Animal Food Products to Support Human Nutrition and to Boost Human Health: The Potential of Feedstuffs Resources and Their Metabolites as Health-Promoters
Abstract
1. Introduction
2. Animal Feedstuffs Classification
2.1. NRC Feed Class 1 (Dry Forages and Roughages) and NRC Feed Class 2 (Pasture, Range Plants, and Forages Fed Fresh; Both with ≥18% of Fiber)
2.2. NRC Feed Class 3 (Silages)
2.3. NRC Feed Class 4 (Energetic Sources)
2.4. NRC Feed Class 5 (Protein Sources)
2.5. NRC Feed Class 6 (Minerals) and NRC Feed Class 7 (Vitamins)
2.6. NRC Feed Class 8 (Additives)
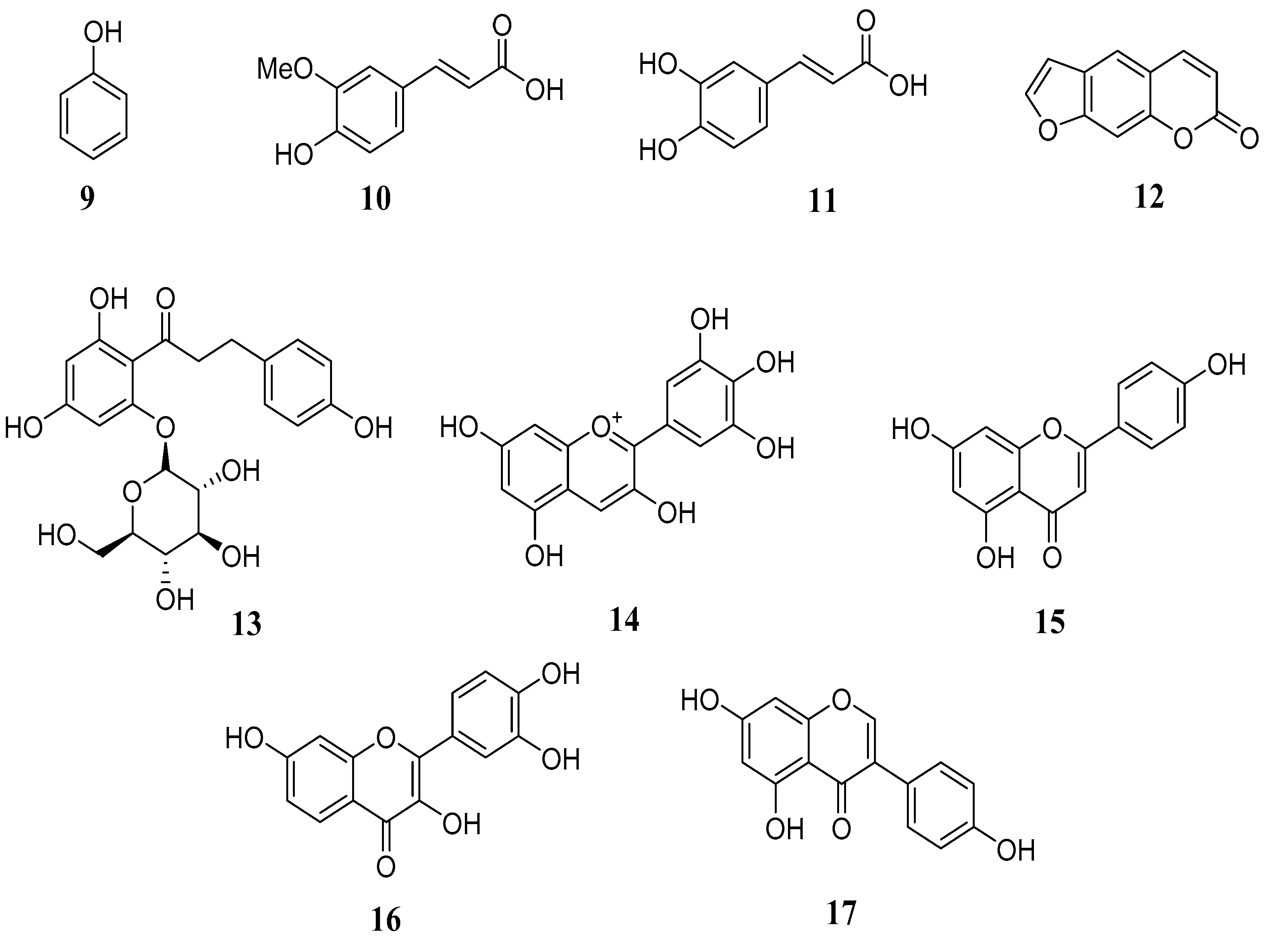
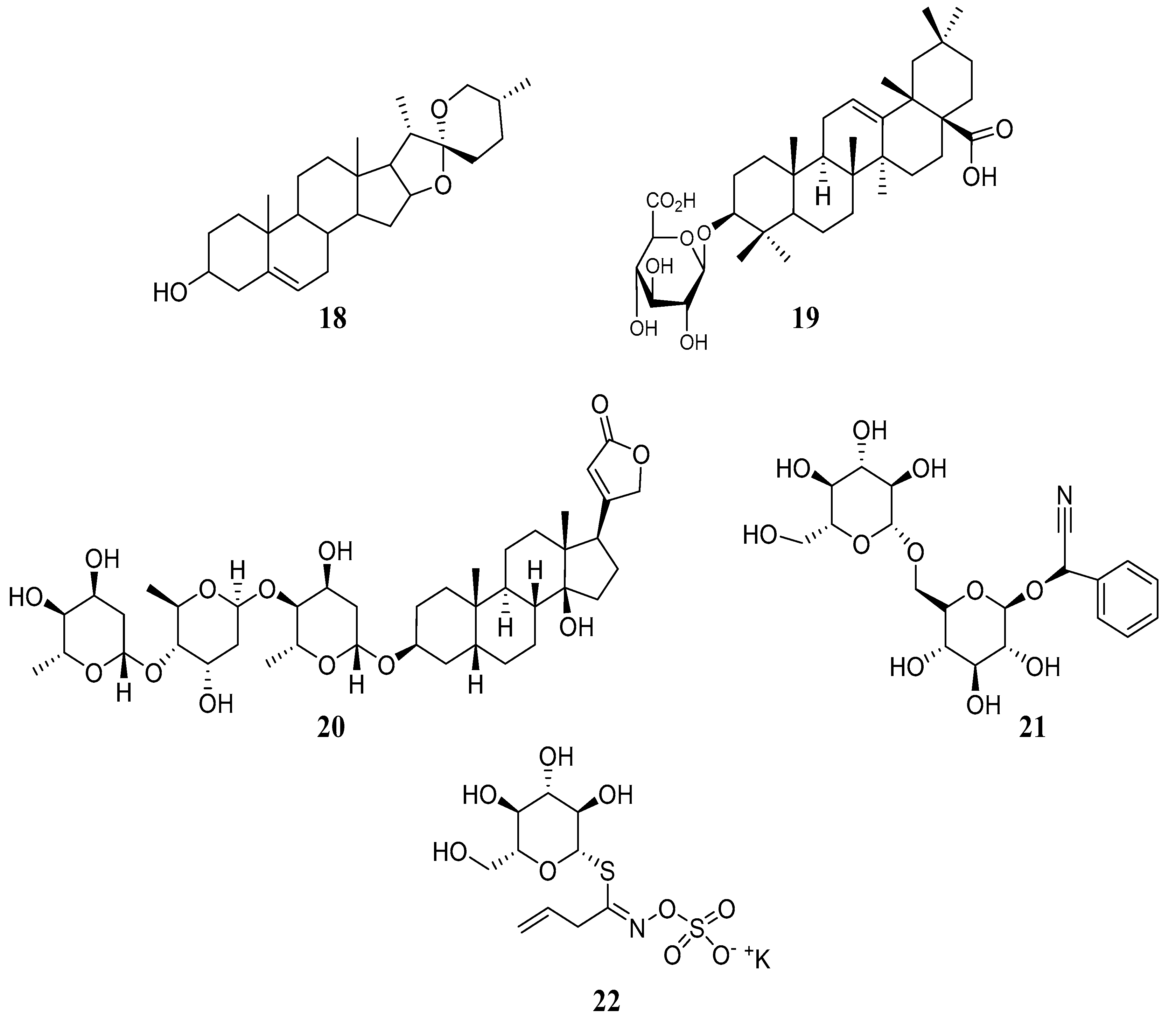
3. Biochemistry of Major Classes of Natural Compounds Found in Animal Feedstuffs
3.1. Terpenes
3.2. Phenolic Compounds
3.3. Glycosides
3.4. Alkaloids
4. Food Products of Animal Origin: Metabolites and Health Benefits and Implications
4.1. Milk and Dairy By-Products
| Metabolite Category | Metabolite | Feedstuffs or Dairy Product | Dose, Concentration, or Treatment | Biological Function of Metabolites, Biochemistry, and Biotransformation | Reference |
|---|---|---|---|---|---|
| Phenols | Tannin | Blood plasma and cheese | Control group and Tannin group supplemented with 150 g/head per day of tannins extract, chestnut and quebracho (60:40). | Serum: tannin supplementation lowered oxidative stress both in spring and in summer. Lowered oxidative stress (IL-1β and higher IL-10). Cheese: improvement of the antioxidant properties. | Santillo et al., 2022 [10] |
| Bioactive peptides | Bioactive peptides | Hydrolysates of camel milk | Hydrolysates at 500 mg/kg of BW | Antidiabetic properties. Hypoglycemic activity and improvement in activity of superoxide dismutase and catalase. Reduced glutathione levels and the attenuation of malondialdehyde. Lower levels of liver function enzymes (aspartate aminotransferase and alanine aminotransferase). Histology of liver and pancreatic tissue displayed absence of lipid accumulation in hepatocytes and preservation of β-cells. | Kilari et al., 2021 [88] |
| Camel whey proteins | Camel whey proteins | Camel milk | Hydrolysates at 500 mg/kg of BW | Antidiabetic properties. Inhibition of DPP-IV (dipeptidyl peptidase IV) and their positive action on hIR (the human insulin receptor) activation and glucose uptake. | Ashraf et al., 2021 [89] |
| Phenolic acids | Anthocyanin | Black rice and purple corn extracted residue | 0, 2, 4, and 6% black rice and purple corn extracted residue | Increase of antioxidant activity and reduction of oxidative stress in plasma. Malondialdehyde (MDA) concentrations in the plasma decreased. | Prommachart, et al., 2021 [90] |
| Phenolic acids | Grape seed and grape marc meal extract | Grape seed and grape marc meal extract | 0, 1% of grape seed and grape marc meal extract or the same total mixed ration supplemented with | Cows fed grape seed and grape marc meal extract had an increased milk and protein yield. Reduced mRNA presence of fibroblast growth factor (FGF) 21. | He et al., 2019 [91] |
| Phenolic acids | Grape seed and grape marc meal extract | Grape seed and grape marc meal extract | 1% grape seed and grape marc meal extract | Cows supplemented with grape seed and grape marc meal extract had a significantly reduced mRNA abundance of fibroblast growth factor (FGF) 21. | Gessner et al., 2015 [92] |
| Phenolic acids | Grape residue silage in the diet | Grape residue silage (0, 50, 75, 100 g/kg DM) | Antioxidant activity in milk was higher with increased dietary levels of grape residue silage. | Santos et al., 2014 [93] | |
| Phenolic acids | Total phenols, total tannins, condensed tannins | Weed species as additives | Tithonia tubiformis (5% of inclusion in the diet of sheep) | Inclusion of T. lucida in the sheep diet resulted in an increase in total phenol content (18%) and an increase in antioxidant activity (30%) | Diaz-Medina et al., 2021 [94] |
| Phenolic acids | Total phenols, total tannins, condensed tannins | Dried by-products | 100 g/day per head of tomato pomace 100 g/day per head of grape marc 75 g/day per head of exhausted myrtle berries | Dried by-products increased antioxidant activity in milk and blood plasma of dairy ewes. Grape marc elevated C18:2n-6. | Buffa et al., 2020 [95] |
| Phenolic acids | Hydrocinnamic acids, flavonoids | Goat milk cheeses | Grazing versus indoor feeding | Grazing feeding increases the quantitative and qualitative antioxidant activity of goats’ milk cheese. Also, the content of some metabolites, such as hydrocinnamic acid, were increased. | Cuchillo et al., 2010 [72] |
