Integrated Metagenomic and Metabolomic Analyses Reveal a Microbiota–Metabolite Axis Associated with Gallstone Pathogenesis
Abstract
1. Introduction
2. Materials and Methods
2.1. Global Burden of Disease (GBD) Analysis
2.2. Meta-Analysis
2.3. GWAS Design
2.4. Data Sources for GWAS Exposures and Outcomes
2.5. Selection of SNPs
2.6. Mouse Model and Sample Collection
2.7. Gallbladder Contractility Assessment
2.8. Bile Collection and Biochemical Analysis
2.9. Metagenomic Sequencing
2.10. Untargeted Metabolomics
2.11. Data Analysis
2.11.1. Microbiome and Metabolomics Data Analysis
2.11.2. Mendelian Randomization Analysis
3. Results
3.1. Global Prevalence and Regional Distribution of Cholelithiasis
3.2. Dietary Inflammatory Index (DII) and Gallstone Risk
3.3. Mendelian Randomization Analysis of Gut Microbiota and Cholelithiasis
3.4. Mendelian Randomization Analysis of Plasma Metabolites and Cholelithiasis
3.5. Mediation Analysis of Plasma Metabolites
3.6. Validation of the Lithogenic Diet–Induced Gallstone Model
3.7. Lithogenic Diet Induces Gut Microbiota Dysbiosis in Gallstone Mice
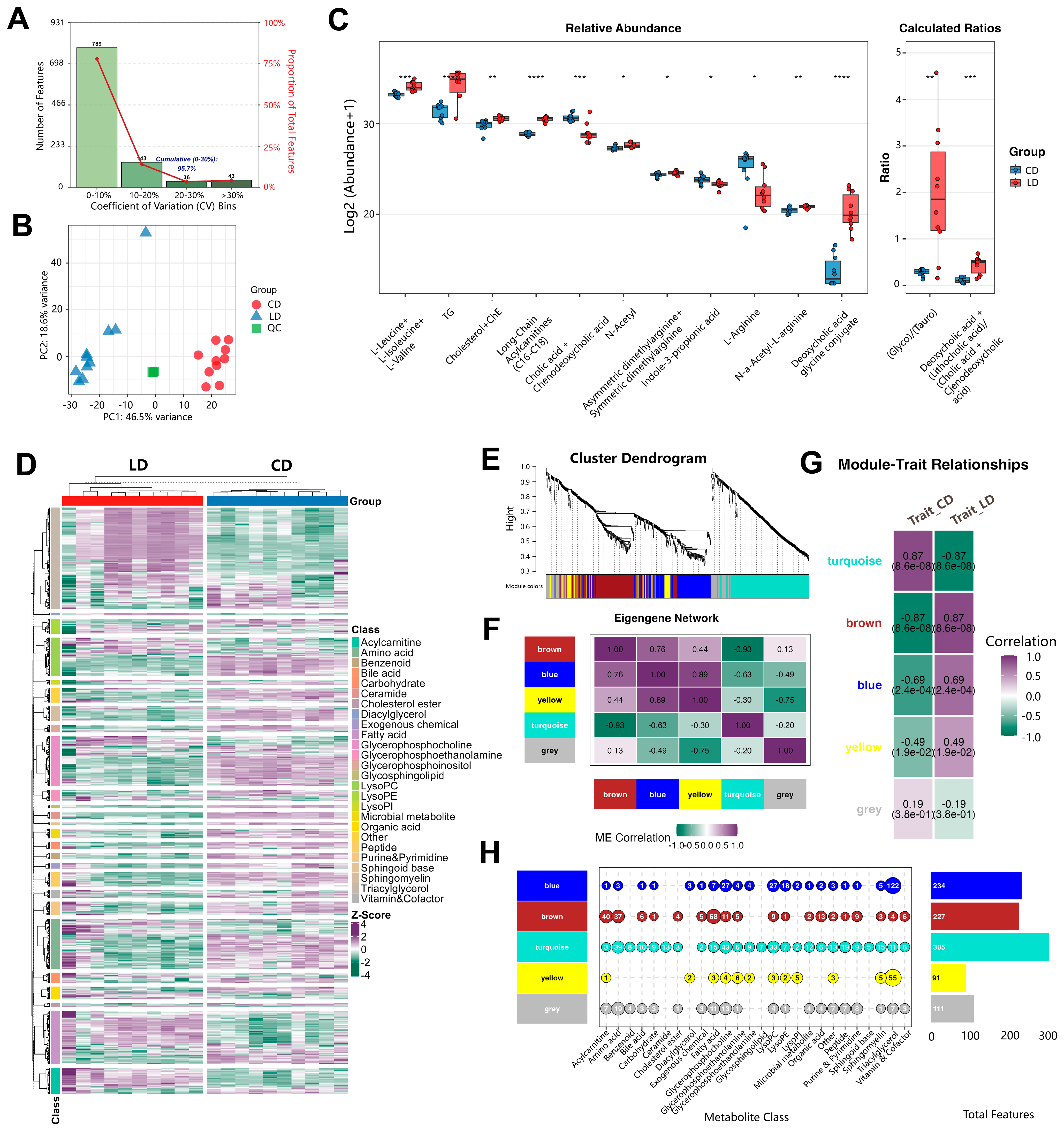
3.8. Lithogenic Diet Alters Metabolome in Gallstone Mice
3.9. Integrated Analysis Reveals Functional Associations Between Microbiota Dysbiosis and Metabolic Networks
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Unalp-Arida, A.; Ruhl, C.E. Increasing gallstone disease prevalence and associations with gallbladder and biliary tract mortality in the US. Hepatology 2023, 77, 1882–1895. [Google Scholar] [CrossRef] [PubMed]
- Lammert, F.; Gurusamy, K.; Ko, C.W.; Miquel, J.F.; Méndez-Sánchez, N.; Portincasa, P.; van Erpecum, K.J.; van Laarhoven, C.J.; Wang, D.Q. Gallstones. Nat. Rev. Dis. Primers 2016, 2, 16024. [Google Scholar] [PubMed]
- Ruhl, C.E.; Everhart, J.E. Gallstone disease is associated with increased mortality in the United States. Gastroenterology 2011, 140, 508–516. [Google Scholar] [CrossRef]
- Shabanzadeh, D.M.; Sørensen, L.T.; Jørgensen, T. Association Between Screen-Detected Gallstone Disease and Cancer in a Cohort Study. Gastroenterology 2017, 152, 1965–1974.e1961. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Jin, Q.; Shi, N.; Wang, F.; Bever, A.M.; Li, J.; Liang, L.; Hu, F.B.; Song, M.; Zeleznik, O.A.; et al. Dietary Inflammatory and Insulinemic Potentials, Plasma Metabolome and Risk of Colorectal Cancer. Metabolites 2023, 13, 744. [Google Scholar] [CrossRef]
- Wang, Z.; Yuan, C.; Zhang, Y.; Abdelaty, N.S.; Chen, C.; Shen, J.; Zhang, L.; Lu, B.; Liu, R.; Li, P. Food inflammation index reveals the key inflammatory components in foods and heterogeneity within food groups: How do we choose food? J. Adv. Res. 2025, 74, 87–98. [Google Scholar]
