Land Use Shapes the Rhizosphere Microbiome and Metabolome of Naturally Growing Barbarea vulgaris
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Trial
2.2. Barbarea vulgaris Rhizosphere Soil Sampling
2.3. Rhizosphere Soil Microbial Phenotypic Structure Assessment
2.4. Mass Spectrometric Assessment of Untargeted Metabolites in Rhizosphere Soil
- (a)
- GC–MS/MS analysis of untargeted metabolites—in that case, to the extract 100 µL of methoxyamine hydrochloride solution (0.02 g·mL−1 in pyridine) was added and allowed to react for 60 min at 30 °C. Next, 100 µL of MSTFA was added, and the mixture was incubated at 50 °C for 30 min. After incubation, 2 µL was injected into a Thermo Finningan gas chromatograph linked to a triple quadruple mass spectrometer (Trace 1310 GC-TSQ 9000 MS) equipped with an SSL injector port set at 250 °C. The metabolites were separated through an HP-5MS capillary column (30 m × 0.25 mm × 0.25 µm; Hewlett Packard, Palo Alto, CA, USA). Helium was used as the carrier gas at a flow rate of 1 mL·min−1. The oven temperature program started at 40 °C for 7 min−1, followed by a ramp of 5 °C·min−1 to 285 °C, held at this final temperature for 7 min. The solvent delay time was set at 3.5 min. The ion source was set at 230 °C and operated in electron ionization mode (EI) at 70 eV. The mass spectrometer was operated in full scan mode with an ion scanning range of 50–550 m/z. Primary data analysis was conducted through Xcalibur 4.0 software, followed by MS-DIAL version 4.9 software according to the procedures described by Lai et al. [24] and Kovacs et al. [7]. Process blanks (extraction solvent without soil sample) were prepared and analyzed alongside samples to identify background contamination, and features detected in blanks at >20% of the average intensity were excluded from downstream analysis. Further, the criterion for compound identification was a mass-spectrum matching score of ≥80%. Each sample’s total ion chromatogram (TIC) was used for peak-area integration. The results were expressed as a percentage of the relative peak area of a peak for each soil sample, which was calculated by dividing the peak area by the total peak area of all identified peaks in each chromatogram.
- (b)
- MALDI TOF/TOF MS analysis of untargeted metabolites—In that case, the remaining part of the extract was dried under a gentle stream of N2, after which it was reconstituted in a 25 µL mixture of 0.05% TFA, water and 2% ammonium hydroxide. Next, 2.5 µL of reconstituted sample was mixed with 2.5 µL of 9-aminoacridine (9-AA matrix solution of 10 mg·mL−1 in 0.1% TFA in acetone). One microliter of this obtained sample mixture was pipetted onto a MALDI-TOF target (MTP 384 polished steel target Bruker Daltonics, Bremen, Germany) and allowed to air dry at room temperature. Mass spectra were acquired with a MALDI TOF/TOF IMS analyser (Autoflex maX, Bruker Daltonics, Bremen, Germany) equipped with a Smartbeam-II laser (Nd:Yag—355 nm). Prior to analysis, calibration was performed in linear mode using a standard mixture of metabolites (lactate, succinate, malate, AMP, ATP, and CoA). The instrument was operated in linear negative ion mode, which provides enhanced sensitivity and broader mass range coverage for low molecular weight metabolites while minimizing fragmentation of labile compounds. For acquisition, the laser intensity was set to 35%, and the laser frequency was set to 500 Hz. Approximately 2000 laser shots were totaled per raster position. Data analysis was performed by Bruker data management software flexAnalysis version 3.4 with the centroid peak detection algorithm and the baseline subtraction TopHat. The R-MetaboList 2 and rMSIfragment tools were subsequently used for metabolite annotation according to the procedures of Baquer et al. [25] and Peris-Diaz et al. [26].
