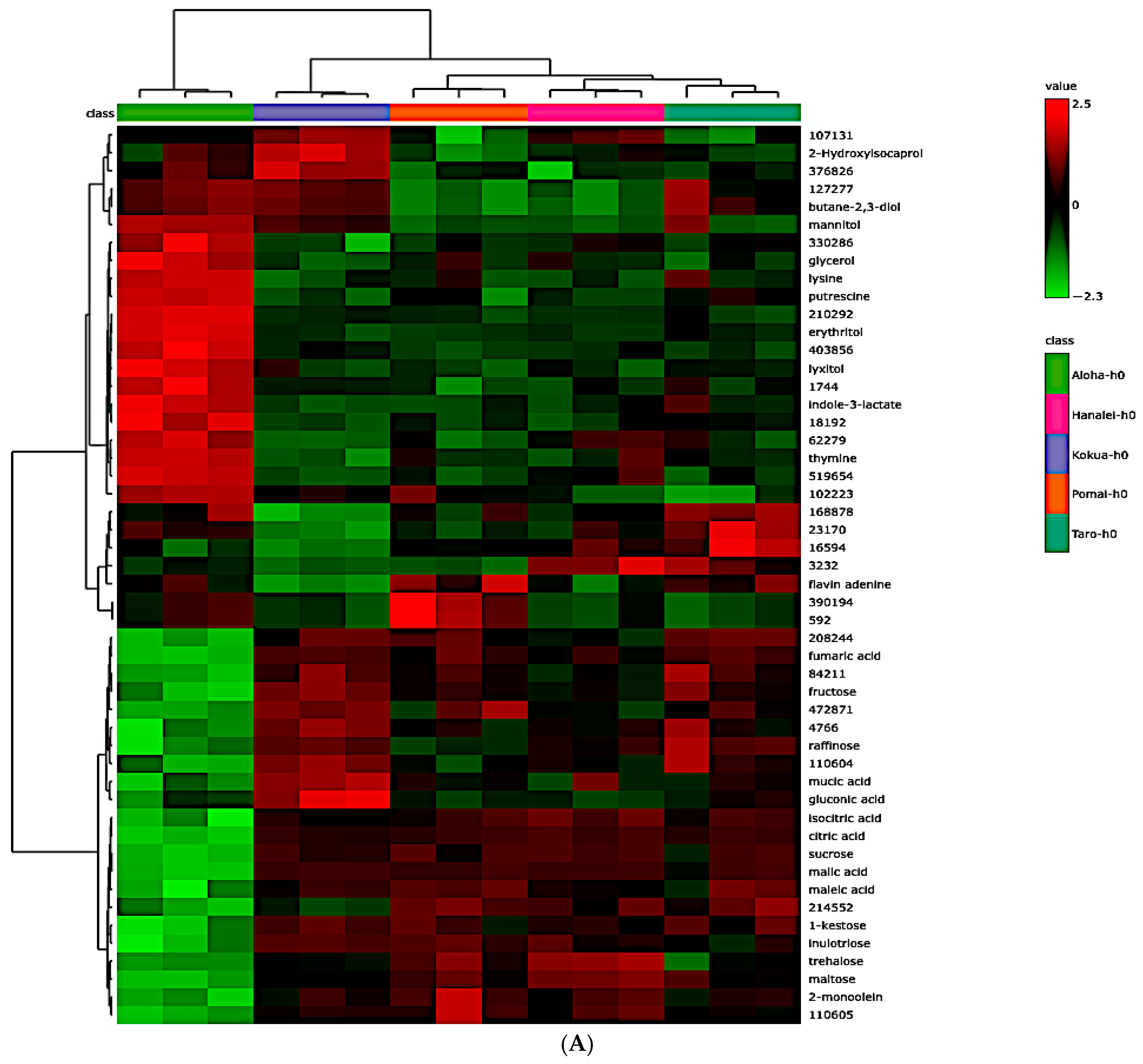
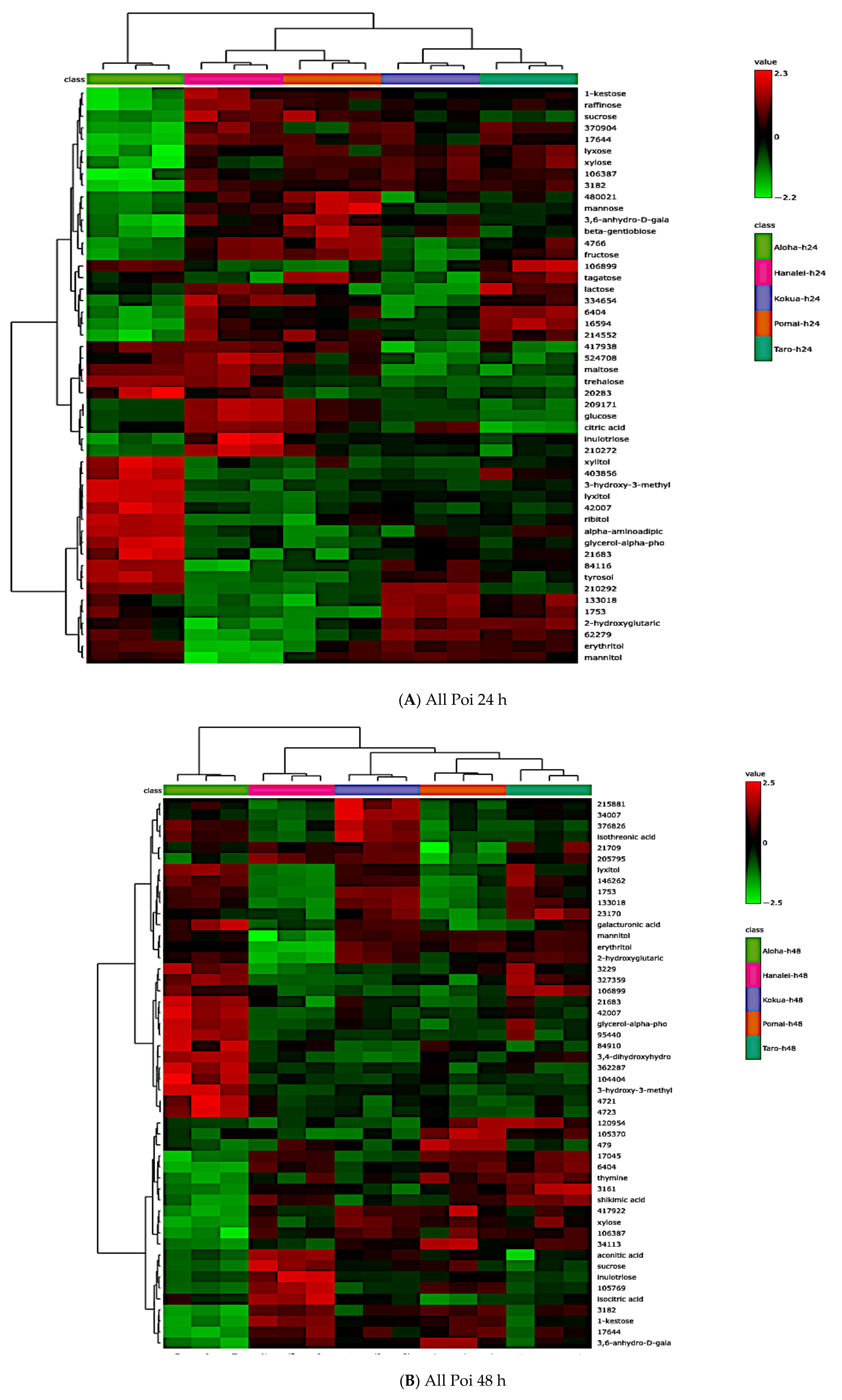
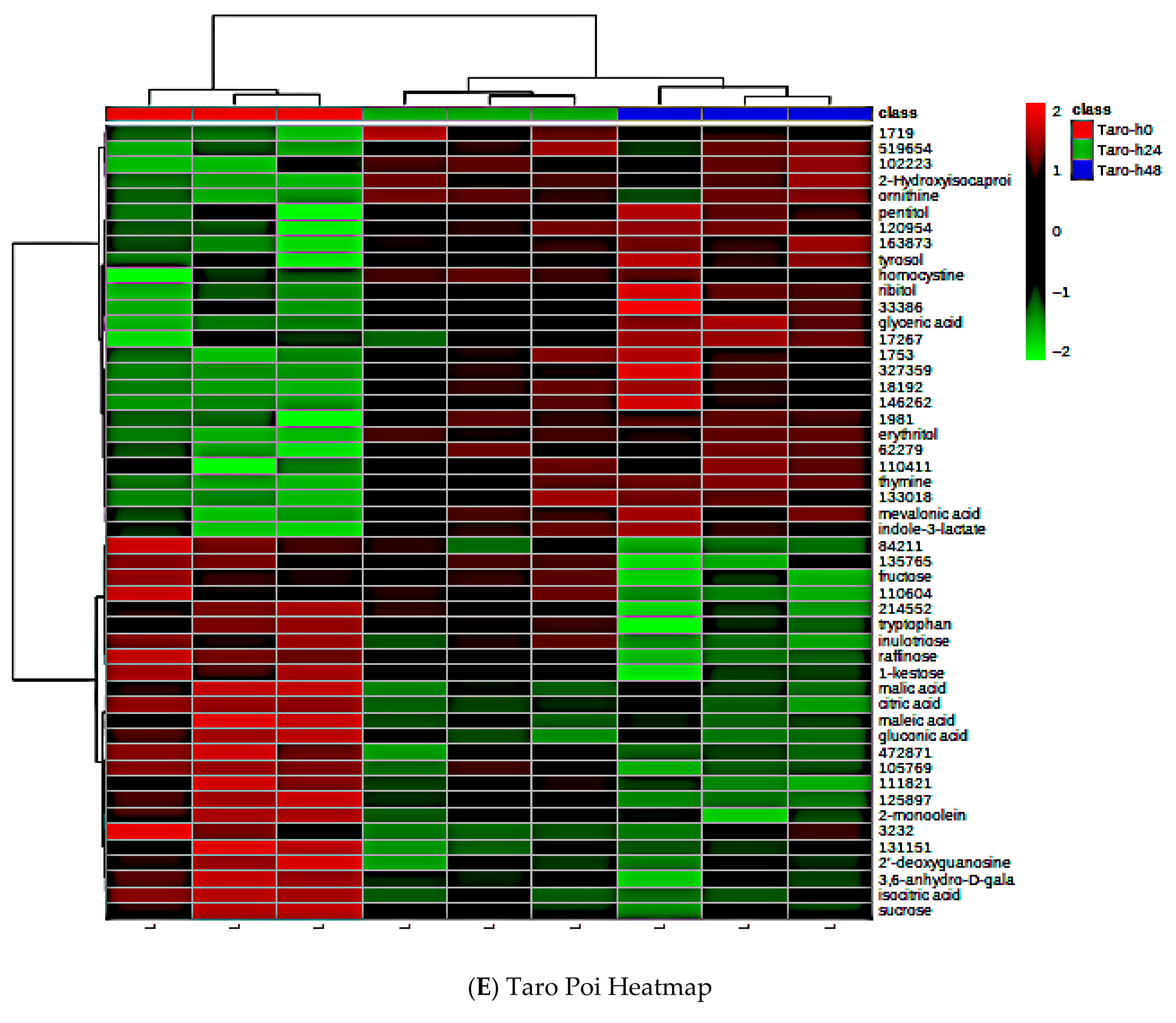
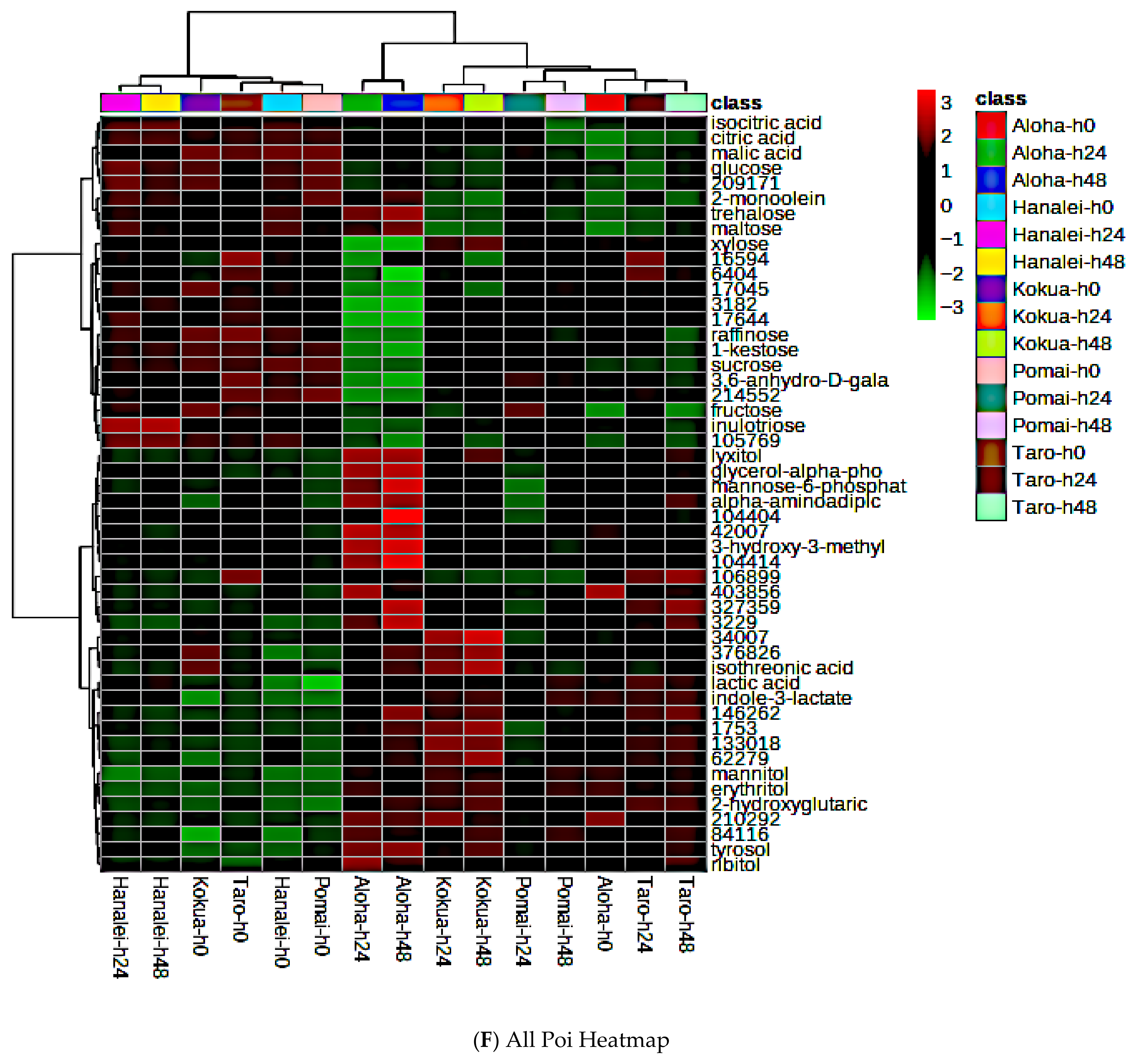
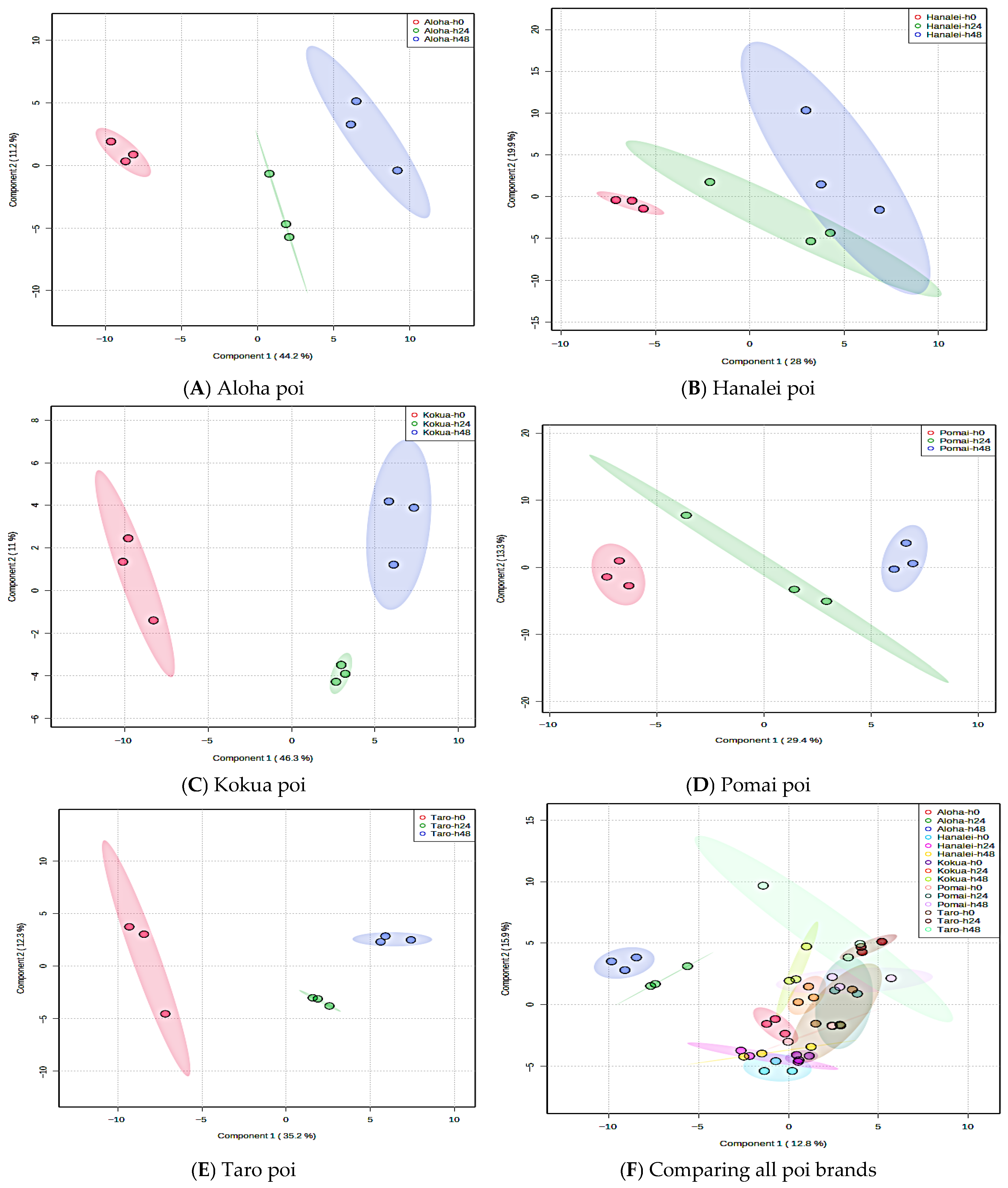
3.7. Global Metabolite Signatures of Five Local Brands of Fresh Poi
3.8. Comparing Global Metabolites in Five Local Poi Brands After 24 h and 48 h of Fermentation
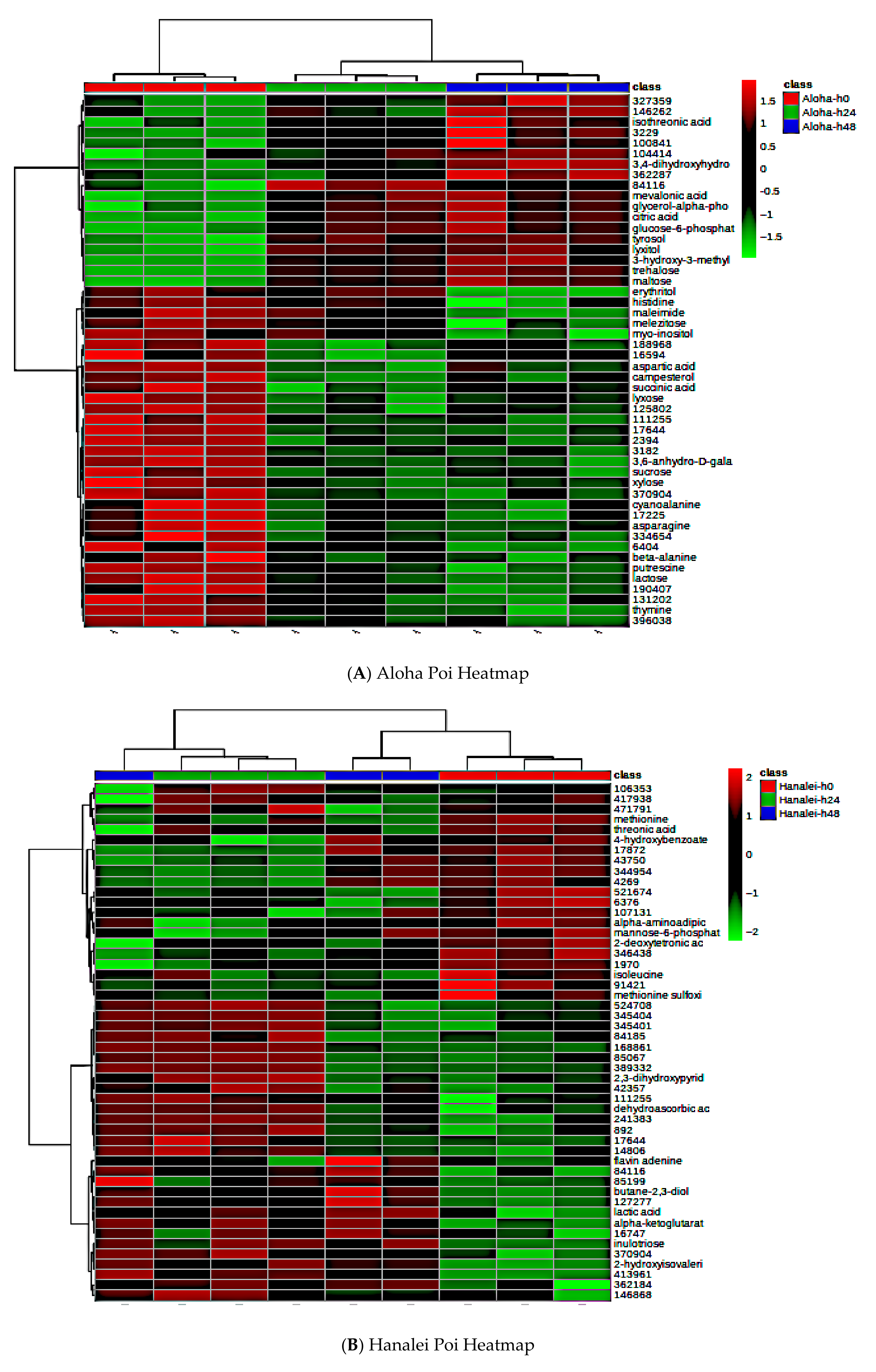
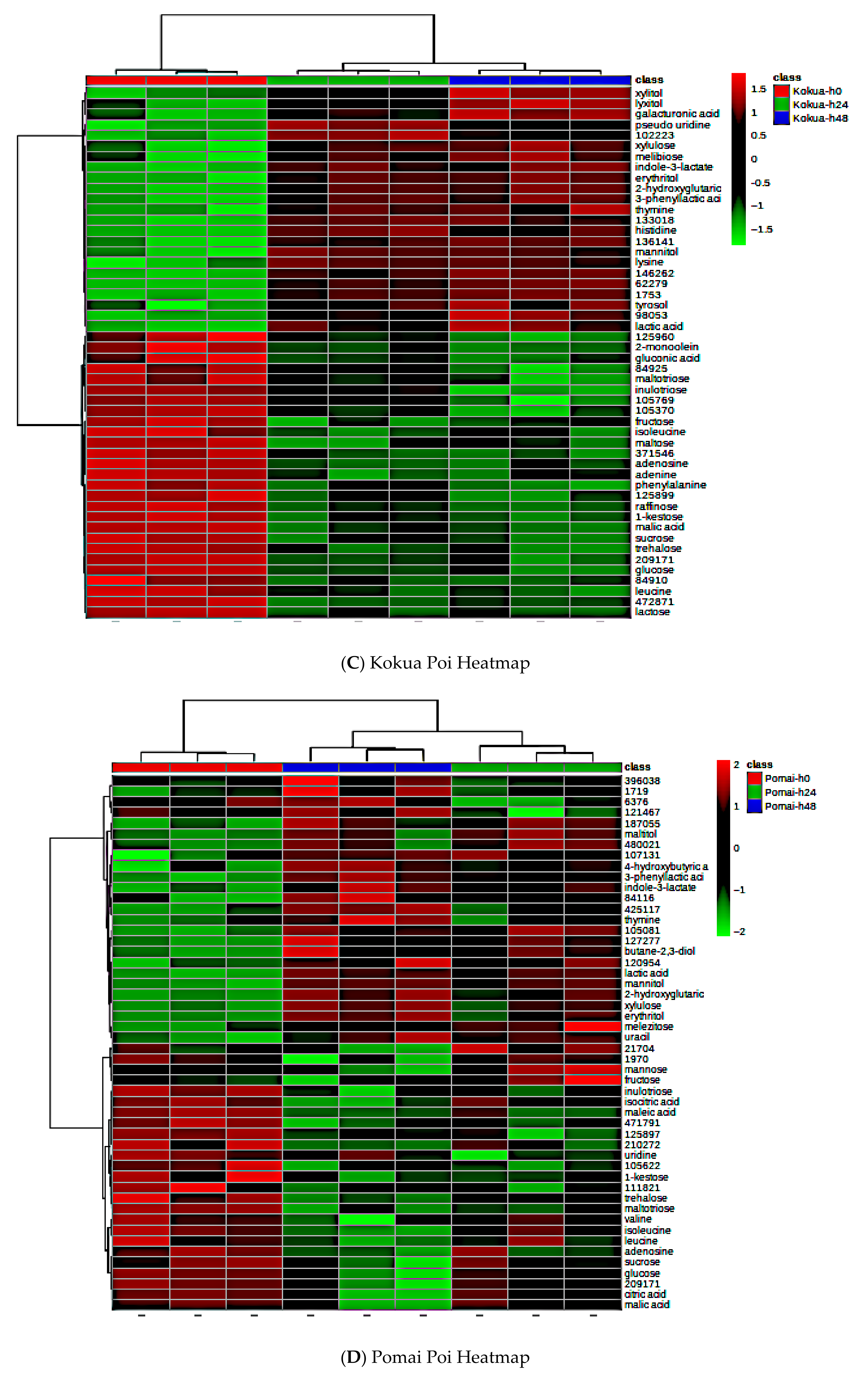
3.9. Effect of Fermentation on Global Metabolite Signatures of Individual Poi Brands
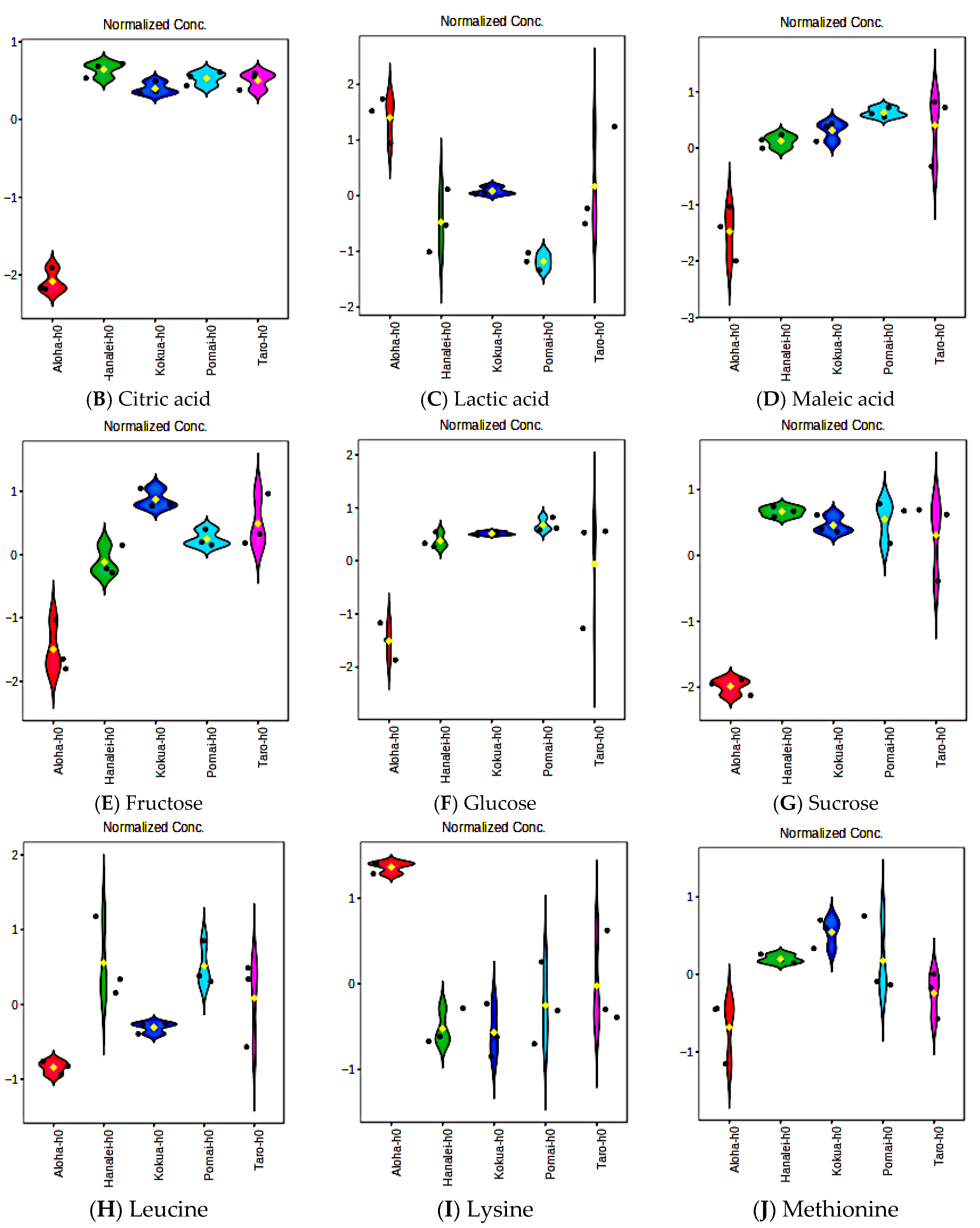
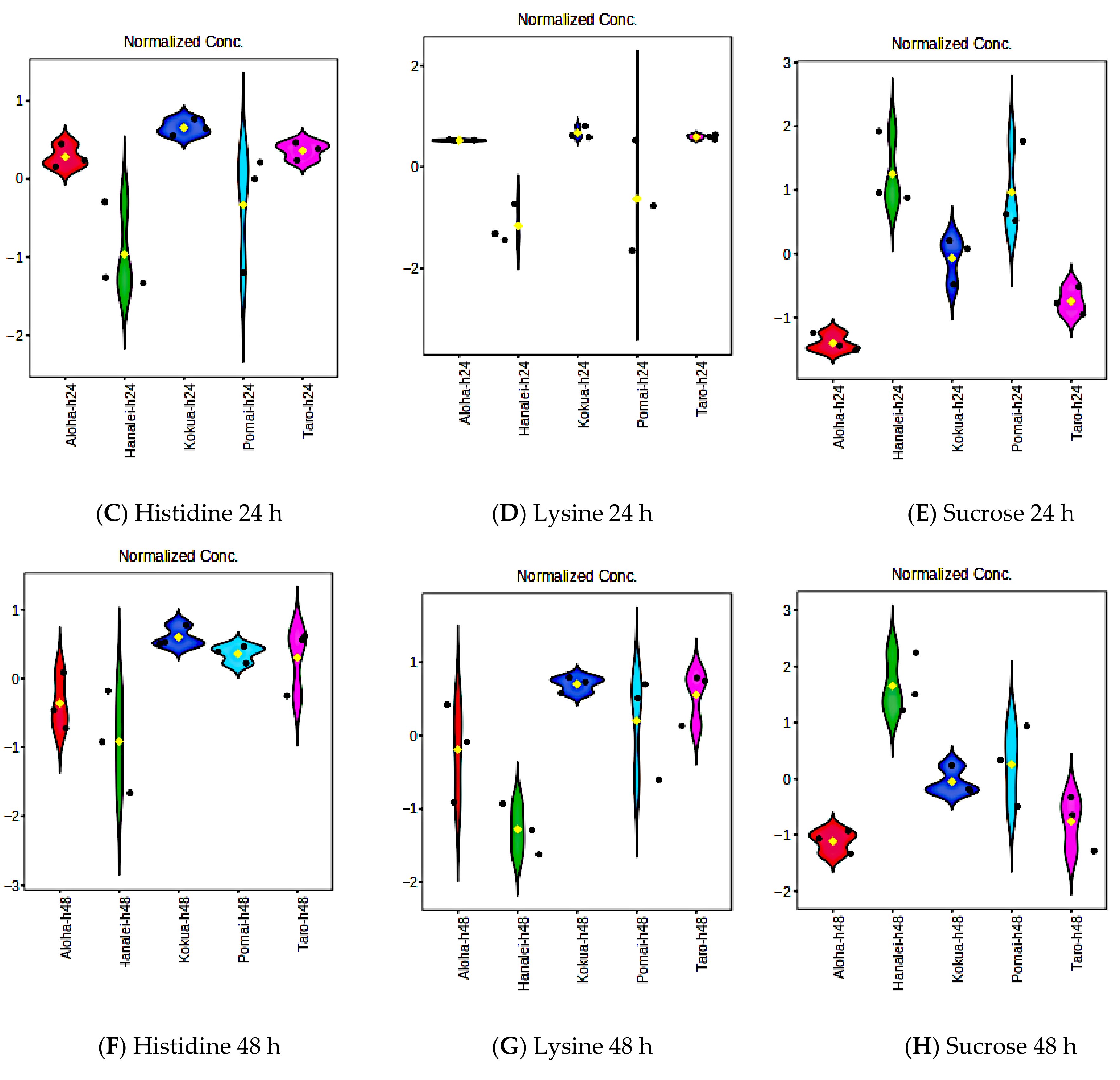
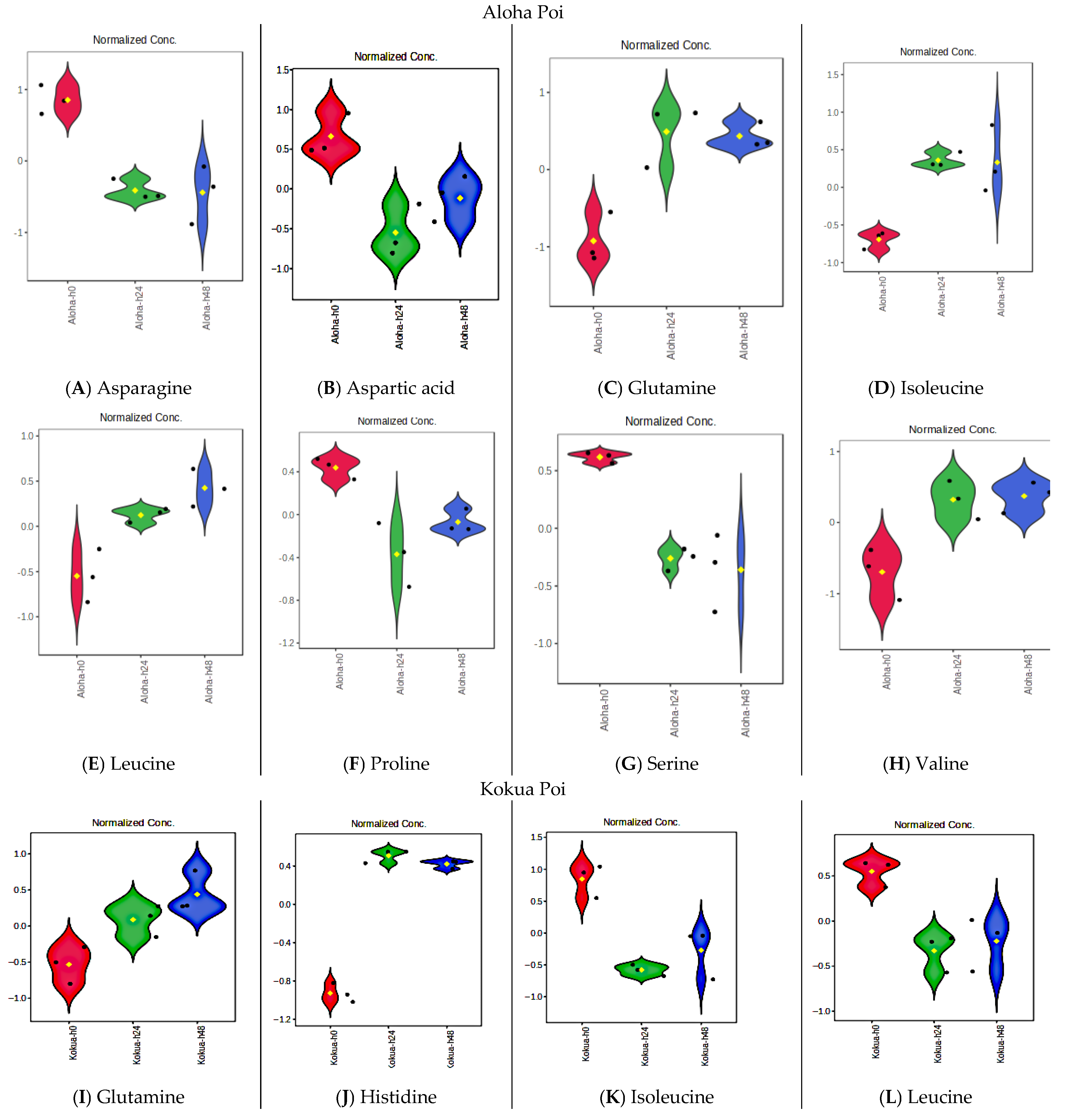
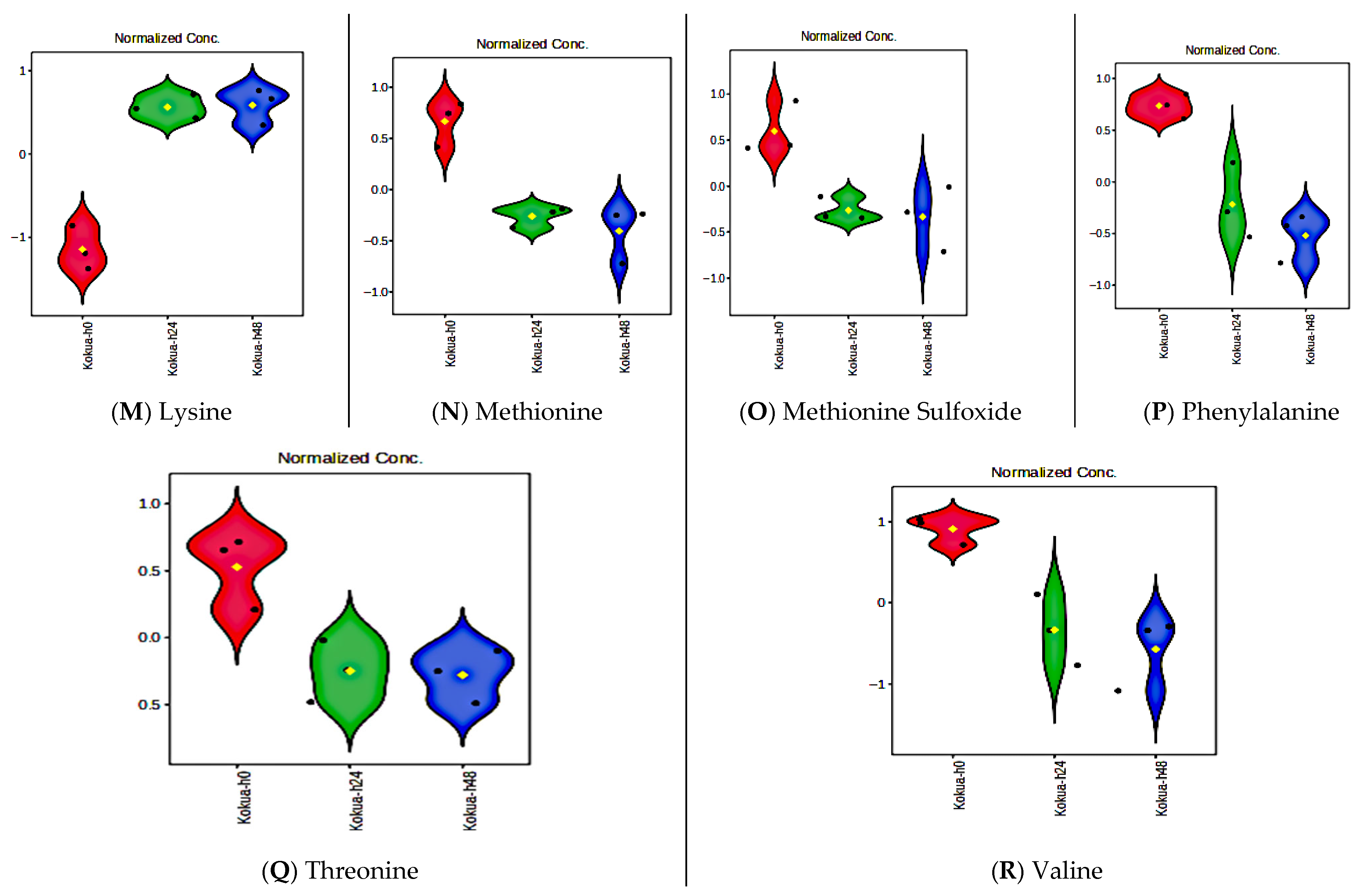
3.10. Fermentation Increases Amino Acid Contents of Poi but Does Not Affect Essential Fatty Acids, Vitamin E, or Flavanols
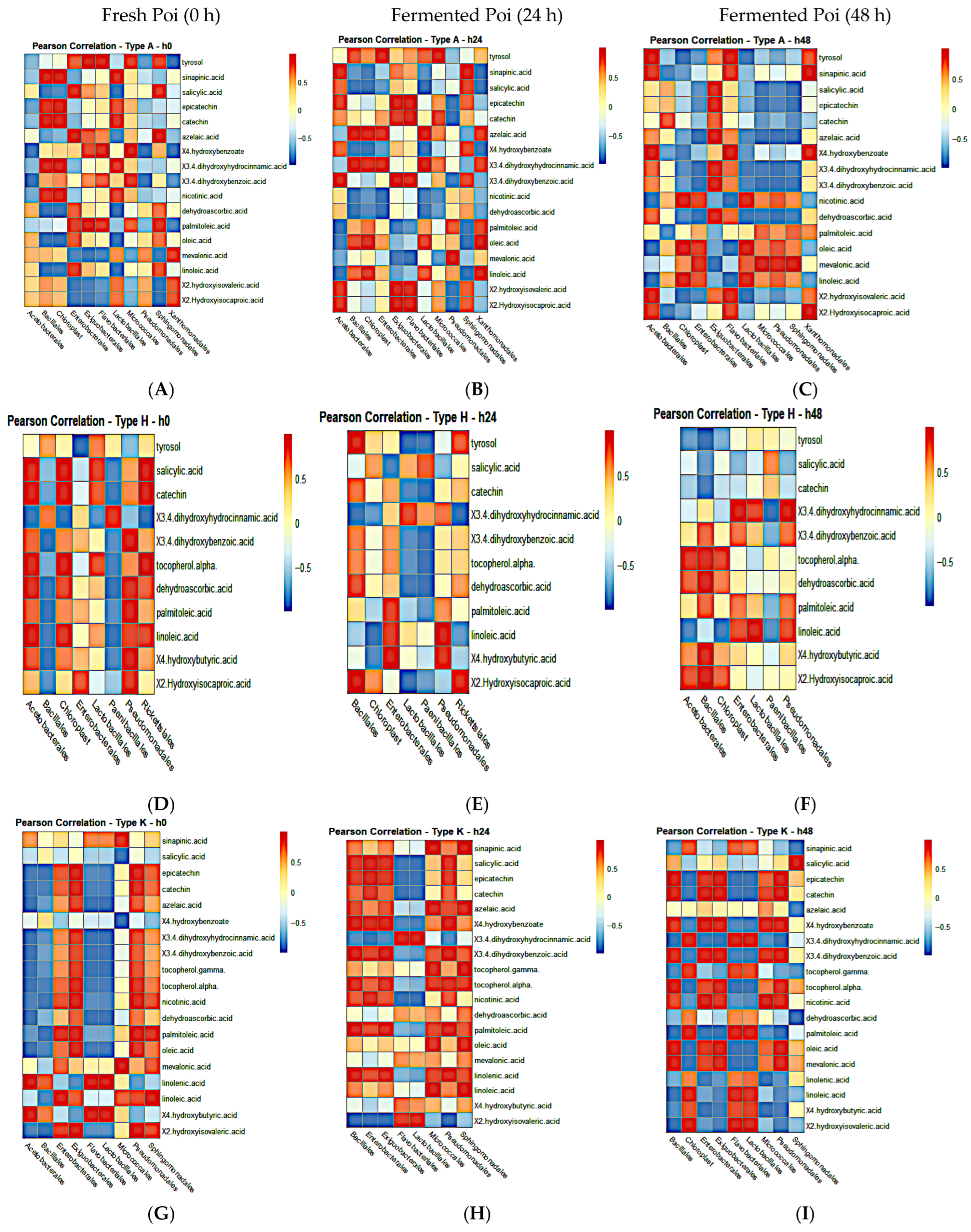
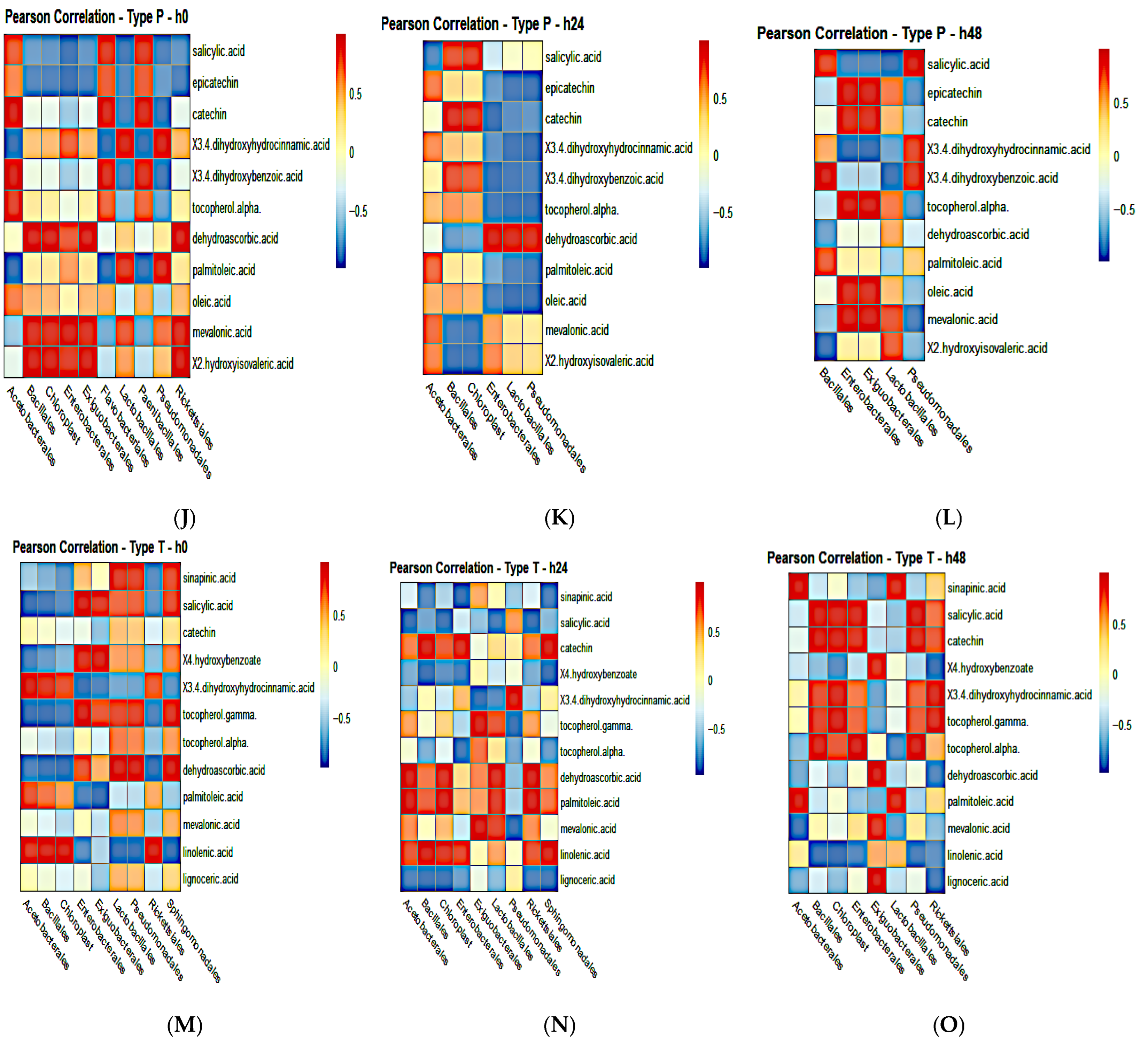
3.11. Pearson Correlation Analysis of Fermenting Bacteria and Global Metabolites in Individual Poi Brands
