Antifouling Lipids from Marine Fungi of the Beibu Gulf
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Material and Lipid Extraction
2.2. Anti-Fouling Bacterial Activity Assay
2.3. Anti-Barnacle Cyprid Larval Settlement Assay
2.4. Antifouling Marine Field Trial
2.5. GC-MS Analysis
2.6. Statistical Analysis
2.7. Ethics Statement
3. Results
3.1. Anti-Fouling Bacterial Activity
3.2. Anti-Barnacle Cyprid Larval Settlement Activity
3.3. Antifouling Marine Field Trial
3.4. Chemical Composition of Selected Lipids Fractions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, C.; Huang, S.Y.; Wang, S.K.; Jiang, B.; Li, H.F.; Wang, K.L. Antifouling natural products from marine fungi. Mycosystema 2021, 40, 1241–1258. [Google Scholar]
- Yan, T.; Li, Z.F.; Hu, L.F.; Li, X.; Cao, W.H.; Luo, W.J.; Cheng, Z.Q. A review on the balanomorph barnacles in the coastal waters of China. Acta Ecol. Sin. 2012, 32, 5230–5241. [Google Scholar] [CrossRef]
- Liu, X.B.; Zou, L.; Li, B.Q.; Martino, P.D.; Rutschof, D.; Yang, J.L.; Maki, J.; Liu, W.; Gu, J.D. Chemical signaling in biofilm-mediated biofouling. Nat. Chem. Biol. 2024, 20, 1406–1419. [Google Scholar] [CrossRef]
- Schultz, M.P.; Bendick, J.A.; Holm, E.R.; Hertel, W.M. Economic impact of biofouling on a naval surface ship. Biofouling 2011, 27, 87–98. [Google Scholar] [CrossRef]
- Demirel, Y.K.; Uzun, D.; Zhang, Y.; Fang, H.C.; Day, A.H.; Turan, O. Effect of barnacle fouling on ship resistance and powering. Biofouling 2017, 33, 819–834. [Google Scholar] [CrossRef] [PubMed]
- He, X.Y.; Bai, X.Q.; Yuan, C.Q.; Ren, K. Research progress of nanomaterial-based coatings for marine antifouling applications. Chin. Surf. Eng. 2023, 36, 37–51. [Google Scholar]
- Fernandes, J.A.; Santos, L.; Vance, T.; Fileman, T.W.; Smith, D.; Bishop, J.D.D.; Viard, F.; Queirós, A.M.; Merino, G.; Buisman, E.; et al. Costs and benefits to European shipping of ballast-water and hull-fouling treatment: Impacts of native and non-indigenous species. Mar. Policy 2016, 64, 148–155. [Google Scholar] [CrossRef]
- Szeto, W.; Leung, M.K.H.; Leung, D.Y.C. Recent developments of titanium dioxide materials for aquatic antifouling application. J. Mar. Sci. Technol. 2021, 26, 301–321. [Google Scholar] [CrossRef]
