Hexavalent Chromium Induces Defense Responses, Hepatocellular Apoptosis, and Lipid Metabolism Alterations in New Zealand Rabbit Livers
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Treatment
2.2. Sample Collection and Processing
2.3. Pathological Examination
2.4. Transmission Electron Microscopy Ultrastructure of Liver Tissue
2.5. TUNEL Staining
2.6. QRT-PCR Validation
2.7. Oil Red O Staining
2.8. Determination of Cr and Essential Trace Element Content in Tissue
2.9. Element Content Determination
2.10. Statistical Analysis
3. Results
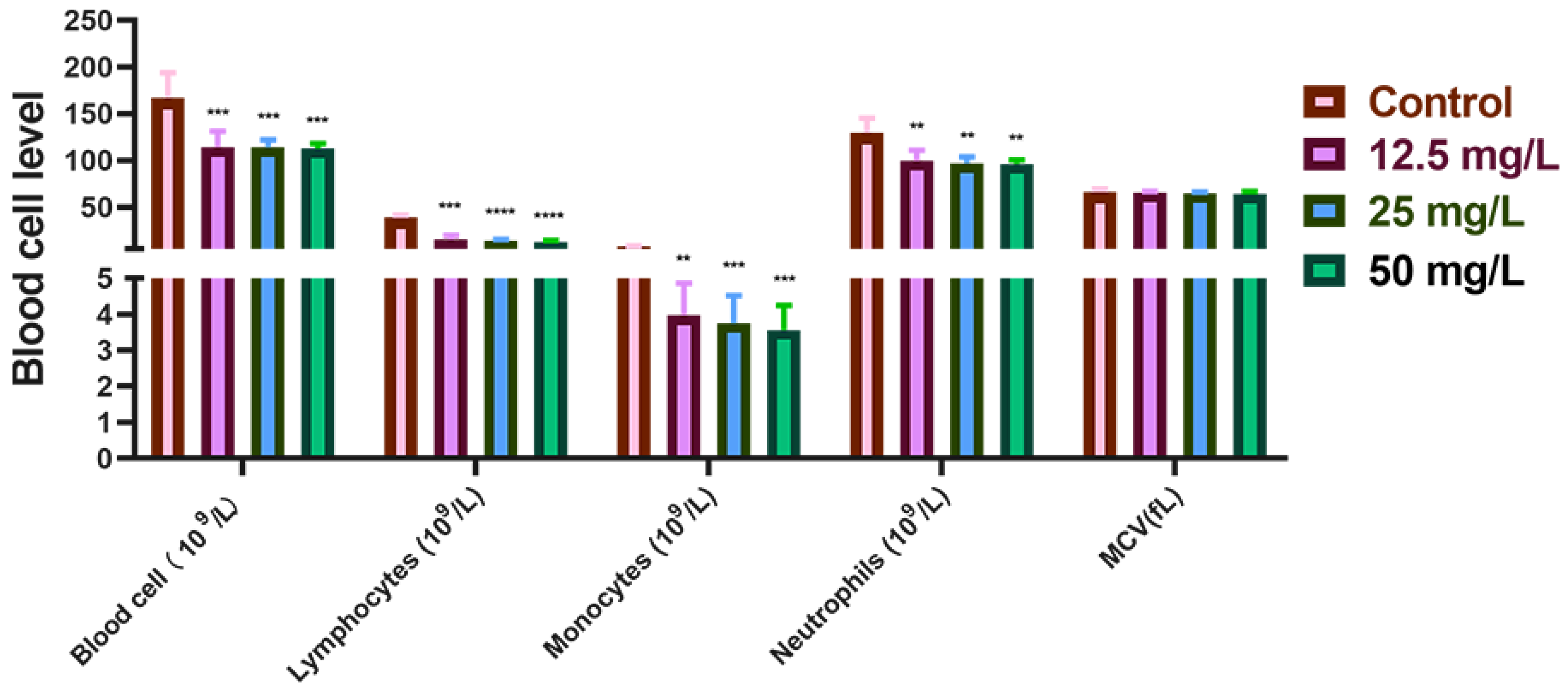
3.1. Cr(VI) Downregulated Hematological Indicators in New Zealand Rabbits
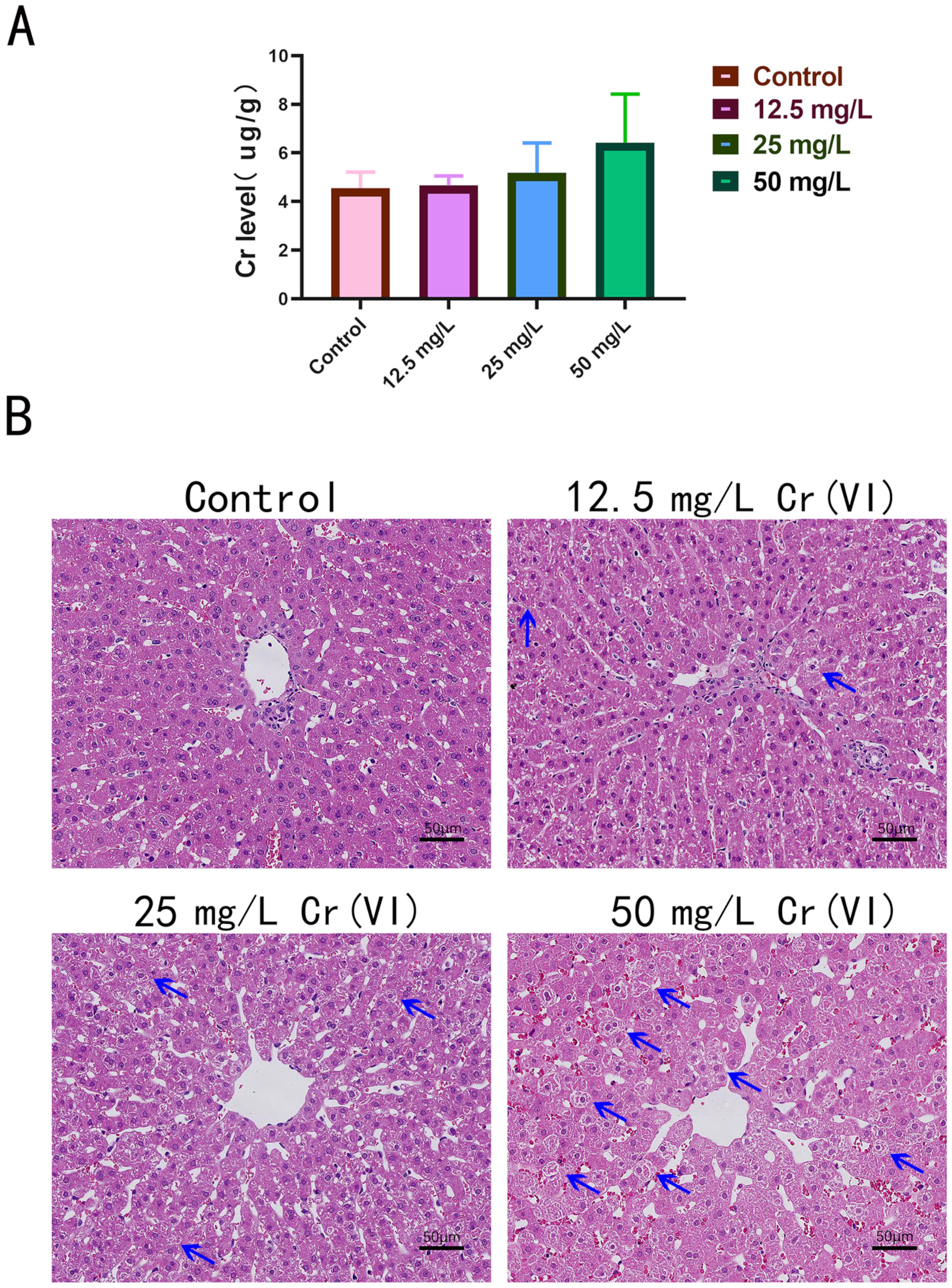
3.2. Cr Accumulation and Pathological Damage in Liver Tissue
3.3. Trace Element Metabolism in Liver Tissue
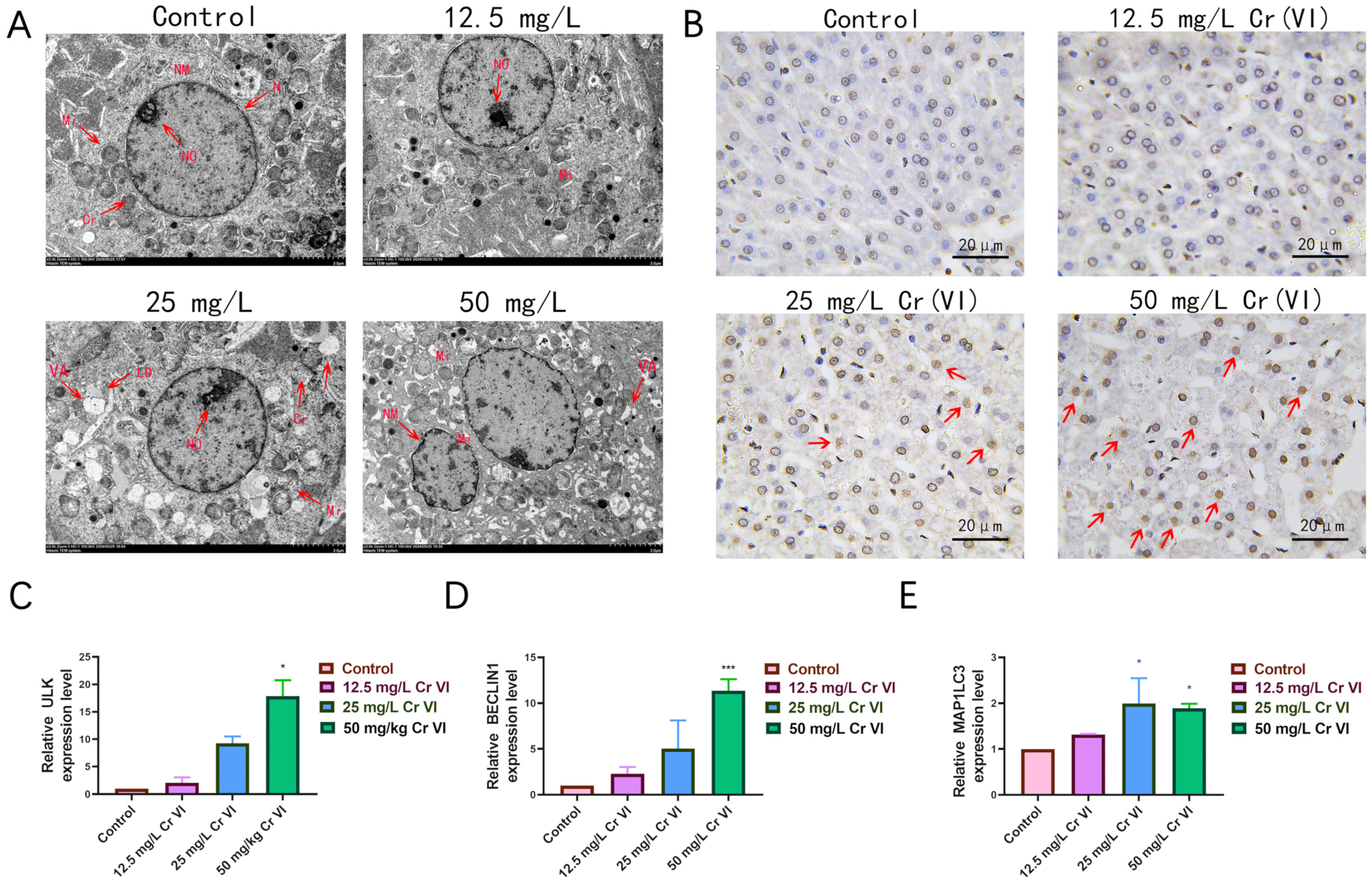
3.4. Ultrastructural Changes in Liver Tissue
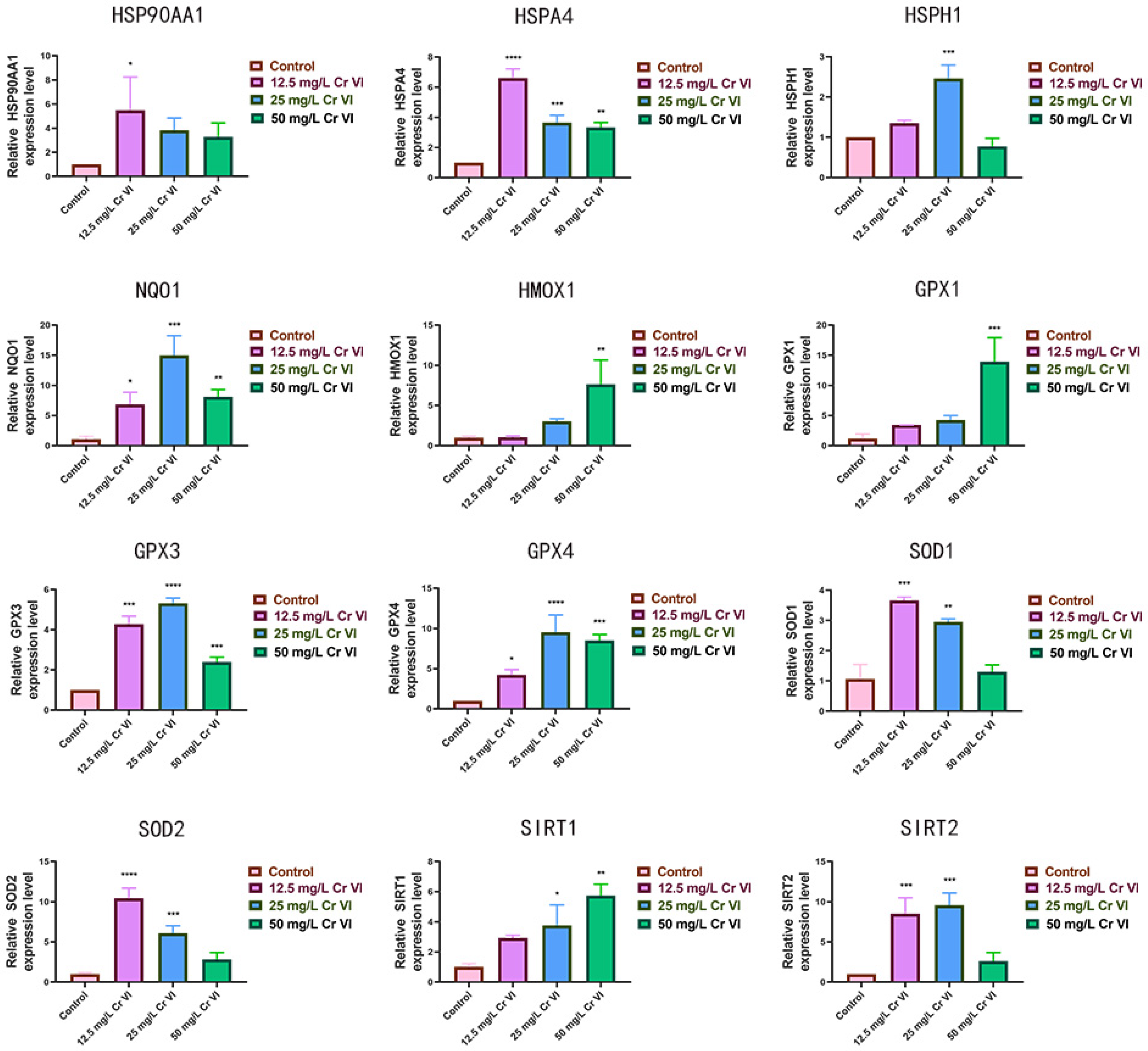
3.5. Alterations in Antioxidant-Related Gene Expression
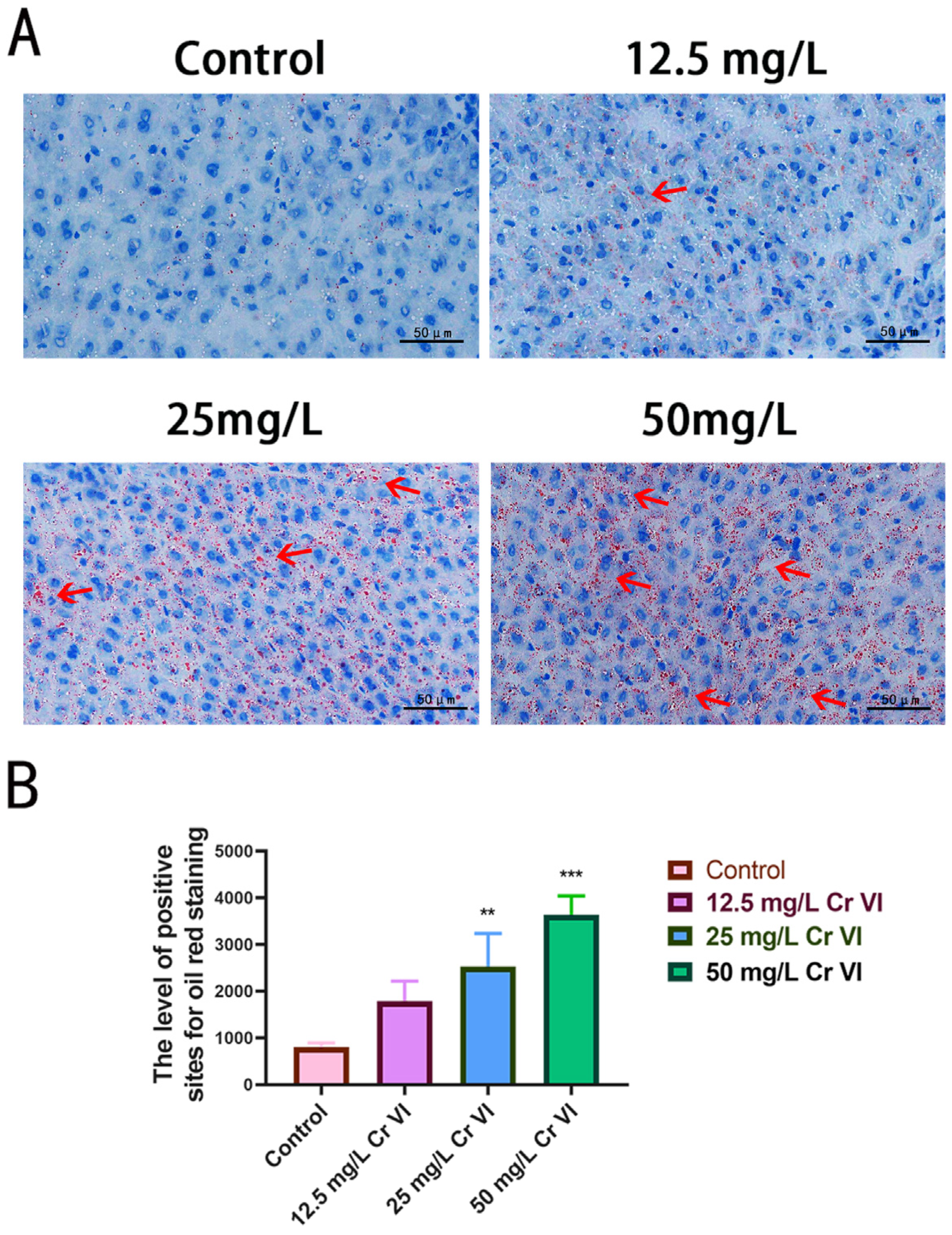
3.6. Lipid Droplet Accumulation in Liver Tissue
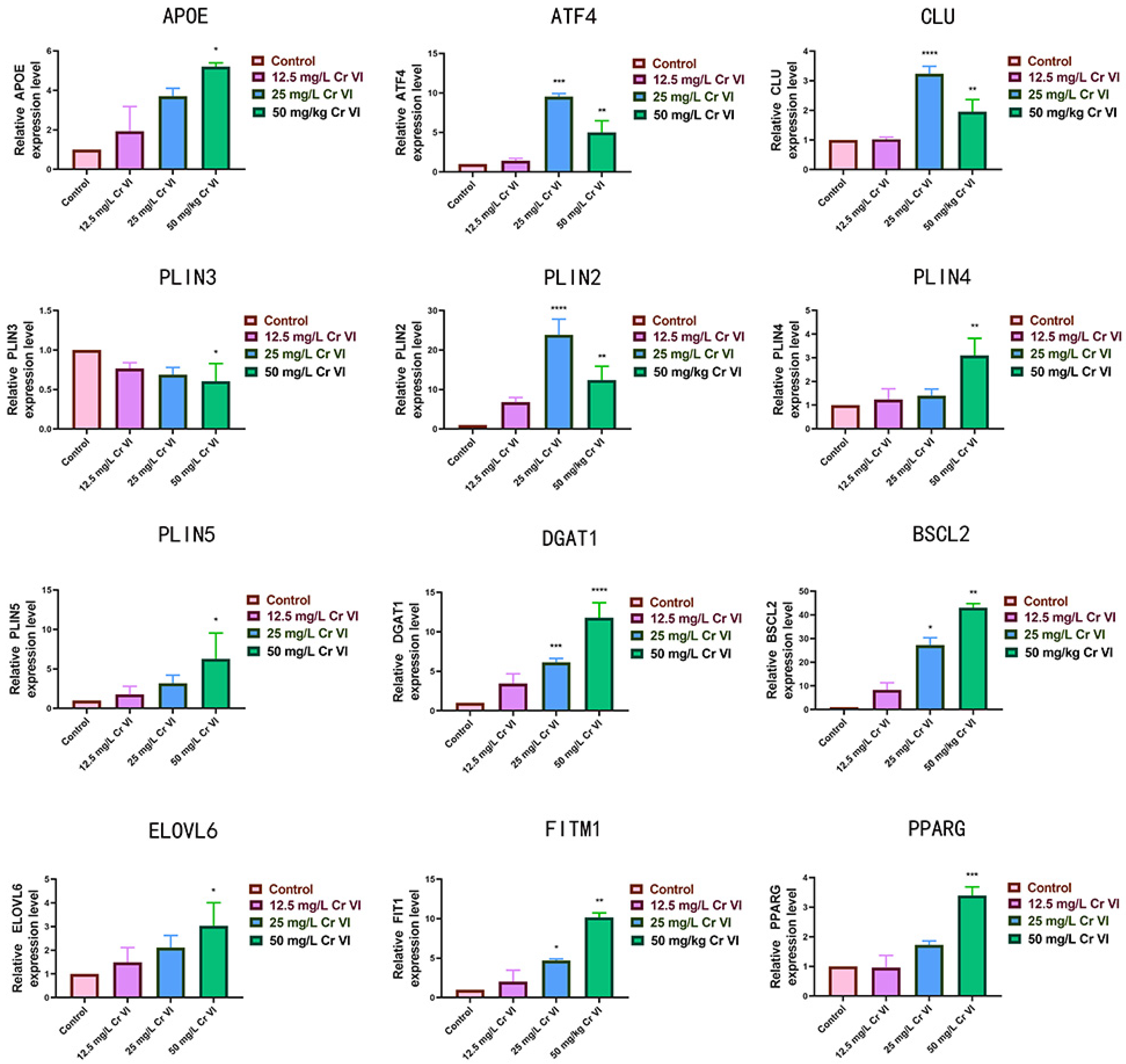
3.7. Changes in Lipid Metabolism-Related Gene Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Cr | Chromium |
| Cr(III) | Trivalent chromium |
| Cr(VI) | Hexavalent chromium |
| ROS | Reactive oxygen species |
| ER | Endoplasmic reticulum |
| ATF4 | Cyclic AMP-dependent transcription factor 4 |
| FASN | Fatty acid synthase |
| ACOX1 | Acyl-CoA oxidase 1 |
| BSCL2 | Berardinelli-Seip congenital lipodystrophy 2 |
| NCBI | National Center for Biotechnology Information |
| IVC | individually ventilated cages |
| TEM | Transmission electron microscopy |
| SOD1 | Superoxide dismutase 1 |
| GPX | Glutathione peroxidase |
| HSPs | Heat shock proteins |
| APOE | Apolipoprotein E |
| CLU | Clusterin |
| PPARG | Peroxisome Proliferator Activated Receptor Gamma |
| ELOVL6 | Elongation of Very Long Chain Fatty Acids Protein 6 |
| DGAT1 | Diacylglycerol O-Acyltransferase 1 |
| PLINs | Perilipins |
| FITM1 | Fat storage inducing transmembrane protein 1 |
| MAFLD | Metabolic-associated fatty liver disease |
| Fe | iron |
| Mn | Manganese |
| Zn | Zinc |
| Se | Selenium |
| Cu | Copper |
| ICP-OES | Inductively coupled plasma optical emission spectroscopy |
| TXNRD | thioredoxin reductases |
| SEPP1 | selenoprotein-P |
| TG | Triglyceride |
| LDL-C | Low-density lipoprotein cholesterol |
References
- Georgaki, M.N.; Tsokkou, S.; Keramas, A.; Papamitsou, T.; Karachrysafi, S.; Kazakis, N. Chromium supplementation and type 2 diabetes mellitus: An extensive systematic review. Env. Geochem. Health 2024, 46, 515. [Google Scholar] [CrossRef]
- Khan, C.; Malik, R.N.; Chen, J. Human exposure to chromite mining pollution, the toxicity mechanism and health impact. Heliyon 2024, 10, e40083. [Google Scholar] [CrossRef] [PubMed]
- Das, S.K.; Tripathi, A.K.; Kandi, S.K.; Mustakim, S.M.; Bhoi, B.; Rajput, P. Ferrochrome slag: A critical review of its properties, environmental issues and sustainable utilization. J. Environ. Manag. 2023, 326, 116674. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Zhao, D.; Ren, F.; Huang, L. Spatiotemporal variation of soil heavy metals in China: The pollution status and risk assessment. Sci. Total Environ. 2023, 871, 161768. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, J.; Ma, J.; Liu, Q.; Shi, T.; Gong, Y.; Yang, S.; Wu, Y. Status of chromium accumulation in agricultural soils across China (1989–2016). Chemosphere 2020, 256, 127036. [Google Scholar] [CrossRef]
- Sahu, K.; Dash, P. Chromium dynamics in soil and detoxification of chromite belts using rhizospheric soil-plant interface. Environ. Monit. Assess. 2025, 197, 654. [Google Scholar] [CrossRef]