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACN | Acetonitrile |
| ADSC | Agriculture Diagnostic Service Center |
| AI | Adequate Intake |
| ALEX-CIS GCTOF | Automated liner exchange cold injection system gas chromatography time of flight |
| AMG | Amyloglucosidase |
| ANOVA | Analysis of variance |
| ASGPB | Advanced Studies in Genomics, Proteomics and Bioinformatics |
| ASV | Amplicon sequence variants |
| bp | Base pair |
| CDRR | Chronic Disease Risk Reduction Level |
| DADA2 | Deficiency of Adenosine Deaminase 2 |
| DNA | Deoxyribonucleic acid |
| dsDNA | Double-stranded Deoxyribonucleic acid |
| DV | Daily value |
| EI | Electron ionization |
| FAME | Fatty acid methyl ester |
| FDR | False discovery rate |
| GC | Gas chromatography |
| GOPOD | Glucose Determination Reagent |
| HCA | Hierarchical cluster analysis |
| IPA | Isopropanol |
| LAB | Lactic acid bacteria |
| LSD | Least Significant Difference |
| MaxEE | Maximum expected error |
| MS | Mass spectrometer |
| MSTFA | N-methyl-N-(trimethylsilyl) trifluoroacetamide |
| NA | Not available |
| NIH | National Institutes of Health |
| NRS | Non-resistant starch |
| OPLS-DA | Orthogonal partial least squares discriminant analysis |
| OTU | Operational Taxonomic Unit |
| PCA | Principal component analysis |
| PCR | Polymerase chain reaction |
| PERMANOVA | Permutational analysis of variance |
| PLS-DA | Partial least squares discriminant analysis |
| RCF | Relative centrifugal force |
| RDA | Recommended Dietary Allowance |
| RDP | Ribosomal Database Project |
| rRNA | Ribosomal ribonucleic acid |
| RS | Resistant starch |
| TMM | Trimmed mean of M |
| TOF | Time-of-flight |
| UHM | UH Mānoa |
| UL | Tolerable Upper Intake Level |
References
- Chair, H.; Traore, R.E.; Duval, M.F.; Rivallan, R.; Mukherjee, A.; Aboagye, L.M.; Van Rensburg, W.J.; Andrianavalona, V.; Pinheiro de Carvalho, M.A.; Saborio, F.; et al. Genetic Diversification and Dispersal of Taro (Colocasia esculenta (L.) Schott). PLoS ONE 2016, 11, e0157712. [Google Scholar] [CrossRef] [PubMed]
