NAD+-Dependent Lysine Acetylation Regulates Glucose Uptake and Fatty Acid Oxidation in Cardiomyocytes
Abstract
1. Introduction
2. Materials and Methods
2.1. Rat Cardiomyocytes Culture
2.2. Confocal Microscopy
2.3. Glucose Uptake Measurement
2.4. Immunoblot Analysis
2.5. Proteomic Analysis
2.6. Pathway Analysis and Bioinformatics
2.7. Fatty Acid Uptake and Oxidation Measurements
2.8. NAD+ and NADH Determination
2.9. Statistics
3. Results
3.1. Effect of Chronic NAM and NR Treatments on Lysine Acetylation and Glucose Uptake
3.2. NAM and NR Treatments Increase the AMPK Signaling Pathway
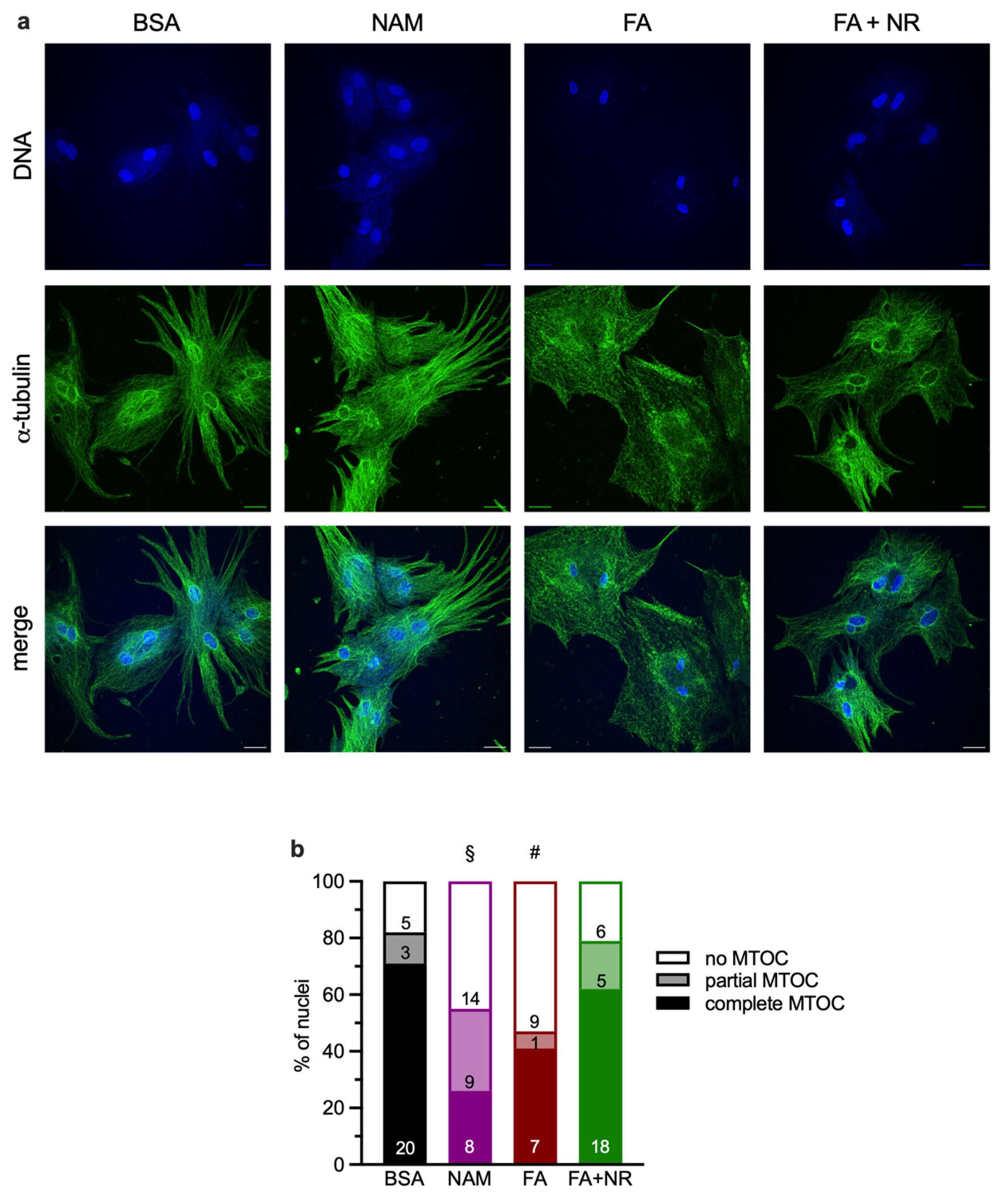
3.3. Chronic NAM or FA Exposure Disrupts Microtubule-Organizing Centers (MTOCs)
3.4. Proteomics Analysis Indicates HADHA Acetylation Is Influenced by Chronic NAM and NR Treatments
3.5. HADHA Acetylation Is Associated with Increased Fatty Acid Oxidation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMPK | AMP-activated protein kinase |
| ANOVA | Analysis of variance |
| BSA | Bovine serum albumin |
| EDTA | Ethylenediaminetetraacetic acid |
| FA | Fatty acid |
| HADHA | Hydroxyacyl-CoA dehydrogenase trifunctional enzyme subunit α |
| HDAC | Histone deacetylase |
| KAT | Lysine acetyl transferase |
| MTOC | Microtubule-organizing center |
| NAD+/NADH | Nicotinamide adenine dinucleotide (oxidized/reduced) |
| NAM | Nicotinamide |
| NR | Nicotinamide riboside |
| PBS | Phosphate-buffered saline |
Appendix A
| Antigen | Conjugation | Company | Catalog Number |
|---|---|---|---|
| Primary antibodies | |||
| Acetylated-lysine | Cell Signaling Technology (Leiden, The Netherlands) | 9441 | |
| AMPKα | Cell Signaling Technology | 2532 | |
| AMPKα; phosphoT172 | Cell Signaling Technology | 2535 | |
| AS160 | Cell Signaling Technology | 2670 | |
| Phospho-(Ser/Thr) Akt substrate | Cell Signaling Technology | 9611 | |
| raptor | Cell Signaling Technology | 2280 | |
| raptor; phosphoS792 | Cell Signaling Technology | 2083 | |
| α-actinin | Sigma-Aldrich (Buchs, Switzerland) | A7811 | |