| Fatty acids | Fatty acids | Goat milk cheeses | Grazing versus indoor feeding | Major polyunsaturated fatty acids in milk and cheese from goats were increased by grazing compared to indoor systems. | Cuchillo et al., 2010 [73] |
| Phenolic acids | Gallic, caffeic, chlorogenic, and ferulic acids, catechin, epicatechin, and quercetin | Goat milk | Inclusion of Acacia farnesiana pods meal in goat diets | A. farnesiana increased the presence of bioactive compounds and the antioxidant activity of goats’ milk, while cholesterol content was reduced. | Delgadillo-Puga et al., 2019 [83] |
| Bioactive compounds | Fatty acids, monoterpene and sesquiterpene, tocopherol, linoleic acid | Cow and goat milk cheeses | Grazing versus indoor feeding | Grazing feeding increases terpenes, tocopherol, and antioxidant activity of cow and goat milk cheeses. Fat and cholesterol contents were diminished. | Galina et al., 2007 [85] |
| Bioactive compounds |
Polyphenol, hydroxycinnamic acids, flavonoids, fatty acids. | Goat milk and cheeses | Grazing versus indoor feeding | Grazing/browsing promote the transference of bioactive compounds from vegetation to animal products. Supplementation with rich-bioactive compound forages increased the bioactive compounds in milk and cheese. The consumption of goat milk prevents obesity, insulin resistance, inflammation, and hepatic steatosis in mice. | Delgadillo-Puga and Cuchillo-Hilario, 2021 [37] |
| Bioactive compounds |
Polyphenol, hydroxycinnamic acids, flavonoids, and fatty acids. | Goat milk and cheeses | Grazing versus indoor feeding | Goat milk intake prevents obesity, reduces fat mass, and increases lean mass in the mice fed with high fat diets. Also, there was a reduction in inflammatory markers, an increase in energy expenditure, and a higher presence of mitochondrial content in the skeletal muscle of mice. | Delgadillo-Puga et al., 2020 [86] |
| Terpenes | d-limonene (95.17 g/100 g orange peel essential oil) | Blood plasma and milk antioxidant activity. | Dietary orange peel essential oil inclusion in lactating dairy ewes’ | Inclusion of 300 mg of orange peel essential oil/kg to ewes increased milk saturated fatty acids. Addition of 450 mg of orange peel essential oil/kg to ewes concentrate improved blood plasma and milk antioxidant activity. | Kotsampasi et al., 2018 [96] |
| Phenolic acids | Anthocyanins | Blood plasma and milk of goats | Anthocyanin-rich purple corn stover silage on goats feeding | Lactating goats fed with anthocyanin-rich purple corn stover silage resulted in higher levels of peonidin and malvidin-3-O-glucoside and a higher level of superoxide dismutase (SOD) in plasma and milk relative to the control diet. | Tian et al., 2019 [97] |
| Phenolic acids | Not determined | Blood plasma and milk of goats | 0% (control), 6%, 12%, and 18% of date palm (Phoenix dactylifera L.) seed | Date palm increased antioxidant capacity in milk and blood of dairy goats. Conjugated linoleic acid (CLA) in milk was also increased. | Sharif et al., 2017 [98] |
| Essential oils |
Carvacrol, p-Cymene, Borneol, Β-Caryophyllene | Blood plasma and milk of goats | Thirty g equivalent to a daily dosage of 1 mL of essential oil of Origanum vulgare ssp. Hirtum. per animal. | Origanum vulgare increases the glutathione peroxidase and glutathione reductase both in blood and milk. | Paraskevakis et al., 2015 [99] |
4.2. Meat and Meat By-Products
| Category | Metabolite | Meat Product or By-Product Added to Feedstuff | Dose, Concentration, or Treatment | Biological Function of Metabolites, Biochemistry, and Biotransformation | Reference |
|---|---|---|---|---|---|
| Acyl-coenzyme A (CoAs) | Acylcarnitines: acetylcarnitine, propionylcarnitine, and 2-methylbutyrylcarnitine | Meat and fish meat | NR | The transportation of long-chain fatty acids into mitochondria requires carnitine to form acylcarnitines, which is also suggested to impact mental health and brain function. | Cheung et al., 2017; Li et al., 2019 [143,144] |
| Amino acids (AA) biomarkers | Urinary carnosine, 1-methylhistidine and 3-methylhistidine. | Meat | 1.5 g/kg/d | In the meat protein-based diet, the main protein sources were pork, beef, and chicken. Urinary and plasma AA may be potentially useful biomarkers for meat protein intake. | Altorf-van der Kuil et al., 2013 [146] |
| Antioxidants | Phenolic acids, anthocyanins, flavonoids, monomeric phenolic compounds such as (+)-catechins and (−)-epicatechin, as well as dimeric, trimeric, and turmeric procyanidins and condensed tannins. ⤉ | Grape Seed Extract (GSE) | 100 and 300 mg GSE/kg | Antioxidant enzymes of rabbits (superoxide dismutase, catalase, glutathione peroxidase, glutathione transferase) and total antioxidant capacity in blood were increased (p ≤ 0.05) by adding dietary GSE. | Hassan et al., 2016 [116] |