- Xu, S.; Yu, C.; Wang, Q.; Liao, J.; Liu, C.; Huang, L.; Liu, Q.; Wen, Z.; Feng, Y. Chromium Contamination and Health Risk Assessment of Soil and Agricultural Products in a Rural Area in Southern China. Toxics 2022, 11, 27. [Google Scholar] [CrossRef]
- Suljević, D.; Sulejmanović, J.; Fočak, M.; Halilović, E.; Pupalović, D.; Hasić, A.; Alijagic, A. Assessing hexavalent chromium tissue-specific accumulation patterns and induced physiological responses to probe chromium toxicity in Coturnix japonica quail. Chemosphere 2021, 266, 129005. [Google Scholar] [CrossRef]
- Aliu, C.; Ajayi, O.O.; Olawuyi, T.S.; Gbadamosi, O.K.; Barbosa, F.J.; Adedire, C.O.; Adeyemi, J.A. Tissue Accumulation, Cytotoxicity, Oxidative Stress, and Immunotoxicity in African Catfish, Clarias gariepinus Exposed to Sublethal Concentrations of Hexavalent Chromium. Biol. Trace Elem. Res. 2024, 202, 2294–2307. [Google Scholar] [CrossRef]
- Yin, J.; Wang, L.; Li, S.; Chen, F.; Hegazy, A.M.; Zhang, X. Accumulation and depuration of dissolved hexavalent chromium and effects on the antioxidant response in bighead carp (Aristichthys nobilis). Environ. Toxicol. Pharmacol. 2020, 80, 103465. [Google Scholar] [CrossRef]
- Huang, H.; Chen, J.; Sun, Q.; Liu, Y.; Tang, Y.; Teng, X. NLRP3 inflammasome is involved in the mechanism of mitigative effect of selenium on lead-induced inflammatory damage in chicken kidneys. Environ. Sci. Pollut. Res. Int. 2021, 28, 10898. [Google Scholar] [CrossRef]
- Qiu, M.; Jiang, C.; Liang, J.; Zhou, Q.; Liu, Y.; Hao, Z.; Liu, Y.; Liu, X.; Teng, X.; Sun, W.; et al. Oxidative Stress, Energy Metabolism Disorder, Mitochondrial Damage, and miR-144 Participated in Molecular Mechanisms of 4-Octylphenol-Caused Cardiac Autophagic Damage in Common Carps (Cyprinus carpio L.). Metabolites 2025, 15, 391. [Google Scholar] [CrossRef]
- Zhou, Q.; Hao, Z.; Qiu, M.; Liu, Y.; Chang, M.; Liu, X.; Wang, Y.; Tang, Y.; Sun, W.; Teng, X.; et al. Amino acid metabolism disorder and oxidative stress took part in EGCG alleviating Mn-caused ferroptosis via miR-9-5p/got1 axis. J. Hazard. Mater. 2025, 489, 137656. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhao, J.L.; Bai, Z.; Du, J.; Shi, Y.; Wang, Y.; Wang, Y.; Liu, Y.; Yu, Z.; Li, M.Y. Polysaccharide from dandelion enriched nutritional composition, antioxidant capacity, and inhibited bioaccumulation and inflammation in Channa asiatica under hexavalent chromium exposure. Int. J. Biol. Macromol. 2022, 201, 557–568. [Google Scholar] [CrossRef] [PubMed]
- Mu, H.; Wang, G.; Huang, B.; Fu, X.; Cheng, S.; Wen, J. Effect of hexavalent chromium exposure on the reproductive status and biomarker responses of female Geloina erosa. Ecotoxicology 2023, 32, 736–745. [Google Scholar] [CrossRef] [PubMed]
- Cuevas-Magaña, M.Y.; Vega-García, C.C.; León-Contreras, J.C.; Hernández-Pando, R.; Zazueta, C.; García-Niño, W.R. Ellagic acid ameliorates hexavalent chromium-induced renal toxicity by attenuating oxidative stress, suppressing TNF-α and protecting mitochondria. Toxicol. Appl. Pharmacol. 2022, 454, 116242. [Google Scholar] [CrossRef]
- Verdonck, J.; Duca, R.C.; Galea, K.S.; Iavicoli, I.; Poels, K.; Töreyin, Z.N.; Vanoirbeek, J.; Godderis, L. Systematic review of biomonitoring data on occupational exposure to hexavalent chromium. Int. J. Hyg. Environ. Health 2021, 236, 113799. [Google Scholar] [CrossRef]
- Alvarez, C.C.; Bravo, G.M.; Hernández, Z.A. Hexavalent chromium: Regulation and health effects. J. Trace Elem. Med. Biol. 2021, 65, 126729. [Google Scholar] [CrossRef]
- Piñeda-Zayas, A.; Lopez-Mateos, L.M.; Palma-Fernández, J.C.; Iglesias-Linares, A. Assessment of metal ion accumulation in oral mucosa cells of patients with fixed orthodontic treatment and cellular DNA damage: A systematic review. Crit. Rev. Toxicol. 2021, 51, 622–633. [Google Scholar] [CrossRef]
