Multi-Omics Decoding of Potential Microbial–Genetic Synergy Underlying Polysaccharide and Glycosidic Polymer Biosynthesis in Two Cultivars of Lilium brownii var. viridulum Baker
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Metabolomic Analysis
2.2.1. Metabolite Extraction and Chromatographic Analysis
2.2.2. Metabolite Identification, Quantification, and Data Processing
2.2.3. Differential Metabolite Screening and Functional Annotation
2.3. Transcriptomic Analysis
2.3.1. RNA Extraction
2.3.2. Library Construction and Sequencing of Transcriptomic
2.3.3. Differential Gene Screening and Functional Annotation
2.4. Soil Metagenomic Analysis
2.4.1. DNA Extraction
2.4.2. Library Construction and Sequencing of Soil Metagenomic
3. Results and Analysis
3.1. Metabolic Differences Between Glycosides in Two Cultivars
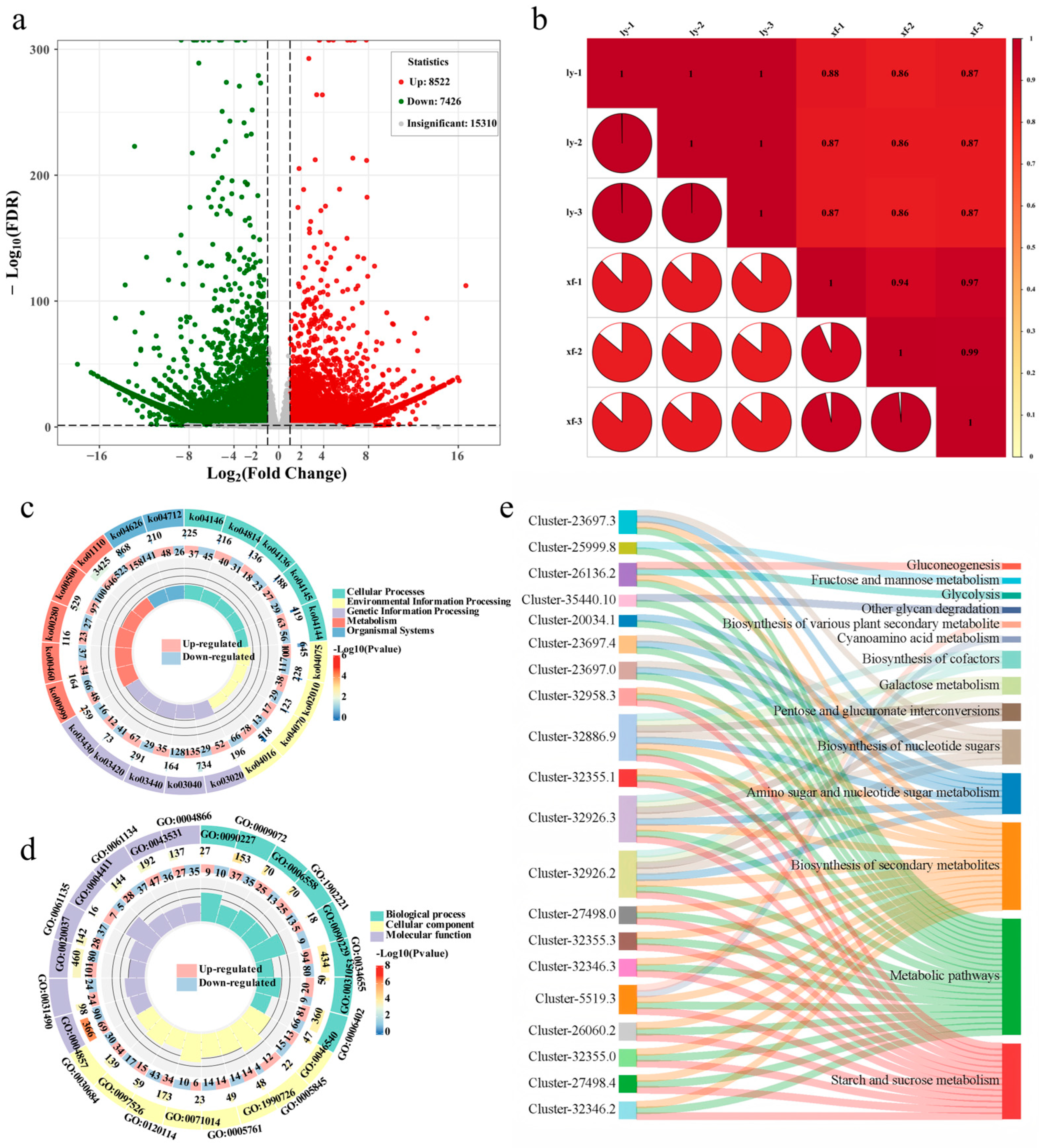
3.2. Transcriptome Profiling Reveals Gene Expression Differences Between Bulbs of Two Cultivars
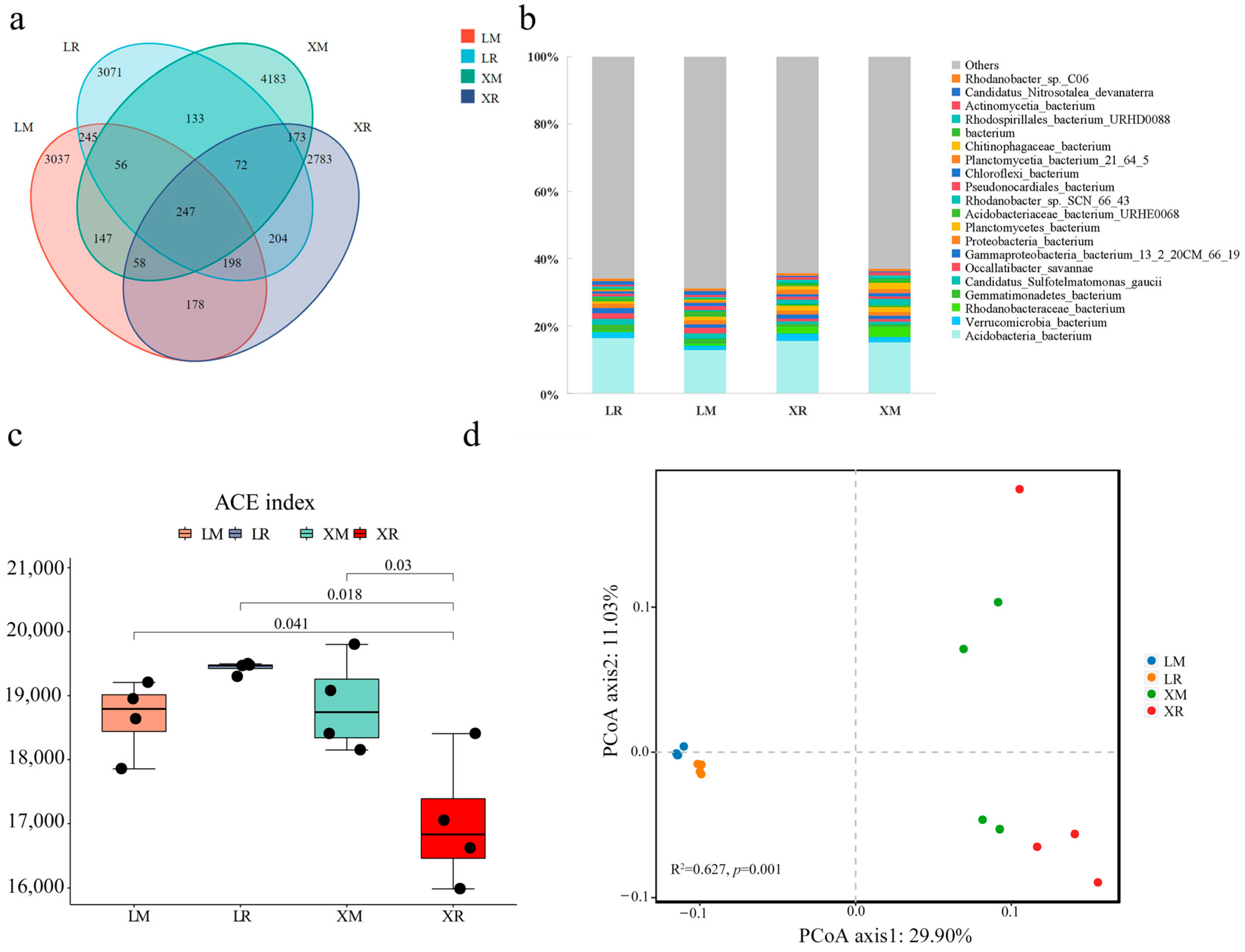
3.3. Diversity and Composition of Soil Microbiota Associated with Two Cultivars
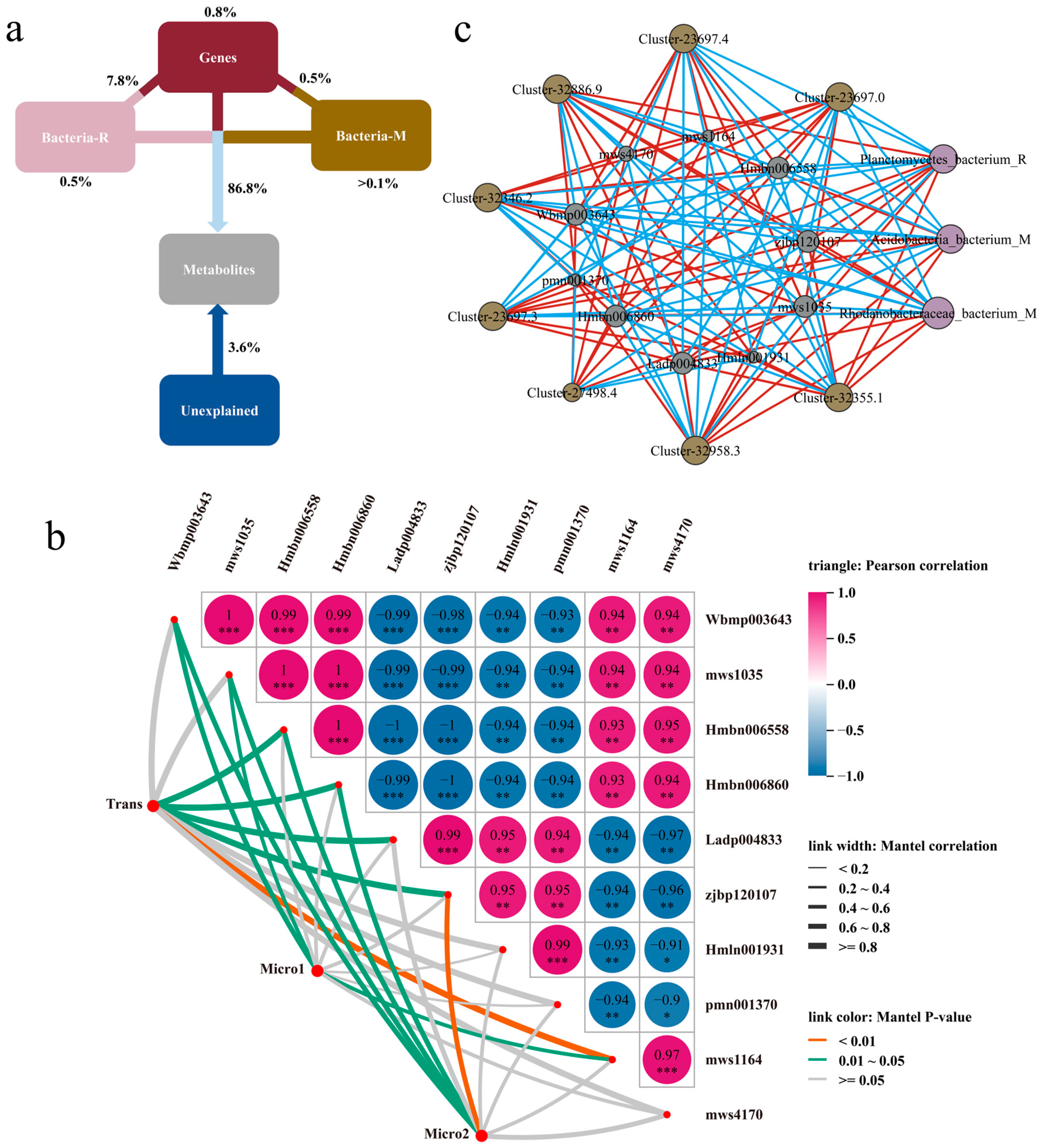
3.4. The Relationship Between Soil Microorganisms, Genetic Factors, and Metabolic Regulation in the Two Cultivars
4. Discussion
4.1. Breeding Based on Differences in Lv Bulbs’ Glycoside Contents
4.2. Regulatory Role of Genetic Background Differences on Glycoside Synthesis
4.3. Potential Regulatory Role of Soil Microorganisms on Glycoside Accumulation in Lv Bulbs
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, X.; Yin, Y.; Xiao, S.; Chen, J.; Zhang, R.; Yang, T. Extraction, structural characterization and immunoactivity of glucomannan type polysaccahrides from Lilium brownii var. viridulum Baker. Carbohydr. Res. 2024, 536, 109046. [Google Scholar] [CrossRef]
- Ma, T.; Wang, Z.; Zhang, Y.; Luo, J.; Kong, L. Bioassay-Guided Isolation of Anti-Inflammatory Components from the Bulbs of Lilium brownii var. viridulum and Identifying the Underlying Mechanism through Acting on the NF-κB/MAPKs Pathway. Molecules 2017, 22, 506. [Google Scholar] [CrossRef]