| Antioxidants | Carnosine | Chicken, red meat, processed meat | NR | Carnosine, a dipeptide of Beta-alanine and L-histidine found in muscles and the brain, exhibits radical scavenging ability primarily due to the imidazole ring on the L-histidine residue. | Boldyrev 1993; Zhou et al., 1999 [140,141] |
| Antioxidants | Gallic acid; catechin and epicatechin. ⤉ | Wine making by-product meal (WBM) | 0.5, 1 and 2% | WBM can replace butylhydroxytoluene (BHT) as a natural antioxidant in beef burgers stored at −20 °C for up to 120 days, at a maximum of 1 g/100 g. Higher WBM levels increase lipid oxidation and decrease sensory quality, while all levels provide enough crude fiber (>3 g/100 g) for dietary fiber labeling. | de Alencar et al., 2022 [122] |
| Antioxidants | Phenolic acids: gallic and protocatechuic acids; Flavanols: catechin, epicatechin, and proanthocyanidin B1 Flavonols: quercetin 3-O-rutinoside, quercetin 3-O-glucoside, and kaempferol 3-O-glucoside. ⤉ | Wine making by-product meal (WBM) | Gallic acid 16.66 mg/100 g 29, 33, and 40 mg/100 g of catechin, epicatechin and proanthocyanidin B1, respectively. Quercetin 3-O-glucoside (48.8 mg/100 g) | Winemaking by-products represent a source of phenolic compounds with antioxidant and anti-cholinesterase activities. | Jara-Palacios et al., 2020 [147] |
| Antioxidants | NR | Fresh plum juice concentrate (FP) | 2.5 and 5% | All plum ingredients reduced TBARS values, inhibited lipid oxidation, and minimally affected tenderness, sensory characteristics, color, and appearance in raw and precooked pork sausage. | Nuñez de Gonzalez et al., 2008 [124] |
| Antioxidants | Hydroxybenzoic acids, hydroxycinnamic acids, flavonoids. ⤉ | Origanum vulgare extract | 13.3, 17.8, and 24.0 mL/kg | 24 mL/kg of oregano extract could be recommended as a natural antioxidant. | Fernandes et al., 2017 [121] |
| Antioxidants | Carvacrol, thymol, p-cymene, rosmarinic acid, α-thujene, α, β-pinene, p-coumaric acid, and γ-terpinene. ⤉ | Origanum vulgare extract | Protein oxidation is initiated by myoglobin, metallic catalysts, or oxidizing lipids reacting with amino acid side chains, leading to carbonyl derivatives and protein carbonylation, or radicals. | Ranucci et al., 2015 [119] | |
| Antioxidants | NR | Origanum vulgare extract ⤉ | 4964, 6630, and 8038 mg/kg | The extract showed antioxidant potential similar to sodium erythorbate at intermediate and high levels, measured by DPPH and FRAP methods. | Fernandes et al., 2017 [121] |
| Bioactive peptides | Neokyotorphyn (alpha 137–141) TSKYR. | Meat by-products: Blood | 0.31 mg/mL | Antibiotical properties with an MIC value of 0.31 mg/mL for S. aureus. | Abou-Diab et al., 2020 [137] |
| Bioactive peptides | I3LRS5, ALDH1A1, A0A4X1VHB8, and ALDOB | Porcine liver protein fraction at pH 4.8 showed antioxidant capacity but antihypertensive inhibition | NR | Two isoforms of aldehyde dehydrogenase (I3LRS5, ALDH1A1) and four peptides from fructose bisphosphate aldolase (A0A4X1VHB8, ALDOB) were correlated with antioxidant and antihypertensive activities. | López-Pedrouso et al., 2023 [132] |
| Bioactive peptides | AEEEYPDL and LGVGG | Iberian dry-cured ham, proteins that are hydrolyzed during processing, is a source of bioactive peptides. | NR | Bioactive peptides in Spanish dry-cured ham have AEEEYPDL and LGVGG α-glucosidase inhibitory activity. | Mora et al., 2020 [133] |
| Bioactive peptides | KA and AAATP | Spanish dry-cured ham extract (SD-CHE) | IC50 6.27 mM and 6.47 mM | Peptides KA and AAATP from SD-CHE exhibit strong DPP-IV inhibitory activity (IC50 values: 6.27 mM and 6.47 mM), indicating their potential for functional products targeting type 2 diabetes. | Gallego et al., 2014 [134] |
| Dipeptide | Carnosine is a pleiotropic histidine-containing dipeptide synthesized from β-alanine and L-histidine | Meat chicken, red meat, processed meat | 8.0 ± 4.3 μg/g | Carnosine in myocardial tissue is promising, potentially beneficial in both healthy and diseased myocardial models. | Creighton [142] et al., 2022 [142] |
| Essential Oils | Hymol 60.9%, p-cymene 10.5%, γ-terpinene 7.6%, and carvacrol 5.8%. ⤉ | Oregano essential oil of Origanum vulgare L. | 1 mL/kg | Improved the oxidative stability of the sheep meat produced. | Simitzis et al., 2008 [118] |
