A Pilot Study on the Effects of Sweet Potato Petiole and Leaf Powder on Gut Microbiota and Aging-Related Biomarkers in an Aged Microminipig Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Experimental Protocols
2.3. Gut Microbiota Analysis from Fecal Samples
2.4. Analysis of Senescence-Associated Cells in Peripheral Blood
3. Results
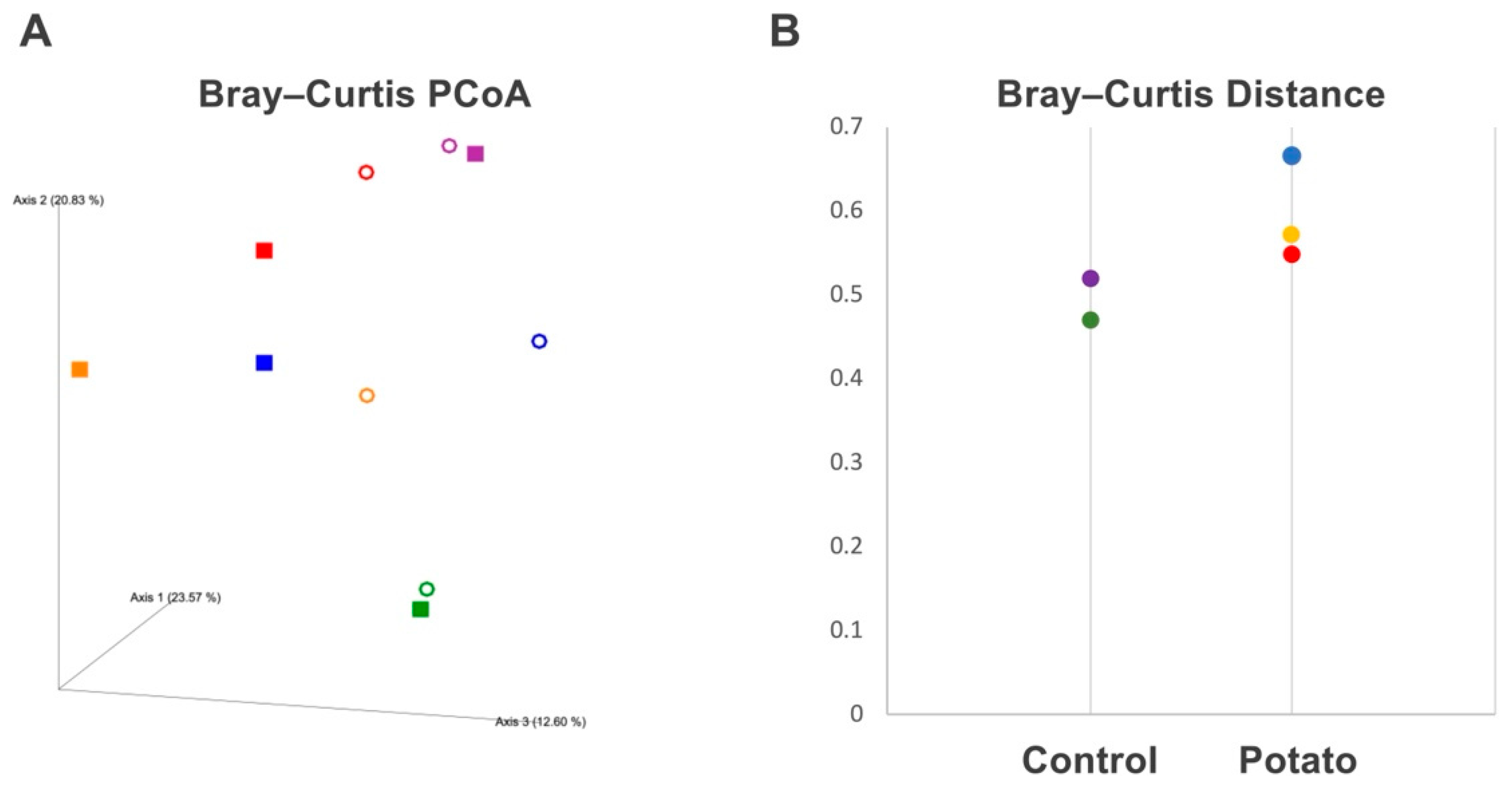
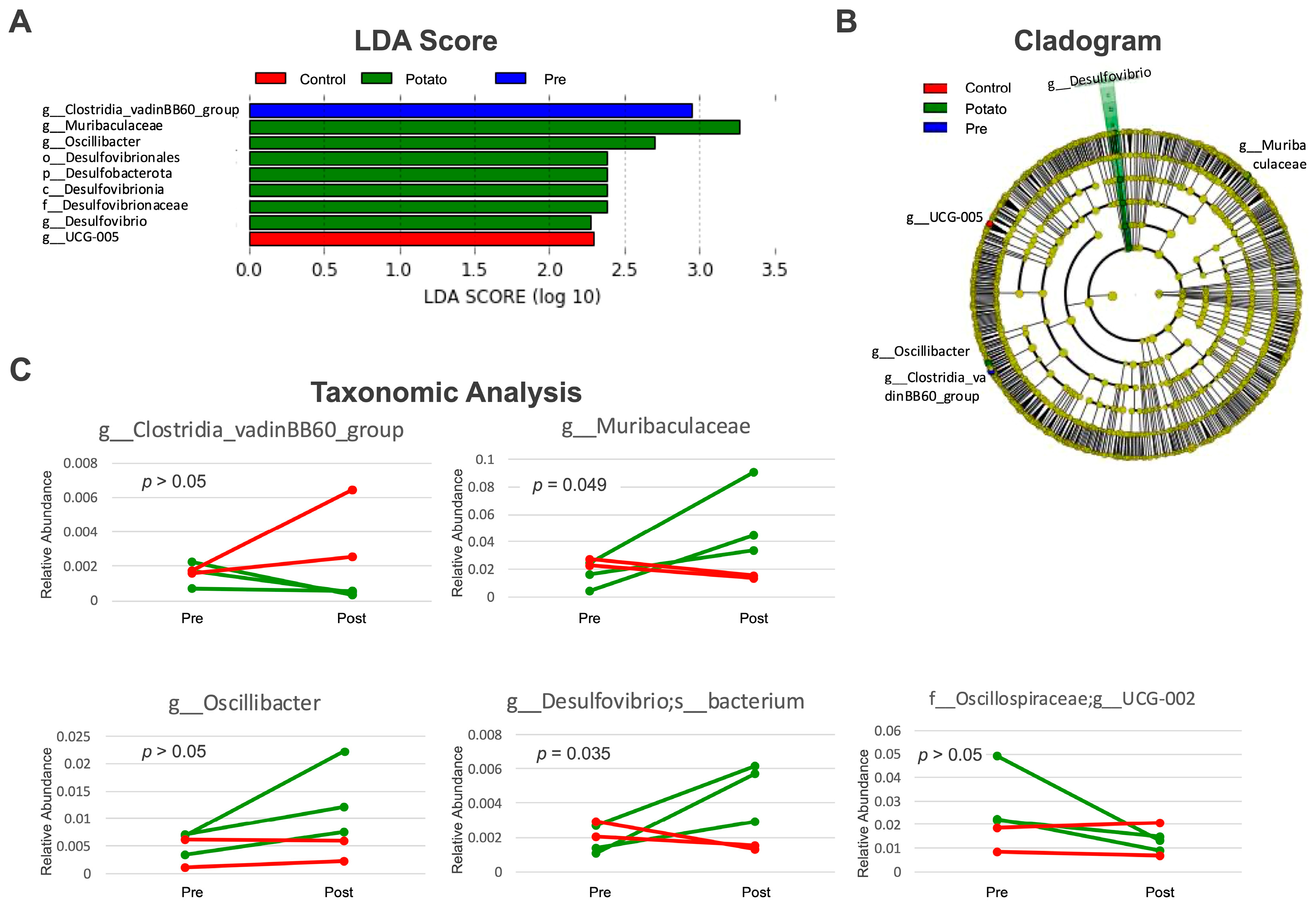
3.1. Gut Microbiota Changes Induced by Sweet Potato Petiole and Leaf
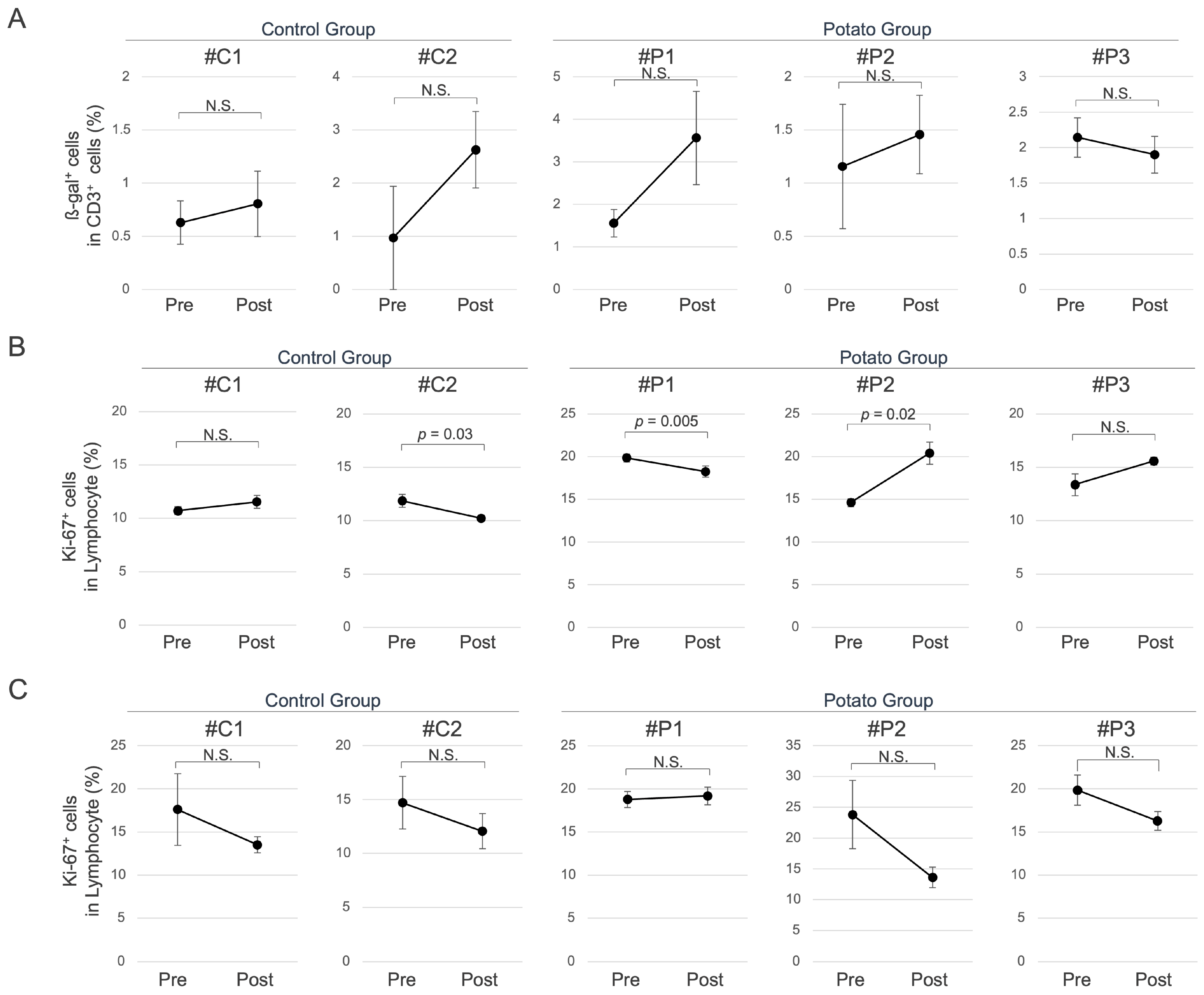
3.2. Senescence-Associated Cellular Changes in Peripheral Blood Induced by Sweet Potato Petiole and Leaf
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nakatani, H. Ageing and shrinking population: The looming demographic challenges of super-aged and super-low fertility society starting from Asia. Glob. Health Med. 2023, 5, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Guillemot, J.R.; Zhang, X.; Warner, M.E. Population Aging and Decline Will Happen Sooner Than We Think. Soc. Sci. 2024, 13, 190. [Google Scholar] [CrossRef]
- Bloom, D.E.; Luca, D.L. Chapter 1—The Global Demography of Aging: Facts, Explanations, Future. In Handbook of the Economics of Population Aging; Piggott, J., Woodland, A., Eds.; North-Holland: Amsterdam, The Netherlands, 2016; Volume 1, pp. 3–56. [Google Scholar]
- Rosenberg, M.; Tomioka, S.; Barber, S.L. Research to inform health systems’ responses to rapid population ageing: A collection of studies funded by the WHO Centre for Health Development in Kobe, Japan. Health Res. Policy Syst. 2022, 20, 128. [Google Scholar] [CrossRef]
- Hong, C.; Sun, L.; Liu, G.; Guan, B.; Li, C.; Luo, Y. Response of Global Health Towards the Challenges Presented by Population Aging. China CDC Wkly. 2023, 5, 884–887. [Google Scholar] [CrossRef] [PubMed]
- Sierra, F. The Emergence of Geroscience as an Interdisciplinary Approach to the Enhancement of Health Span and Life Span. Cold Spring Harb Perspect Med. 2016, 6, a025163. [Google Scholar] [CrossRef]
- Polidori, M.C. Geroscience in the continuum from healthy longevity to frailty. Z. Gerontol. Geriatr. 2024, 57, 361–364. [Google Scholar] [CrossRef]