- Lebot, V. Tropical root and tuber crops: Cassava, sweet potato, yams and aroids. In Crop Production Science in Horticulture; CABI: Wallingford, UK; Cambridge, MA, USA, 2009; p. 17. [Google Scholar]
- Rao, V.R. The global diversity of taro: Ethnobotany and conservation. In The Global Diversity of Taro: Ethnobotany and Conservation; Bioversity International: Rome, Italy, 2010. [Google Scholar]
- Kapoor, B.; Singh, S.; Kumar, P. Taro (Colocasia esculenta): Zero wastage orphan food crop for food and nutritional security. S. Afr. J. Bot. 2022, 145, 157–169. [Google Scholar]
- Okonkwo, C.A.C. Taro. In Genetic Improvement of Vegetable Crops; Elsevier: Amsterdam, The Netherlands, 1993; pp. 709–715. [Google Scholar]
- Simsek, S.; El, S.N. In vitro starch digestibility, estimated glycemic index and antioxidant potential of taro (Colocasia esculenta L. Schott) corm. Food Chem. 2015, 168, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Watson, R.R.; Preedy, V.R. Bioactive Foods in Promoting Health; Academic Press: Cambridge, MA, USA, 2010. [Google Scholar]
- World Production of Taro (Cocoyam). UNdata. FAOSTAT (Food and Agriculture Organization). 2024. Available online: https://data.un.org/Data.aspx?q=taro&d=FAO&f=itemCode%3a136 (accessed on 25 August 2025).
- Wood, L. Global Taro Market 2020–2025: North America Dominates Global Imports ResearchAndMarkets.com. 2020. Available online: https://www.businesswire.com/news/home/20200819005251/en/Global-Taro-Market-2020-2025-North-America-Dominates-Global-Imports---ResearchAndMarkets.com (accessed on 25 August 2025).
- Taro Statistics, State of Hawaii 2021; United States Department of Agriculture, Hawaii Department of Agriculture, Market Analysis and News Branch: Honolulu, HI, USA, 2021. Available online: https://hdoa.hawaii.gov/wp-content/uploads/2022/11/Taro-Stats-2021_SOH_10.31.2022R.pdf (accessed on 14 November 2024).