| Essential Oils | Carvacrol, thymol, p-cymene, linalool, phenolic acids, flavonoids, and triterpenoids. ⤉ | Oregano essential oil and sweet chestnut wood extract. | 0.2% | Improved the oxidative stability of the pork meat produced. | Ranucci et al., 2015 [119] |
| Essential Oils | Oregano essential oils (OEO) extract. ⤉ | Oregano essential oil | 130 and 230 mg/d OEO | Dietary with OEO increases antioxidant capacity and enzyme activities and reduces pH, cooking loss, and malondialdehyde content. It also enhanced polyunsaturated fatty acids, conjugated linoleic acid, and essential amino acids in the Longissimus thoracis muscle. | Jara-Palacios [147] et al., 2020 [147] |
| Essential Oils | Oregano essential oil: thymol 60.9%, p-cymene 10.5%, γ-terpinene 7.6%, and carvacrol 5.8%. Sage essential oil: eucalyptol 49.4%, camphor 8.5% and α-pinene 5.4%. ⤉ | Origanum majorana, Origanum vulgare, and Salvia officilalis | 3% | Meat beef and pork essential oil treatments significantly reduced the oxidation. | Fasseas et al., 2008 [120] |
| Fatty acids | Stearic acid and oleic acid ⤉ | Rambouillet lambs | 50 and 100 g | Including chia seeds in lambs’ diets increased the bodyweight of neither the meat carcasses nor the non-meat components. It tended to increase the oleic acid and decrease the stearic acid in the meat. | Uribe-Martínez et al., 2023 [148] |
| Fiber | NR | Goat meat | 25 and 55% | Consumers preferred meat from kids fed a diet with 55% forage cactus, which resulted in lower lipid content and higher levels of monounsaturated fatty acids in goat meat. | Pinheiro et al., 2023 [149] |
| Fiber | Linoleic, conjugated linoleic, eicosapentaenoic, docosahexaenoic ⤉ | Lamb meat | ≤28 g/kg DM | Microalgae Spirullina platensis, Schizochytrium sp.MIA supplementation increased (p < 0.05) the content of linoleic, conjugated linoleic, eicosapentaenoic, docosahexaenoic, and total ω-3 FAs in meat. | Orzuna-Orzuna et al., 2023 [150] |
| Fiber | NR | Lamb meat | 2 and 4 g/kg DM | Ddetected better animal performance and physicochemical characteristics in meat from lambs supplemented with microalgae | Alghonaim et al., 2022 [151] |
| Fiber | NR | Sausages with orange peel flour or maguey leaf (SWOPFML) | 3% | 150 people were assessed to taste SWOPFML, which had significantly higher levels of bitter, astringent, and spicy notes than the control. | Chaparro et al., 2013 [126] |
| Fiber | NR | Cooked sausage | 2% | Cooked sausages formulated with functional ingredients (CP or P fiber) showed attributes such as color, sweetness, astringency, bitterness, pork meat aroma, and a firm, pliable texture. | Díaz-Vela et al., 2017 [125] |
| Fiber | NR | Adding pomegranate peel flour (PPF) to chorizos resulted in a tougher texture, crumbly consistency, and less intense color. | 2 and 4% | PPF is a functional ingredient that can replace some fat in raw meat products such as chorizo, enhancing texture, coloration, and shelf life through its polyphenols and dietary fiber, which lower water activity and promote a lower pH. | Maillard-Berdeja et al., 2022 [127] |
| Peptides from pork | Dipeptidyl peptidase IV (DPPV-IV) inhibitor | Pork meat | Bioactive peptides regulate type 2 diabetes by influencing enzymes in carbohydrates metabolism, insulin secretion, and incretin hormones such as GIP and GLP-1, thereby impacting postprandial blood glucose levels. | Martini et al., 2019 [135] | |
| Peptides from slaughterhouse | Anserine and carnosine chelate copper; anserine’s stability in serum and resistance to degradation are due to methylation. Anserine is found in the skeletal muscle and brain of mammals and birds. | Chicken slaughterhouse by-products (CSBP) | CSBP hydrolysates show diverse health benefits, including antioxidant, antidiabetic, anti-inflammatory, and cardioprotective properties. Studies on antioxidant and antihypertensive effects suggest potential therapeutic applications research into their bioactive peptide content and health implications. | Ibarz-Blanch et al., 2023 [139] | |
| Peptides as biomarkers | Anserine (β-alanyl-3 methylhistidine) is a dipeptide derived from carnosine, consisting of β-alanine and methylated 3-methylhistidine. | Anserine and carnosine are abundant in poultry, particularly in chicken and turkey. | Anserine (beta-alanyl-3-methyl-L-histidine) supplementation improved memory functions in AD-model mice by exerting a protective effect on the neurovascular units, which are composed of endothelial cells, pericytes, and supporting glial cells. | Creighton et al., 2022; Kaneko et al., 2017 [142,152] | |