- Petr, M.A.; Matiyevskaya, F.; Osborne, B.; Berglind, M.; Reves, S.; Zhang, B.; Ezra, M.B.; Carmona-Marin, L.M.; Syadzha, M.F.; Mediavilla, M.C.; et al. Pharmacological interventions in human aging. Ageing Res. Rev. 2024, 95, 102213. [Google Scholar] [CrossRef]
- Herrera, A.P.; Snipes, S.A.; King, D.W.; Torres-Vigil, I.; Goldberg, D.S.; Weinberg, A.D. Disparate inclusion of older adults in clinical trials: Priorities and opportunities for policy and practice change. Am. J. Public Health 2010, 100 (Suppl. S1), S105–S112. [Google Scholar] [CrossRef] [PubMed]
- Provencher, V.; Mortenson, W.B.; Tanguay-Garneau, L.; Belanger, K.; Dagenais, M. Challenges and strategies pertaining to recruitment and retention of frail elderly in research studies: A systematic review. Arch. Gerontol. Geriatr. 2014, 59, 18–24. [Google Scholar] [CrossRef]
- Ridda, I.; Lindley, R.; MacIntyre, R.C. The challenges of clinical trials in the exclusion zone: The case of the frail elderly. Australas. J. Ageing 2008, 27, 61–66. [Google Scholar] [CrossRef]
- Mitchell, S.J.; Scheibye-Knudsen, M.; Longo, D.L.; de Cabo, R. Animal models of aging research: Implications for human aging and age-related diseases. Annu. Rev. Anim. Biosci. 2015, 3, 283–303. [Google Scholar] [CrossRef] [PubMed]
- Holtze, S.; Gorshkova, E.; Braude, S.; Cellerino, A.; Dammann, P.; Hildebrandt, T.B.; Hoeflich, A.; Hoffmann, S.; Koch, P.; Terzibasi Tozzini, E.; et al. Alternative Animal Models of Aging Research. Front. Mol. Biosci. 2021, 8, 660959. [Google Scholar] [CrossRef]
- Tohyama, S.; Kobayashi, E. Age-Appropriateness of Porcine Models Used for Cell Transplantation. Cell Transplant. 2019, 28, 224–228. [Google Scholar] [CrossRef] [PubMed]
- Shirakawa, K.; Kobayashi, E.; Ichihara, G.; Kitakata, H.; Katsumata, Y.; Sugai, K.; Hakamata, Y.; Sano, M. H(2) Inhibits the Formation of Neutrophil Extracellular Traps. JACC Basic Transl. Sci. 2022, 7, 146–161. [Google Scholar] [CrossRef]
- Santulli, G.; Borras, C.; Bousquet, J.; Calza, L.; Cano, A.; Illario, M.; Franceschi, C.; Liotta, G.; Maggio, M.; Molloy, W.D.; et al. Models for preclinical studies in aging-related disorders: One is not for all. Transl. Med. UniSa 2015, 13, 4–12. [Google Scholar]