- Wu, W.; Pei, Y.; Wang, J.; Liang, Q.; Chen, W. Association of dietary quality indicators with gallstones in the US: NHANES 2017–2020. BMC Public Health 2025, 25, 976. [Google Scholar] [CrossRef]
- Gurusamy, K.S.; Davidson, B.R. Gallstones. BMJ 2014, 348, g2669. [Google Scholar] [CrossRef]
- Lensu, S.; Pekkala, S. Gut Microbiota, Microbial Metabolites and Human Physical Performance. Metabolites 2021, 11, 716. [Google Scholar] [CrossRef]
- Min, B.H.; Devi, S.; Kwon, G.H.; Gupta, H.; Jeong, J.J.; Sharma, S.P.; Won, S.M.; Oh, K.K.; Yoon, S.J.; Park, H.J.; et al. Gut microbiota-derived indole compounds attenuate metabolic dysfunction-associated steatotic liver disease by improving fat metabolism and inflammation. Gut Microbes 2024, 16, 2307568. [Google Scholar] [CrossRef]
- Cai, J.; Rimal, B.; Jiang, C.; Chiang, J.Y.L.; Patterson, A.D. Bile acid metabolism and signaling, the microbiota, and metabolic disease. Pharmacol. Ther. 2022, 237, 108238. [Google Scholar] [CrossRef]
- Mori, H.; Svegliati Baroni, G.; Marzioni, M.; Di Nicola, F.; Santori, P.; Maroni, L.; Abenavoli, L.; Scarpellini, E. Farnesoid X Receptor, Bile Acid Metabolism, and Gut Microbiota. Metabolites 2022, 12, 647. [Google Scholar] [CrossRef] [PubMed]
- Georgescu, D.; Lighezan, D.F.; Ionita, I.; Hadaruga, N.; Buzas, R.; Rosca, C.I.; Ionita, M.; Suceava, I.; Mitu, D.A.; Ancusa, O.E. Cholesterol Gallstones and Long-Term Use of Statins: Is Gut Microbiota Dysbiosis Bridging over Uncertainties? Diagnostics 2024, 14, 1234. [Google Scholar] [CrossRef] [PubMed]
- Bugajska, J.; Berska, J.; Pasternak, A.; Sztefko, K. Biliary Amino Acids and Telocytes in Gallstone Disease. Metabolites 2023, 13, 753. [Google Scholar] [CrossRef]
- Liu, Y.; Li, H.; Sun, T.; Sun, G.; Jiang, B.; Liu, M.; Wang, Q.; Li, T.; Cao, J.; Zhao, L.; et al. Gut microbiome and metabolome characteristics of patients with cholesterol gallstones suggest the preventive potential of prebiotics. iMeta 2025, 4, e70000. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Shao, W.; Liu, Q.; Liu, N.; Wang, Q.; Xu, J.; Zhang, X.; Weng, Z.; Lu, Q.; Jiao, L.; et al. Gut microbiota promotes cholesterol gallstone formation by modulating bile acid composition and biliary cholesterol secretion. Nat. Commun. 2022, 13, 252. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, H.; Huang, W.; Guo, X.; Yu, L.; Shan, J.; Deng, X.; Liu, J.; Li, W.; Shen, W.; et al. Bacteroides fragilis alleviates necrotizing enterocolitis through restoring bile acid metabolism balance using bile salt hydrolase and inhibiting FXR-NLRP3 signaling pathway. Gut Microbes 2024, 16, 2379566. [Google Scholar] [CrossRef]