- Helmkampf, M.; Wolfgruber, T.K.; Bellinger, M.R.; Paudel, R.; Kantar, M.B.; Miyasaka, S.C.; Kimball, H.L.; Brown, A.; Veillet, A.; Read, A.; et al. Phylogenetic Relationships, Breeding Implications, and Cultivation History of Hawaiian Taro (Colocasia esculenta) Through Genome-Wide SNP Genotyping. J. Hered. 2018, 109, 272–282. [Google Scholar] [CrossRef]
- Gupta, K.; Kumar, A.; Tomer, V.; Kumar, V.; Saini, M. Potential of Colocasia leaves in human nutrition: Review on nutritional and phytochemical properties. J. Food Biochem. 2019, 43, e12878. [Google Scholar] [CrossRef]
- Mitharwal, S.; Kumar, A.; Chauhan, K.; Taneja, N.K. Nutritional, phytochemical composition and potential health benefits of taro (Colocasia esculenta L.) leaves: A review. Food Chem. 2022, 383, 132406. [Google Scholar] [CrossRef]
- Brown, A.C.; Reitzenstein, J.E.; Liu, J.; Jadus, M.R. The anti-cancer effects of poi (Colocasia esculenta) on colonic adenocarcinoma cells In vitro. Phytother. Res. 2005, 19, 767–771. [Google Scholar] [CrossRef]
- Brown, A.C.; Valiere, A. Probiotics and medical nutrition therapy. Nutr. Clin. Care 2004, 7, 56–68. [Google Scholar]
- Trumbull, R. Hawaii, Poi is the Staff of Life. New York Times. 1982. Available online: https://www.nytimes.com/1982/10/31/travel/in-hawaii-poi-is-the-staff-of-life.html (accessed on 20 March 2023).
- Brown, A.C.; Valiere, A. The medicinal uses of poi. Nutr. Clin. Care 2004, 7, 69–74. [Google Scholar]
- Glaser, J.; Lawrence, R.A.; Harrison, A.; Ball, M.R. Poi, its use as a food for normal, allergic and potentially allergic children. Ann. Allery 1967, 25, 496–500. [Google Scholar]
- Roth, R.; Worth, R.M.; Lichton, I.J. Use of poi in the prevention of allergic disease in potentially allergic infants. Ann. Allergy 1967, 25, 501–506. [Google Scholar]
- Maier, T.V.; Lucio, M.; Lee, L.H.; VerBerkmoes, N.C.; Brislawn, C.J.; Bernhardt, J.; Lamendella, R.; McDermott, J.E.; Bergeron, N.; Heinzmann, S.S.; et al. Impact of Dietary Resistant Starch on the Human Gut Microbiome, Metaproteome, and Metabolome. mBio 2017, 8, e01343-17. [Google Scholar] [CrossRef]
- Pereira, P.R.; Corrêa, A.C.N.T.F.; Vericimo, M.A.; Paschoalin, V.M.F. Tarin, a Potential Immunomodulator and COX—Inhibitor Lectin Found in Taro (Colocasia esculenta). Compr. Rev. Food Sci. Food Saf. 2018, 17, 878–891. [Google Scholar] [CrossRef] [PubMed]
- Saxby, S.; Tipton, L.; Lee, C.; Wang, L.; Zhang, H.; Jia, W.; Boushey, C.; Li, Y. Prebiotic Potential of Taro (Colocasia esculenta) to Modulate Gut Bacteria Composition and Short Chain Fatty Acid Production. Curr. Dev. Nutr. 2020, 4, 1582. [Google Scholar] [CrossRef]
- Surono, I.S.; Venema, K. Modulation of Gut Microbiota Profile and Short-Chain Fatty Acids of Rats Fed with Taro Flour or Taro Starch. Int. J. Microbiol. 2020, 2020, 8893283. [Google Scholar] [CrossRef]
- Nkhata, S.G.; Ayua, E.; Kamau, E.H.; Shingiro, J.B. Fermentation and germination improve nutritional value of cereals and legumes through activation of endogenous enzymes. Food Sci. Nutr. 2018, 6, 2446–2458. [Google Scholar] [CrossRef]
- Pranoto, Y.; Anggrahini, S.; Efendi, Z. Effect of natural and Lactobacillus plantarum fermentation on in-vitro protein and starch digestibilities of sorghum flour. Food Biosci. 2013, 2, 46–52. [Google Scholar] [CrossRef]
- Sharma, N.; Angural, S.; Rana, M.; Puri, N.; Kondepudi, K.K.; Gupta, N. Phytase producing lactic acid bacteria: Cell factories for enhancing micronutrient bioavailability of phytate rich foods. Trends Food Sci. Technol. 2020, 96, 1–12. [Google Scholar] [CrossRef]
- Huang, A.S.; Lam, S.Y.; Nakayama, T.M.; Lin, H. Microbiological and chemical changes in poi stored at 20 °C. J. Agric. Food Chem. 1994, 42, 45–48. [Google Scholar] [CrossRef]
- Climate in The Hawaiian Islands; Hawaii Tourism Authority: Honolulu, HI, USA, 2024; Available online: https://www.gohawaii.com/trip-planning/weather#:~:text=The%20average%20daytime%20summer%20temperature,F%20lower%20than%20the%20daytime (accessed on 11 December 2024).
- Method 3050Bacid Digestion of Sediments Sludges Soils 10 Scope Application Washington, D.C.; U.S. Environmental Protection Agency: Washington, DC, USA, 1996.
- Nerurkar, P.V.; Yokoyama, J.; Ichimura, K.; Kutscher, S.; Wong, J.; Bittenbender, H.C.; Deng, Y. Medium Roasting and Brewing Methods Differentially Modulate Global Metabolites, Lipids, Biogenic Amines, Minerals, and Antioxidant Capacity of Hawai’i-Grown Coffee (Coffea arabica). Metabolites 2023, 13, 412. [Google Scholar] [CrossRef]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glockner, F.O. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2013, 41, e1. [Google Scholar] [CrossRef] [PubMed]
- Straub, D.; Blackwell, N.; Langarica-Fuentes, A.; Peltzer, A.; Nahnsen, S.; Kleindienst, S. Interpretations of Environmental Microbial Community Studies Are Biased by the Selected 16S rRNA (Gene) Amplicon Sequencing Pipeline. Front. Microbiol. 2020, 11, 550420. [Google Scholar] [CrossRef] [PubMed]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 2011, 17, 10. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glockner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- Cajka, T.; Fiehn, O. Toward Merging Untargeted and Targeted Methods in Mass Spectrometry-Based Metabolomics and Lipidomics. Anal. Chem. 2016, 88, 524–545. [Google Scholar] [CrossRef]
- Fiehn, O. Metabolomics by Gas Chromatography-Mass Spectrometry: Combined Targeted and Untargeted Profiling. Curr. Protoc. Mol. Biol. 2016, 114, 30.4.1–30.4.32. [Google Scholar] [CrossRef]
- Granda, M.L.; Prince, D.K.; Fiehn, O.; Chen, Y.; Rajabi, T.; Yeung, C.K.; Hoofnagle, A.N.; Kestenbaum, B. Metabolomic Profiling Identifies New Endogenous Markers of Tubular Secretory Clearance. Kidney360 2023, 4, 23–31. [Google Scholar] [CrossRef]
- Johnson, C.M.; Rosario, R.; Brito, A.; Agrawal, K.; Fanter, R.; Lietz, G.; Haskell, M.; Engle-Stone, R.; Newman, J.W.; La Frano, M.R. Multiassay nutritional metabolomics profiling of low vitamin A status versus adequacy is characterized by reduced plasma lipid mediators among lactating women in the Philippines: A pilot study. Nutr. Res. 2022, 104, 118–127. [Google Scholar] [CrossRef]
- Sud, M.; Fahy, E.; Cotter, D.; Azam, K.; Vadivelu, I.; Burant, C.; Edison, A.; Fiehn, O.; Higashi, R.; Nair, K.S.; et al. Metabolomics Workbench: An international repository for metabolomics data and metadata, metabolite standards, protocols, tutorials and training, and analysis tools. Nucleic Acids Res. 2016, 44, D463–D470. [Google Scholar] [CrossRef]
- Aranaz, P.; Ramos-Lopez, O.; Cuevas-Sierra, A.; Martinez, J.A.; Milagro, F.I.; Riezu-Boj, J.I. A predictive regression model of the obesity-related inflammatory status based on gut microbiota composition. Int. J. Obes. 2021, 45, 2261–2268. [Google Scholar] [CrossRef]
- Chong, J.; Liu, P.; Zhou, G.; Xia, J. Using MicrobiomeAnalyst for comprehensive statistical, functional, and meta-analysis of microbiome data. Nat. Protoc. 2020, 15, 799–821. [Google Scholar] [CrossRef] [PubMed]
- Cuevas-Sierra, A.; Romo-Hualde, A.; Aranaz, P.; Goni, L.; Cuervo, M.; Martinez, J.A.; Milagro, F.I.; Riezu-Boj, J.I. Diet- and sex-related changes of gut microbiota composition and functional profiles after 4 months of weight loss intervention. Eur. J. Nutr. 2021, 60, 3279–3301. [Google Scholar] [CrossRef] [PubMed]
- Nurient Recommendations and Databases; National Academy of Sciences: Washington, DC, USA. Available online: https://ods.od.nih.gov/HealthInformation/nutrientrecommendations.aspx (accessed on 10 October 2024).
- Poi. Food Data Central Food Details. 2019. Available online: https://fdc.nal.usda.gov/food-details/170431/nutrients (accessed on 15 January 2025).
- Derstine, V.; Rada, E.L. Some dietetic factors influencing the market for poi in Hawaii, with emphasis on a survey of the use of poi by the medical profession and allied institutions. 1952. Available online: http://hdl.handle.net/10125/16475 (accessed on 14 November 2024).
- Mulville, K.; Kai, J.; Kearney, J.M.; Ng-Osorio, J.; Boushey, C.J.; Fialkowski, M.K. A Qualitative Analysis of a Caregivers’ Experience of Complementary Feeding in a Population of Native Hawaiian, Other Pacific Islander and Filipino Infants: The Timing of the Introduction of Complementary Foods, and the Role of Transgenerational Experience. Nutrients 2022, 14, 3268. [Google Scholar] [CrossRef] [PubMed]
- Gerrano, A.S.; Mathew, I.; Shayanowako, A.I.; Amoo, S.; Mellem, J.J.; Van Rensburg, W.J.; Bairu, M.W.; Venter, S.L. Variation in mineral element composition of landrace taro (Colocasia esculenta) corms grown under dryland farming system in South Africa. Heliyon 2021, 7, e06727. [Google Scholar] [CrossRef]
- Karuma, A.N.; Njuguna, J.W.; Gicheru, P.; Kaburu, F. Proximate Analysis and Mineral Profile of Taro (Colocasia esculenta) in Embu Kenya. Trop. Subtrop. Agroecosyst. 2024, 27, 61. [Google Scholar] [CrossRef]
- Taro, Cooked, without Salt. Food Data Central Food Details; 2019. Available online: https://fdc.nal.usda.gov/food-details/168486/nutrients (accessed on 10 March 2025).
- Billions Worldwide Consume Inadequate Levels of Micronutrients Critical to Human Health; Harvard T.H. Chan School of Public Health: Boston, MA, USA, 2024; Available online: https://hsph.harvard.edu/news/billions-worldwide-consume-inadequate-levels-of-micronutrients-critical-to-human-health/ (accessed on 11 February 2025).