| Secondary antibodies | |||
| Rabbit IgG | Horse radish peroxidase | Cell Signaling Technology | 7074 |
| Mouse IgG | Horse radish peroxidase | Cell Signaling Technology | 7076 |
References
- Alegria, J.R.; Miller, T.D.; Gibbons, R.J.; Yi, Q.-L.; Yusuf, S. Infarct size, ejection fraction, and mortality in diabetic patients with acute myocardial infarction treated with thrombolytic therapy. Am. Heart J. 2007, 154, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Marso, S.P.; Miller, T.; Rutherford, B.D.; Gibbons, R.J.; Qureshi, M.; Kalynych, A.; Turco, M.; Schultheiss, H.P.; Mehran, R.; Krucoff, M.W.; et al. Comparison of Myocardial Reperfusion in Patients Undergoing Percutaneous Coronary Intervention in ST-Segment Elevation Acute Myocardial Infarction With Versus Without Diabetes Mellitus (from the EMERALD Trial). Am. J. Cardiol. 2007, 100, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Lopaschuk, G.D.; Ussher, J.R.; Folmes, C.D.L.; Jaswal, J.S.; Stanley, W.C. Myocardial Fatty Acid Metabolism in Health and Disease. Physiol. Rev. 2010, 90, 207–258. [Google Scholar] [CrossRef]
- Hsu, C.-P.; Zhai, P.; Yamamoto, T.; Maejima, Y.; Matsushima, S.; Hariharan, N.; Shao, D.; Takagi, H.; Oka, S.; Sadoshima, J. Silent Information Regulator 1 Protects the Heart From Ischemia/Reperfusion. Circulation 2010, 122, 2170–2182. [Google Scholar] [CrossRef]
- Nadtochiy, S.M.; Urciuoli, W.; Zhang, J.; Schafer, X.; Munger, J.; Brookes, P.S. Metabolomic profiling of the heart during acute ischemic preconditioning reveals a role for SIRT1 in rapid cardioprotective metabolic adaptation. J. Mol. Cell. Cardiol. 2015, 88, 64–72. [Google Scholar] [CrossRef]
- Yamamoto, T.; Byun, J.; Zhai, P.; Ikeda, Y.; Oka, S.; Sadoshima, J. Nicotinamide Mononucleotide, an Intermediate of NAD+ Synthesis, Protects the Heart from Ischemia and Reperfusion. PLoS ONE 2014, 9, e98972. [Google Scholar] [CrossRef]