- Lunney, J.K.; Van Goor, A.; Walker, K.E.; Hailstock, T.; Franklin, J.; Dai, C. Importance of the pig as a human biomedical model. Sci. Transl. Med. 2021, 13, eabd5758. [Google Scholar] [CrossRef]
- Gutierrez, K.; Dicks, N.; Glanzner, W.G.; Agellon, L.B.; Bordignon, V. Efficacy of the porcine species in biomedical research. Front. Genet. 2015, 6, 293. [Google Scholar] [CrossRef]
- Wei, J.; Zhang, W.; Li, J.; Jin, Y.; Qiu, Z. Application of the transgenic pig model in biomedical research: A review. Front. Cell Dev. Biol. 2022, 10, 1031812. [Google Scholar] [CrossRef]
- Kaneko, N.; Itoh, K.; Sugiyama, A.; Izumi, Y. Microminipig, a Non-rodent Experimental Animal Optimized for Life Science Research: Preface. J. Pharmacol. Sci. 2011, 115, 112–114. [Google Scholar] [CrossRef]
- Kangawa, A.; Nishimura, T.; Nishimura, T.; Otake, M.; Enya, S.; Yoshida, T.; Shibata, M. Spontaneous Age-Related Histopathological Changes in Microminipigs. Toxicol. Pathol. 2019, 47, 817–832. [Google Scholar] [CrossRef]
- Fraga, C.G.; Croft, K.D.; Kennedy, D.O.; Tomas-Barberan, F.A. The effects of polyphenols and other bioactives on human health. Food Funct. 2019, 10, 514–528. [Google Scholar] [CrossRef]
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and Human Health: The Role of Bioavailability. Nutrients 2021, 13, 273. [Google Scholar] [CrossRef]
- Wan, M.L.Y.; Co, V.A.; El-Nezami, H. Dietary polyphenol impact on gut health and microbiota. Crit. Rev. Food Sci. Nutr. 2021, 61, 690–711. [Google Scholar] [CrossRef] [PubMed]
- Rana, A.; Samtiya, M.; Dhewa, T.; Mishra, V.; Aluko, R.E. Health benefits of polyphenols: A concise review. J. Food Biochem. 2022, 46, e14264. [Google Scholar] [CrossRef] [PubMed]
- Escobar-Puentes, A.A.; Palomo, I.; Rodriguez, L.; Fuentes, E.; Villegas-Ochoa, M.A.; Gonzalez-Aguilar, G.A.; Olivas-Aguirre, F.J.; Wall-Medrano, A. Sweet Potato (Ipomoea batatas L.) Phenotypes: From Agroindustry to Health Effects. Foods 2022, 11, 1058. [Google Scholar] [CrossRef]