| Polyphenolics | Curcumin, carvacrol, thymol, cinnamaldehyde. ⤉ | Curcumin or commercial microencapsulated phytogenic supplement | CU-with 50 mg/kg of curcumin | Curcumin with or without a phytogenic agent improved meat quality, with increased antioxidant levels and reduction of lipid peroxidation. | Galli et al., 2020 [115] |
| Polyphenolics | Phenolic acid, anthocyanins, and flavonoids, including monomeric phenolic compounds, such as (+)-catechins, (−)-epicatechin, and (−)-epicatechin-3-O-flattened dimeric, trimeric, and turmeric procyanidins. ⤉ | Grape seed extract (GSE) | 100 and 300 mg GSE/kg | Antioxidant enzymes of rabbits (superoxide dismutase, catalase, glutathione peroxidase, glutathione transferase) and total antioxidant capacity in blood were increased (p ≤ 0.05) by adding dietary GSE. | Hassan et al., 2016 [116] |
| Polyphenolics | Phenolic acid, anthocyanins, and flavonoids. ⤉ | Wine-making by-product meal (WBM) contains concentrated phenolic compounds, as quantified in its crude extract. | 0.5, 1 and 3% | In analysis of the WBM extract by HPLC, 25 phenolic compounds were observed, one of which was below the limit of quantification (procyanidin A2), totaling 9.51 mg phenolic compounds per gram of extract. The flavonol group was the major (36.4%), followed by anthocyanins and tannins with 24.07% and 13.5%, respectively. | de Alencar et al., 2022 [122] |
| Polyphenolics | NR | Plum puree, prunes (dried plum), and plum extracts. | 3% plum extract | Treatment had a reduced (p < 0.05) TBARS value of 0.84 mg MDA/kg meat after 7 days of storage at 4 °C. | Ahmad et al., 2015 [123] |
| Probiotics | NR | LAB: Lb. plantarum, Lb. paraplantarum, Lb. brevis, Lb. rhamnosus, Lb. sakei, Lb. zeae, Lb. paracasei, Ent. faecalis, Ent. faecium, Leuc. mesenteroides, Ped. pentosaceus, Ped. acidilactici, W. cibaria, W. viridescens, Lb. sake, Lb. curvatus, and Lb. plantarum | NR | Dried fermented sausage (salami, salsiccia, soppressata, alheiras, botillo, chorizo, salchicón, pepperoni). | Martín et al., 2006; Tamang et al., 2016 [120,121] |
| Probiotics | NR | Analysis of auto-aggregation ability of LAB. Six thermotolerant lactic acid bacteria were isolated from cooked meat products (Vienna sausages) | 62.6, 71.9, and 87.7% | E. faecium UAM1 showed significantly higher adherence (around 20%) to human Caco-2 cells compared to P. pentosaceus strains (2–5%) and Lactobacillus acidophilus LA-5 (6%). These findings suggest that E. faecium UAM1 has probiotic potential and may competitively colonize the intestinal tract. | Hernández-Alcántara et al., 2018 [128] |
| Digestive byproduct | Trimethylamine N-oxide (TMAO) is a small colorless amine oxide generated from choline, betaine, and carnitine by gut microbial metabolism. | Red meat, poultry, or fish. | NR | TMAO and its precursor choline in plasma predict cardiovascular disease risk in individuals undergoing cardiac evaluation. TMAO’s proatherogenic effects stem from the gut microbiota transforming phosphatidylcholine found in foods. | Wang et al., 2011 [153] |
| Tannins | Hydrolysable tannins (HTs) and condensed tannins (CTs). ⤉ | Essential nutrient complex (ENC) extracted from chestnut wood (Castanea sativa) | 0.5 and 1% ENC | ENC did not negatively affect carcass or meat traits in rabbits. It demonstrated antioxidant benefits at 0.5% inclusion but exhibited pro-oxidant effects at 1%. ENC had minimal impact on the fatty acid profile of rabbit meat. | Liu et al., 2009 [154] |
| Amino acids (AA) biomarkers | Urinary carnosine, 1-methylhistidine and 3-methylhistidine | - | 1.5 g/kg per d | Urinary and plasma amino acids are potentially valuable biomarkers for assessing meat protein intake in diets primarily consisting of pork, beef, and chicken as main protein sources. | Altorf-van del kuil et al., 2013 [146] |
4.3. Eggs and Egg By-Products
4.4. Fish and Fish By-Products
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kussmann, M.; Abe Cunha, D.H.; Berciano, S. Bioactive compounds for human and planetary health. Front. Nutr. 2023, 10, 1193848. [Google Scholar] [CrossRef] [PubMed]
- Dixit, V.; Joseph, K.S.W.; Bajrang, C.P.; Dayal, D.; Chaubey, K.K.; Pal, A.K.; Xavier, J.; Manjunath, B.T.; Bachheti, R.K. Functional foods: Exploring the health benefits of bioactive compounds from plant and animal sources. J. Food Qual. 2023, 2023, 22. [Google Scholar] [CrossRef]
- Tedeschi, L.O.; Muir, J.P.; Naumann, H.D.; Norris, A.B.; Ramírez-Restrepo, C.A.; Mertens-Talcott, S.U. Nutritional aspects of ecologically relevant phytochemicals in ruminant production. Front. Vet. Sci. 2021, 8, 628445. [Google Scholar] [CrossRef] [PubMed]
- Besharati, M.; Maggiolino, A.; Palangi, V.; Kaya, A.; Jabbar, M.; Eseceli, H.; De Palo, P.; Lorenzo, J.M. Tannin in ruminant nutrition: Review. Molecules 2022, 27, 8273. [Google Scholar] [CrossRef] [PubMed]