- Xia, Y.; Xu, Y.; Liu, Q.; Zhang, J.; Zhang, Z.; Jia, Q.; Tang, Q.; Jing, X.; Li, J.; Chen, J.; et al. Glutaredoxin 1 regulates cholesterol metabolism and gallstone formation by influencing protein S-glutathionylation. Metabolism 2023, 145, 155610. [Google Scholar] [CrossRef]
- Ye, X.; Huang, D.; Dong, Z.; Wang, X.; Ning, M.; Xia, J.; Shen, S.; Wu, S.; Shi, Y.; Wang, J.; et al. FXR Signaling-Mediated Bile Acid Metabolism Is Critical for Alleviation of Cholesterol Gallstones by Lactobacillus Strains. Microbiol. Spectr. 2022, 10, e0051822. [Google Scholar] [CrossRef]
- Boef, A.G.; Dekkers, O.M.; le Cessie, S. Mendelian randomization studies: A review of the approaches used and the quality of reporting. Int. J. Epidemiol. 2015, 44, 496–511. [Google Scholar] [CrossRef]
- Larsson, S.C.; Butterworth, A.S.; Burgess, S. Mendelian randomization for cardiovascular diseases: Principles and applications. Eur. Heart J. 2023, 44, 4913–4924. [Google Scholar] [CrossRef]
- Skrivankova, V.W.; Richmond, R.C.; Woolf, B.A.R.; Yarmolinsky, J.; Davies, N.M.; Swanson, S.A.; VanderWeele, T.J.; Higgins, J.P.T.; Timpson, N.J.; Dimou, N.; et al. Strengthening the Reporting of Observational Studies in Epidemiology Using Mendelian Randomization: The STROBE-MR Statement. JAMA 2021, 326, 1614–1621. [Google Scholar] [CrossRef]
- Qin, Y.; Havulinna, A.S.; Liu, Y.; Jousilahti, P.; Ritchie, S.C.; Tokolyi, A.; Sanders, J.G.; Valsta, L.; Brożyńska, M.; Zhu, Q.; et al. Combined effects of host genetics and diet on human gut microbiota and incident disease in a single population cohort. Nat. Genet. 2022, 54, 134–142. [Google Scholar] [CrossRef]
- Kurki, M.I.; Karjalainen, J.; Palta, P.; Sipilä, T.P.; Kristiansson, K.; Donner, K.M.; Reeve, M.P.; Laivuori, H.; Aavikko, M.; Kaunisto, M.A.; et al. FinnGen provides genetic insights from a well-phenotyped isolated population. Nature 2023, 613, 508–518. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Lu, T.; Pettersson-Kymmer, U.; Stewart, I.D.; Butler-Laporte, G.; Nakanishi, T.; Cerani, A.; Liang, K.Y.H.; Yoshiji, S.; Willett, J.D.S.; et al. Genomic atlas of the plasma metabolome prioritizes metabolites implicated in human diseases. Nat. Genet. 2023, 55, 44–53. [Google Scholar] [CrossRef]
- Fu, H.; Zhao, S.; Song, S.; Xie, Q. Gut microbiota causally affects drug-induced liver injury via plasma metabolites: A Mendelian randomization study. Front. Microbiol. 2024, 15, 1432049. [Google Scholar] [CrossRef]