2.5. Statistical Analysis
3. Results
3.1. Microbiome Composition of the Rhizosphere Soil of Barbarea vulgaris Growing Under Different Land Uses
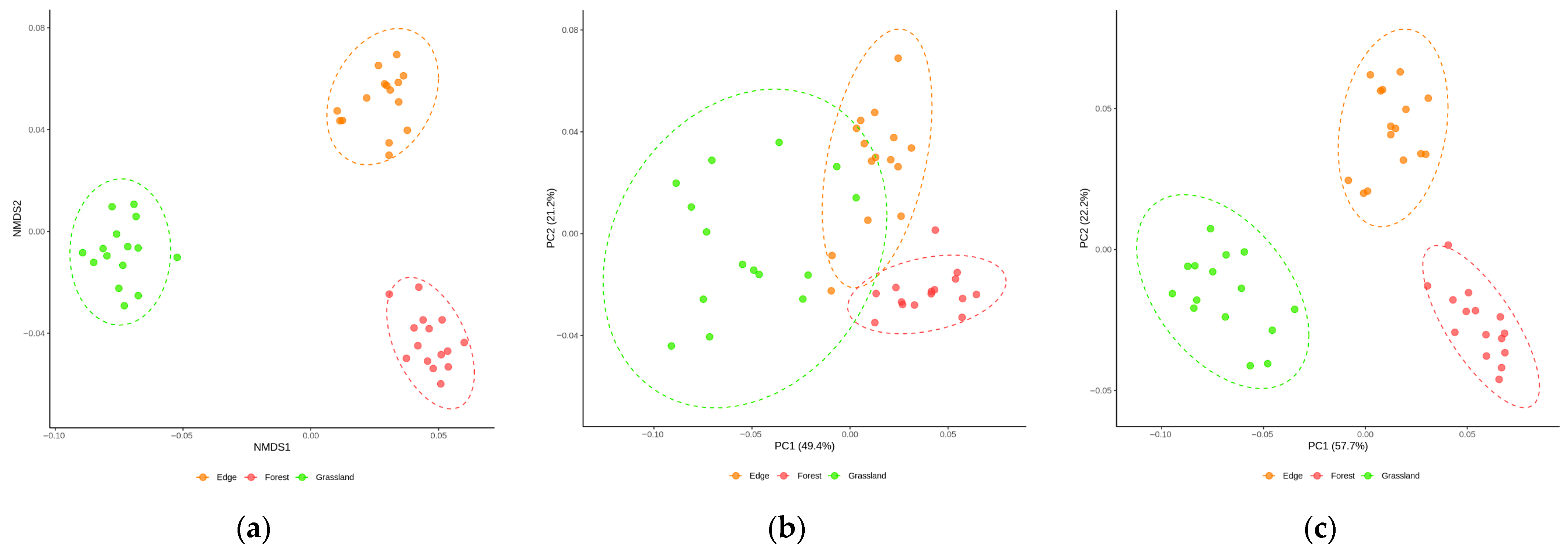
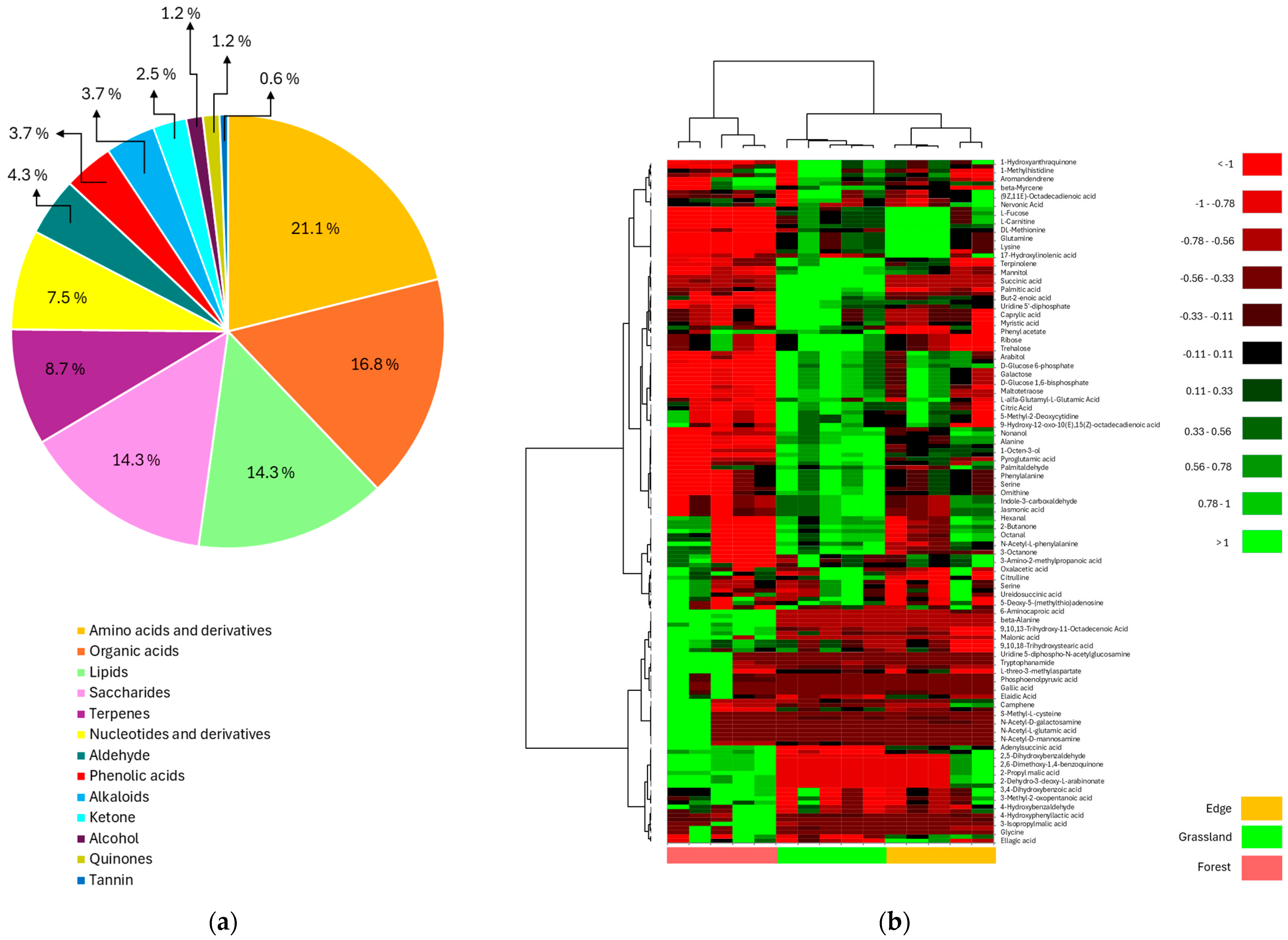
3.2. Untargeted Metabolite Analysis of Rhizosphere Soil of Barbarea vulgaris Grown Under Different Land Uses
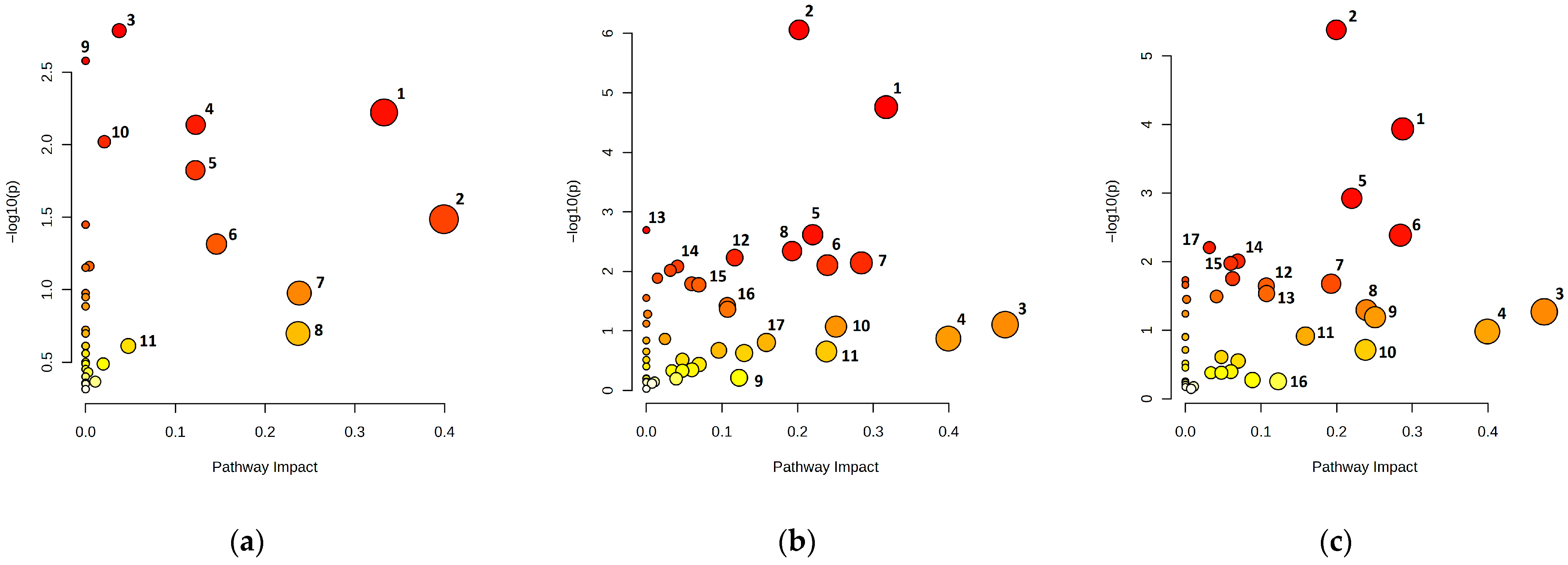
3.3. Metabolic Pathway Analysis of the Barbarea vulgaris Rhizosphere Soil Across Land Use Types
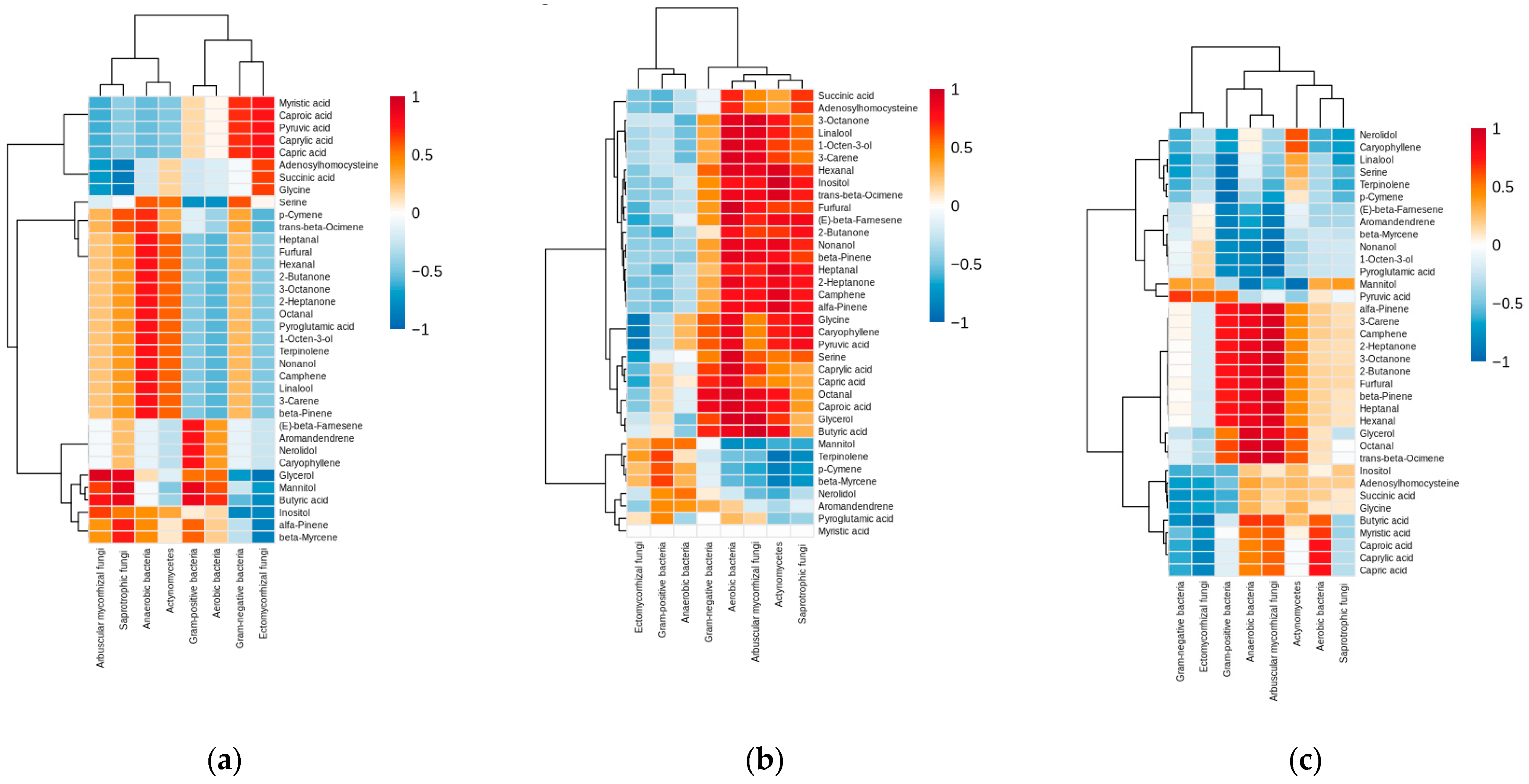
3.4. Correlations Between the Microbiota and Metabolites of Barbarea vulgaris Rhizosphere Soil
3.5. Co-Inertia Analysis of Land Use and the Microbiome-Metabolome of Barbarea vulgaris Rhizosphere Soil
4. Discussion
4.1. Land-Use-Driven Microbiome–Metabolome Coupling in the Barbarea vulgaris Rhizosphere
4.2. Ecological Implications
4.3. Current Research Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bouwmeester, H.; Dong, L.; Wippel, K.; Hofland, T.; Smilde, A. The chemical interaction between plants and the rhizosphere microbiome. Trends Pant Sci. 2025, 30, 1002–1019. [Google Scholar] [CrossRef] [PubMed]
- Alawiye, T.T.; Babalola, O.O. Bacterial diversity and community structure in typical plant rhizosphere. Diversity 2019, 11, 179. [Google Scholar] [CrossRef]
- Wankhade, A.; Wilkinson, E.; Britt, D.W.; Kaundal, A. A Review of Plant–Microbe Interactions in the Rhizosphere and the Role of Root Exudates in Microbiome Engineering. Appl. Sci. 2025, 15, 7127. [Google Scholar] [CrossRef]
- Xue, P.; Minasny, B.; McBratney, A.B. Land-use affects soil microbial co-occurrence networks and their putative functions. Appl. Soil Ecol. 2022, 169, 104184. [Google Scholar] [CrossRef]
- Wang, D.; Deng, S.; Yang, H.; Li, N.; Feng, Q.; Liu, J.; Yin, H. The microbial network exhibits higher complexity in the rhizosphere than in bulk soils along elevational gradients in the alpine forests. Appl. Soil Ecol. 2025, 213, 106264. [Google Scholar] [CrossRef]
- Liu, X.; Fan, X.; Zhang, M.; Zhang, H.; Yue, Y.; Wu, J.; Teng, W.; Mu, N.; Teng, K.; Wen, H. Insights into the interlinkages between rhizosphere soil extracellular enzymes and microbiome assemblages across soil profiles in grasslands. Appl. Soil Ecol. 2025, 211, 106139. [Google Scholar] [CrossRef]
- Kovacs, E.D.; Rusu, T.; Kovacs, M.H. Sustainable soil volatilome: Discrimination of land uses through GC–MS-identified volatile organic compounds. Separations 2025, 12, 92. [Google Scholar] [CrossRef]