- Krusinski, L.; Sergin, S.; Jambunathan, V.; Rowntree, J.E.; Fenton, J.I. Attention to the details: How variations in U.S. Grass-fed cattle-feed supplementation and finishing date influence human health. Front. Sustain. Food Syst. 2022, 6, 851494. [Google Scholar] [CrossRef]
- Simitzis, P.E.; Deligeorgis, S.G. Agroindustrial by-products and animal products: A Great alternative for Improving food-quality Characteristics and Preserving Human Health. In Food Quality: Balancing Health and Disease; Holban, A.M., Grumezescu, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 253–290. [Google Scholar]
- Cuchillo, H.M.; Puga, D.C.; Wrage-Mönning, N.; Espinosa, M.J.G.; Montaño, B.S.; Navarro-Ocaña, A.; Ledesma, J.A.; Diaz, M.M.; Pérez-Gil, R.F. Chemical composition, antioxidant activity and bioactive compounds of vegetation species ingested by goats on semiarid rangelands. J. Anim. Feed Sci. 2013, 22, 106–115. [Google Scholar] [CrossRef]
- Maestri, E.; Pavlicevic, M.; Montorsi, M.; Marmiroli, N. Meta-analysis for correlating structure of bioactive peptides in foods of animal origin with regard to effect and stability. Compr. Rev. Food Sci. Food Saf. 2019, 18, 3–30. [Google Scholar] [CrossRef]
- Prasad, A.; Kothari, N. Cow products: Boon to human health and food security. Trop. Anim. Health Prod. 2021, 54, 12. [Google Scholar] [CrossRef]
- Santillo, A.; Ciliberti, M.G.; Ciampi, F.; Luciano, G.; Natalello, A.; Menci, R.; Caccamo, M.; Sevi, A.; Albenzio, M. Feeding tannins to dairy cows in different seasons improves the oxidative status of blood plasma and the antioxidant capacity of cheese. J. Dairy Sci. 2022, 105, 8609–8620. [Google Scholar] [CrossRef]
- Hashem, N.M.; Gonzalez-Bulnes, A.; Simal-Gandara, J. Polyphenols in farm animals: Source of reproductive gain or waste? Antioxidants 2020, 9, 1023. [Google Scholar] [CrossRef]
- Nehme, R.; Andrés, S.; Pereira, R.B.; Ben Jemaa, M.; Bouhallab, S.; Ceciliani, F.; López, S.; Rahali, F.Z.; Ksouri, R.; Pereira, D.M.; et al. Essential oils in livestock: From health to food quality. Antioxidants 2021, 10, 330. [Google Scholar] [CrossRef]
- Uushona, T.; Chikwanha, O.C.; Tayengwa, T.; Katiyatiya, C.L.F.; Strydom, P.E.; Mapiye, C. Nutraceutical and preservative potential of Acacia mearnsii and Acacia dealbata leaves for ruminant production and product quality enhancement. J. Agric. Sci. 2022, 159, 743–756. [Google Scholar] [CrossRef]
- van Vliet, S.; Provenza, F.D.; Kronberg, S.L. Health-promoting phytonutrients are higher in grass-fed meat and milk. Front. Sustain. Food Syst. 2021, 4, 555426. [Google Scholar] [CrossRef]
- Zekrumah, M.; Begua, P.; Razak, A.; Wahab, J.; Moffo, N.; Ivane, A.; Oman, M.; Elrashied, H.; Zou, X.; Zhang, D. Role of dietary polyphenols in non-communicable chronic disease prevention, and interactions in food systems: An overview. Nutrition 2023, 112, 112034. [Google Scholar] [CrossRef] [PubMed]
- Bešlo, D.; Golubić, N.; Rastija, V.; Agić, D.; Karnaš, M.; Šubarić, D.; Lučić, B. Antioxidant activity, metabolism, and bioavailability of polyphenols in the diet of animals. Antioxidants 2023, 12, 1141. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, I.; Wilairatana, P.; Saqib, F.; Nasir, B.; Wahid, M.; Latif, M.F.; Iqbal, A.; Naz, R.; Mubarak, M.S. Plant polyphenols and their potential benefits on cardiovascular health: A review. Molecules 2023, 28, 6403. [Google Scholar] [CrossRef]
- Sun, W.; Shahrajabian, M.H. Therapeutic potential of phenolic compounds in medicinal plants-natural health products for human health. Molecules 2023, 28, 1845. [Google Scholar] [CrossRef]
- Essa, M.M.; Bishir, M.; Bhat, A.; Chidambaram, S.B.; Al-Balushi, B.; Hamdan, H.; Govindarajan, N.; Freidland, R.P.; Qoronfleh, M.W. Functional foods and their impact on health. J. Food Sci. Technol. 2023, 60, 820–834. [Google Scholar] [CrossRef]
- Mueller-Harvey, I.; Bee, G.; Dohme-Meier, F.; Hoste, H.; Karonen, M.; Kölliker, R.; Lüscher, A.; Niderkorn, V.; Pellikaan, W.F.; Salminen, J.P.; et al. Benefits of condensed tannins in forage legumes fed to ruminants: Importance of structure, concentration, and diet composition. Crop Sci. 2019, 59, 861–885. [Google Scholar] [CrossRef]
- Leroy, F.; Abraini, F.; Beal, T.; Dominguez-Salas, P.; Gregorini, P.; Manzano, P.; Rowntree, J.; van Vliet, S. Animal board invited review: Animal source foods in healthy, sustainable, and ethical diets—An argument against drastic limitation of livestock in the food system. Animal 2022, 16, 100457. [Google Scholar] [CrossRef]