- Tumolo, M.; Ancona, V.; De Paola, D.; Losacco, D.; Campanale, C.; Massarelli, C.; Uricchio, V.F. Chromium Pollution in European Water, Sources, Health Risk, and Remediation Strategies: An Overview. Int. J. Environ. Res. Public Health 2020, 17, 5438. [Google Scholar] [CrossRef]
- Singh, V.; Singh, N.; Verma, M.; Kamal, R.; Tiwari, R.; Sanjay, C.M.; Rai, S.N.; Kumar, A.; Singh, A.; Singh, M.P.; et al. Hexavalent-Chromium-Induced Oxidative Stress and the Protective Role of Antioxidants against Cellular Toxicity. Antioxidants 2022, 11, 2375. [Google Scholar] [CrossRef] [PubMed]
- Kurmangaliyeva, S.; Baktikulova, K.; Tkachenko, V.; Seitkhanova, B.; Shapambayev, N.; Rakhimzhanova, F.; Almagambetova, A.; Kurmangaliyev, K. An Overview of Hexavalent Chromium-Induced Necroptosis, Pyroptosis, and Ferroptosis. Biol. Trace Elem. Res. 2025, 203, 2619–2635. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.; Zhang, Y.; Hong, S.; Zhang, Q.; Xu, J.; Hu, G.; Zhu, X.; Yuan, F.; Yu, S.; Wang, T.; et al. Relationships between blood chromium exposure and liver injury: Exploring the mediating role of systemic inflammation in a chromate-exposed population. J. Environ. Sci. 2024, 143, 224–234. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, N.; Zhang, X.; Zhang, L.; Jia, G.; Yu, S. Low-Dose Hexavalent Chromium Exposure Induces Endoplasmic Reticulum Stress-Mediated Apoptosis in Rat Liver. Biol. Trace Elem. Res. 2024, 202, 4136–4145. [Google Scholar] [CrossRef]
- Li, N.; Li, X.; Zhang, X.; Zhang, L.; Wu, H.; Yu, Y.; Jia, G.; Yu, S. Low-dose hexavalent chromium induces mitophagy in rat liver via the AMPK-related PINK1/Parkin signaling pathway. PeerJ 2024, 12, e17837. [Google Scholar] [CrossRef]
- Li, L.; Chen, H.; Bi, R.; Xie, L. Bioaccumulation, subcellular distribution, and acute effects of chromium in Japanese medaka (Oryzias latipes). Environ. Toxicol. Chem. 2015, 34, 2611–2617. [Google Scholar] [CrossRef]
- Zhou, X.; Yang, X.; Liu, X.; Jiang, L.; Jiang, Y. Hexavalent chromium (Cr6+) induces oxidative stress, inflammatory response, apoptosis, and DNA damage in the liver of largemouth bass by inhibiting the Nrf2-Keap1 signal pathway. Fish. Physiol. Biochem. 2025, 51, 53. [Google Scholar] [CrossRef]
- Yu, Z.; Xu, S.F.; Zhao, J.L.; Zhao, L.; Zhang, A.Z.; Li, M.Y. Toxic effects of hexavalent chromium (Cr6+) on bioaccumulation, apoptosis, oxidative damage and inflammatory response in Channa asiatica. Environ. Toxicol. Pharmacol. 2021, 87, 103725. [Google Scholar] [CrossRef]
- Pipitone, R.M.; Ciccioli, C.; Infantino, G.; La Mantia, C.; Parisi, S.; Tulone, A.; Pennisi, G.; Grimaudo, S.; Petta, S. MAFLD: A multisystem disease. Ther. Adv. Endocrinol. Metab. 2023, 14, 1859452467. [Google Scholar] [CrossRef]
- Badmus, O.O.; Hillhouse, S.A.; Anderson, C.D.; Hinds, T.D.; Stec, D.E. Molecular mechanisms of metabolic associated fatty liver disease (MAFLD): Functional analysis of lipid metabolism pathways. Clin. Sci. 2022, 136, 1347–1366. [Google Scholar] [CrossRef]
- Fromenty, B.; Roden, M. Mitochondrial alterations in fatty liver diseases. Hepatol. J. 2023, 78, 415–429. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, W.; Liu, X.; Song, X.; Chai, L. Probing the effects of hexavalent chromium exposure on histology and fatty acid metabolism in liver of Bufo gargarizans tadpoles. Chemosphere 2020, 243, 125437. [Google Scholar] [CrossRef]
- Luo, M.; Huang, S.; Zhang, J.; Zhang, L.; Mehmood, K.; Jiang, J.; Zhang, N.; Zhou, D. Effect of selenium nanoparticles against abnormal fatty acid metabolism induced by hexavalent chromium in chicken’s liver. Environ. Sci. Pollut. Res. Int. 2019, 26, 21828–21834. [Google Scholar] [CrossRef]