- Martin, A.S.; Abraham, D.M.; Hershberger, K.A.; Bhatt, D.P.; Mao, L.; Cui, H.; Liu, J.; Liu, X.; Muehlbauer, M.J.; Grimsrud, P.A.; et al. Nicotinamide mononucleotide requires SIRT3 to improve cardiac function and bioenergetics in a Friedreich’s ataxia cardiomyopathy model. JCI Insight 2017, 2, e93885. [Google Scholar] [CrossRef]
- Nadtochiy, S.M.; Wang, Y.T.; Nehrke, K.; Munger, J.; Brookes, P.S. Cardioprotection by nicotinamide mononucleotide (NMN): Involvement of glycolysis and acidic pH. J. Mol. Cell. Cardiol. 2018, 121, 155–162. [Google Scholar] [CrossRef]
- Hsu, C.-P.; Oka, S.; Shao, D.; Hariharan, N.; Sadoshima, J. Nicotinamide Phosphoribosyltransferase Regulates Cell Survival Through NAD+ Synthesis in Cardiac Myocytes. Circ. Res. 2009, 105, 481–491. [Google Scholar] [CrossRef]
- Viglino, C.; Khoramdin, B.; Praplan, G.; Montessuit, C. Pleiotropic effects of chronic phorbol ester treatment to improve glucose transport in insulin-resistant cardiomyocytes. J. Cell. Biochem. 2017, 118, 4716–4727. [Google Scholar] [CrossRef] [PubMed]
- Eppenberger-Eberhardt, M.; Flamme, I.; Kurer, V.; Eppenberger, H.M. Reexpression of α-smooth muscle actin isoform in cultured adult rat cardiomyocytes. Dev. Biol. 1990, 139, 269–278. [Google Scholar] [CrossRef]
- Claycomb, W.C.; Palazzo, M.C. Culture of the terminally differentiated adult cardiac muscle cell: A light and scanning electron microscope study. Dev. Biol. 1980, 80, 466–482. [Google Scholar] [CrossRef]
- Montessuit, C.; Papageorgiou, I.; Lerch, R. Nuclear receptors agonists improve insulin responsiveness in cultured cardiomyocytes through enhanced signaling and preserved cytoskeletal architecture. Endocrinology 2008, 149, 1064–1074. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Montessuit, C.; Papageorgiou, I.; Campos, L.; Lerch, R. Retinoic acids increase expression of GLUT4 in dedifferentiated and hypertrophied cardiac myocytes. Basic Res. Cardiol. 2006, 101, 27–35. [Google Scholar] [CrossRef][Green Version]