- Hong, X.; Luo, J.; Guo, C.; Kong, L. New steroidal saponins from the bulbs of Lilium brownii var. viridulum. Carbohydr. Res. 2012, 361, 19–26. [Google Scholar] [CrossRef]
- Guan, Y.; Lu, S.; Sun, Y.; Zhang, R.; Lu, X.; Pang, L.; Wang, L. Effect of Tea Tree Essential Oil on the Quality, Antioxidant Activity, and Microbiological Safety of Lightly Processed Lily (Lilium brownii var. viridulum) during Storage. Foods 2024, 13, 2106. [Google Scholar] [CrossRef]
- Chang, N.; Zheng, L.; Xu, Y.; Wang, C.; Li, H.; Wang, Y. Integrated transcriptomic and metabolomic analysis reveals the molecular profiles of dynamic variation in Lilium brownii var. viridulum suffering from bulb rot. Front. Genet. 2024, 15, 1432997. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, Z.; Hou, C.; Gong, X.; Liu, Z.; Shi, Y. Identification and Characterization of Volatile Organic Compounds Based on GC-IMS Technology in Different Organs of Lilium brownii var. viridulum and After Bud-Removal and Non-Bud-Removal Treatments. Molecules 2025, 30, 1238. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Jin, J.; Zhou, R.; Fang, L.; Liu, H.; Zhong, C. Integrative analysis of metabolome and transcriptome provide new insights into the bitter components of Lilium lancifolium and Lilium brownii. J. Pharm. Biomed. Anal. 2022, 215, 114778. [Google Scholar] [CrossRef] [PubMed]
- Tu, J.; Zhao, X.; Yang, Y.; Yi, Y.; Wang, H.; Wei, B.; Zeng, L. Two Bacillus spp. Strains Improve the Structure and Diversity of the Rhizosphere Soil Microbial Community of Lilium brownii var. viridulum. Microorganisms 2023, 11, 1229. [Google Scholar] [CrossRef]
- Wei, W.; Guo, T.; Fan, W.; Ji, M.; Fu, Y.; Lian, C. Integrative analysis of metabolome and transcriptome provides new insights into functional components of Lilii Bulbus. Chin. Herb. Med. 2024, 16, 435–448. [Google Scholar] [CrossRef]