- Jin, H.C.; Tian, L.M.; Bing, W.; Zhao, J.; Ren, L. Bioinspired marine antifouling coatings: Status, prospects, and future. Prog. Mater. Sci. 2022, 124, 100889. [Google Scholar] [CrossRef]
- Miftah, J.A.; Hanif, N.; Tan, L.T.; Muljani, S.; Mubarik, N.R. The next generation of integrated eco-friendly anti-biofouling and anti-biocorrosion marine natural products. Chem. Biodivers. 2025, e02263. [Google Scholar] [CrossRef]
- Varrella, S.; Barone, G.; Corinaldesi, C.; Giorgetti, A.; Nomaki, H.; Nunoura, T.; Rastelli, E.; Tangherlini, M.; Danovaro, R.; Dell’Anno, A. Fungal abundance and diversity in the Mariana Trench, the deepest ecosystem on earth. J. Fungi 2024, 16, 73. [Google Scholar] [CrossRef]
- Ghattavi, S.; Homaei, A.; Fernandes, P. Marine natural products for biofouling elimination in marine environments. Biocatal. Agric. Biotechnol. 2024, 61, 103385. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Xu, X.Y.; Peng, J.; Ma, C.F.; Nong, X.H.; Bao, J.; Zhang, G.Z.; Qi, S.H. Antifouling potentials of eight deep-sea-derived fungi from the South China Sea. J. Ind. Microbiol. Biotechnol. 2014, 41, 741–748. [Google Scholar] [CrossRef]
- Liu, L.L.; Wu, C.H.; Qian, P.Y. Marine natural products as antifouling molecules—A mini-review (2014–2020). Biofouling 2020, 36, 1210–1226. [Google Scholar] [CrossRef] [PubMed]
- Morgan, R.N.; Ali, A.A.; Alshahrani, M.Y.; Aboshanab, K.M. New insights on biological activities, chemical compositions, and classifications of marine actinomycetes antifouling agents. Microorganisms 2023, 11, 2444. [Google Scholar] [CrossRef]
- Bhattarai, H.D.; Ganti, V.S.; Paudel, B.; Lee, Y.; Lee, H.K.; Hong, Y.; Shin, H.W. Isolation of antifouling compounds from the marine bacterium, Shewanella oneidensis SCH0402. World J. Microbiol. Biotechnol. 2007, 23, 243–249. [Google Scholar] [CrossRef]
- Escobar, A.; Pérez, M.; Sathicq, A.; García, M.; Paola, A.; Romanelli, G.; Blustein, G. Alkyl 2-furoates obtained by green chemistry procedures as suitable new antifoulants for marine protective coatings. J. Coat. Technol. Res. 2019, 16, 159–166. [Google Scholar] [CrossRef]
- Chu, C.I.; Ouyang, D.J.; Chen, R.K.; Chen, Q.V.; Liang, H.Y.; Li, Q.S.; Jin, L. Study on the antibacterial activity of the extract of Melscope pielcifolia. Shandong Chem. Ind. 2024, 53, 30–32, 36. [Google Scholar]