- Jia, J.; Zhang, M.; Cao, Z.; Hu, X.; Lei, S.; Zhang, Y.; Kang, X. The rabbit model for spinal tuberculosis: An overview. J. Orthop. Surg. 2024, 32, 793538575. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Zhou, J.; Zhang, R.; Liang, Y.; Lai, L.; Li, Z. Genome-edited rabbits: Unleashing the potential of a promising experimental animal model across diverse diseases. Zool. Res. 2024, 45, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Mathur, A.K.; Chandra, S.V.; Tandon, S.K. Comparative toxicity of trivalent and hexavalent chromium to rabbits II. Morphological changes in some organs. Toxicology 1977, 8, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Yousef, M.I.; El-Demerdash, F.M.; Kamil, K.I.; Elaswad, F.A. Ameliorating effect of folic acid on chromium(VI)-induced changes in reproductive performance and seminal plasma biochemistry in male rabbits. Reprod. Toxicol. 2006, 21, 322–328. [Google Scholar] [CrossRef]
- El-Demerdash, F.M.; Yousef, M.I.; Elaswad, F.A. Biochemical study on the protective role of folic acid in rabbits treated with chromium (VI). J. Environ. Sci. Health B 2006, 41, 731–746. [Google Scholar] [CrossRef]
- Zhao, X.J.; Bao, C.F.; Wang, L.; Liu, X.L. Effects of chromium-contaminated groundwater on rat liver function and expression of Caspase-3 and PARP. Chin. J. Mod. Med. 2019, 29, 7–11. [Google Scholar]
- Lv, Y.G.; Cai, W.T.; Yang, L.; Bian, C.; Li, J.J.; Wang, M.G. Research on the pollution characteristics and migration transformation rules of hexavalent chromium in groundwater contaminated by a chromium slag pile. Hydrol. Eng. Geol. 2024, 51, 180–190. [Google Scholar]
- Liu, Y.; Qiu, M.; Hao, Z.; Liu, Y.; Wang, S.; Chang, M.; Liu, X.; Sun, W.; Teng, X.; Wang, X. The mechanism of lycopene alleviating cadmium-inhibited glucose uptake ability of epithelioma papulosum cyprini cells: miR-375, oxidative stress, and actin cytoskeleton dysfunction. J. Environ. Manag. 2025, 380, 125143. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yang, J.; Wang, W.; Bao, X.; Chang, D.; Hao, Z.; Qiu, M.; Liu, Y.; Teng, X.L.Z. Mechanisms of nanoplastic-induced energy metabolism reprogramming in juvenile Sepia esculenta: mRNA profile, miRNA/mRNA network, and ceRNA network. Environ. Chem. Ecotoxicol. 2025, 7, 1836–1849. [Google Scholar] [CrossRef]
- Zadoorian, A.; Du, X.; Yang, H. Lipid droplet biogenesis and functions in health and disease. Nat. Rev. Endocrinol. 2023, 19, 443–459. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Li, S.; Ye, S.; Tang, S.; Hu, D.; Wei, L.; Xiao, F. Hexavalent chromium inhibits the formation of neutrophil extracellular traps and promotes the apoptosis of neutrophils via AMPK signaling pathway. Ecotoxicol. Environ. Saf. 2021, 223, 112614. [Google Scholar] [CrossRef]
- García-Rodríguez, M.C.; Carvente-Juárez, M.M.; Altamirano-Lozano, M.A. Antigenotoxic and apoptotic activity of green tea polyphenol extracts on hexavalent chromium-induced DNA damage in peripheral blood of CD-1 mice: Analysis with differential acridine orange/ethidium bromide staining. Oxid. Med. Cell Longev. 2013, 2013, 486419. [Google Scholar] [CrossRef]
- Lou, J.; Yu, S.; Feng, L.; Guo, X.; Wang, M.; Branco, A.T.; Li, T.; Lemos, B. Environmentally induced ribosomal DNA (rDNA) instability in human cells and populations exposed to hexavalent chromium [Cr (VI)]. Environ. Int. 2021, 153, 106525. [Google Scholar] [CrossRef]
- Bertinato, J.; Griffin, P. A low chromium diet increases body fat, energy intake and circulating triglycerides and insulin in male and female rats fed a moderately high-fat, high-sucrose diet from peripuberty to young adult age. PLoS ONE 2023, 18, e281019. [Google Scholar] [CrossRef]