- Drake, V. Micronutrient Inadequacies in the US Population: An Overview 2017; Linus Pauling Institute: Corvallis, OR, USA, 2018; Available online: https://lpi.oregonstate.edu/mic/micronutrient-inadequacies/overview (accessed on 15 February 2025).
- Kiani, A.K.; Dhuli, K.; Donato, K.; Aquilanti, B.; Velluti, V.; Matera, G.; Iaconelli, A.; Connelly, S.T.; Bellinato, F.; Gisondi, P.; et al. Main nutritional deficiencies. J. Prev. Med. Hyg. 2022, 63 (Suppl. S3), E93–E101. [Google Scholar]
- Bhaskaram, P. Immunobiology of mild micronutrient deficiencies. Br. J. Nutr. 2001, 85 (Suppl. S2), S75–S80. [Google Scholar] [CrossRef]
- Ward, E. Addressing nutritional gaps with multivitamin and mineral supplements. Nutr. J. 2014, 13, 72. [Google Scholar] [CrossRef]
- Arredondo, M.; Martinez, R.; Nunez, M.T.; Ruz, M.; Olivares, M. Inhibition of iron and copper uptake by iron, copper and zinc. Biol. Res. 2006, 39, 95–102. [Google Scholar] [CrossRef]
- Fosmire, G.J. Zinc toxicity. Am. J. Clin. Nutr. 1990, 51, 225–227. [Google Scholar] [CrossRef] [PubMed]
- Sandstead, H.H. Zinc interference with copper metabolism. JAMA 1978, 240, 2188. [Google Scholar] [CrossRef] [PubMed]
- Spencer, H.; Norris, C.; Williams, D. Inhibitory effects of zinc on magnesium balance and magnesium absorption in man. J. Am. Coll. Nutr. 1994, 13, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Wapnir, R.A.; Balkman, C. Inhibition of copper absorption by zinc. Effect of histidine. Biol. Trace Elem. Res. 1991, 29, 193–202. [Google Scholar] [CrossRef]
- Danneskiold-Samsoe, N.B.; Dias de Freitas Queiroz Barros, H.; Santos, R.; Bicas, J.L.; Cazarin, C.B.B.; Madsen, L.; Kristiansen, K.; Pastore, G.M.; Brix, S.; Marostica Junior, M.R. Interplay between food and gut microbiota in health and disease. Food Res. Int. 2019, 115, 23–31. [Google Scholar] [CrossRef]
- Lesmes, U.; Beards, E.J.; Gibson, G.R.; Tuohy, K.M.; Shimoni, E. Effects of resistant starch type III polymorphs on human colon microbiota and short chain fatty acids in human gut models. J. Agric. Food Chem. 2008, 56, 5415–5421. [Google Scholar] [CrossRef]
- Sajilata, M.G.; Singhal, R.S.; Kulkarni, P.R. Resistant Starch-A Review. Compr. Rev. Food Sci. Food Saf. 2006, 5, 1–17. [Google Scholar] [CrossRef]
- Setiarto, R.H.B.; Jenie, B.S.L.; Faridah, D.N.; Saskiawan, I.; Sulistiani, S. Effect of Lactic Acid Bacteria fermentation and autoclaving-cooling for resistant starch and prebiotic properties of modified taro flour. Int. Food Res. J. 2018, 25, 1691–1697. [Google Scholar]
- Kanazawa, S.; Sanabria, M.; Monteiro, M. Influence of the fermentation methods on the resistant starch formation by X-ray diffraction. Appl. Sci. 2021, 3, 191. [Google Scholar] [CrossRef]
- Wardana, A.A.; Surono, I.S. Resistant starch content, pasting properties, and structure of modified taro (Colocasia esculenta L. Schott) starch granule by steam cooking. J. Phys. 2019, 1363, 12008. [Google Scholar] [CrossRef]
- Wardana, A.A.; Surono, I.S. Effects of various cooling temperatures on resistant starch formation and pasting behaviour of autoclaved taro flour. Earth Environ. Sci. 2022, 998, 12053. [Google Scholar]
- Simsek, S.; El, S.N. Production of resistant starch from taro (Colocasia esculenta L. Schott) corm and determination of its effects on health by in vitro methods. Carbohydr. Polym. 2012, 90, 1204–1209. [Google Scholar] [CrossRef]
- Hill, D.; Sugrue, I.; Arendt, E.; Hill, C.; Stanton, C.; Ross, R.P. Recent advances in microbial fermentation for dairy and health. F1000Research 2017, 6, 751. [Google Scholar] [CrossRef]
- MacDonald, T.T.; Bell, I. Probiotics and the immune response to vaccines. Proc. Nutr. Soc. 2010, 69, 442–446. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.; Teixeira, J.; Rodrigues, A. Production of dextransucrase, dextran and fructose from sucrose using Leuconostoc mesenteroides NRRL B512. Biochem. Eng. J. 2000, 4, 177–188. [Google Scholar] [CrossRef]
- Wagar, L.E.; Champagne, C.P.; Buckley, N.D.; Raymond, Y.; Green-Johnson, J.M. Immunomodulatory properties of fermented soy and dairy milks prepared with lactic acid bacteria. J. Food Sci. 2009, 74, M423–M430. [Google Scholar] [CrossRef] [PubMed]
- Zapasnik, A.; Sokolowska, B.; Bryla, M. Role of Lactic Acid Bacteria in Food Preservation and Safety. Foods 2022, 11, 1283. [Google Scholar] [CrossRef]
- Allen, O.N.; Allen, E.K. The Manufacture of Poi from Taro in Hawaii: With Special Emphasis Upon Its Fermentation; CTAHR Bulletin 70; Hawaii Agricultural Experiment Station: Honolulu, HI, USA, 1933. [Google Scholar]
- Choi, S.S.; Kim, Y.; Han, K.S.; You, S.; Oh, S.; Kim, S.H. Effects of Lactobacillus strains on cancer cell proliferation and oxidative stress in vitro. Lett. Appl. Microbiol. 2006, 42, 452–458. [Google Scholar] [CrossRef]
- Kwak, S.H.; Cho, Y.M.; Noh, G.M.; Om, A.S. Cancer Preventive Potential of Kimchi Lactic Acid Bacteria (Weissella cibaria, Lactobacillus plantarum). J. Cancer Prev. 2014, 19, 253–258. [Google Scholar] [CrossRef]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef]
- Worley, B.; Powers, R. Multivariate Analysis in Metabolomics. Curr. Metabolomics 2013, 1, 92–107. [Google Scholar]

| Nutrition Facts * | Aloha Brand | Hanalei Brand | Kokua Brand | Pomai Brand | Taro Brand |
|---|---|---|---|---|---|
| Serving size (g) | 90 | 104 | NA | NA | 82 |
| Total fat (g) | 0 | 0.5 | NA | NA | 0 |
| Saturated fat (g) | 0 | 0 | NA | NA | 0 |
| Total carbohydrate (g) | 19 | 14 | NA | NA | 12 |
| Dietary fiber (g) | 2 | 1 | NA | NA | 1 |
| Sugars (g) | 0 | 1 | NA | NA | 0 |
| Protein (g) | 1 | 0 | NA | NA | 0 |
| Vitamin A | 0% | NA | NA | NA | NA |
| Vitamin C | 0% | NA | NA | NA | NA |
| Vitamin D | NA | 0 mcg | NA | NA | 0 mcg |
| Calcium | 2% DV | 20 mg | NA | NA | 16 mg/2% DV |
| Iron | 4% DV | 0.8 mg DV | NA | NA | 1 mg |
| Potassium | NA | 120 mg DV | NA | NA | 107 mg DV |
| Laboratory-measured moisture content (%) ** | 80.74 ± 1.131 a | 83.89 ± 0.683 a,c | 76.37 ± 0.191 b | 84.44 ± 2.060 c | 81.20 ± 1.506 a,c |
| Minerals | Minerals—Average RDA, AI, and UL (mg/day) in Males and Females (19–70 y) | Minerals in Aloha Poi mg/100 g (n = 3) | Minerals in Hanalei Poi mg/100 g (n = 3) | Minerals in Kokua Poi mg/100 g (n = 3) | Minerals in Pomai Poi mg/100 g (n = 3) | Minerals in Taro Poi mg/100 g (n = 3) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 h | 24 h | 48 h | 0 h | 24 h | 48 h | 0 h | 24 h | 48 h | 0 h | 24 h | 48 h | 0 h | 24 h | 48 h | ||
| P 1 | 700 * 1250 ** | 255.1 ± 45.33 a,b,d | 255.7 ± 26.23 a,b,d | 259 ± 36.77 a,b,d | 207.1 ± 3.678 a | 217.4 ± 15.68 a | 225.1 ± 15.19 a,c | 294.6 ± 10.99 b | 288.5 ± 3.433 b,c | 291.1 ± 9.157 b,c | 205.6 ± 10.47 d | 201.5 ± 14.5 d | 196.1 ± 10.3 d | 258.4 ± 33.84 a,d | 242.2 ± 25.9 a,d | 248.4 ± 29.24 a,d |
| K 2 | 2600 * 4700 ** | 998.5 ± 196.7 a | 974.9 ± 129.7 a | 1014 ± 199 a | 1321 ± 80.64 a,b | 1380 ± 51.28 a,b | 1403 ± 91.61 a,b | 1691 ± 28.08 b | 1667 ± 16.6 b | 1682 ± 34.82 b | 1320 ± 285.4 a,b | 1309 ± 375.6 a,b | 1258 ± 309.7 a,b | 1629 ± 241.2 b | 1521 ± 166.2 a,b | 1541 ± 130.9 a,b |
| Ca 1 | 1000 * 1300 ** | 204.8 ± 36.52 a | 204.1 ± 19.24 a | 205.7 ± 26.29 a | 162.1 ± 22.87 a | 168.3 ± 18 a | 168.3 ± 21.54 a | 181.2 ± 19.65 a | 177.9 ± 7.16 a | 175.4 ± 8.571 a | 163.9 ± 29.42 a | 168.6 ± 28.51 a | 166.4 ± 26.18 a | 185.1 ± 41.14 a | 175.6 ± 38.44 a | 179.6 ± 43.05 a |