- Sokol, N.W.; Foley, M.M.; Blazewicz, S.J.; Bhattacharyya, A.; DiDonato, N.; Estera-Molina, K.; Firestone, M.; Greenlon, A.; Hungate, B.A.; Kimbrel, J.; et al. The path from root input to mineral-associated soil carbon is dictated by habitat-specific microbial traits and soil moisture. Soil Biol. Biochem. 2024, 193, 109367. [Google Scholar] [CrossRef]
- Gupta, V.V.S.R.; Tiedje, J.M. Ranking environmental and edaphic attributes driving soil microbial community structure and activity with special attention to spatial and temporal scales. mLife 2024, 3, 21–41. [Google Scholar] [CrossRef]
- Yusuf, A.; Li, M.; Zhang, S.Y.; Odedishemi-Ajibade, F.; Luo, R.F.; Wu, Y.X.; Zhang, T.T.; Yunusa Ugya, A.; Zhang, Y.; Duan, S. Harnessing plant–microbe interactions: Strategies for enhancing resilience and nutrient acquisition for sustainable agriculture. Front. Plant Sci. 2025, 16, 1503730. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, P.; Sharma, N.; Tapwal, A.; Kumar, A.; Verma, G.S.; Meena, M.; Seth, C.S.; Swapnil, P. Soil Microbiome: Diversity, Benefits and Interactions with Plants. Sustainability 2025, 15, 14643. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, H.; Kang, Y.; Wen, T. Small molecule metabolites drive plant rhizosphere microbial community assembly patterns. Front. Microbiol. 2025, 16, 1503537. [Google Scholar] [CrossRef]
- Chen, Q.; Song, Y.; An, Y.; Lu, Y.; Zhong, G. Mechanisms and Impact of Rhizosphere Microbial Metabolites on Crop Health, Traits, Functional Components: A Comprehensive Review. Molecules 2024, 29, 5922. [Google Scholar] [CrossRef]
- Fadiji, A.E.; Barmukh, R.; Varshney, R.K.; Singh, B.K. Exploring the connectivity between rhizosphere microbiomes and the plant genes: A way forward for sustainable increase in primary productivity. J. Sustain. Agric. Environ. 2023, 2, 424–443. [Google Scholar] [CrossRef]
- Luo, C.; He, Y.; Chen, Y. Rhizosphere microbiome regulation: Unlocking the potential for plant growth. Curr. Res. Microb. Sci. 2025, 8, 100322. [Google Scholar] [CrossRef]
- Marfil-Santana, M.D.; Martínez-Cárdenas, A.; Ruíz-Hernández, A.; Vidal-Torres, M.; Márquez-Velázquez, N.A.; Figueroa, M.; Prieto-Davó, A. A meta-omics analysis unveils the shift in microbial community structures and metabolomics profiles in mangrove sediments treated with a selective actinobacterial isolation procedure. Molecules 2021, 26, 7332. [Google Scholar] [CrossRef]