- Takamura, H.; Yorisue, T.; Tanaka, K.; Kadota, I. Antifouling activity of xylemin, its structural analogs, and related polyamines. Chem. Biodivers. 2025, 22, e202403213. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.; Han, S.S.; Wang, J.J.; Lin, H.S.; Cao, W.H. An overview of fouling ascidians. Acta Ecol. Sin. 2017, 37, 6647–6655. [Google Scholar] [CrossRef]
- Qiu, Q.; Gu, Y.; Ren, Y.; Ding, H.; Hu, C.; Wu, D.; Mou, J.; Wu, Z.; Dai, D. R Research progress on eco-friendly natural antifouling agents and their antifouling mechanisms. Chem. Eng. J. 2024, 495, 153638. [Google Scholar] [CrossRef]
- Dafforn, K.A.; Lewis, J.A.; Johnston, E.L. Antifouling strategies: History and regulation, ecological impacts and mitigation. Mar. Pollut. Bull. 2011, 62, 453–465. [Google Scholar] [CrossRef]
- Chen, L.; Lam, J.C.W. SeaNine 211 as antifouling biocide: A coastal pollutant of emerging concern. J. Environ. Sci. 2017, 61, 68–79. [Google Scholar] [CrossRef]
- Konstantinou, I.K.; Albanis, T.A. Worldwide occurrence and effects of antifouling paint booster biocides in the aquatic environment: A review. Environ. Int. 2004, 30, 235–248. [Google Scholar] [CrossRef]
- Gao, M.; Wang, K.; Su, R.G.; Li, X.Z.; Lu, W. Antifouling potential of bacteria isolated from a marine biofilm. J. Ocean Univ. China 2014, 13, 799–804. [Google Scholar] [CrossRef]
- Martín-Rodríguez, A.J.; Reyes, F.; Martín, J.; Pérez-Yépez, J.; León-Barrios, M.; Couttolenc, A.; Espinoza, C.; Trigos, A.; Martín, V.S.; Norte, M.; et al. Inhibition of bacterial quorum sensing by extracts from aquatic fungi: First report from marine endophytes. Mar. Drugs 2014, 12, 5503–5526. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Qian, P.Y. Review on molecular mechanisms of antifouling compounds: An update since 2012. Mar. Drugs 2017, 15, 264. [Google Scholar] [CrossRef]
- Hao, R.; Li, T.; Zhou, X.; Guo, W.; Liu, J.; Guo, L.; Wang, Y. Strategies and challenges in marine antifouling coatings: Current innovations and future outlook. J. Coat. Technol. Res. 2025. [Google Scholar] [CrossRef]


| No. | Strain Number | Species | Source |
|---|---|---|---|
| 1 | GXIMD00527 | Fusarium incarnatum | Beihai Zhulin salt field |
| 2 | GXIMD00543 | Aspergillus carneus | Sponge, Weizhou Island, Beihai |
| 3 | GXIMD00545 | Curvularia lunata | Beihai seawater |
| 4 | GXIMD00533 | Cladosporium cladosporioides | Beihai Zhulin salt field |