- Shaw, P.; Mondal, P.; Dey, B.A.; Bandyopadhyay, A.; Sudarshan, M.; Chakraborty, A.; Chattopadhyay, A. Environmentally Relevant Hexavalent Chromium Disrupts Elemental Homeostasis and Induces Apoptosis in Zebrafish Liver. Bull. Env. Contam. Toxicol. 2022, 108, 716–724. [Google Scholar] [CrossRef]
- Shen, J.; Kom, M.C.; Huang, H.; Fu, G.; Xie, Y.; Gao, Y.; Tang, Y.; Yan, J.; Jin, L. Role of NF-κB signaling pathway in hexavalent chromium-induced hepatotoxicity. Env. Toxicol. 2023, 38, 1361–1371. [Google Scholar] [CrossRef]
- Boşgelmez, I.İ.; Güvendik, G. N-Acetyl-L-Cysteine Protects Liver and Kidney Against Chromium(VI)-Induced Oxidative Stress in Mice. Biol. Trace Elem. Res. 2017, 178, 44–53. [Google Scholar] [CrossRef]
- Kotyzová, D.; Hodková, A.; Bludovská, M.; Eybl, V. Effect of chromium (VI) exposure on antioxidant defense status and trace element homeostasis in acute experiment in rat. Toxicol. Ind. Health 2015, 31, 1044–1050. [Google Scholar] [CrossRef]
- Huang, X.; Yuan, J.; Gu, J.; Abbas, Y.; Yuan, Y.; Liu, Z.; Zou, H.; Bian, J. Protective effect of honokiol on cadmium-induced liver injury in chickens. Poult. Sci. 2024, 103, 104066. [Google Scholar] [CrossRef]
- Chen, L.; Min, J.; Wang, F. Copper homeostasis and cuproptosis in health and disease. Signal Transduct. Target. Ther. 2022, 7, 378. [Google Scholar] [CrossRef] [PubMed]
- Xue, Q.; Kang, R.; Klionsky, D.J.; Tang, D.; Liu, J.; Chen, X. Copper metabolism in cell death and autophagy. Autophagy 2023, 19, 2175–2195. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Cai, Q.; Liang, R.; Zhang, D.; Liu, X.; Zhang, M.; Xiong, Y.; Xu, M.; Liu, Q.; Li, P.; et al. Copper homeostasis and copper-induced cell death in the pathogenesis of cardiovascular disease and therapeutic strategies. Cell Death Dis. 2023, 14, 105. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Li, Y.Y.; Liu, X. Copper homeostasis and copper-induced cell death: Novel targeting for intervention in the pathogenesis of vascular aging. Biomed. Pharmacother. 2023, 169, 115839. [Google Scholar] [CrossRef]
- Li, Y.; Du, Y.; Zhou, Y.; Chen, Q.; Luo, Z.; Ren, Y.; Chen, X.; Chen, G. Iron and copper: Critical executioners of ferroptosis, cuproptosis and other forms of cell death. Cell Commun. Signal 2023, 21, 327. [Google Scholar] [CrossRef]
- Bjørklund, G.; Aaseth, J.; Skalny, A.V.; Suliburska, J.; Skalnaya, M.G.; Nikonorov, A.A.; Tinkov, A.A. Interactions of iron with manganese, zinc, chromium, and selenium as related to prophylaxis and treatment of iron deficiency. J. Trace Elem. Med. Biol. 2017, 41, 41–53. [Google Scholar] [CrossRef]
- Bai, Y.Z.; Zhang, Y.; Zhang, S.Q. New horizons for the role of selenium on cognitive function: Advances and challenges. Metab. Brain Dis. 2024, 39, 1255–1268. [Google Scholar] [CrossRef]
- Demircan, K.; Chillon, T.S.; Bang, J.; Gladyshev, V.N.; Schomburg, L. Selenium, diabetes, and their intricate sex-specific relationship. Trends Endocrinol. Metab. 2024, 35, 781–792. [Google Scholar] [CrossRef]
- Shi, Z.; Han, Z.; Chen, J.; Zhou, J.C. Endoplasmic reticulum-resident selenoproteins and their roles in glucose and lipid metabolic disorders. Biochim. Biophys. Acta Mol. Basis Dis. 2024, 1870, 167246. [Google Scholar] [CrossRef] [PubMed]
- Souza, L.; Campos, R.O.; Braga, J.F.; Jesus, J.; Ramos, H.E.; Anunciação, S.M.; Cassemiro, J.F.; Rende, P.; Hecht, F. Selenium nutritional status and thyroid dysfunction. Arch. Endocrinol. Metab. 2025, 69, e230348. [Google Scholar] [CrossRef] [PubMed]
- Hao, Z.; Qiu, M.; Liu, Y.; Liu, Y.; Chang, M.; Liu, X.; Wang, Y.; Sun, W.; Teng, X.; Tang, Y. Co-exposure to ammonia and lipopolysaccharide-induced impaired energy metabolism via the miR-1599/HK2 axis and triggered autophagy, ER stress, and apoptosis in chicken cardiomyocytes. Poult. Sci. 2025, 104, 104965. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Bao, X.; Liu, X.; Wang, Y.; Zhu, X.; Zhang, Y.; Wang, Z.; Maslennikov, S.; Whiteside, M.; Wang, W. Transcriptome analysis provides preliminary insights into the response of Sepia esculenta to high salinity stress. Agric. Commun. 2024, 2, 100064. [Google Scholar] [CrossRef]