- Wu, G.; Zhang, M.; Han, P.; Guo, D.; Shi, Y.; Mu, D.; Li, X.; Wu, X. Microbial community succession patterns and metabolite profiles in cigar tobacco during different mildew stages. Ind. Crop. Prod. 2024, 222, 120005. [Google Scholar] [CrossRef]
- Reinula, I.; Träger, S.; Järvine, H.T.; Kuningas, V.M.; Kaldra, M.; Aavik, T. Beware of the impact of land use legacy on genetic connectivity: A case study of the long-lived perennial Primula veris. Biol. Conserv. 2024, 292, 110518. [Google Scholar] [CrossRef]
- Luo, X.; Tong, Z.; Xie, Y.; An, R.; Yang, Z.; Liu, Y. Land Use Change under Population Migration and Its Implications for Human–Land Relationship. Land 2022, 11, 934. [Google Scholar] [CrossRef]
- Nirhamo, A.; Aakala, T.; Kouki, J. Forest biodiversity in boreal Europe: Species richness and turnover among old-growth forests, managed forests and clearcut sites. Biol. Conserv. 2025, 306, 111147. [Google Scholar] [CrossRef]
- Kovacs, E.D.; Kovacs, M.H.; Barcelo, D.; Pereira, P. Nonsteroidal anti-inflammatory drugs impact the microbial community in three different soil types—A laboratory experiment. Case Stud. Chem. Environ. Eng. 2024, 10, 100833. [Google Scholar] [CrossRef]
- Kovacs, E.D.; Silaghi-Dumitrescu, L.; Roman, C.; Tian, D. Structural and metabolic profiling of Lycopersicon esculentum rhizosphere microbiota artificially exposed at commonly used non-steroidal anti-inflammatory drugs. Microorganisms 2022, 10, 254. [Google Scholar] [CrossRef]
- Blight, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Lai, Z.; Tsugawa, H.; Wohlgemuth, G.; Mehta, S.; Mueller, M.; Zheng, Y.; Ogiwara, A.; Meissen, J.; Showalter, M.; Takeuchi, K.; et al. Identifying metabolites by integrating metabolome databases with mass spectrometry cheminformatics. Nat. Methods 2018, 15, 53–56. [Google Scholar] [CrossRef]
- Baquer, G.; Semente, L.; Rafols, P.; Martin-Saiz, L.; Bookmeyer, C.; Fernandez, J.A.; Correig, X.; Garcia-Altares, M. rMSIfragment: Improving MALDI-MSI lipidomics through automated in-source fragment annotation. J. Cheminform. 2023, 15, 80. [Google Scholar] [CrossRef] [PubMed]
- Peris-Diaz, M.D.; Sweeney, S.R.; Rodak, O.; Sentandreu, E.; Tiziani, S. R-MetaboList 2: A flexible tool for metabolite annotation from high-resolution data-independent acquisition mass spectrometry analysis. Metabolites 2019, 9, 187. [Google Scholar] [CrossRef]
- Gul, S.; Whalen, J.K. Chapter six—Perspectives and strategies to increase the microbial-derived soil organic matter that persists in agroecosystems. Adv. Agron. 2022, 175, 347–401. [Google Scholar] [CrossRef]