- Wen, Z.; Yang, M.; Lu, G.; Han, M.; Song, Y.; Sun, Y. Microbial Alliances: Unveiling the Effects of a Bacterial and Fungal Cross-Kingdom SynCom on Bacterial Dynamics, Rhizosphere Metabolites, and Soybean Resilience in Acidic Soils. J. Agric. Food Chem. 2025, 73, 18013–18031. [Google Scholar] [CrossRef]
- Liu, X.; Qiao, Y.; Wu, X.; Chen, X.; Yang, F.; Li, H. Comprehensive metabolomic and microbial analysis of tobacco rhizosphere soil responses to crop rotation and fertilization. Front. Plant Sci. 2025, 16, 1595870. [Google Scholar] [CrossRef] [PubMed]
- Schwabe, R.; Dittrich, C.; Kadner, J.; Rudi Senges, C.; Bandow, J.; Tischler, D.; Wiche, O. Secondary metabolites released by the rhizosphere bacteria Arthrobacter oxydans and Kocuria rosea enhance plant availability and soil-plant transfer of germanium (Ge) and rare earth elements (REEs). Chemosphere 2021, 285, 131466. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Xu, F.; Qiang, X.; Liu, H.; Wang, L.; Jiang, L. Regulated cleavage and translocation of FERONIA control immunity in Arabidopsis roots. Nat. Plants 2024, 10, 1761–1774. [Google Scholar] [CrossRef] [PubMed]
- Quecine, M.; Kidarsa, T.; Goebel, N.; Shaffer, B.; Henkels, M.; Zabriskie, T.; Loper, J. An Interspecies Signaling System Mediated by Fusaric Acid Has Parallel Effects on Antifungal Metabolite Production by Pseudomonas protegens Strain Pf-5 and Antibiosis of Fusarium spp. Appl. Environ. Microbiol. 2015, 82, 1372–1382. [Google Scholar] [CrossRef]
- Zhang, N.; Zhu, X.; Tao, X.; Li, J.; Tang, Q.; Liu, X. Interspecies signaling modulates the biosynthesis of antimicrobial secondary metabolites related to biological control activities of Pseudomonas fluorescens 2P24. Microbiol. Spectr. 2025, 13, e0188624. [Google Scholar] [CrossRef]
- Kim, Y.; Anderson, A. Rhizosphere pseudomonads as probiotics improving plant health. Mol. Plant Pathol. 2018, 19, 2349–2359. [Google Scholar] [CrossRef]
- Yang, F.; Jiang, H.; Chang, G.; Liang, S.; Ma, K.; Cai, Y. Effects of Rhizosphere Microbial Communities on Cucumber Fusarium wilt Disease Suppression. Microorganisms 2023, 11, 1576. [Google Scholar] [CrossRef]