- Chen, W.P.; Mao, T.J.; Fan, L.; Zhou, Y.H.; Yu, J.; Jin, Y.; Hou, P.C. Effect of purple sweet potato on lipid metabolism and oxidative stress in hyperlipidemic rats. Zhejiang Da Xue Xue Bao Yi Xue Ban 2011, 40, 360–364. [Google Scholar] [CrossRef]
- Insanu, M.; Amalia, R.; Fidrianny, I. Potential Antioxidative Activity of Waste Product of Purple Sweet Potato (Ipomoea batatas Lam.). Pak. J. Biol. Sci. 2022, 25, 681–687. [Google Scholar] [CrossRef]
- Han, K.H.; Matsumoto, A.; Shimada, K.; Sekikawa, M.; Fukushima, M. Effects of anthocyanin-rich purple potato flakes on antioxidant status in F344 rats fed a cholesterol-rich diet. Br. J. Nutr. 2007, 98, 914–921. [Google Scholar] [CrossRef]
- Li, C.; Feng, Y.; Li, J.; Lian, R.; Qin, L.; Wang, C. Extraction, purification, structural characterization, and hepatoprotective effect of the polysaccharide from purple sweet potato. J. Sci. Food Agric. 2023, 103, 2196–2206. [Google Scholar] [CrossRef]
- Herawati, E.R.N.; Santosa, U.; Sentana, S.; Ariani, D. Protective Effects of Anthocyanin Extract from Purple Sweet Potato (Ipomoea batatas L.) on Blood MDA Levels, Liver and Renal Activity, and Blood Pressure of Hyperglycemic Rats. Prev. Nutr. Food Sci. 2020, 25, 375–379. [Google Scholar] [CrossRef]
- Hisamuddin, A.S.B.; Naomi, R.; Bin Manan, K.A.; Bahari, H.; Othman, F.; Embong, H.; Ismail, A.; Ahmed, Q.U.; Jumidil, S.H.; Hussain, M.K.; et al. The role of lutein-rich purple sweet potato leaf extract on the amelioration of diabetic retinopathy in streptozotocin-induced Sprague-Dawley rats. Front. Pharmacol. 2023, 14, 1175907. [Google Scholar] [CrossRef]
- Kinoshita, A.; Nagata, T.; Furuya, F.; Nishizawa, M.; Mukai, E. White-skinned sweet potato (Ipomoea batatas L.) acutely suppresses postprandial blood glucose elevation by improving insulin sensitivity in normal rats. Heliyon 2023, 9, e14719. [Google Scholar] [CrossRef]
- Mi, W.; Hu, Z.; Zhao, S.; Wang, W.; Lian, W.; Lu, P.; Shi, T. Purple sweet potato anthocyanins normalize the blood glucose concentration and restore the gut microbiota in mice with type 2 diabetes mellitus. Heliyon 2024, 10, e31784. [Google Scholar] [CrossRef]