- Qiu, M.; Hao, Z.; Liu, Y.; Liu, Y.; Chang, M.; Lin, X.; Liu, X.; Dong, N.; Sun, W.; Teng, X. ROS acted as an initial role in selenium nanoparticles alleviating insecticide chlorpyrifos-induced oxidative stress, pyroptosis, and intestinal barrier dysfunction in porcine intestinal epithelial cells. Pestic. Biochem. Physiol. 2025, 211, 106418. [Google Scholar] [CrossRef]
- Song, C.; Zhang, A.; Zhang, M.; Song, Y.; Huangfu, H.; Jin, S.; Sun, Y.; Zhang, C.; Shi, D.; Wang, J.; et al. Nrf2/PINK1-mediated mitophagy induction alleviates sodium fluoride-induced hepatic injury by improving mitochondrial function, oxidative stress, and inflammation. Ecotoxicol. Env. Saf. 2023, 252, 114646. [Google Scholar] [CrossRef]
- Nie, Z.; Xiao, C.; Wang, Y.; Li, R.; Zhao, F. Heat shock proteins (HSPs) in non-alcoholic fatty liver disease (NAFLD): From molecular mechanisms to therapeutic avenues. Biomark. Res. 2024, 12, 120. [Google Scholar] [CrossRef]
- Tower, J. Hsps and aging. Trends Endocrinol. Metab. 2009, 20, 216–222. [Google Scholar] [CrossRef]
- Zuehlke, A.D.; Beebe, K.; Neckers, L.; Prince, T. Regulation and function of the human HSP90AA1 gene. Gene 2015, 570, 8–16. [Google Scholar] [CrossRef]
- Bălănescu, A.; Stan, I.; Codreanu, I.; Comănici, V.; Bălănescu, E.; Bălănescu, P. Circulating Hsp90 Isoform Levels in Overweight and Obese Children and the Relation to Nonalcoholic Fatty Liver Disease: Results from a Cross-Sectional Study. Dis. Markers 2019, 2019, 9560247. [Google Scholar] [CrossRef]
- Rodriguez, A.; Von Salzen, D.; Holguin, B.A.; Bernal, R.A. Complex Destabilization in the Mitochondrial Chaperonin Hsp60 Leads to Disease. Front. Mol. Biosci. 2020, 7, 159. [Google Scholar] [CrossRef]
- Weng, S.W.; Wu, J.C.; Shen, F.C.; Chang, Y.H.; Su, Y.J.; Lian, W.S.; Tai, M.H.; Su, C.H.; Chuang, J.H.; Lin, T.K.; et al. Chaperonin counteracts diet-induced non-alcoholic fatty liver disease by aiding sirtuin 3 in the control of fatty acid oxidation. Diabetologia 2023, 66, 913–930. [Google Scholar] [CrossRef]
- Ross, D.; Siegel, D. The diverse functionality of NQO1 and its roles in redox control. Redox Biol. 2021, 41, 101950. [Google Scholar] [CrossRef] [PubMed]
- Chhetri, J.; Dilek, J.; Davies, N.; Jacobson, G.; Dallmann, R.; Gueven, N. NQO1 protects against clioquinol toxicity. Front. Pharmacol. 2022, 13, 1000278. [Google Scholar] [CrossRef] [PubMed]
- Preethi, S.; Arthiga, K.; Patil, A.B.; Spandana, A.; Jain, V. Review on NAD(P)H dehydrogenase quinone 1 (NQO1) pathway. Mol. Biol. Rep. 2022, 49, 8907–8924. [Google Scholar] [CrossRef] [PubMed]
- Ryter, S.W. Heme Oxygenase-1: An Anti-Inflammatory Effector in Cardiovascular, Lung, and Related Metabolic Disorders. Antioxidants 2022, 11, 555. [Google Scholar] [CrossRef]
- Flohé, L.; Toppo, S.; Orian, L. The glutathione peroxidase family: Discoveries and mechanism. Free Radic. Biol. Med. 2022, 187, 113–122. [Google Scholar] [CrossRef]
- Saxena, P.; Selvaraj, K.; Khare, S.K.; Chaudhary, N. Superoxide dismutase as multipotent therapeutic antioxidant enzyme: Role in human diseases. Biotechnol. Lett. 2022, 44, 1–22. [Google Scholar] [CrossRef]
- Sayed, A.M.; Hassanein, E.; Salem, S.H.; Hussein, O.E.; Mahmoud, A.M. Flavonoids-mediated SIRT1 signaling activation in hepatic disorders. Life Sci. 2020, 259, 118173. [Google Scholar] [CrossRef]
- Amer, S.A.; Omar, A.E.; El-Hack, M.E.A. Effects of Selenium- and Chromium-Enriched Diets on Growth Performance, Lipid Profile, and Mineral Concentration in Different Tissues of Growing Rabbits. Biol. Trace Elem. Res. 2019, 187, 92–99. [Google Scholar] [CrossRef]
