LC-MS/MS Detection of Tryptophan, Kynurenine, Kynurenic Acid, and Quinolinic Acid in Urine Samples from Drug-Positive and Illicit Drug-Negative Patients with a Known History of Substance Use Disorder
Abstract
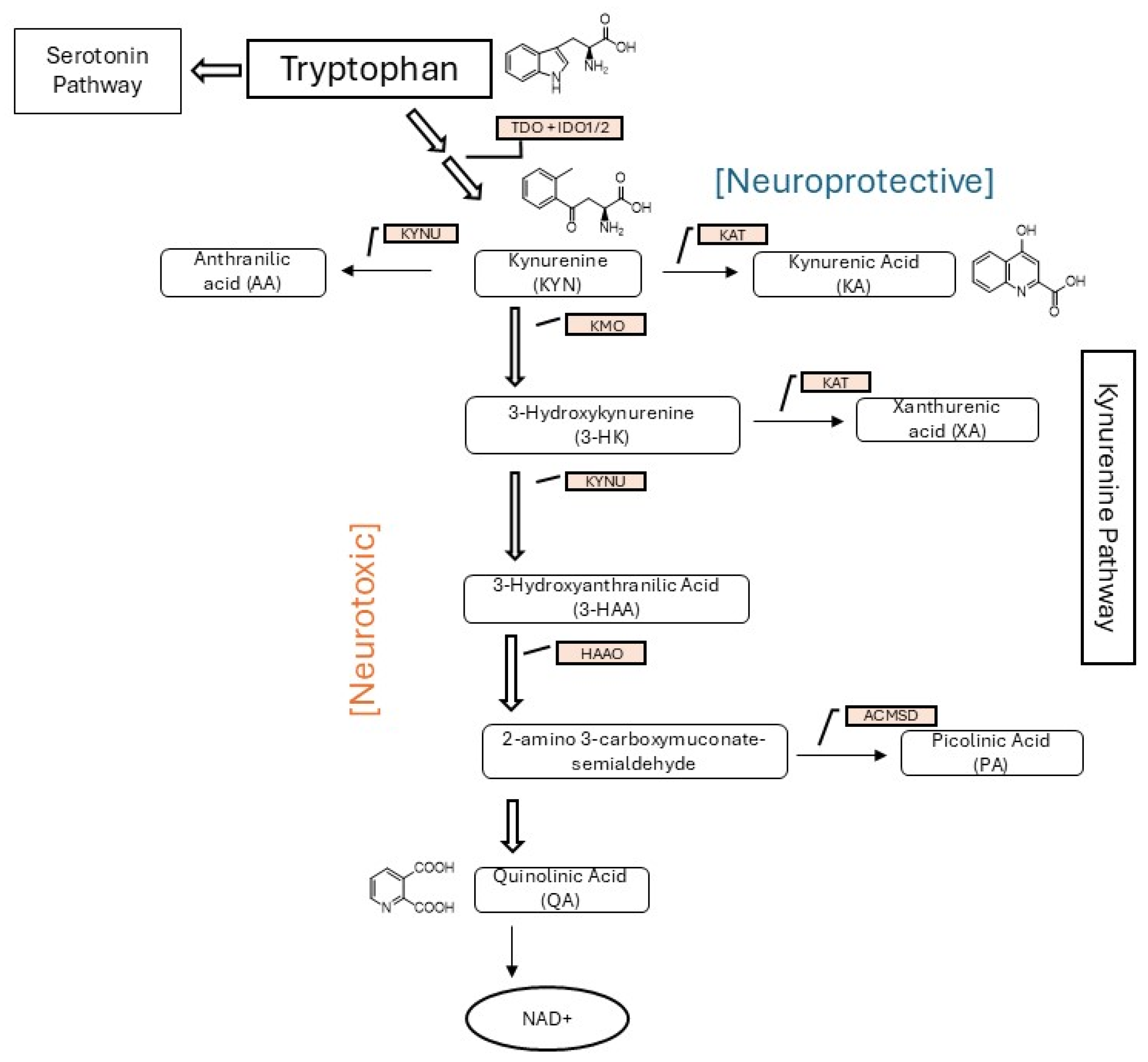
1. Introduction
2. Materials and Methods
2.1. Preparation of Standards
2.2. Extraction Procedure
2.3. Analyte Separation and Instrument Acquisition Parameters
2.4. Samples
2.5. Method Validation
2.5.1. Percent of Expected Concentration and Precision
2.5.2. Linearity
2.5.3. Analytical Sensitivity
2.5.4. Carry-Over
2.5.5. Matrix Effects
2.5.6. Interference Assessment
2.5.7. Stability
2.5.8. Data Analysis
3. Results
3.1. Percent of Expected Concentration and Precision
3.2. Linearity
3.3. Analytical Sensitivity
3.4. Carry-Over
3.5. Matrix Effects
3.6. Interference Assessment
3.7. Stability
3.8. Clinical Performance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Laing, R.; Donnelly, C.A. Evolution of an epidemic: Understanding the opioid epidemic in the United States and the impact of the COVID-19 pandemic on opioid-related mortality. PLoS ONE 2024, 19, e0306395. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Sun, L.; Yin, X.; Chen, H.; Yang, L.; Yang, X. Burden of drug use disorders in the United States from 1990 to 2021 and its projection until 2035: Results from the GBD study. BMC Public Health 2024, 24, 1639. [Google Scholar] [CrossRef] [PubMed]
- Morales-Puerto, N.; Giménez-Gómez, P.; Pérez-Hernández, M.; Abuin-Martínez, C.; de Biedma-Elduayen, L.G.; Vidal, R.; Gutiérrez-López, M.D.; O’Shea, E.; Colado, M.I. Addiction and the kynurenine pathway: A new dancing couple? Pharmacol. Ther. 2021, 223, 107807. [Google Scholar] [CrossRef] [PubMed]
- Savitz, J. The kynurenine pathway: A finger in every pie. Mol. Psychiatry 2020, 25, 131–147. [Google Scholar] [CrossRef] [PubMed]
- Kirby, L.G.; Zeeb, F.D.; Winstanley, C.A. Contributions of serotonin in addiction vulnerability. Neuropharmacology 2011, 61, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Müller, C.P.; Homberg, J.R. The role of serotonin in drug use and addiction. Behav. Brain Res. 2015, 277, 146–192. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.; Gray, J.A.; Roth, B.L. The expanded biology of serotonin. Annu. Rev. Med. 2009, 60, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Nair, M.P.N.; Schwartz, S.A.; Mahajan, S.D.; Tsiao, C.; Chawda, R.P.; Whitney, R.; Sykes, B.B.D.; Hewitt, R. Drug abuse and neuropathogenesis of HIV infection: Role of DC-SIGN and IDO. J. Neuroimmunol. 2004, 157, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Donlon, J.; Kumari, P.; Varghese, S.P.; Bai, M.; Florentin, O.D.; Frost, E.D.; Banks, J.; Vadlapatla, N.; Kam, O.; Shad, M.U.; et al. Integrative Pharmacology in the Treatment of Substance Use Disorders. J. Dual Diagn. 2024, 20, 132–177. [Google Scholar] [CrossRef] [PubMed]
- Gunn, J.; Hill, M.M.; Cotten, B.M.; Deer, T.R. An Analysis of Biomarkers in Patients with Chronic Pain. Pain Physician 2020, 23, E41–E49. [Google Scholar] [CrossRef] [PubMed]
- CLSI. Liquid Chromatography-Mass Spectrometry Methods C62, 2nd ed.; CLSI: Berwyn, PA, USA, 2022. [Google Scholar]
- Food and Drug Administration. Bioanalytical Method Validation: Guidance for Industry; FDA: Silver Spring, MD, USA, 2018. [Google Scholar]
- Matuszewski, B.K.; Constanzer, M.L.; Chavez-Eng, C.M. Strategies for the assessment of matrix effect in quantitative bioanalytical methods based on HPLC-MS/MS. Anal. Chem. 2003, 75, 3019–3030. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.S.; Seo, H.S.; Kim, K.H.; Pyo, H.; Chung, B.C.; Lee, J. Urinary Profiling of Tryptophan and Its Related Metabolites in Patients with Metabolic Syndrome by Liquid Chromatography–Electrospray Ionization/Mass Spectrometry. Anal. Bioanal. Chem. 2017, 409, 5501–5512. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Geng, X. Research progress on the kynurenine pathway in the prevention and treatment of Parkinson’s disease. J. Enzym. Inhib. Med. Chem. 2023, 38, 2225800. [Google Scholar] [CrossRef] [PubMed]
- Pearson, K.; Beier, K.; Mardis, T.; Munoz, B.; Zaidi, A. The Neurochemistry of Depression: The Good, The Bad and The Ugly. Mo. Med. 2024, 121, 68–75. [Google Scholar] [PubMed]
- Maciejczyk, M.; Ptaszyńska-Sarosiek, I.; Niemcunowicz-Janica, A.; Szeremeta, M.; Waszkiewicz, N.; Kułak-Bejda, A.; Cwalina, U.; Nesterowicz, M.; Zalewska, A. Do Circulating Redox Biomarkers Have Diagnostic Significance in Alcohol-Intoxicated People? Int. J. Mol. Sci. 2022, 23, 11808. [Google Scholar] [CrossRef] [PubMed]