- Majid, M.; Nasir, B.; Zahra, S.S.; Khan, M.R.; Mirza, B.; Haq, I.U. Ipomoea batatas L. Lam. ameliorates acute and chronic inflammations by suppressing inflammatory mediators, a comprehensive exploration using in vitro and in vivo models. BMC Complement. Altern. Med. 2018, 18, 216. [Google Scholar] [CrossRef]
- Sun, R.; Kan, J.; Cai, H.; Hong, J.; Jin, C.; Zhang, M. In vitro and in vivo ameliorative effects of polyphenols from purple potato leaves on renal injury and associated inflammation induced by hyperuricemia. J. Food Biochem. 2022, 46, e14049. [Google Scholar] [CrossRef]
- Zhao, J.; Yu, J.; Zhi, Q.; Yuan, T.; Lei, X.; Zeng, K.; Ming, J. Anti-aging effects of the fermented anthocyanin extracts of purple sweet potato on Caenorhabditis elegans. Food Funct. 2021, 12, 12647–12658. [Google Scholar] [CrossRef]
- Hoffman, J.M.; Valencak, T.G. A short life on the farm: Aging and longevity in agricultural, large-bodied mammals. Geroscience 2020, 42, 909–922. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Contreras, C.; Vazquez-Gomez, M.; Torres-Rovira, L.; Gonzalez, J.; Porrini, E.; Gonzalez-Colaco, M.; Isabel, B.; Astiz, S.; Gonzalez-Bulnes, A. Characterization of Ageing- and Diet-Related Swine Models of Sarcopenia and Sarcopenic Obesity. Int. J. Mol. Sci. 2018, 19, 823. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zou, Q.; Lv, D.; Raza, M.A.; Wang, X.; Chen, Y.; Xi, X.; Li, P.; Wen, A.; Zhu, L.; et al. Comprehensive transcriptional profiling of aging porcine liver. PeerJ 2019, 7, e6949. [Google Scholar] [CrossRef] [PubMed]
- Lim, M.Y.; Song, E.J.; Kang, K.S.; Nam, Y.D. Age-related compositional and functional changes in micro-pig gut microbiome. Geroscience 2019, 41, 935–944. [Google Scholar] [CrossRef]
- Schachtschneider, K.M.; Schook, L.B.; Meudt, J.J.; Shanmuganayagam, D.; Zoller, J.A.; Haghani, A.; Li, C.Z.; Zhang, J.; Yang, A.; Raj, K.; et al. Epigenetic clock and DNA methylation analysis of porcine models of aging and obesity. Geroscience 2021, 43, 2467–2483. [Google Scholar] [CrossRef] [PubMed]
- Qiao, C.; Liu, C.; Ding, R.; Wang, S.; He, M. Unveiling the Metabolic Trajectory of Pig Feces Across Different Ages and Senescence. Metabolites 2024, 14, 558. [Google Scholar] [CrossRef]