- Peng, Y.; Li, H.; Shen, K.; Pan, W.; Zhang, J.; Zhou, D. Nano-selenium alleviating the lipid metabolism disorder of LMH cells induced by potassium dichromate via down-regulating ACACA and FASN. Env. Sci. Pollut. Res. Int. 2021, 28, 69426–69435. [Google Scholar] [CrossRef]
- Treguier, Y.; Bull-Maurer, A.; Roingeard, P. Apolipoprotein E, a Crucial Cellular Protein in the Life cycle of Hepatitis Viruses. Int. J. Mol. Sci. 2022, 23, 3676. [Google Scholar] [CrossRef]
- Qi, G.; Mi, Y.; Shi, X.; Gu, H.; Brinton, R.D.; Yin, F. ApoE4 Impairs Neuron-Astrocyte Coupling of Fatty Acid Metabolism. Cell Rep. 2021, 34, 108572. [Google Scholar] [CrossRef] [PubMed]
- Oleszycka, E.; Kwiecień, K.; Grygier, B.; Cichy, J.; Kwiecińska, P. The many faces of DGAT1. Life Sci. 2025, 362, 123322. [Google Scholar] [CrossRef] [PubMed]
- Griseti, E.; Bello, A.A.; Bieth, E.; Sabbagh, B.; Iacovoni, J.S.; Bigay, J.; Laurell, H.; Copic, A. Molecular mechanisms of perilipin protein function in lipid droplet metabolism. FEBS Lett. 2024, 598, 1170–1198. [Google Scholar] [CrossRef] [PubMed]
- Fader, K.C.; Romano, P.S.; Vanrell, M.C.; Pocognoni, C.A.; Jacob, J.; Caruso, B.; Delgui, L.R. Biogenesis and Breakdown of Lipid Droplets in Pathological Conditions. Front. Cell Dev. Biol. 2021, 9, 826248. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, J.; Yang, Q.; Li, X.; Qiu, Y.; Zhang, Y.; Liu, M.; Zhu, A.J. Therapeutic siRNA targeting PLIN2 ameliorates steatosis, inflammation, and fibrosis in steatotic liver disease models. J. Lipid Res. 2024, 65, 100635. [Google Scholar] [CrossRef]
- Mishra, A.; Liu, S.; Promes, J.; Harata, M.; Sivitz, W.; Fink, B.; Bhardwaj, G.; O’Neill, B.T.; Kang, C.; Sah, R.; et al. Perilipin 2 downregulation in β cells impairs insulin secretion under nutritional stress and damages mitochondria. J. Clin. Investig. 2021, 6, e144341. [Google Scholar] [CrossRef]
- Cortini, M.; Ilieva, E.; Massari, S.; Bettini, G.; Avnet, S.; Baldini, N. Uncovering the protective role of lipid droplet accumulation against acid-induced oxidative stress and cell death in osteosarcoma. Biochim. Biophys. Acta Mol. Basis Dis. 2025, 1871, 167576. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Z.; Wang, D.; Nie, K.; Wu, W.; Gao, Y.; Chen, S.; Jiang, X.; Tang, Y.; Su, H.; et al. Berberine Attenuates Nonalcoholic Hepatic Steatosis by Regulating Lipid Droplet-Associated Proteins: In Vivo, In Vitro and Molecular Evidence. J. Cell Mol. Med. 2025, 29, e70524. [Google Scholar] [CrossRef]
- Berthier, A.; Johanns, M.; Zummo, F.P.; Lefebvre, P.; Staels, B. PPARs in liver physiology. Biochim. Biophys. Acta Mol. Basis Dis. 2021, 1867, 166097. [Google Scholar] [CrossRef]
- Monroy-Ramirez, H.C.; Galicia-Moreno, M.; Sandoval-Rodriguez, A.; Meza-Rios, A.; Santos, A.; Armendariz-Borunda, J. PPARs as Metabolic Sensors and Therapeutic Targets in Liver Diseases. Int. J. Mol. Sci. 2021, 22, 8298. [Google Scholar] [CrossRef] [PubMed]
- Russo-Savage, L.; Schulman, I.G. Liver X receptors and liver physiology. Biochim. Biophys. Acta Mol. Basis Dis. 2021, 1867, 166121. [Google Scholar] [CrossRef] [PubMed]
- Magré, J.; Prieur, X. Seipin Deficiency as a Model of Severe Adipocyte Dysfunction: Lessons from Rodent Models and Teaching for Human Disease. Int. J. Mol. Sci. 2022, 23, 740. [Google Scholar] [CrossRef] [PubMed]
- Klug, Y.A.; Ferreira, J.V.; Carvalho, P. A unifying mechanism for seipin-mediated lipid droplet formation. FEBS Lett. 2024, 598, 1116–1126. [Google Scholar] [CrossRef]
- Eguchi, A.; Iwasa, M. The Role of Elevated Liver-Type Fatty Acid-Binding Proteins in Liver Diseases. Pharm. Res. 2021, 38, 89–95. [Google Scholar] [CrossRef]
- Nowowiejska, J.; Baran, A.; Flisiak, I. Fatty Acid-Binding Proteins in Psoriasis-A Review. Metabolites 2022, 12, 833. [Google Scholar] [CrossRef]