- Zebrowski, D.C.; Vergarajauregui, S.; Wu, C.C.; Piatkowski, T.; Becker, R.; Leone, M.; Hirth, S.; Ricciardi, F.; Falk, N.; Giessl, A.; et al. Developmental alterations in centrosome integrity contribute to the post-mitotic state of mammalian cardiomyocytes. eLife 2015, 4, e05563. [Google Scholar] [CrossRef] [PubMed]
- Asrih, M.; Pellieux, C.; Papageorgiou, I.; Lerch, R.; Montessuit, C. Role of ERK1/2 activation in microtubule stabilization and glucose transport in cardiomyocytes. Am. J. Physiol. Endocrinol. Metab. 2011, 301, E836–E843. [Google Scholar] [CrossRef][Green Version]
- Manning, N.J.; Olpin, S.E.; Pollitt, R.J.; Webley, J. A comparison of [9,10-3H]palmitic and [9,10-3H]myristic acids for the detection of defects of fatty acid oxidation in intact cultured fibroblasts. J. Inherit. Metab. Dis. 1990, 13, 58–68. [Google Scholar] [CrossRef]
- Benjamini, Y.; Yekutieli, D. False Discovery Rate-Adjusted Multiple Confidence Intervals for Selected Parameters. J. Acoust. Soc. Am. 2005, 100, 71–81. [Google Scholar] [CrossRef]
- Asrih, M.; Lerch, R.; Papageorgiou, I.; Pellieux, C.; Montessuit, C. Differential regulation of stimulated glucose transport by free fatty acids and PPARα or -δ agonists in cardiac myocytes. Am. J. Physiol. Endocrinol. Metab. 2012, 302, E872–E884. [Google Scholar] [CrossRef]
- Viglino, C.; Foglia, B.; Montessuit, C. Chronic AICAR treatment prevents metabolic changes in cardiomyocytes exposed to free fatty acids. Pflügers Arch. Eur. J. Physiol. 2019, 471, 1219–1234. [Google Scholar] [CrossRef]
- Vanni, E.; Lindner, K.; Gavin, A.-C.; Montessuit, C. Differential intracellular management of fatty acids impacts on metabolic stress-stimulated glucose uptake in cardiomyocytes. Sci. Rep. 2023, 13, 14805. [Google Scholar] [CrossRef]
- Kramer, H.F.; Witczak, C.A.; Taylor, E.B.; Fujii, N.; Hirshman, M.F.; Goodyear, L.J. AS160 Regulates Insulin- and Contraction-stimulated Glucose Uptake in Mouse Skeletal Muscle. J. Biol. Chem. 2006, 281, 31478–31485. [Google Scholar] [CrossRef]