| 5 | GXIMD00547 | Trichoderma brevicompactum | Qinzhou seawater |
| 6 | GXIMD00548 | Aspergillus iizukae | Beihai Zhulin salt field |
| 7 | GXIMD00519 | Aspergillus carneus | Coral, Weizhou Island, Beihai |
| 8 | GXIMD00544 | Aspergillus fumigatus | Beihai Zhulin salt field |
| 9 | GXIMD00541 | Fusarium chlamydosporum | Beihai Zhulin salt field |
| 10 | GXIMD00502 | Aspergillus niger | Coral, Weizhou Island, Beihai |
| Fungal Lipid Fraction | Strain Number | VR | VP | MJ | AM |
|---|---|---|---|---|---|
| 1 | GXIMD00519 | 8.19 ± 0.75 | — b | — | 6.90 ± 0.54 |
| 2 | GXIMD00541 | 7.00 ± 0.22 | — | — | — |
| 3 | GXIMD00545 | 8.08 ± 0.37 | 7.21 ± 0.11 | 7.15 ± 0.33 | 7.99 ± 0.30 |
| 4 | GXIMD00502 | — | 6.47 ± 0.23 | — | — |
| 5 | GXIMD00547 | — | — | — | — |
| 6 | GXIMD00543 | 7.58 ± 0.71 | — | 7.73 ± 0.21 | 6.66 ± 0.38 |
| 7 | GXIMD00544 | — | 7.62 ± 0.22 | — | — |
| 8 | GXIMD00527 | — | 6.84 ± 0.02 | — | — |
| 9 | GXIMD00548 | — | — | 7.24 ± 0.12 | 6.34 ± 0.06 |
| 10 | GXIMD00533 | — | — | — | — |
| Positive control | Penicillin | 18.16 ± 0.11 | 12.45 ± 0.22 | 17.18 ± 0.04 | 9.47 ± 0.07 |
| Chloramphenicol | 14.88 ± 0.05 | — | 16.23 ± 0.14 | — |
| Strain Number | EC50 (μg/mL) a | LC50 (μg/mL) a |
|---|---|---|
| GXIMD00543 | 5.50 ± 0.25 | >50 |
| GXIMD00541 | 1.81 ± 0.23 | >50 |
| GXIMD00533 | 13.92 ± 0.41 | >50 |
| GXIMD00527 | 0.23 ± 0.13 | >50 |
| GXIMD00548 | 0.21 ± 0.13 | >50 |
| GXIMD00519 | 0.59 ± 0.27 | 50 |
| GXIMD00547 | 3.89 ± 0.32 | >50 |
| seaNine-211 | 1.95 ± 0.05 | 33.93 ± 2.06 |
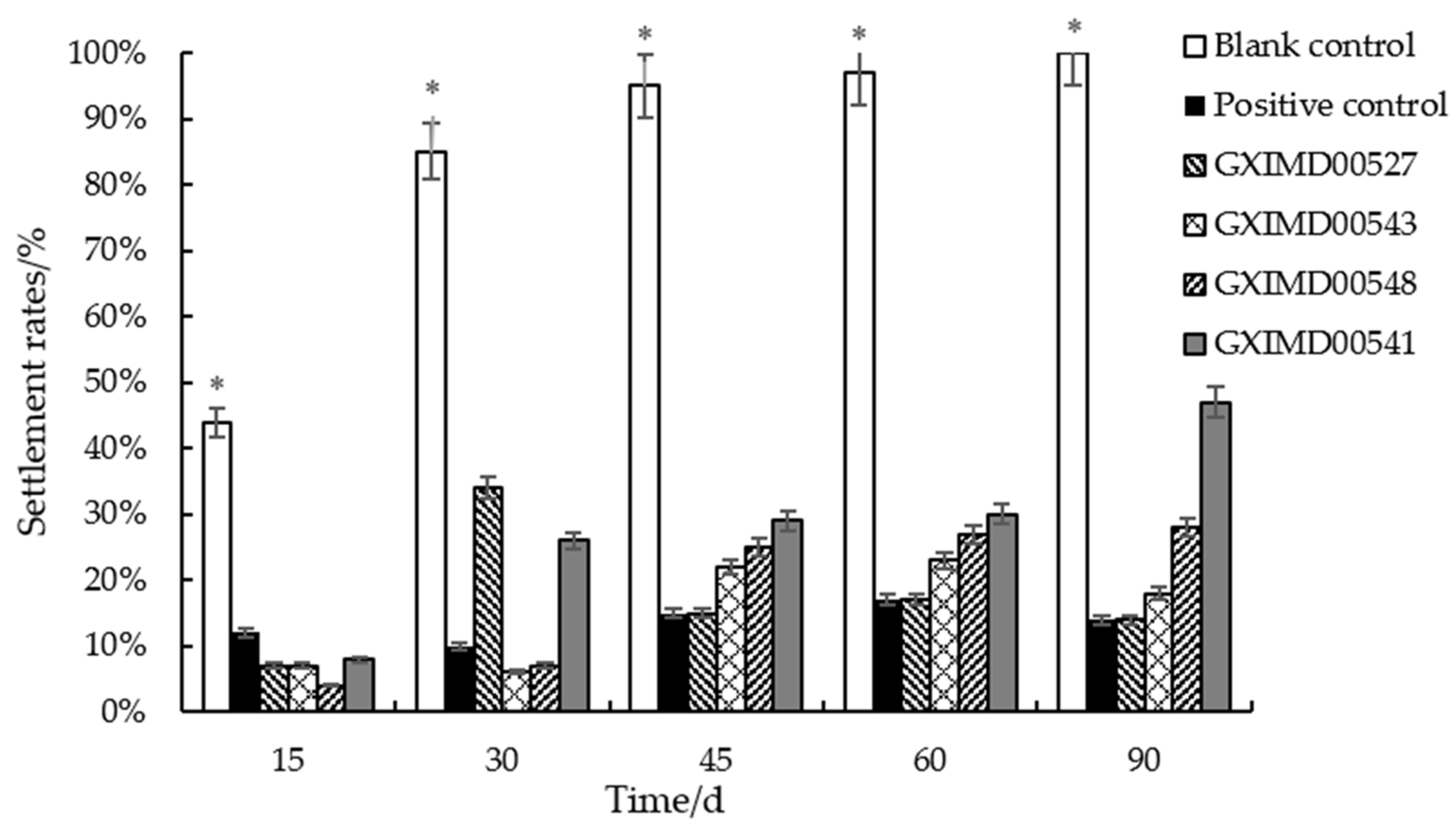
| Sample Number | Settlement Rates (%) | ||||
|---|---|---|---|---|---|
| 15 d | 30 d | 45 d | 60 d | 90 d | |
| GXIMD00543 | 7 | 6 | 22 | 23 | 18 |
| GXIMD00541 | 8 | 26 | 29 | 30 | 47 |
| GXIMD00533 | 27 | 48 | 67 | 68 | 49 |
| GXIMD00527 | 7 | 43 | 15 | 17 | 20 |
| GXIMD00548 | 4 | 7 | 25 | 27 | 28 |
| GXIMD00519 | 9 | 15 | 23 | 19 | 72 |
| GXIMD00547 | 10 | 17 | 55 | 60 | 57 |
| Positive control | 12 | 10 | 15 | 17 | 14 |
| Blank control | 44 | 85 | 95 | 97 | 100 |
| Compound | Molecular Formula | Relative Content (%) | |||
|---|---|---|---|---|---|
| GXIMD00527 | GXIMD00548 | GXIMD00543 | |||
| 1 | 2,4-Di-tert-butylphenol | C14H22O | — a | 1.47 | 0.31 |
| 2 | Methyl palmitate | C17H34O2 | 2.73 | 2.33 | 19.35 |
| 3 | Palmitic acid | C16H32O2 | 28.35 | 19.59 | 7.9 |