- Sanna, S.; van Zuydam, N.R.; Mahajan, A.; Kurilshikov, A.; Vich Vila, A.; Võsa, U.; Mujagic, Z.; Masclee, A.A.M.; Jonkers, D.; Oosting, M.; et al. Causal relationships among the gut microbiome, short-chain fatty acids and metabolic diseases. Nat. Genet. 2019, 51, 600–605. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Wang, C.; Li, W. Genetic insights into the effect of trace elements on cardiovascular diseases: Multi-omics Mendelian randomization combined with linkage disequilibrium score regression analysis. Front. Immunol. 2024, 15, 1459465. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Cai, P.; Hu, A.; Li, J.; Li, X.; Dang, Y. The role of 1400 plasma metabolites in gastric cancer: A bidirectional Mendelian randomization study and metabolic pathway analysis. Medicine 2024, 103, e40612. [Google Scholar] [CrossRef]
- Wang, Q.; Dai, H.; Hou, T.; Hou, Y.; Wang, T.; Lin, H.; Zhao, Z.; Li, M.; Zheng, R.; Wang, S.; et al. Dissecting Causal Relationships Between Gut Microbiota, Blood Metabolites, and Stroke: A Mendelian Randomization Study. J. Stroke 2023, 25, 350–360. [Google Scholar] [CrossRef]
- Xu, S.; Liu, Y.; Wang, Q.; Liu, F.; Xian, Y.; Xu, F.; Liu, Y. Gut microbiota in combination with blood metabolites reveals characteristics of the disease cluster of coronary artery disease and cognitive impairment: A Mendelian randomization study. Front. Immunol. 2023, 14, 1308002. [Google Scholar] [CrossRef] [PubMed]
- Papadimitriou, N.; Dimou, N.; Tsilidis, K.K.; Banbury, B.; Martin, R.M.; Lewis, S.J.; Kazmi, N.; Robinson, T.M.; Albanes, D.; Aleksandrova, K.; et al. Physical activity and risks of breast and colorectal cancer: A Mendelian randomisation analysis. Nat. Commun. 2020, 11, 597. [Google Scholar] [CrossRef] [PubMed]
- Pierce, B.L.; Burgess, S. Efficient design for Mendelian randomization studies: Subsample and 2-sample instrumental variable estimators. Am. J. Epidemiol. 2013, 178, 1177–1184. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Gill, D.; Giovannucci, E.L.; Larsson, S.C. Obesity, Type 2 Diabetes, Lifestyle Factors, and Risk of Gallstone Disease: A Mendelian Randomization Investigation. Clin. Gastroenterol. Hepatol. 2022, 20, e529–e537. [Google Scholar] [CrossRef]
- Zhu, Q.; Xing, Y.; Fu, Y.; Chen, X.; Guan, L.; Liao, F.; Zhou, X. Causal association between metabolic syndrome and cholelithiasis: A Mendelian randomization study. Front. Endocrinol. 2023, 14, 1180903. [Google Scholar] [CrossRef]
- Wang, B.; Xiong, Y.; Li, R.; Zhang, S. Depression increases the risk of gallstone: A cross-sectional study and Mendelian randomization analysis. J. Affect. Disord. 2024, 362, 606–614. [Google Scholar] [CrossRef]
- Wang, B.; Bai, X.; Yang, Y.; Yang, H. Possible linking and treatment between Parkinson’s disease and inflammatory bowel disease: A study of Mendelian randomization based on gut-brain axis. J. Transl. Med. 2025, 23, 45. [Google Scholar] [CrossRef]
- Carey, M.C. Critical tables for calculating the cholesterol saturation of native bile. J. Lipid Res. 1978, 19, 945–955. [Google Scholar] [CrossRef]