- Yan, C.; Xia, X.; He, J.; Ren, Z.; Xu, D.; Xiong, Y.; Zuo, B. MyoD Is a Novel Activator of Porcine FIT1 Gene by Interacting with the Canonical E-Box Element during Myogenesis. Int. J. Mol. Sci. 2015, 16, 25014–25030. [Google Scholar] [CrossRef]
- Gao, H.; Jin, Z.; Bandyopadhyay, G.; Wang, G.; Zhang, D.; Rocha, K.; Liu, X.; Zhao, H.; Kisseleva, T.; Brenner, D.A.; et al. Aberrant iron distribution via hepatocyte-stellate cell axis drives liver lipogenesis and fibrosis. Cell Metab. 2022, 34, 1201–1213. [Google Scholar] [CrossRef]
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| APOE | GCAGGAGGCGACTGACC | GCAGGTAATCCCAGAAGCGG |
| BSCL2 | AGTCACAAGTGACAGAGCCAG | CTGGCGGCTCCTCTTCCT |
| FITM1 | TTGCAAAAACGGTGCTGAACTTAT | ATGTGCTGGTCACCCTTCAC |
| PLIN3 | GCCGCAGATTGCGTCG | CCGACACCTTGGATGACACA |
| PLIN4 | GTCAGTGGAGGAGTGTGGTC | ACTGCCAGCTGAGCTTGTTC |
| PLIN5 | CTCAACTTTCCTGCCCGTCA | CTGATCACCGGACATTCTGCT |
| PLIN2 | AGTGTCCGACAGCTTCCTCA | CAGTGAAATCAAATGCCAGCCAG |
| PPARG | GGTGGTTGAGAGCAGTGGAT | CTGTGTCAACCATGATGACTTCTTT |
| DGAT1 | CCACTGGGAGCTGAGATGC | CCAGGAACAACCGTGCATTG |
| CLU | CGTGGAGTTCATCACAGGAGG | TGGCACTTAGCACACTGGTC |
| HSP90AA1 | GCCCAGAGTGCTGAATACCC | TAACAGGTGCCCTGCTTCTC |
| HSPA4 | TCCAGTGCCTCTCTAGTGGA | AGCTTGAGAAGTCACGCCG |
| HSPD1 | GTAAGCCCCTGGTCATAATTGCT | TCCCTCTTCTCCAAATACTGCAC |
| SOD1 | CACCATCCACTTCGAGCAGA | CTGCACTCGTACAGCCTTGT |
| SOD2 | GCAAGGAACAACAGGCCTTA | AACAGCCAGAGAGCACGAC |
| HMOX1 | GCCGAGGGTTTTAAGCTGGT | CAGCTCCTCCGGGAAGTAGA |
| NQO1 | CTTCAACCCCGTCCTTTCCA | ACTGCAGCGGGAACTGAAATA |
| SIRT1 | TAACTGGAGCTGGGGTGTCT | GACGGTTGAAACTGTCCAGG |
| SIRT2 | GGTGCAAGAGGCTCAGGATT | AGCGGTCGCTCTGAATGAAG |
| GPX1 | AACCAGTTTGGGCATCAGGAGA | GGCCTTGGCGCCGTT |
| GPX3 | GGGGCCAAGAGAAGTCCAA | TCTTGTAGTGCATTCAGTTCCACG |
| GPX4 | CGGGAGGCAGGAGCC | GGTGAAGTTCCACTTGATGGC |
| MAP1LC3 | GGATCACAGTCCCTTCCTCG | CTTACAACGGTCGGCAAAGC |
| BECLIN1 | GCCGAAGACTGAAGGTCACT | ACGTTGAGCTGAGTGTCCAG |
| ULK | ACCGTGGGCAAGTTCGAG | ACCGTGGGCAAGTTCGAG |
| Actin | CAGTGGCCGTACAACTGGTAT | AAACGCAAGATCGCATGTGG |
| Cu (μg/g) | Zn (μg/g) | Fe (μg/g) | Mn (μg/g) | Se (μg/g) | |
|---|---|---|---|---|---|
| Control | 1.79 ± 0.588 | 15.29 ± 1.326 | 1061 ± 226.7 | 250.4 ± 17.32 | 3.981 ± 0.262 |
| 12.5 mg/L | 1.82 ± 0.599 | 15.47 ± 1.896 | 641.3 ± 57.38 (*) | 247.5 ± 25.86 | 3.938 ± 0.324 |
| 25 mg/L | 2.56 ± 0.417 | 14.39 ± 0.015 | 517.4 ± 140.9 (**) | 219.6 ± 27.25 | 3.661 ± 0.217 |
| 50 mg/L | 1.51 ± 0.199 | 14.35 ± 1.154 | 464.6 ± 142.6 (***) | 214.6 ± 23.89 | 3.642 ± 0.322 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, J.; Zhang, L.; Li, X.; Li, X.; Zhao, P.; Ren, X.; Song, Y. Hexavalent Chromium Induces Defense Responses, Hepatocellular Apoptosis, and Lipid Metabolism Alterations in New Zealand Rabbit Livers. Metabolites 2025, 15, 637. https://doi.org/10.3390/metabo15100637
Yuan J, Zhang L, Li X, Li X, Zhao P, Ren X, Song Y. Hexavalent Chromium Induces Defense Responses, Hepatocellular Apoptosis, and Lipid Metabolism Alterations in New Zealand Rabbit Livers. Metabolites. 2025; 15(10):637. https://doi.org/10.3390/metabo15100637
Chicago/Turabian StyleYuan, Junzhao, Lei Zhang, Xiuqing Li, Xinfeng Li, Pandeng Zhao, Xiaoli Ren, and Yuzhen Song. 2025. "Hexavalent Chromium Induces Defense Responses, Hepatocellular Apoptosis, and Lipid Metabolism Alterations in New Zealand Rabbit Livers" Metabolites 15, no. 10: 637. https://doi.org/10.3390/metabo15100637
APA StyleYuan, J., Zhang, L., Li, X., Li, X., Zhao, P., Ren, X., & Song, Y. (2025). Hexavalent Chromium Induces Defense Responses, Hepatocellular Apoptosis, and Lipid Metabolism Alterations in New Zealand Rabbit Livers. Metabolites, 15(10), 637. https://doi.org/10.3390/metabo15100637