- Mittal, A.; Dabur, R. Detection of New Human Metabolic Urinary Markers in Chronic Alcoholism and Their Reversal by Aqueous Extract of Tinospora cordifolia Stem. Alcohol Alcohol. 2015, 50, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; Dabur, R. Protective Effects of Tinospora cordifolia on Hepatic and Gastrointestinal Toxicity Induced by Chronic and Moderate Alcoholism. Alcohol Alcohol. 2016, 51, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Gowda, S.; Desai, P.B.; Kulkarni, S.S.; Hull, V.V.; Math, A.A.K.; Vernekar, S.N. Markers of renal function tests. N. Am. J. Med. Sci. 2010, 2, 170–173. [Google Scholar] [PubMed]

| TRP | KYN | KA | QA | TRP-D5 | KYN-D4 | KA-D5 | QA-D3 | |
|---|---|---|---|---|---|---|---|---|
| CAS # | 73-22-3 | 2922-83-0 | 492-27-3 | 89-00-9 | 62595-11-3 | 2672568-86-2 | 350820-13-2 | 138946-42-6 |
| Molecular Weight | 204.23 | 208.21 | 189.17 | 167.12 | 209.26 | 212.2 | 194.2 | 170.14 |
| Precursor ion [m/z] | 205.2 | 209.2 | 190.2 | 168.1 | 210.3 | 213.2 | 195.2 | 171.1 |
| Quantifier Ion [m/z] | 188 | 192 | 144 | 124 | 192.1 | 96.1 | 149 | 153 |
| Qualifier Ion [m/z] | 118 | 94.1 | 89 | 150 | - | - | - | - |
| Collision Energy [eV] | 5 | 5 | 17 | 5 | 5 | 5 | 17 | 5 |
| Retention Time (RT) [Min] | 3.318 | 2.447 | 3.535 | 1.007 | 3.29 | 2.411 | 3.459 | 0.97 |
| Low QC | High QC | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ng/mL | ME% | Extraction Recovery (%) | % of Expected Concentration | CV% | Accuracy (%) | CV% | ||||||||
| Analyte | R2 | Weight | LOD | Linear Range | Low QC | High QC | Intra-Day | Inter-Day | Intra-Day | Inter-Day | ||||
| Tryptophan | 0.997 | 1/x | 6 | 98 to 100,000 | 1500 | 10,000 | 295% | 106% | 9.60% | 6.34% | 4.55% | 5.50% | 2.12% | 10.25% |
| Kynurenine | 0.999 | 1/x | 1 | 3 to 3000 | 200 | 2500 | 332% | 107% | −11.10% | 1.17% | 6.88% | −2.80% | 4.49% | 8.44% |
| Kynurenic Acid | 0.999 | 1/x | 1 | 7 to 7200 | 500 | 7000 | 102% | 97% | −0.90% | 1.55% | 6.48% | −1.10% | 3.33% | 8.45% |
| Quinolinic Acid | 0.998 | 1/x | 4 | 63 to 64,000 | 625 | 5000 | 315% | 100% | 1.50% | 9.32% | 12.46% | −2.70% | 2.41% | 4.84% |
| Kynurenine | ng/mL | RF | RT | Tryptophan | ng/mL | RF | RT | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample | Initial Run | RT | RF | % Bias | % Bias | Sample | Initial Run | RT | RF | % Bias | % Bias |