- Samaniego, T.; Ramirez, J.; Solórzano, R. Litter Decomposition Rates of Four Species of Agroecological Importance in the Peruvian Coast and Andean Highland. Nitrogen 2024, 5, 772–789. [Google Scholar] [CrossRef]
- Wegner, R.; Plassmann, M.; Sauerland, L.; Carter, A.; Monteux, S.; Oburger, E.; Wild, B. Back to the roots: Characterizing root exudates of dominant tundra plants to improve the understanding of plant-soil interactions in a changing arctic. Soil Biol. Biochem. 2025, 209, 109897. [Google Scholar] [CrossRef]
- Shen, X.; Yang, F.; Xiao, C.; Zhou, Y. Increased contribution of root exudates to soil carbon input during grassland degradation. Soil Biol. Biochem. 2020, 146, 107817. [Google Scholar] [CrossRef]
- Maciel-Rodríguez, M.; Moreno-Valencia, F.D.; Plascencia-Espinosa, M. The Role of Plant Growth-Promoting Bacteria in Soil Restoration: A Strategy to Promote Agricultural Sustainability. Microorganisms 2025, 13, 1799. [Google Scholar] [CrossRef]
- Du, X.F.; Liu, H.W.; Li, Y.B.; Li, B.; Han, X.; Li, Y.H.; Mahamood, M.; Li, Q. Soil community richness and composition jointly influence the multifunctionality of soil along the forest-steppe ecotone. Ecol. Indic. 2022, 139, 108900. [Google Scholar] [CrossRef]
- Marfo, T.D.; Datta, R.; Vranova, V.; Ekielski, A. Ecotone dynamics and stability from soil perspective: Forest-Agriculture land transition. Agriculture 2019, 9, 228. [Google Scholar] [CrossRef]
- Ortiz-Colin, P.; Hulshof, C.M. Ecotones as Windows into Organismal-to-Biome Scale Responses across Neotropical Forests. Plants 2024, 13, 2396. [Google Scholar] [CrossRef]
- Aslan, C.E.; Zachmann, L.; Epanchin-Niell, R.S.; Brunson, M.W.; Veloz, S.; Sikes, B.A. Soil characteristics and bare ground cover differ among jurisdictions and disturbance histories in Western US protected area-centered ecosystems. Front. Ecol. Evol. 2022, 10, 1053548. [Google Scholar] [CrossRef]
- Canarini, A.; Kaiser, C.; Merchant, A.; Richter, A.; Wanek, W. Root exudation of primary metabolites: Mechanisms and their roles in plant responses to environmental stimuli. Front. Plant Sci. 2019, 10, 157. [Google Scholar] [CrossRef]
- Dussarrat, T.; Latorre, C.; Barros Santos, M.C.; Aguado-Norese, C.; Prigent, S.; Díaz, F.P.; Rolin, D.; González, M.; Müller, C.; Gutiérrez, R.A.; et al. Rhizochemistry and soil bacterial community are tailored to natural stress gradients. Soil Biol. Biochem. 2025, 202, 109662. [Google Scholar] [CrossRef]