| 4 | Methyl 9,10-octadecadienoate | C19H34O2 | — | 2.65 | — |
| 5 | Methyl 9-octadecenoate | C19H36O2 | 1.38 | 1.77 | — |
| 6 | Methyl 8-methyl nonanoate | C11H22O2 | — | 1.27 | — |
| 7 | Linoleic acid | C18H32O2 | — | 67.48 | — |
| 8 | 2,5-Di-tert-butylphenol | C14H22O | 1.12 | — | — |
| 9 | Ethyl palmitate | C18H36O2 | 0.6 | — | — |
| 10 | Methyl linoleate | C19H34O2 | 1.7 | — | — |
| 11 | Methyl stearate | C19H38O2 | 1.43 | — | 3.67 |
| 12 | Dodecyl-9-alkynyl chloroacetate | C14H23ClO2 | 34.8 | — | 17.19 |
| 13 | cis-13-Octadecenoic acid | C18H34O2 | 15.11 | — | — |
| 14 | Stearic acid | C18H36O2 | 8.96 | — | 0.86 |
| 15 | Butyl palmitate | C20H40O2 | 1.5 | — | 0.72 |
| 16 | 2-Chloroethyl linoleate | C20H35ClO2 | 0.72 | — | — |
| 17 | 2,2,2-Trifluoroethanol | C20H35F3O2 | 0.52 | — | — |
| 18 | Butyl octadecanoate | C22H44O2 | 0.54 | — | — |
| 19 | Oleic acid | C18H34O2 | — | — | 3.66 |
| 20 | Methyl 12-methyltridecanoate | C15H30O2 | — | — | 0.76 |
| 21 | Methyl pentadecanoate | C16H32O2 | — | — | 0.31 |
| 22 | Methyl 11,14-octadetadienoate | C19H34O2 | — | — | 28.17 |
| 23 | (E)-9-Octadecenoic acid methyl ester | C19H36O2 | — | — | 14.5 |
| 24 | Phenazocine | C22H27NO | — | — | 1.02 |
| 25 | Ethyl linoleate | C20H36O2 | — | — | 0.77 |
| 26 | 2-Hydroxy-1-(hydroxymethyl)ethyl ester cetanoate | C19H38O4 | — | — | 0.41 |
| Total identified | 99.46 | 95.56 | 99.60 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, M.; Jiang, W.; Huang, H.; Lu, L.; Su, Z.; Luo, X.; Gao, C.; Liu, Y.; Xu, X. Antifouling Lipids from Marine Fungi of the Beibu Gulf. Metabolites 2025, 15, 721. https://doi.org/10.3390/metabo15110721
Qi M, Jiang W, Huang H, Lu L, Su Z, Luo X, Gao C, Liu Y, Xu X. Antifouling Lipids from Marine Fungi of the Beibu Gulf. Metabolites. 2025; 15(11):721. https://doi.org/10.3390/metabo15110721
Chicago/Turabian StyleQi, Mengfan, Wang Jiang, Huaqing Huang, Lu Lu, Zhiwei Su, Xiaowei Luo, Chenghai Gao, Yonghong Liu, and Xinya Xu. 2025. "Antifouling Lipids from Marine Fungi of the Beibu Gulf" Metabolites 15, no. 11: 721. https://doi.org/10.3390/metabo15110721
APA StyleQi, M., Jiang, W., Huang, H., Lu, L., Su, Z., Luo, X., Gao, C., Liu, Y., & Xu, X. (2025). Antifouling Lipids from Marine Fungi of the Beibu Gulf. Metabolites, 15(11), 721. https://doi.org/10.3390/metabo15110721