- Manck-Götzenberger, J.; Requena, N. Arbuscular mycorrhiza Symbiosis Induces a Major Transcriptional Reprogramming of the Potato SWEET Sugar Transporter Family. Front. Plant Sci. 2016, 7, 487. [Google Scholar] [CrossRef]
- Chen, H.; Huh, J.; Yu, Y.; Ho, L.; Chen, L.; Tholl, D. The Arabidopsis vacuolar sugar transporter SWEET2 limits carbon sequestration from roots and restricts Pythium infection. Plant J. 2015, 83, 1046–1058. [Google Scholar] [CrossRef]
- Zhang, N.; Chen, X.; Liang, J.; Zhao, C.; Xiang, J.; Luo, L. Rhizocompartmental microbiomes of arrow bamboo (Fargesia nitida) and their relation to soil properties in Subalpine Coniferous Forests. PeerJ 2023, 11, e16488. [Google Scholar] [CrossRef]
- Lee, H.; Cho, G.; Chung, S.; Whang, K. Streptomyces panaciradicis sp. nov., a β-glucosidase-producing bacterium isolated from ginseng rhizoplane. Int. J. Syst. Evol. Microbiol. 2014, 64, 3816–3820. [Google Scholar] [CrossRef]
- Li, L.; Qiao, Y.; Qi, X.; Liu, W.; Xu, W.; Dong, S. Sucrose promotes branch-thorn occurrence of Lycium ruthenicum through dual effects of energy and signal. Tree Physiol. 2023, 43, 1187–1200. [Google Scholar] [CrossRef]
- Sun, W.; Cheng, S.; Chao, Y.; Lin, S.; Yang, T.; Ho, Y. Sugars and sucrose transporters in pollinia of Phalaenopsis aphrodite (Orchidaceae). J. Exp. Bot. 2023, 74, 2556–2571. [Google Scholar] [CrossRef] [PubMed]
- Zang, X.; Liu, M.; Fan, Y.; Xu, J.; Xu, X.; Li, H. The structural and functional contributions of β-glucosidase-producing microbial communities to cellulose degradation in composting. Biotechnol. Biofuels Bioprod. 2018, 11, 51. [Google Scholar] [CrossRef] [PubMed]
- Halmi, M.; Simarani, K. Response of soil microbial glycoside hydrolase family 6 cellulolytic population to lignocellulosic biochar reveals biochar stability toward microbial degradation. J. Environ. Qual. 2024, 53, 546–551. [Google Scholar] [CrossRef] [PubMed]
- Zang, X.; Liu, M.; Wang, H.; Fan, Y.; Zhang, H.; Liu, J. The distribution of active β-glucosidase-producing microbial communities in composting. Can. J. Microbiol. 2017, 63, 998–1008. [Google Scholar] [CrossRef]