- Kanai, M.; Akiyama, M.; Takahashi, A.; Matoba, N.; Momozawa, Y.; Ikeda, M.; Iwata, N.; Ikegawa, S.; Hirata, M.; Matsuda, K.; et al. Genetic analysis of quantitative traits in the Japanese population links cell types to complex human diseases. Nat. Genet. 2018, 50, 390–400. [Google Scholar] [CrossRef]
- Orrù, V.; Steri, M.; Sidore, C.; Marongiu, M.; Serra, V.; Olla, S.; Sole, G.; Lai, S.; Dei, M.; Mulas, A.; et al. Complex genetic signatures in immune cells underlie autoimmunity and inform therapy. Nat. Genet. 2020, 52, 1036–1045. [Google Scholar] [CrossRef]
- Zagkos, L.; Dib, M.J.; Pinto, R.; Gill, D.; Koskeridis, F.; Drenos, F.; Markozannes, G.; Elliott, P.; Zuber, V.; Tsilidis, K.; et al. Associations of genetically predicted fatty acid levels across the phenome: A mendelian randomisation study. PLoS Med. 2022, 19, e1004141. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhu, H.; Pan, L.; Zhang, M.; Wan, X.; Xu, H.; Hua, R.; Zhu, M.; Gao, P. Systemic inflammatory regulators and risk of acute-on-chronic liver failure: A bidirectional mendelian-randomization study. Front. Cell Dev. Biol. 2023, 11, 1125233. [Google Scholar] [CrossRef]
- Luo, Y.; Gao, X.; Xiao, M.; Yang, F.; Zhu, X.; Qiao, G.; Xiang, C.; Tao, J. Association between dietary inflammatory index and gallstones in US adults. Front. Nutr. 2024, 11, 1403438. [Google Scholar] [CrossRef]
- Jiang, C.; Shao, Y. Characterizing the relationships between dietary indices, gallstone prevalence and the need for gallbladder surgery in the general US population. Front. Nutr. 2024, 11, 1392960. [Google Scholar] [CrossRef]
- Cheng, J.; Zhuang, Q.; Wang, W.; Li, J.; Zhou, L.; Xu, Y.; Zhang, H.; Zhang, Z.; Zhou, F.; Yang, D.; et al. Association of pro-inflammatory diet with increased risk of gallstone disease: A cross-sectional study of NHANES January 2017-March 2020. Front. Nutr. 2024, 11, 1344699. [Google Scholar] [CrossRef]
- Wang, X.; Yu, W.; Jiang, G.; Li, H.; Li, S.; Xie, L.; Bai, X.; Cui, P.; Chen, Q.; Lou, Y.; et al. Global Epidemiology of Gallstones in the 21st Century: A Systematic Review and Meta-Analysis. Clin. Gastroenterol. Hepatol. 2024, 22, 1586–1595. [Google Scholar] [CrossRef]
- Sánchez-García, M.; León-Wu, K.; de Miguel-Ibáñez, R.; López-Juárez, N.; Ramírez-Rentería, C.; Espinosa-Cárdenas, E.; Sosa-Eroza, E.; García-Sáenz, M.R. Metabolic Changes in Patients with Premature Ovarian Insufficiency: Adipose Tissue Focus–A Narrative Review. Metabolites 2025, 15, 242. [Google Scholar] [CrossRef]
- Li, Q.; Cui, Y.; Xu, B.; Wang, Y.; Lv, F.; Li, Z.; Li, H.; Chen, X.; Peng, X.; Chen, Y.; et al. Main active components of Jiawei Gegen Qinlian decoction protects against ulcerative colitis under different dietary environments in a gut microbiota-dependent manner. Pharmacol. Res. 2021, 170, 105694. [Google Scholar] [CrossRef] [PubMed]