| Sample 1 | 4459 | 3907 | 3976 | −11% | −12% | Sample 1 | 12,991 | 14,697 | 13,246 | 2% | 13% |
| Sample 2 | 943 | 923 | 925 | −2% | −2% | Sample 2 | 1611 | 1652 | 1602 | −1% | 3% |
| Sample 3 | 3356 | 3416 | 3433 | 2% | 2% | Sample 3 | 6504 | 6619 | 6596 | 1% | 2% |
| Sample 4 | 482 | 485 | 465 | −3% | 1% | Sample 4 | 21,940 | 22,405 | 22,843 | 4% | 2% |
| Sample 5 | 5921 | 5830 | 5803 | −2% | −2% | Sample 5 | 65 | 69 | 62 | −5% | 6% |
| Sample 6 | 6874 | 6745 | 6717 | −2% | −2% | Sample 6 | 4908 | 4621 | 4616 | −6% | −6% |
| Sample 7 | 952 | 967 | 1004 | 5% | 2% | Sample 7 | 38,604 | 41,220 | 40,419 | 5% | 7% |
| Sample 8 | 147 | 148 | 145 | −1% | 1% | Sample 8 | 13,030 | 12,460 | 12,833 | −2% | −4% |
| Sample 9 | 151 | 147 | 148 | −2% | −2% | Sample 9 | 2654 | 2672 | 2669 | 1% | 1% |
| Sample 10 | 806 | 802 | 779 | −3% | −1% | Sample 10 | 20 | 20 | 20 | 4% | 5% |
| Kynurenic Acid | RF | RT | Quinolinic Acid | RF | RT | ||||||
| Sample | Initial Run | RT | RF | % Bias | % Bias | Sample | Initial Run | RT | RF | % Bias | % Bias |
| Sample 1 | 8570 | 6737 | 6718 | −22% | −21% | Sample 1 | 3709 | 2883 | 2978 | −20% | −22% |
| Sample 2 | 4479 | 4458 | 4446 | −1% | 0% | Sample 2 | 1930 | 1617 | 1600 | −17% | −16% |
| Sample 3 | 785 | 778 | 796 | 1% | −1% | Sample 3 | 2821 | 2234 | 2253 | −20% | −21% |
| Sample 4 | 10,853 | 9337 | 8854 | −18% | −14% | Sample 4 | 6107 | 6063 | 5925 | −3% | −1% |
| Sample 5 | 1208 | 1179 | 1189 | −2% | −2% | Sample 5 | 13,052 | 12,027 | 14,536 | 11% | −8% |
| Sample 6 | 6260 | 6289 | 6300 | 1% | 0% | Sample 6 | 8023 | 7037 | 6415 | −20% | −12% |
| Sample 7 | 3861 | 3836 | 3906 | 1% | −1% | Sample 7 | 3321 | 3409 | 3403 | 2% | 3% |
| Sample 8 | 2403 | 2391 | 2346 | −2% | −1% | Sample 8 | 1219 | 1223 | 1199 | −2% | 0% |
| Sample 9 | 667 | 662 | 663 | 0% | −1% | Sample 9 | 8656 | 8569 | 8299 | −4% | −1% |
| Sample 10 | 427 | 417 | 421 | −1% | −2% | Sample 10 | 6460 | 5084 | 5179 | −20% | −21% |
| Illicit Drug-Negative | Drug-Positive | |||||||
|---|---|---|---|---|---|---|---|---|
| TRP | KYN | KA | QA | TRP | KYN | KA | QA | |
| Mean | 3.64 | 2.66 | 3.61 | 4.58 | 6.27 | 1.02 | 2.89 | 13.41 |