- Othibeng, K.; Nephali, L.; Myoli, A.; Buthelezi, N.; Jonker, W.; Huyser, J.; Tugizimana, F. Metabolic Circuits in Sap Extracts Reflect the Effects of a Microbial Biostimulant on Maize Metabolism under Drought Conditions. Plants 2022, 11, 510. [Google Scholar] [CrossRef]
- Nie, X.; Chen, X.; Lu, X.; Yang, S.; Wang, X.; Liu, F. Metagenomics Insights into the Role of Microbial Communities in Mycotoxin Accumulation During Maize Ripening and Storage. Foods 2025, 14, 1378. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Y.; Shi, X.; Xu, L.; Zhang, L.; Zhang, L. The succession of GH6 cellulase-producing microbial communities and temporal profile of GH6 gene abundance during vermicomposting of maize stover and cow dung. Bioresour. Technol. 2022, 344, 126242. [Google Scholar] [CrossRef]
- Liu, Z.; Moore, R.; Gao, Y.; Chen, P.; Yu, L.; Zhang, M.; Sun, J. Comparison of Phytochemical Profiles of Wild and Cultivated American Ginseng Using Metabolomics by Ultra-High Performance Liquid Chromatography-High-Resolution Mass Spectrometry. Molecules 2022, 28, 9. [Google Scholar] [CrossRef]
- Gou, N.; Chen, C.; Huang, M.; Zhang, Y.; Bai, H.; Li, H. Transcriptome and Metabolome Analyses Reveal Sugar and Acid Accumulation during Apricot Fruit Development. Int. J. Mol. Sci. 2023, 24, 16992. [Google Scholar] [CrossRef]
- Hu, H.; Gutierrez-Gonzalez, J.; Liu, X.; Yeats, T.; Garvin, D.F.; Hoekenga, O. Heritable temporal gene expression patterns correlate with metabolomic seed content in developing hexaploid oat seed. Plant Biotechnol. J. 2020, 18, 1211–1222. [Google Scholar] [CrossRef] [PubMed]
- Ammah, A.; Do, D.; Bissonnette, N.; Gévry, N.; Ibeagha-Awemu, E. Co-Expression Network Analysis Identifies miRNA-mRNA Networks Potentially Regulating Milk Traits and Blood Metabolites. Int. J. Mol. Sci. 2018, 19, 2500. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Mi, B.; Lv, H.; Liu, J.; Xiong, Y.; Hu, L. Shared KEGG pathways of icariin-targeted genes and osteoarthritis. J. Cell. Biochem. 2019, 120, 7741–7750. [Google Scholar] [CrossRef] [PubMed]