| Mg 1 | 310–320 * (female) 400–420 * (males) 420 ** | 228.7 ± 30.24 a | 229.5 ± 12.09 a | 233.2 ± 17.92 a | 148.9 ± 14.81 b | 157.6 ± 12.49 b | 161.2 ± 10.88 b | 169.3 ± 6.574 b | 166.5 ± 4.775 b | 166.4 ± 6.574 b | 167.3 ± 11.82 b | 169.3 ± 11.42 b | 161.6 ± 6.499 b | 201.6 ± 22.36 a | 189.6 ± 17.62 a,b | 193.3 ± 19.57 a,b |
| Na 4 | 2300 * 2300 ** | 74.52 ± 7.582 a,b,c | 89.7 ± 38.09 a,c | 73.42 ± 10.51 a,b,c | 53.55 ± 11.18 a,b | 57.8 ± 9.769 a,b,c | 57.86 ± 10.12 a,b,c | 104.5 ± 24.06 c | 97.35 ± 15.16 a,c | 97.97 ± 15.39 a,c | 32 ± 11.54 b | 34.9 ± 16.87 b | 30.51 ± 11.71 b | 57.24 ± 7.063 a,b | 52.38 ± 3.741 a,b | 51.51 ± 6.335 a,b |
| Fe 1 | 18 * (female) 8 * (males) 18 ** | 10.99 ± 4.451 a | 10.84 ± 3.405 a | 12.03 ± 4.655 a | 6.287 ± 0.516 a | 6.746 ± 0.792 a | 6.773 ± 0.866 a | 10.16 ± 2.041 a | 10.31 ± 1.458 a | 10.74 ± 0.973 a | 8.001 ± 1.012 a | 7.838 ± 2.328 a | 7.811 ± 1.095 a | 8.138 ± 0.97 a | 7.765 ± 1.079 a | 7.962 ± 1.163 a |
| Mn 2 | 2.3 ** | 6.923 ± 4.247 a | 6.806 ± 3.834 a | 6.982 ± 3.919 a | 2.736 ± 2.369 a | 2.812 ± 2.347 a | 2.883 ± 2.379 a | 2.466 ± 0.148 a | 2.394 ± 0.129 a | 2.367 ± 0.117 a | 3.107 ± 0.239 a | 3.15 ± 0.384 a | 3.041 ± 0.427 a | 6.238 ± 1.111 a | 5.903 ± 1.369 a | 6.003 ± 1.154 a |
| Zn 1 | 8 * (female) 11 * (males) 11 ** | 8.69 ± 2.869 a,c | 7.717 ± 1.972 a,c | 7.036 ± 1.786 a,c | 3.148 ± 1.153 b | 4.304 ± 0.881 a,b | 4.093 ± 1.683 a,b | 10.46 ± 2.453 c | 10.06 ± 2.349 c,d | 10.94 ± 3.754 c | 4.88 ± 0.686 a,d | 5.593 ± 1.101 a,c,d | 5.141 ± 1.104 a,c,e | 5.547 ± 0.299 a,c,d | 4.785 ± 0.744 a,d | 4.777 ± 0.584 a,d |
| Cu 1 | 0.9 ** | 5.747 ± 5.663 a | 2.89 ± 0.441 a | 2.741 ± 0.549 a | 3.287 ± 1.092 a | 3.495 ± 0.635 a | 3.4 ± 0.547 a | 2.528 ± 0.777 a | 2.419 ± 0.994 a | 2.647 ± 1.711 a | 3.65 ± 0.324 a | 3.518 ± 0.662 a | 3.449 ± 0.305 a | 3.925 ± 0.479 a | 4.086 ± 0.589 a | 3.75 ± 0.468 a |
| B 3 | 1.5 *** | 0.677 ± 0.153 a,b | 0.603 ± 0.209 a,b | 0.655 ± 0.099 a,b | 0.401 ± 0.017 a | 0.447 ± 0.059 a | 0.453 ± 0.039 a | 0.746 ± 0.04 b,c | 0.751 ± 0.1 b,c | 0.825 ± 0.158 b | 0.517 ± 0.013 a,c | 0.508 ± 0.037 a,c | 0.49 ± 0.076 a,c | 0.625 ± 0.139 a,b | 0.524 ± 0.072 a,b | 0.509 ± 0.078 a |
| Mo | 0.19 ± 0.24 a | 0.06 ± 0.10 a | 0.07 ± 0.08 a | 0.03 ± 0.03 a | 0.04 ± 0.03 a | 0.05 ± 0.05 a | 0.18 ± 0.09 a | 0.13 ± 0.06 a | 0.16 ± 0.15 a | 0.07 ± 0.03 a | 0.05 ± 0.03 a | 0.03 ± 0.04 a | 0.21 ± 0.30 a | 0.05 ± 0.04 a | 0.05 ± 0.04 a | |
| Poi Type | Chao1 | Shannon | Simpson | Beta Diversity |
|---|---|---|---|---|
| p-Value (F-Value) | p-Value (F-Value); R-Squared | |||
| Aloha Poi | 0.1517 (2.625) | 0.054635 (4.9061) | 0.72472 (0.33988) | 0.135 (2.3467); 0.43891 |
| Hanalei Poi | 0.00036443 (39) | 0.27816 (1.60) | 0.5652 (0.62845) | 0.143 (1.7287); 0.36557 |
| Kokua Poi | 0.49205 (0.8) | 0.94387 (0.058322) | 0.93635 (0.066494) | 0.718 (0.1632); 0.051592 |
| Pomai Poi | 0.031491 (6.5) | 0.0032237 (17.308) | 0.0018695 (21.353) | 0.035 (15.118); 0.83441 |
| Taro Poi | 0.46704 (0.86667) | 0.37857 (1.147) | 0.47933 (0.83334) | 0.062 (2.3996); 0.44441 |
| Poi Type | Bacteria | log2FC | logCPM | p-Values | FDR |
|---|---|---|---|---|---|
| Aloha (24 h vs. 0 h) | Acetobacterales | 2.6945 | 18.791 | 0.0019061 | 0.026685 |
| Aloha (48 h vs. 0 h) | Acetobacterales | 2.6894 | 18.791 | 0.0019396 | 0.027155 |
| Hanalei (24 h vs. 0 h) | Bacillales | 5.0053 | 20.965 | 4.6694 × 10−5 | 3.7355 × 10−4 |
| Lactobacillales | 9.947 | 19.499 | 1.0571 × 10−4 | 4.2284 × 10−4 | |
| Paenibacillales | 9.2845 | 15.897 | 1.8001 × 10−4 | 4.8003 × 10−4 | |
| Rickettsiales | −3.8424 | 9.5277 | 0.012311 | 0.024622 | |
| Hanalei (48 h vs. 0 h) | Bacillales | 5.8261 | 20.965 | 5.0368 × 10−6 | 4.0295 × 10−5 |
| Lactobacillales | 9.2047 | 19.499 | 2.2913 × 10−4 | 9.1651 × 10−4 | |
| Paenibacillales | 5.1236 | 15.897 | 0.014407 | 0.023961 | |
| Rickettsiales | −6.1149 | 9.5277 | 4.668 × 10−4 | 0.0012448 | |
| Chloroplast | −4.2247 | 12.479 | 0.014975 | 0.023961 | |
| Kokua (24 h vs. 0 h) | Staphylococcales | 4.1499 | 8.6133 | 4.7759 × 10−4 | 0.0057311 |
| Pomai (24 h vs. 0 h) | Lactobacillales | 5.4469 | 21.905 | 0.0023267 | 0.016287 |
| Pseudomonadales | −5.2523 | 13.232 | 0.013722 | 0.048025 | |
| Pomai (48 h vs. 0 h) | Pseudomonadales | −5.4531 | 13.232 | 0.011211 | 0.04091 |
| Acetobacterales | −6.6853 | 8.9206 | 0.011689 | 0.04091 | |
| Lactobacillales | 3.9097 | 21.905 | 0.019219 | 0.044845 | |
| Taro (24 h vs. 0 h) | Chloroplast | −7.1425 | 18.653 | 1.5086 × 10−4 | 0.0019612 |
| Rickettsiales | −6.5874 | 14.251 | 7.3087 × 10−4 | 0.0047507 | |
| Veillonellales_Selenomon | −7.8672 | 8.2966 | 0.0038517 | 0.016691 | |
| Taro (48 h vs. 0 h) | Chloroplast | −7.0509 | 18.653 | 1.7311 × 10−4 | 0.0022504 |
| Bacillales | −7.1661 | 9.8968 | 0.016601 | 0.043163 | |
| Rickettsiales | −6.5583 | 14.251 | 7.6878 × 10−4 | 0.0049971 | |
| Veillonellales_Selenomon | −6.5424 | 8.2966 | 0.012738 | 0.0414 | |
| Acetobacterales | 6.7157 | 15.951 | 0.0064293 | 0.02786 | |
| Lactobacillales | 3.2676 | 19.818 | 0.022279 | 0.048271 |
| Poi Type | Bacteria | log2FC | logCPM | p-Values | FDR |
|---|---|---|---|---|---|
| Aloha (48 h vs. 0 h) | Erwinia | −7.3815 | 9.6925 | 3.7464 × 10−4 | 0.014986 |
| Lacticaseibacillus | 4.8789 | 9.9958 | 7.5536 × 10−4 | 0.015107 | |
| Liquorilactobacillus | 3.8105 | 16.004 | 0.0029049 | 0.038733 | |
| Ameyamaea | 4.7979 | 11.043 | 0.0054701 | 0.045588 | |
| Gluconobacter | 3.5008 | 14.47 | 0.0056986 | 0.045588 | |
| Hanalei (24 h vs. 0 h) | Not_Assigned | −4.6964 | 13.539 | 0.0017626 | 0.0098498 |
| Streptococcus | 11.659 | 19.252 | 0.001947 | 0.0098498 | |
| Paenibacillus | 7.9309 | 15.999 | 0.0026863 | 0.0098498 | |
| Bacillus | 5.5402 | 20.343 | 0.023304 | 0.042725 | |
| Leuconostoc | 3.4136 | 12.256 | 0.016102 | 0.042725 | |
| Geobacillus | −3.2936 | 14.119 | 0.022835 | 0.042725 | |
| Hanalei (48 h vs. 0 h) | Not_Assigned | −6.4615 | 13.539 | 7.0012 × 10−5 | 7.7013 × 10−4 |
| Streptococcus | 10.221 | 19.252 | 0.004495 | 0.018986 | |
| Bacillus | 5.5857 | 20.343 | 0.022453 | 0.049396 | |
| Leuconostoc | 3.4245 | 12.256 | 0.015767 | 0.043359 | |
| Geobacillus | −4.2101 | 14.119 | 0.005178 | 0.018986 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stillwell, N.; Khadka, V.S.; Nerurkar, P.V. Impact of Natural Fermentation on Mineral Composition, Resistant and Non-Resistant Starches, Microbial Diversity, and Global Metabolite Profiles in Commercial Poi from Hawai‘i. Metabolites 2025, 15, 748. https://doi.org/10.3390/metabo15110748
Stillwell N, Khadka VS, Nerurkar PV. Impact of Natural Fermentation on Mineral Composition, Resistant and Non-Resistant Starches, Microbial Diversity, and Global Metabolite Profiles in Commercial Poi from Hawai‘i. Metabolites. 2025; 15(11):748. https://doi.org/10.3390/metabo15110748
Chicago/Turabian StyleStillwell, Nyan, Vedbar S. Khadka, and Pratibha V. Nerurkar. 2025. "Impact of Natural Fermentation on Mineral Composition, Resistant and Non-Resistant Starches, Microbial Diversity, and Global Metabolite Profiles in Commercial Poi from Hawai‘i" Metabolites 15, no. 11: 748. https://doi.org/10.3390/metabo15110748
APA StyleStillwell, N., Khadka, V. S., & Nerurkar, P. V. (2025). Impact of Natural Fermentation on Mineral Composition, Resistant and Non-Resistant Starches, Microbial Diversity, and Global Metabolite Profiles in Commercial Poi from Hawai‘i. Metabolites, 15(11), 748. https://doi.org/10.3390/metabo15110748