- Bavineni, M.; Wassenaar, T.M.; Agnihotri, K.; Ussery, D.W.; Lüscher, T.F.; Mehta, J.L. Mechanisms linking preterm birth to onset of cardiovascular disease later in adulthood. Eur. Heart J. 2019, 40, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- Aljabbari, A.; Kihara, S.; Mota-Santiago, P.; Rades, T.; Boyd, B.J. Adsorption of bile salts onto crystalline ritonavir particles under simulated gastrointestinal conditions. Colloids Surf. B Biointerfaces 2025, 245, 114283. [Google Scholar] [CrossRef]
- Gultekin, H.; Erdem, S.R.; Emre-Aydingoz, S.; Tuncer, M. The role of nitric oxide in the electrical field stimulation-induced contractions of sphincter of oddi and gallbladder strips in Guinea pigs. J. Pharmacol. Sci. 2006, 101, 240–244. [Google Scholar] [CrossRef]
- Trautwein, E.A.; Siddiqui, A.; Hayes, K.C. Modeling plasma lipoprotein-bile lipid relationships: Differential impact of psyllium and cholestyramine in hamsters fed a lithogenic diet. Metabolism 1993, 42, 1531–1540. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.H.; Portincasa, P.; Mendez-Sanchez, N.; Uribe, M.; Wang, D.Q. Effect of ezetimibe on the prevention and dissolution of cholesterol gallstones. Gastroenterology 2008, 134, 2101–2110. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Fang, S.; Wei, H.; He, M.; Fu, H.; Xiong, X.; Zhou, Y.; Wu, J.; Gao, J.; Yang, H.; et al. Prevotella copri increases fat accumulation in pigs fed with formula diets. Microbiome 2021, 9, 175. [Google Scholar] [CrossRef]
- Hankir, M.K.; Seyfried, F.; Schellinger, I.N.; Schlegel, N.; Arora, T. Leaky Gut as a Potential Culprit for the Paradoxical Dysglycemic Response to Gastric Bypass-Associated Ileal Microbiota. Metabolites 2021, 11, 153. [Google Scholar] [CrossRef]
- Fan, M.; Zhang, J.; Tsai, C.W.; Orlando, B.J.; Rodriguez, M.; Xu, Y.; Liao, M.; Tsai, M.F.; Feng, L. Structure and mechanism of the mitochondrial Ca2+ uniporter holocomplex. Nature 2020, 582, 129–133. [Google Scholar] [CrossRef]
- Sun, D.; Niu, Z.; Zheng, H.X.; Wu, F.; Jiang, L.; Han, T.Q.; Wei, Y.; Wang, J.; Jin, L. A Mitochondrial DNA Variant Elevates the Risk of Gallstone Disease by Altering Mitochondrial Function. Cell. Mol. Gastroenterol. Hepatol. 2021, 11, 1211–1226.e1215. [Google Scholar] [CrossRef]
- Xie, S.; Li, J.; Lyu, F.; Xiong, Q.; Gu, P.; Chen, Y.; Chen, M.; Bao, J.; Zhang, X.; Wei, R.; et al. Novel tripeptide RKH derived from Akkermansia muciniphila protects against lethal sepsis. Gut 2023, 73, 78–91. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Shi, J.; Gu, C.; Yuan, J.; Huang, C.; Li, X.; Zhou, K.; Qi, J. Akkermansia muciniphila: A next-generation gut probiotic supporting neurorepair and functional recovery. Neural Regen. Res. 2025. [Google Scholar] [CrossRef]