- Li, S.; Ahmed, W.; Jiang, T.; Yang, D.; Yang, L.; Hu, X.; Zhao, M.; Peng, X.; Yang, Y.; Zhang, W.; et al. Amino acid metabolism pathways as key regulators of nitrogen distribution in tobacco: Insights from transcriptome and WGCNA analyses. BMC Plant Biol. 2025, 25, 393. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, G.; Zhao, P.; Zhou, Q.; Zhao, X. The abundance of certain metabolites responds to drought stress in the highly drought tolerant plant Caragana korshinskii. Acta Physiol. Plant. 2017, 39, 116. [Google Scholar] [CrossRef]
- Walker, R.P.; Chen, Z.H.; Famiani, F. Gluconeogenesis in Plants: A Key Interface between Organic Acid/Amino Acid/Lipid and Sugar Metabolism. Molecules 2021, 26, 5129. [Google Scholar] [CrossRef]
- Nunes-Nesi, A.; Fernie, A.R.; Stitt, M. Metabolic and signaling aspects underpinning the regulation of plant carbon nitrogen interactions. Mol. Plant 2010, 3, 973–996. [Google Scholar] [CrossRef] [PubMed]
- Shtaida, N.; Khozin-Goldberg, I.; Boussiba, S. The role of pyruvate hub enzymes in supplying carbon precursors for fatty acid synthesis in photosynthetic microalgae. Photosynth. Res. 2015, 125, 407–422. [Google Scholar] [CrossRef]
- Jardine, K.J.; Honeker, L.K.; Zhang, Z.; Kwatcho Kengdo, S.; Yang, Y.; Roscioli, J.; Riley, W.J. Evolutionary and functional relationships between plant and microbial C1 metabolism in terrestrial ecosystems. New Phytol. 2025, 248, 1132–1153. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Xu, X.; Yang, K.; Zhu, C.; Liu, Y.; Gao, Z. Multifaceted analyses reveal carbohydrate metabolism mainly affecting the quality of postharvest bamboo shoots. Front. Plant Sci. 2022, 13, 1021161. [Google Scholar] [CrossRef]
- Chen, L.; Meng, Y.; Bai, Y.; Yu, H.; Qian, Y.; Zhang, D.; Zhou, Y. Starch and sucrose metabolism and plant hormone signaling pathways play crucial roles in Aquilegia salt stress adaptation. Int. J. Mol. Sci. 2023, 24, 3948. [Google Scholar] [CrossRef] [PubMed]
- Stincone, A.; Prigione, A.; Cramer, T.; Wamelink, M.M.C.; Campbell, K.; Cheung, E.; Olin-Sandoval, V.; Grüning, N.M.; Krüger, A.; Tauqeer Alam, M.; et al. The return of metabolism: Biochemistry and physiology of the pentose phosphate pathway. Biol. Rev. 2015, 90, 927–963. [Google Scholar] [CrossRef]
- Kruger, N.J.; Von Schaewen, A. The oxidative pentose phosphate pathway: Structure and organization. Curr. Opin. Plant Biol. 2003, 6, 236–246. [Google Scholar] [CrossRef]