- Fukushima, Y.; Ohie, T.; Yonekawa, Y.; Yonemoto, K.; Aizawa, H.; Mori, Y.; Watanabe, M.; Takeuchi, M.; Hasegawa, M.; Taguchi, C.; et al. Coffee and green tea as a large source of antioxidant polyphenols in the Japanese population. J. Agric. Food Chem. 2009, 57, 1253–1259. [Google Scholar] [CrossRef]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. BMJ Open Sci. 2020, 4, e100115. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef] [PubMed]
- Langille, M.G.; Zaneveld, J.; Caporaso, J.G.; McDonald, D.; Knights, D.; Reyes, J.A.; Clemente, J.C.; Burkepile, D.E.; Vega Thurber, R.L.; Knight, R.; et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat. Biotechnol. 2013, 31, 814–821. [Google Scholar] [CrossRef]
- Yang, C.; Mai, J.; Cao, X.; Burberry, A.; Cominelli, F.; Zhang, L. ggpicrust2: An R package for PICRUSt2 predicted functional profile analysis and visualization. Bioinformatics 2023, 39, btad470. [Google Scholar] [CrossRef]
- Li, J.; Han, X.; Zhang, X.; Wang, S. Spatiotemporal evolution of global population ageing from 1960 to 2017. BMC Public Health 2019, 19, 127. [Google Scholar] [CrossRef]
- Cheng, X.; Yang, Y.; Schwebel, D.C.; Liu, Z.; Li, L.; Cheng, P.; Ning, P.; Hu, G. Population ageing and mortality during 1990–2017: A global decomposition analysis. PLoS Med. 2020, 17, e1003138. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, B.; Zhang, X.; Akbar, M.T.; Wu, T.; Zhang, Y.; Zhi, L.; Shen, Q. Exploration of the Muribaculaceae Family in the Gut Microbiota: Diversity, Metabolism, and Function. Nutrients 2024, 16, 2660. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Huang, D.; Sun, Z.; Chen, X. Effects of intestinal Desulfovibrio bacteria on host health and its potential regulatory strategies: A review. Microbiol. Res. 2024, 284, 127725. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Wang, T.; Wang, Y.; Li, Y.; Sun, Y.; Qiu, H.J. Short-chain fatty acids: Key antiviral mediators of gut microbiota. Front. Immunol. 2025, 16, 1614879. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, T.; Lin, K.C.; Chang, C.C.; Hsia, C.W.; Manubolu, M.; Huang, W.C.; Sheu, J.R.; Hsia, C.H. Targeting MAPK/NF-kappaB Pathways in Anti-Inflammatory Potential of Rutaecarpine: Impact on Src/FAK-Mediated Macrophage Migration. Int. J. Mol. Sci. 2021, 23, 92. [Google Scholar] [CrossRef]