- Gaudet, P.; Logie, C.; Lovering, R.; Kuiper, M.; Lægreid, A.; Thomas, P. Gene Ontology representation for transcription factor functions. Biochim. Biophys. Acta-Gene Regul. Mech. 2021, 1864, 194752. [Google Scholar] [CrossRef]
- Finn, D. A metagenomic alpha-diversity index for microbial functional biodiversity. FEMS Microbiol. Ecol. 2024, 100, fiae019. [Google Scholar] [CrossRef]
- Glassmire, A.; Zehr, L.; Wetzel, W. Disentangling dimensions of phytochemical diversity: Alpha and beta have contrasting effects on an insect herbivore. Ecology 2020, 101, e03158. [Google Scholar] [CrossRef]
- Shen, Y.; Wu, S.; Chen, Y.; Li, X.; Zhu, Q.; Nakayama, K. Alterations in gut microbiome and metabolomics in chronic hepatitis B infection-associated liver disease and their impact on peripheral immune response. Gut Microbes. 2023, 15, 2155018. [Google Scholar] [CrossRef]
- La Monica, M.; Raub, B.; Hartshorn, S.; Gustat, A.; Grdic, J.; Kirby, T.; Townsend, J.; Sandrock, J.; Ziegenfuss, T. The effects of AG1® supplementation on the gut microbiome of healthy adults: A randomized, double-blind, placebo-controlled clinical trial. J. Int. Soc. Sports Nutr. 2024, 21, 2409682. [Google Scholar] [CrossRef]
- Singavarapu, B.; Du, J.; Beugnon, R.; Cesarz, S.; Eisenhauer, N.; Xue, K. Functional Potential of Soil Microbial Communities and Their Subcommunities Varies with Tree Mycorrhizal Type and Tree Diversity. Microbiol. Spectr. 2023, 11, e0457822. [Google Scholar] [CrossRef]
- Kellogg, J.; Kvalheim, O.; Cech, N. Composite score analysis for unsupervised comparison and network visualization of metabolomics data. Anal Chim Acta. 2020, 1095, 38–47. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, G.; Yan, Q.; Ahmad, B.; Pei, J.; Huang, L. Quality variation and salt-alkali-tolerance mechanism of Cynomorium songaricum: Interacting from microbiome-transcriptome-metabolome. Sci. Total Environ. 2024, 919, 170801. [Google Scholar] [CrossRef] [PubMed]