- Villalba, J.J.; Costes-Thiré, M.; Ginane, C. Phytochemicals in animal health: Diet selection and trade-offs between costs and benefits. Proc. Nutr. Soc. 2017, 76, 113–121. [Google Scholar] [CrossRef] [PubMed]
- N.R.C. National Research Council. United States-Canadian Tables of Feed Composition: Nutritional Data for United States and Canadian Feeds. Third Revision; The National Academies Press: Cambridge, MA, USA, 1982. [Google Scholar] [CrossRef]
- Okunade, S.A.; Isah, O.A.; Aderinboye, R.Y.; Olafadehan, O.A. Assessment of chemical composition and in vitro degradation profile of some Guinea Savannah browse plants of Nigeria. Trop. Subtrop. Agroecosystems 2014, 17, 529–538. [Google Scholar]
- Hloucalová, P.; Skládanka, J.; Horký, P.; Klejdus, B.; Pelikán, J.; Knotová, D. Determination of phytoestrogen content in fresh-cut legume forage. Animals 2016, 6, 43. [Google Scholar] [CrossRef] [PubMed]
- Antunović, Z.; Novoselec, J.; Klir Šalavardić, Ž.; Steiner, Z.; Šperanda, M.; Jakobek Barron, L.; Ronta, M.; Pavić, V. Influence of red corn rich in anthocyanins on productive traits, blood metabolic profile, and antioxidative status of fattening lambs. Animals 2022, 12, 612. [Google Scholar] [CrossRef]
- Dabbou, S.; Gasco, L.; Rotolo, L.; Pozzo, L.; Tong, J.M.; Dong, X.F.; Rubiolo, P.; Schiavone, A.; Gai, F. Effects of dietary alfalfa flavonoids on the performance, meat quality and lipid oxidation of growing rabbits. Asian-Australas. J. Anim. Sci. 2018, 31, 270–277. [Google Scholar] [CrossRef]
- Dadáková, K.; Trnková, A.; Kašparovská, J.; Křížová, L.; Lochman, J.; Kašparovský, T. In vitro metabolism of red clover isoflavones in rumen fluid. J. Anim. Physiol. Anim. Nutr. 2020, 104, 1647–1654. [Google Scholar] [CrossRef]
- Pecoraro, B.M.; Leal, D.F.; Frias-De-Diego, A.; Browning, M.; Odle, J.; Crisci, E. The health benefits of selenium in food animals: A review. .J. Animal Sci. Biotechnol. 2022, 13, 58. [Google Scholar] [CrossRef]
- Gu, X.; Gao, C.Q. New horizons for selenium in animal nutrition and functional foods. Anim. Nutr. 2022, 11, 80–86. [Google Scholar] [CrossRef]
- Shastak, Y.; Pelletier, W. The role of vitamin A in non-ruminant immunology. Front. Anim. Sci. 2023, 4, 1197802. [Google Scholar] [CrossRef]
- Pandey, A.K.; Kumar, P.; Saxena, M.J. Feed additives in animal health. In Nutraceuticals in Veterinary Medicine; Gupta, R.C., Srivastava, A., Lall, R., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 345–362. [Google Scholar]
- Juárez-Silva, M.E.; Cuchillo-Hilario, M.; Villarreal-Delgado, E. Dietary supplementation of inulin or flavomycin and type of cut of rabbit meat: Changes on fatty acid profile and sensorial characteristics. Rev. Mex. Cienc. Pecu. 2019, 10, 552–570. [Google Scholar] [CrossRef]
- Weiss, C.P.; Gentry, W.W.; Meredith, C.M.; Meyer, B.E.; Cole, N.A.; Tedeschi, L.O.; McCollum, F.T.; Jennings, J.S. Effects of roughage inclusion and particle size on digestion and ruminal fermentation characteristics of beef steers. J. Anim. Sci. 2017, 95, 1707–1714. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-Domínguez, S.; Rodríguez-Martínez, R.E.; Díaz-Martínez, M.; Magaña-Gallegos, E.; Cuchillo-Hilario, M. Potential application of pelagic Sargassum spp. in animal feeding. J. Appl. Phycol. 2023, 35, 433–444. [Google Scholar] [CrossRef]
- Jha, R.; Fouhse, J.M.; Tiwari, U.P.; Li, L.; Willing, B.P. Dietary fiber and intestinal health of monogastric animals. Front. Vet. Sci. 2019, 6, 48. [Google Scholar] [CrossRef] [PubMed]
- Delgadillo-Puga, C.; Cuchillo-Hilario, M. Reviewing the benefits of grazing/browsing semiarid rangeland feed resources and the transference of bioactivity and pro-healthy properties to goat milk and cheese: Obesity, insulin resistance, inflammation and hepatic steatosis prevention. Animals 2021, 11, 2942. [Google Scholar] [CrossRef]
- Ding, H.; Ao, C.; Zhang, X. Potential use of garlic products in ruminant feeding: A review. Anim. Nutr. 2023, 14, 343–355. [Google Scholar] [CrossRef]
- Rao, I.; Peters, M.; Castro, A.; Schultze-Kraft, R.; White, D.; Fisher, M.; Miles, J.; Blümmel, M.; Bungenstab, D.; Tapasco, J.; et al. LivestockPlus: Sustainable intensification of tropical forage-based systems for improving livelihood and environmental benefits. Trop. Grassl. Forr. Trop. 2015, 3, 59–82. [Google Scholar] [CrossRef]
- Teixeira, D.A.A.; Costa, K.A.d.P.; Dias, M.B.d.C.; Guimarães, K.C.; Epifanio, P.S.; Fernandes, P.B. Protein and carbohydrate fractionation of silages made from maize, Urochloa species and their mixtures. Trop. Grassl. Forrajes Trop. 2022, 10, 134–142. [Google Scholar] [CrossRef]