- Han, S.; Georgiev, P.; Ringel, A.E.; Sharpe, A.H.; Haigis, M.C. Age-associated remodeling of T cell immunity and metabolism. Cell Metab. 2023, 35, 36–55. [Google Scholar] [CrossRef]
- Goyani, P.; Christodoulou, R.; Vassiliou, E. Immunosenescence: Aging and Immune System Decline. Vaccines 2024, 12, 1314. [Google Scholar] [CrossRef]
- Hisamuddin, A.S.B.; Naomi, R.; Bin Manan, K.A.; Bahari, H.; Yazid, M.D.; Othman, F.; Embong, H.; Hadizah Jumidil, S.; Hussain, M.K.; Zakaria, Z.A. Phytochemical component and toxicological evaluation of purple sweet potato leaf extract in male Sprague-Dawley rats. Front. Pharmacol. 2023, 14, 1132087. [Google Scholar] [CrossRef]
- Huffman, D.M.; Justice, J.N.; Stout, M.B.; Kirkland, J.L.; Barzilai, N.; Austad, S.N. Evaluating Health Span in Preclinical Models of Aging and Disease: Guidelines, Challenges, and Opportunities for Geroscience. J. Gerontol. A Biol. Sci. Med. Sci. 2016, 71, 1395–1406. [Google Scholar] [CrossRef]
- Wang, Q.; Zhu, B.T.; Lei, P. Animal models of Alzheimer’s disease: Current strategies and new directions. Zool. Res. 2024, 45, 1385–1407. [Google Scholar] [CrossRef]
| ID | Age | Sex | Group |
|---|---|---|---|
| #C1 | 8 years old | Female | Control |
| #C2 | 9 years old | Male | Control |
| #P1 | 8 years old | Female | Potato |
| #P2 | 13 years old | Female | Potato |
| #P3 | 9 years old | Male | Potato |
| KEGG ID | Pathway Name | Mapped Number |
|---|---|---|
| ko01100 | Metabolic pathways | 52 |
| ko00195 | Photosynthesis | 27 |
| ko01110 | Biosynthesis of secondary metabolites | 14 |
| ko00190 | Oxidative phosphorylation | 11 |
| ko00860 | Porphyrin metabolism | 7 |
| ko00910 | Nitrogen metabolism | 3 |
| ko02010 | ABC transporters | 3 |
| ko01240 | Biosynthesis of cofactors | 3 |
| ko00130 | Ubiquinone and other terpenoid-quinone biosynthesis | 2 |
| ko00906 | Carotenoid biosynthesis | 2 |
| ko00900 | Terpenoid backbone biosynthesis | 2 |