- Ma, Q.; Xu, M.; Liu, M.; Cao, X.; Hill, P.W.; Chadwick, D.R.; Wu, L.; Jones, D.L. Organic and inorganic sulfur and nitrogen uptake by co-existing grassland plant species competing with soil microorganisms. Soil Biol. Biochem. 2022, 168, 108627. [Google Scholar] [CrossRef]
- Nikiforova, V.J.; Kopka, J.; Tolstikov, V.; Fiehn, O.; Hopkins, L.; Hawkesford, M.J.; Hesse, H.; Hoefgen, R. Systems Rebalancing of Metabolism in Response to Sulfur Deprivation, as Revealed by Metabolome Analysis of Arabidopsis Plants. Plant Physiol. 2005, 138, 304–318. [Google Scholar] [CrossRef]
- Mondal, S.; Karmakar, S.; Panda, D.; Pramanik, K.; Bose, B.; Singhal, R.K. Crucial plant processes under heat stress and tolerance through heat shock proteins. Plant Stress 2023, 10, 100227. [Google Scholar] [CrossRef]
- Lv, J.; Yang, S.; Zhou, W.; Liu, Z.; Tan, J.; Wei, M. Microbial regulation of plant secondary metabolites: Impact, mechanisms and prospects. Microbiol. Res. 2024, 283, 127688. [Google Scholar] [CrossRef] [PubMed]
- Marks, B.B.; Nogueira, M.A.; Hungria, M. Microbial Secondary Metabolites and Their Use in Achieving Sustainable Agriculture: Present Achievements and Future Challenges. Agronomy 2025, 15, 1350. [Google Scholar] [CrossRef]
- Zhang, T.; Dong, X.; Yang, J.; Li, Z.; Zhu, J. Effects of near-natural forest management on soil microbial communities in the temperate-subtropical transition zone in China. Microorganisms 2025, 13, 1906. [Google Scholar] [CrossRef] [PubMed]
- Vivian, J.; Chazdon, R.L.; Catling, A.A.; Lee, D.J. Early evidences of links between soil microbes and forest restoration through multiple ecosystem metrics. Front. For. Glob. Change 2025, 8, 1540513. [Google Scholar] [CrossRef]
- Buschmann, H.; Edwards, P.J.; Dietz, H. Variations in growth pattern and response to slug damage among native and invasive provenances of four perennial Brassicaceae species. J. Ecol. 2005, 93, 322–334. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, X.; Lin, Q.; Li, G.; Kong, W. Using a combination of PLFA and DNA-based sequencing analyses to detect shifts in the soil microbial community composition after a simulated spring precipitation in a semi-arid grassland in China. Sci. Total Environ. 2019, 657, 1237–1245. [Google Scholar] [CrossRef]
- Li, J.; Li, Y.T.; Yang, X.D.; Zhang, J.J.; Lin, Z.A.; Zhao, B.Q. Microbial community structure and functional metabolic diversity are associated with organic carbon availability in an agricultural soil. J. Integr. Agric. 2015, 14, 2500–2511. [Google Scholar] [CrossRef]