- Farcuh, M.; Li, B.; Rivero, R.; Shlizerman, L.; Sadka, A.; Blumwald, E. Sugar metabolism reprogramming in a non-climacteric bud mutant of a climacteric plum fruit during development on the tree. J. Exp. Bot. 2017, 68, 5813–5828. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ravipati, A.; Koyyalamudi, S.; Jeong, S.; Reddy, N.; Smith, P. Antioxidant and anti-inflammatory activities of selected medicinal plants containing phenolic and flavonoid compounds. J. Agric. Food Chem. 2011, 59, 12361–12367. [Google Scholar] [CrossRef] [PubMed]
- Karimi, A.; Moradi, M.; Alidadi, S.; Hashemi, L. Anti-adenovirus activity, antioxidant potential, and phenolic content of black tea (Camellia sinensis Kuntze) extract. J. Integr. Complement. Med. 2016, 13, 357–363. [Google Scholar] [CrossRef]
- Xu, C.; Wei, L.; Huang, S.; Yang, C.; Wang, Y.; Yuan, H. Drought Resistance in Qingke Involves a Reprogramming of the Phenylpropanoid Pathway and UDP-Glucosyltransferase Regulation of Abiotic Stress Tolerance Targeting Flavonoid Biosynthesis. J. Agric. Food Chem. 2021, 69, 3992–4005. [Google Scholar] [CrossRef]
- Klimek-Szczykutowicz, M.; Szopa, A.; Blicharska, E.; Dziurka, M.; Komsta, Ł.; Ekiert, H. Bioaccumulation of selected macro- and microelements and their impact on antioxidant properties and accumulation of glucosinolates and phenolic acids in in vitro cultures of Nasturtium officinale (watercress) microshoots. Food Chem. 2019, 300, 125184. [Google Scholar] [CrossRef]
- Almuhayawi, M.; AbdElgawad, H.; AlJaouni, S.; Selim, S.; Hassan, A.; Khamis, G. Elevated CO2 improves glucosinolate metabolism and stimulates anticancer and anti-inflammatory properties of broccoli sprouts. Food Chem. 2020, 328, 127102. [Google Scholar] [CrossRef]
- Guo, X.; Shakeel, M.; Wang, D.; Qu, C.; Yang, S.; Ahmad, S.; Song, Z. Metabolome and transcriptome profiling unveil the mechanisms of light-induced anthocyanin synthesis in rabbiteye blueberry (Vaccinium ashei: Reade). BMC Plant Biol. 2022, 22, 223. [Google Scholar] [CrossRef]