| ko00770 | Pantothenate and CoA biosynthesis | 2 |
| ko01232 | Nucleotide metabolism | 2 |
| ko01054 | Nonribosomal peptide structures | 1 |
| ko02020 | Two-component system | 1 |
| ko00650 | Butanoate metabolism | 1 |
| ko01230 | Biosynthesis of amino acids | 1 |
| ko00230 | Purine metabolism | 1 |
| ko01250 | Biosynthesis of nucleotide sugars | 1 |
| ko00240 | Pyrimidine metabolism | 1 |
| ko00660 | C5-Branched dibasic acid metabolism | 1 |
| ko00290 | Valine, leucine and isoleucine biosynthesis | 1 |
| ko00410 | beta-Alanine metabolism | 1 |
| ko03060 | Protein export | 1 |
| ko01210 | 2-Oxocarboxylic acid metabolism | 1 |
| ko00541 | Biosynthesis of various nucleotide sugars | 1 |
| ko00970 | Aminoacyl-tRNA biosynthesis | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sugai, K.; Miyamoto, Y.; Sato, T.; Hakamata, Y.; Murakoshi, T.; Kobayashi, S.; Iwamoto, S.; Kobayashi, E. A Pilot Study on the Effects of Sweet Potato Petiole and Leaf Powder on Gut Microbiota and Aging-Related Biomarkers in an Aged Microminipig Model. Metabolites 2025, 15, 713. https://doi.org/10.3390/metabo15110713
Sugai K, Miyamoto Y, Sato T, Hakamata Y, Murakoshi T, Kobayashi S, Iwamoto S, Kobayashi E. A Pilot Study on the Effects of Sweet Potato Petiole and Leaf Powder on Gut Microbiota and Aging-Related Biomarkers in an Aged Microminipig Model. Metabolites. 2025; 15(11):713. https://doi.org/10.3390/metabo15110713
Chicago/Turabian StyleSugai, Kazuhisa, Yoshiaki Miyamoto, Toshiyuki Sato, Yoji Hakamata, Toshiyuki Murakoshi, Shou Kobayashi, Sadahiko Iwamoto, and Eiji Kobayashi. 2025. "A Pilot Study on the Effects of Sweet Potato Petiole and Leaf Powder on Gut Microbiota and Aging-Related Biomarkers in an Aged Microminipig Model" Metabolites 15, no. 11: 713. https://doi.org/10.3390/metabo15110713
APA StyleSugai, K., Miyamoto, Y., Sato, T., Hakamata, Y., Murakoshi, T., Kobayashi, S., Iwamoto, S., & Kobayashi, E. (2025). A Pilot Study on the Effects of Sweet Potato Petiole and Leaf Powder on Gut Microbiota and Aging-Related Biomarkers in an Aged Microminipig Model. Metabolites, 15(11), 713. https://doi.org/10.3390/metabo15110713