- Thong, F.S.L.; Bilan, P.J.; Klip, A. The Rab GTPase-Activating Protein AS160 Integrates Akt, Protein Kinase C, and AMP-Activated Protein Kinase Signals Regulating GLUT4 Traffic. Diabetes 2007, 56, 414–423. [Google Scholar] [CrossRef]
- Randle, P.J.; Garland, P.B.; Hales, C.N.; Newsholme, E.A. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet 1963, 281, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Hue, L.; Taegtmeyer, H. The Randle cycle revisited: A new head for an old hat. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E578–E591. [Google Scholar] [CrossRef] [PubMed]
- Ketema, E.B.; Lopaschuk, G.D. Post-translational Acetylation Control of Cardiac Energy Metabolism. Front. Cardiovasc. Med. 2021, 8, 723996. [Google Scholar] [CrossRef] [PubMed]
- Renguet, E.; Bultot, L.; Beauloye, C.; Horman, S.; Bertrand, L. The Regulation of Insulin-Stimulated Cardiac Glucose Transport via Protein Acetylation. Front. Cardiovasc. Med. 2018, 5, 70. [Google Scholar] [CrossRef]
- Fukushima, A.; Zhang, L.; Huqi, A.; Lam, V.H.; Rawat, S.; Altamimi, T.; Wagg, C.S.; Dhaliwal, K.K.; Hornberger, L.K.; Kantor, P.F.; et al. Acetylation contributes to hypertrophy-caused maturational delay of cardiac energy metabolism. JCI Insight 2018, 3, e99239. [Google Scholar] [CrossRef]
- Abdellatif, M.; Sedej, S.; Kroemer, G. NAD+ Metabolism in Cardiac Health, Aging, and Disease. Circulation 2021, 144, 1795–1817. [Google Scholar] [CrossRef]
- Diguet, N.; Trammell, S.A.J.; Tannous, C.; Deloux, R.; Piquereau, J.; Mougenot, N.; Gouge, A.; Gressette, M.; Manoury, B.; Blanc, J.; et al. Nicotinamide Riboside Preserves Cardiac Function in a Mouse Model of Dilated Cardiomyopathy. Circulation 2018, 137, 2256–2273. [Google Scholar] [CrossRef]
- Vignier, N.; Chatzifrangkeskou, M.; Morales Rodriguez, B.; Mericskay, M.; Mougenot, N.; Wahbi, K.; Bonne, G.; Muchir, A. Rescue of biosynthesis of nicotinamide adenine dinucleotide protects the heart in cardiomyopathy caused by lamin A/C gene mutation. Hum. Mol. Genet. 2018, 27, 3870–3880. [Google Scholar] [CrossRef] [PubMed]