| Ancestry | Participants | Consortium | GWAS ID | Ref | |
|---|---|---|---|---|---|
| Gut microbiota (473) | European | 5959 | FINRISK | GCST90032172-GCST90032644 | [23] |
| Plasma metabolites (1400) | European | 8288 | CLSA | GCST90199621-GCST90201020 | [25] |
| Cholelithiasis | European | 44,582 cases 397,583 controls | FinnGen R11 | K11_CHOLELITH | [24] |
| Study | Country | Source of Subjects | Morbidity Risk (%) | Odds Ratio (95% CI) | |
|---|---|---|---|---|---|
| High DII | Low DII | ||||
| Luo et al. [43] | USA | Cross-sectional + PSM | 11.4 | 10.0 | 1.14 (1.01–1.29) |
| Jiang et al. [44] | USA | Cross-sectional | 11.0 | 10.0 | 1.10 (1.01–1.19) |
| Wu et al. [7] | USA | Cross-sectional | 14.4 | 10.0 | 1.44 (1.08–1.91) |
| Cheng et al. [45] | USA | Cross-sectional | 15.2 | 10.0 | 1.52 (1.19–1.93) |
| Mediators | Gut Microbiota to Cholelithiasis | |||||
|---|---|---|---|---|---|---|
| GM 1 to Mediator | Mediator to Cholelithiasis | Total Effect | Mediation Effect | |||
| GM | Beta1 | Beta2 | Effect | Proportion (95% CI) | ||
| N-acetylarginine | Akkermansia muciniphila B | 0.072 | 0.12 | −0.035 | 0.0087 | 25.10 (0–60.38)% |
| Glycodeoxycholate | CAG-448 sp000433415 | −0.19 | 0.14 | −0.098 | −0.027 | 27.96 (0–63.11)% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, H.; Luo, K.; Jin, Y.; Sun, X.; Zhang, X.; Zhao, Y.; Muhammad, Y.; Huang, A.; Yin, P.; Zhang, G. Integrated Metagenomic and Metabolomic Analyses Reveal a Microbiota–Metabolite Axis Associated with Gallstone Pathogenesis. Metabolites 2025, 15, 714. https://doi.org/10.3390/metabo15110714
Bai H, Luo K, Jin Y, Sun X, Zhang X, Zhao Y, Muhammad Y, Huang A, Yin P, Zhang G. Integrated Metagenomic and Metabolomic Analyses Reveal a Microbiota–Metabolite Axis Associated with Gallstone Pathogenesis. Metabolites. 2025; 15(11):714. https://doi.org/10.3390/metabo15110714
Chicago/Turabian StyleBai, He, Kai Luo, Yuzhu Jin, Xu Sun, Xudong Zhang, Yuting Zhao, Yaqoob Muhammad, Anliang Huang, Peiyuan Yin, and Guixin Zhang. 2025. "Integrated Metagenomic and Metabolomic Analyses Reveal a Microbiota–Metabolite Axis Associated with Gallstone Pathogenesis" Metabolites 15, no. 11: 714. https://doi.org/10.3390/metabo15110714
APA StyleBai, H., Luo, K., Jin, Y., Sun, X., Zhang, X., Zhao, Y., Muhammad, Y., Huang, A., Yin, P., & Zhang, G. (2025). Integrated Metagenomic and Metabolomic Analyses Reveal a Microbiota–Metabolite Axis Associated with Gallstone Pathogenesis. Metabolites, 15(11), 714. https://doi.org/10.3390/metabo15110714