- Sun, C.; Deng, L.; Du, M.; Zhao, J.; Chen, Q.; Huang, T. A Transcriptional Network Promotes Anthocyanin Biosynthesis in Tomato Flesh. Mol. Plant 2020, 13, 42–58. [Google Scholar] [CrossRef]
- Su, J.; Zhang, C.; Zhu, L.; Yang, N.; Yang, J.; Ma, B. MdFRK2-mediated sugar metabolism accelerates cellulose accumulation in apple and poplar. Biotechnol. Biofuels 2021, 14, 137. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, Y.; Lu, W.; Ghergurovich, J.; Guo, L.; Blair, I. Upregulation of Antioxidant Capacity and Nucleotide Precursor Availability Suffices for Oncogenic Transformation. Cell Metab. 2021, 33, 94–109.e8. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, T.; Xue, Y.; Liu, F.; Zheng, R.; Zhang, Z.; Zheng, Q.; Wang, P. Multi-Omics Decoding of Potential Microbial–Genetic Synergy Underlying Polysaccharide and Glycosidic Polymer Biosynthesis in Two Cultivars of Lilium brownii var. viridulum Baker. Metabolites 2025, 15, 712. https://doi.org/10.3390/metabo15110712
Chang T, Xue Y, Liu F, Zheng R, Zhang Z, Zheng Q, Wang P. Multi-Omics Decoding of Potential Microbial–Genetic Synergy Underlying Polysaccharide and Glycosidic Polymer Biosynthesis in Two Cultivars of Lilium brownii var. viridulum Baker. Metabolites. 2025; 15(11):712. https://doi.org/10.3390/metabo15110712
Chicago/Turabian StyleChang, Tao, Yajie Xue, Fan Liu, Ran Zheng, Zaiqi Zhang, Qinfang Zheng, and Putao Wang. 2025. "Multi-Omics Decoding of Potential Microbial–Genetic Synergy Underlying Polysaccharide and Glycosidic Polymer Biosynthesis in Two Cultivars of Lilium brownii var. viridulum Baker" Metabolites 15, no. 11: 712. https://doi.org/10.3390/metabo15110712
APA StyleChang, T., Xue, Y., Liu, F., Zheng, R., Zhang, Z., Zheng, Q., & Wang, P. (2025). Multi-Omics Decoding of Potential Microbial–Genetic Synergy Underlying Polysaccharide and Glycosidic Polymer Biosynthesis in Two Cultivars of Lilium brownii var. viridulum Baker. Metabolites, 15(11), 712. https://doi.org/10.3390/metabo15110712