- Sukhodub, A.; Du, Q.; Jovanović, S.; Jovanović, A. Nicotinamide-rich diet protects the heart against ischaemia-reperfusion in mice: A crucial role for cardiac SUR2A. Pharmacol. Res. 2010, 61, 564–570. [Google Scholar] [CrossRef] [PubMed]
- Tong, D.L.; Zhang, D.X.; Xiang, F.; Teng, M.; Jiang, X.P.; Hou, J.M.; Zhang, Q.; Huang, Y.S. Nicotinamide pretreatment protects cardiomyocytes against hypoxia-induced cell death by improving mitochondrial stress. Pharmacology 2012, 90, 11–18. [Google Scholar] [CrossRef]
- Tong, D.; Schiattarella, G.G.; Jiang, N.; Altamirano, F.; Szweda, P.A.; Elnwasany, A.; Lee, D.I.; Yoo, H.; Kass, D.A.; Szweda, L.I.; et al. NAD+ Repletion Reverses Heart Failure With Preserved Ejection Fraction. Circ. Res. 2021, 128, 1629–1641. [Google Scholar] [CrossRef] [PubMed]
- Nie, H.; Zhang, Y.; Yu, H.; Xiao, H.; Li, T.; Yang, Q. Oral delivery of carrier-free dual-drug nanocrystal self-assembled microspheres improved NAD+ bioavailability and attenuated cardiac ischemia/reperfusion injury in mice. Drug Deliv. 2021, 28, 433–444. [Google Scholar] [CrossRef]
- Landry, J.; Slama, J.T.; Sternglanz, R. Role of NAD+ in the Deacetylase Activity of the SIR2-like Proteins. Biochem. Biophys. Res. Commun. 2000, 278, 685–690. [Google Scholar] [CrossRef]
- De Loof, M.; Renguet, E.; Ginion, A.; Bouzin, C.; Horman, S.; Beauloye, C.; Bertrand, L.; Bultot, L. Enhanced protein acetylation initiates fatty acid-mediated inhibition of cardiac glucose transport. Am. J. Physiol. Heart Circ. Physiol. 2023, 324, H305–H317. [Google Scholar] [CrossRef]
- Renguet, E.; De Loof, M.; Fourny, N.; Ginion, A.; Bouzin, C.; Poüs, C.; Horman, S.; Beauloye, C.; Bultot, L.; Bertrand, L. α-Tubulin acetylation on Lysine 40 controls cardiac glucose uptake. Am. J. Physiol. Heart Circ. Physiol. 2022, 322, H1032–H1043. [Google Scholar] [CrossRef]
- Russell, R.R.; Li, J.; Coven, D.L.; Pypaert, M.; Zechner, C.; Palmeri, M.; Giordano, F.J.; Mu, J.; Birnbaum, M.J.; Young, L.H. AMP-activated protein kinase mediates ischemic glucose uptake and prevents postischemic cardiac dysfunction, apoptosis, and injury. J. Clin. Investig. 2004, 114, 495–503. [Google Scholar] [CrossRef]