- El-Azaz, J.; Moore, B.; Maeda, H.A. Re-tuning of aromatic amino acid biosynthesis before and after the evolution of tyrosine-derived grass lignin. Plant Physiol. 2025, 199, kiaf366. [Google Scholar] [CrossRef]
- Cannell, N.; Emms, D.M.; Hetherinton, A.J.; MacKay, J.; Kelly, S.; Dolan, L.; Sweetlove, L.J. Multiple metabolic innovations and losses are associated with major transitions in land plant evolution. Curr. Biol. 2020, 30, 1783–1800. [Google Scholar] [CrossRef]

| Microbial Ratios | Mean ± SE | F-Value | Significance | Pairwise Comparison (Tukey HSD) | ||||
|---|---|---|---|---|---|---|---|---|
| F | E | G | F vs. E | E vs. G | G vs. F | |||
| Aerobe/Anaerobe | 3.13 ± 0.08 | 3.11 ± 0.1 | 3.32 ± 0.11 | 1.16 | ns | ns | ns | ns |
| G+/G− | 0.4 ± 0.006 | 0.36 ± 0.009 | 0.29 ± 0.013 | 26.661 | *** | * | *** | *** |
| F/B | 0.083 ± 0.001 | 0.085 ± 0.003 | 0.089 ± 0.003 | 1.455 | ns | ns | ns | ns |
| F/PLFA | 0.077 ± 0.002 | 0.077 ± 0.002 | 0.082 ± 0.003 | 1.417 | ns | ns | ns | ns |
| B/PLFA | 0.923 ± 0.002 | 0.085 ± 0.003 | 0.918 ± 0.003 | 1.417 | ns | ns | ns | ns |
| Land Use | Pairwise Co-Inertia | RV | First Axis Variance (%) | Strength |
|---|---|---|---|---|
| Grassland | Microbiota vs. Soil properties | 0.51 | 48.3 | * |
| Metabolites vs. Soil properties | 0.6 | 71.5 | * | |
| Microbiota vs. Metabolites | 0.5 | 62.8 | * | |
| Edge | Microbiota vs. Soil properties | 0.81 | 79.2 | ** |
| Metabolites vs. Soil properties | 0.74 | 89.2 | * | |
| Microbiota vs. Metabolites | 0.72 | 84.9 | * | |
| Forest | Microbiota vs. Soil properties | 0.68 | 58.2 | * |
| Metabolites vs. Soil properties | 0.91 | 81.2 | *** | |
| Microbiota vs. Metabolites | 0.71 | 66.2 | * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kovacs, E.D.; Kovacs, M.H. Land Use Shapes the Rhizosphere Microbiome and Metabolome of Naturally Growing Barbarea vulgaris. Metabolites 2025, 15, 684. https://doi.org/10.3390/metabo15110684
Kovacs ED, Kovacs MH. Land Use Shapes the Rhizosphere Microbiome and Metabolome of Naturally Growing Barbarea vulgaris. Metabolites. 2025; 15(11):684. https://doi.org/10.3390/metabo15110684
Chicago/Turabian StyleKovacs, Emoke Dalma, and Melinda Haydee Kovacs. 2025. "Land Use Shapes the Rhizosphere Microbiome and Metabolome of Naturally Growing Barbarea vulgaris" Metabolites 15, no. 11: 684. https://doi.org/10.3390/metabo15110684
APA StyleKovacs, E. D., & Kovacs, M. H. (2025). Land Use Shapes the Rhizosphere Microbiome and Metabolome of Naturally Growing Barbarea vulgaris. Metabolites, 15(11), 684. https://doi.org/10.3390/metabo15110684