| Median | 1.49 | 0.547 | 2.212 | 4.086 | 5.75 | 0.674 | 2.518 | 5.116 |
| Minimum | 0.03 | 0.04 | 0.68 | 0.31 | 0.01 | 0.03 | 0.22 | 1.93 |
| Maximum | 19.87 | 40.17 | 67.88 | 11.49 | 23.7 | 26.16 | 17.34 | 429.24 |
| Standard Deviation | 4.39 | 4.72 | 8.53 | 2.18 | 4.75 | 2.54 | 2.31 | 46.6 |
| Compound | Reference Interval (µg/mg) | % Illicit Drug Neg | % Drug Pos | Reference |
|---|---|---|---|---|
| TRP | 8.38 +/− 5.35 | 6% | 6% | [14] * |
| KYN | 0.63 +/− 1.09 | 11% | 35% | [14] * |
| KA | 10.04 +/− 8.44 | 25% | 26% | [14] * |
| QA | 0.0–6.3 | 21% | 36% | [10] # |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Contella, L.; Farrell, C.L.; Boccuto, L.; Litwin, A.H.; Flanagan, H.; Melanson, S.E.F.; Tolan, N.V.; Snyder, M.L.; Greene, D.N. LC-MS/MS Detection of Tryptophan, Kynurenine, Kynurenic Acid, and Quinolinic Acid in Urine Samples from Drug-Positive and Illicit Drug-Negative Patients with a Known History of Substance Use Disorder. Metabolites 2025, 15, 749. https://doi.org/10.3390/metabo15110749
Contella L, Farrell CL, Boccuto L, Litwin AH, Flanagan H, Melanson SEF, Tolan NV, Snyder ML, Greene DN. LC-MS/MS Detection of Tryptophan, Kynurenine, Kynurenic Acid, and Quinolinic Acid in Urine Samples from Drug-Positive and Illicit Drug-Negative Patients with a Known History of Substance Use Disorder. Metabolites. 2025; 15(11):749. https://doi.org/10.3390/metabo15110749
Chicago/Turabian StyleContella, Lindsey, Christopher L. Farrell, Luigi Boccuto, Alain H. Litwin, Hunter Flanagan, Stacy E. F. Melanson, Nicole V. Tolan, Marion L. Snyder, and Dina N. Greene. 2025. "LC-MS/MS Detection of Tryptophan, Kynurenine, Kynurenic Acid, and Quinolinic Acid in Urine Samples from Drug-Positive and Illicit Drug-Negative Patients with a Known History of Substance Use Disorder" Metabolites 15, no. 11: 749. https://doi.org/10.3390/metabo15110749
APA StyleContella, L., Farrell, C. L., Boccuto, L., Litwin, A. H., Flanagan, H., Melanson, S. E. F., Tolan, N. V., Snyder, M. L., & Greene, D. N. (2025). LC-MS/MS Detection of Tryptophan, Kynurenine, Kynurenic Acid, and Quinolinic Acid in Urine Samples from Drug-Positive and Illicit Drug-Negative Patients with a Known History of Substance Use Disorder. Metabolites, 15(11), 749. https://doi.org/10.3390/metabo15110749