- Russell, R.R.; Bergeron, R.; Shulman, G.I.; Young, L.H. Translocation of myocardial GLUT-4 and increased glucose uptake through activation of AMPK by AICAR. Am. J. Physiol. Heart Circ. Physiol. 1999, 277, H643–H649. [Google Scholar] [CrossRef]
- Ginion, A.; Auquier, J.; Benton, C.R.; Mouton, C.; Vanoverschelde, J.-L.; Hue, L.; Horman, S.; Beauloye, C.; Bertrand, L. Inhibition of the mTOR/p70S6K pathway is not involved in the insulin-sensitizing effect of AMPK on cardiac glucose uptake. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H469–H477. [Google Scholar] [CrossRef] [PubMed]
- Kramer, H.F.; Witczak, C.A.; Fujii, N.; Jessen, N.; Taylor, E.B.; Arnolds, D.E.; Sakamoto, K.; Hirshman, M.F.; Goodyear, L.J. Distinct Signals Regulate AS160 Phosphorylation in Response to Insulin, AICAR, and Contraction in Mouse Skeletal Muscle. Diabetes 2006, 55, 2067–2076. [Google Scholar] [CrossRef]
- Steinbusch, L.K.M.; Schwenk, R.W.K.; Ouwens, D.M.; Diamant, M.; Glatz, J.F.C.; Luiken, J.J.F.P. Subcellular trafficking of the substrate transporters GLUT4 and CD36 in cardiomyocytes. Cell. Mol. Life Sci. 2011, 68, 2525–2538. [Google Scholar] [CrossRef]
- Vazquez, E.J.; Berthiaume, J.M.; Kamath, V.; Achike, O.; Buchanan, E.; Montano, M.M.; Chandler, M.P.; Miyagi, M.; Rosca, M.G. Mitochondrial complex I defect and increased fatty acid oxidation enhance protein lysine acetylation in the diabetic heart. Cardiovasc. Res. 2015, 107, 453–465. [Google Scholar] [CrossRef]
- Lundby, A.; Lage, K.; Weinert, B.T.; Bekker-Jensen, D.B.; Secher, A.; Skovgaard, T.; Kelstrup, C.D.; Dmytriyev, A.; Choudhary, C.; Lundby, C.; et al. Proteomic Analysis of Lysine Acetylation Sites in Rat Tissues Reveals Organ Specificity and Subcellular Patterns. Cell Rep. 2012, 2, 419–431. [Google Scholar] [CrossRef]
- Vadvalkar, S.S.; Matsuzaki, S.; Eyster, C.A.; Giorgione, J.R.; Bockus, L.B.; Kinter, C.S.; Kinter, M.; Humphries, K.M. Decreased Mitochondrial Pyruvate Transport Activity in the Diabetic Heart: Role of Mitochondrial Pyruvate Carrier 2 (MPC2) Acetylation. J. Biol. Chem. 2017, 292, 4423–4433. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Xu, J.; Qin, X.; Hou, Z.; Guo, Y.; Liu, Z.; Wu, J.; Zheng, H.; Zhang, X.; Gao, F. Glucose oxidation positively regulates glucose uptake and improves cardiac function recovery after myocardial reperfusion. Am. J. Physiol. Endocrinol. Metab. 2017, 313, E577–E585. [Google Scholar] [CrossRef] [PubMed]
- Asrih, M.; Gardier, S.; Papageorgiou, I.; Montessuit, C. Dual effect of the heart-targeting cytokine cardiotrophin-1 on glucose transport in cardiomyocytes. J. Mol. Cell. Cardiol. 2013, 56, 106–115. [Google Scholar] [CrossRef]
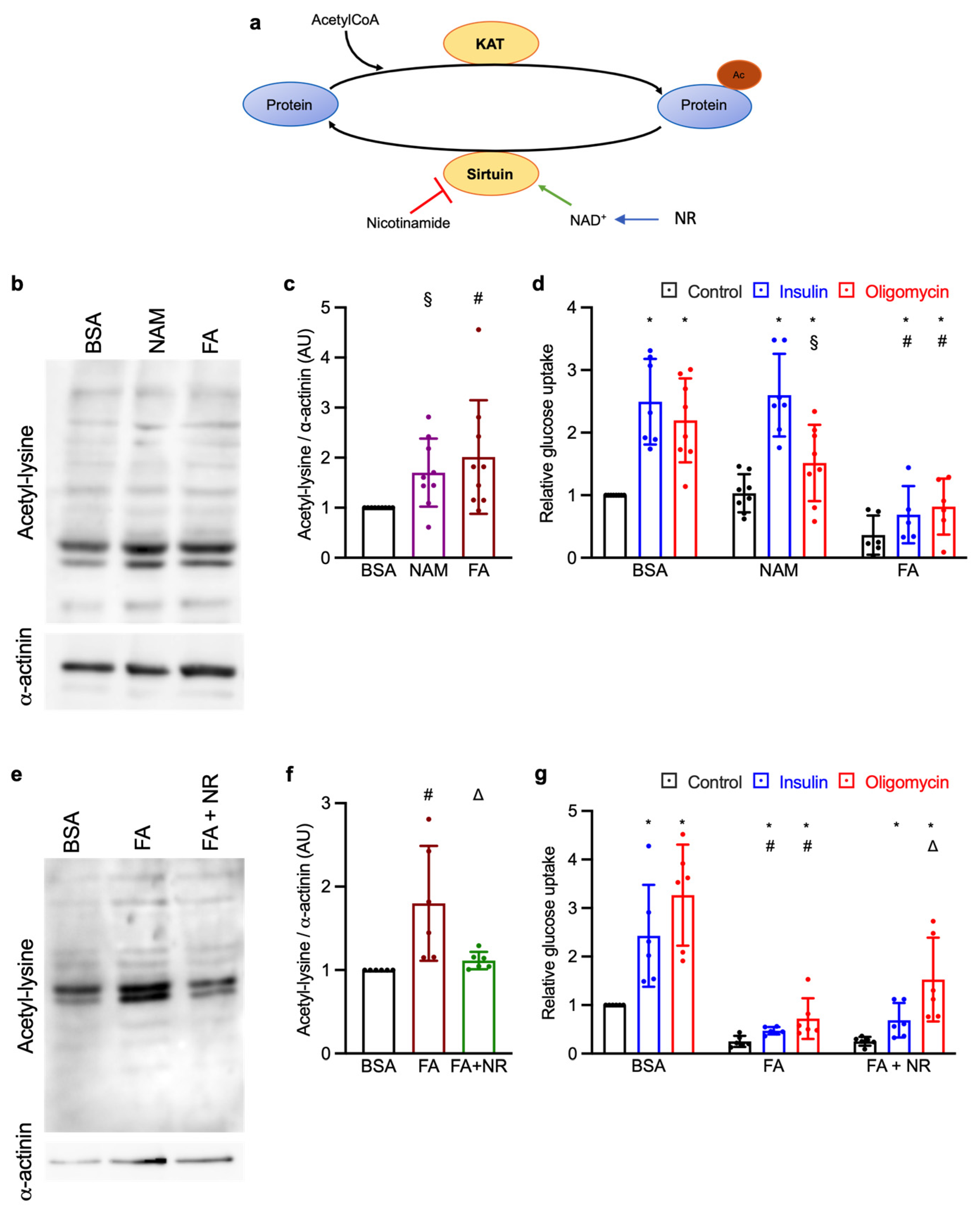

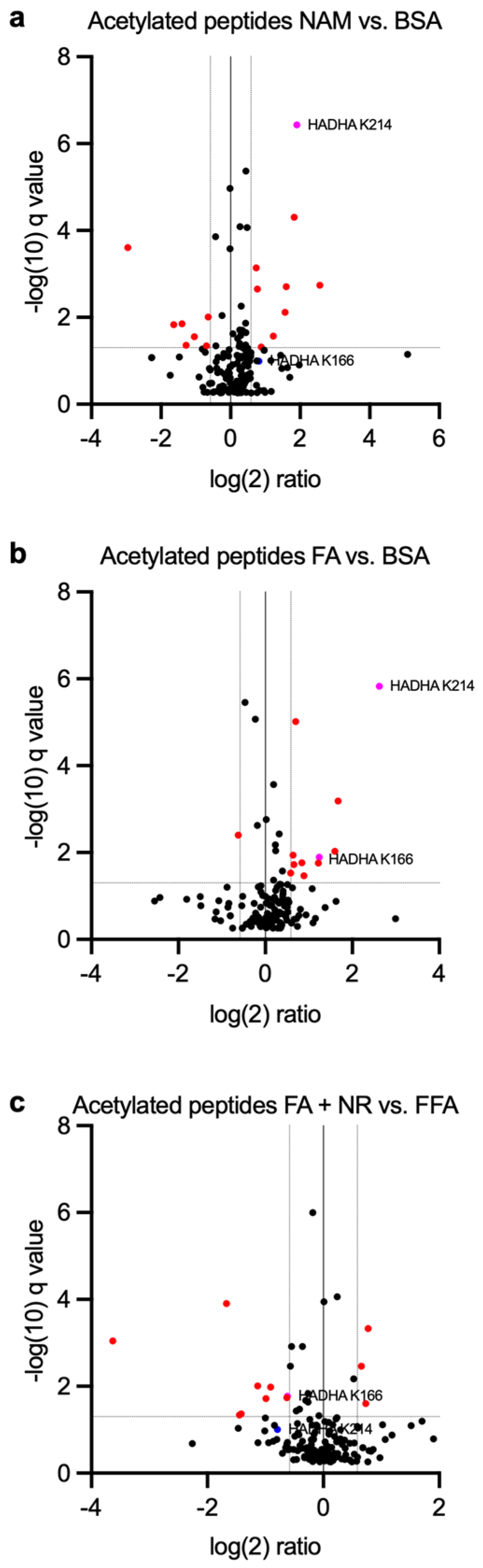


| NAM vs. BSA | FA vs. BSA | FA + NR vs. BSA | |
|---|---|---|---|
| HADHA K166 | Fold change 2.35 q = 0.102 | Fold change 2.36 q = 0.0129 | Fold change 0.654 q = 0.0170 |
| HADHA K214 | Fold change 3.76 q = 3.67 × 10−7 | Fold change 6.15 q = 1.49 × 10−6 | Fold change 0.578 q = 0.099 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vanni, E.; Montessuit, C. NAD+-Dependent Lysine Acetylation Regulates Glucose Uptake and Fatty Acid Oxidation in Cardiomyocytes. Metabolites 2025, 15, 636. https://doi.org/10.3390/metabo15100636
Vanni E, Montessuit C. NAD+-Dependent Lysine Acetylation Regulates Glucose Uptake and Fatty Acid Oxidation in Cardiomyocytes. Metabolites. 2025; 15(10):636. https://doi.org/10.3390/metabo15100636
Chicago/Turabian StyleVanni, Ettore, and Christophe Montessuit. 2025. "NAD+-Dependent Lysine Acetylation Regulates Glucose Uptake and Fatty Acid Oxidation in Cardiomyocytes" Metabolites 15, no. 10: 636. https://doi.org/10.3390/metabo15100636
APA StyleVanni, E., & Montessuit, C. (2025). NAD+-Dependent Lysine Acetylation Regulates Glucose Uptake and Fatty Acid Oxidation in Cardiomyocytes. Metabolites, 15(10), 636. https://doi.org/10.3390/metabo15100636