- Holguín, V.A.; Cuchillo, H.M.; Mazabel, J.; Quintero, S.; Mora-Delgado, J. Effect of a Pennisetum purpureum and Tithonia diversifolia silage mixture on in vitro ruminal fermentation and methane emission in a RUSITEC system. Rev. Mex. Cienc. Pecu. 2020, 11, 19–37. [Google Scholar] [CrossRef]
- Holguín, V.A.; Cuchillo-Hilario, M.; Mazabel, J.; Quintero, S.A.; Martens, S.D.; Mora-Delgado, J. In vitro methane production and fermentative parameters of wild sunflower and elephant grass silage mixtures, either inoculated or not with epiphytic lactic acid bacteria strains. Rev. Mex. Cienc. Pecu. 2021, 12, 789–810. [Google Scholar] [CrossRef]
- Holguín, V.A.; Cuchillo, H.M.; Mazabel, J.; Martens, S.D. In-vitro assessment for ensilabillity of Tithonia diversifolia alone or with Pennisetum purpureum using epiphytic lactic acid bacteria strains as inocula. Acta Sci. Anim. Sci 2018, 40, e37940. [Google Scholar] [CrossRef]
- Muck, R.E.; Nadeau, E.M.G.; McAllister, T.A.; Contreras-Govea, F.E.; Santos, M.C.; Kung, L. Silage review: Recent advances and future uses of silage additives. J. Dairy Sci. 2018, 101, 3980–4000. [Google Scholar] [CrossRef] [PubMed]
- Przybylska-Balcerek, A.; Frankowski, J.; Stuper-Szablewska, K. The influence of weather conditions on bioactive compound content in sorghum grain. Eur. Food Res. Technol. 2020, 246, 13–22. [Google Scholar] [CrossRef]
- Niderkorn, V.; Jayanegara, A. Opportunities offered by plant bioactive compounds to improve silage quality, animal health and product quality for sustainable ruminant production: A review. Agronomy 2021, 11, 86. [Google Scholar] [CrossRef]
- Onjai-Uea, N.; Paengkoum, S.; Taethaisong, N.; Thongpea, S.; Paengkoum, P. Enhancing milk quality and antioxidant status in lactating dairy goats through the dietary incorporation of purple napier grass silage. Animals 2024, 14, 811. [Google Scholar] [CrossRef]
- Usman, I.; Saif, H.; Imran, A.; Afzaal, M.; Saeed, F.; Azam, I.; Afzal, A.; Ateeq, H.; Islam, F.; Shah, Y.A.; et al. Innovative applications and therapeutic potential of oilseeds and their by-products: An eco-friendly and sustainable approach. Food Sci. Nutr. 2023, 11, 2599–2609. [Google Scholar] [CrossRef]
- Morya, S.; Menaa, F.; Jiménez-López, C.; Lourenço-Lopes, C.; BinMowyna, M.N.; Alqahtani, A. Nutraceutical and pharmaceutical behavior of bioactive compounds of miracle oilseeds: An overview. Foods 2022, 11, 1824. [Google Scholar] [CrossRef]
- Siyuan, S.; Tong, L.; Liu, R.H. Corn phytochemicals and their health benefits. Food Sci. Hum. Wellness 2018, 7, 185–195. [Google Scholar] [CrossRef]
- van Vliet, S.; Kronberg, S.L.; Provenza, F.D. Plant-based meats, human health, and climate change. Front. Sustain. Food Syst. 2020, 4, 128. [Google Scholar] [CrossRef]
- Pexas, G.; Doherty, B.; Kyriazakis, I. The future of protein sources in livestock feeds: Implications for sustainability and food safety. Front. Sustain. Food Syst. 2023, 7, 1188467. [Google Scholar] [CrossRef]
- Lima, M.; Costa, R.; Rodrigues, I.; Lameiras, J.; Botelho, G. A narrative review of alternative protein sources: Highlights on meat, fish, egg and dairy analogues. Foods 2022, 11, 2053. [Google Scholar] [CrossRef]
- Sarangi, P.K.; Vivekanand, V.; Mohanakrishna, G.; Pattnaik, B.; Muddapur, U.M.; Aminabhavi, T.M. Production of bioactive phenolic compounds from agricultural by-products towards bioeconomic perspectives. J. Clean. Prod. 2023, 414, 137460. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, X.; Xu, Y.; Yang, J.; Du, L.; Li, K.; Zhou, Y. Milk consumption and multiple health outcomes: Umbrella review of systematic reviews and meta-analyses in humans. Nutr. Metab. 2021, 18, 7. [Google Scholar] [CrossRef] [PubMed]
- Neill, H.R.; Gill, C.I.R.; McDonald, E.J.; McRoberts, W.C.; Pourshahidi, L.K. Vitamin d biofortification of pork may offer a food-based strategy to increase vitamin d intakes in the UK population. Front. Nutr. 2021, 8, 777364. [Google Scholar] [CrossRef] [PubMed]
- Alem, W.T. Effect of herbal extracts in animal nutrition as feed additives. Heliyon 2024, 10, e24973. [Google Scholar] [CrossRef]
- Valenzuela-Grijalva, N.V.; Pinelli-Saavedra, A.; Muhlia-Almazan, A.; Domínguez-Díaz, D.; González-Ríos, H. Dietary inclusion effects of phytochemicals as growth promoters in animal production. J. Anim. Sci. Technol. 2017, 59, 8. [Google Scholar] [CrossRef]
- Lillehoj, H.; Liu, Y.; Calsamiglia, S.; Fernandez-Miyakawa, M.E.; Chi, F.; Cravens, R.L.; Oh, S.; Gay, C.G. Phytochemicals as antibiotic alternatives to promote growth and enhance host health. Vet. Res. 2018, 49, 76. [Google Scholar] [CrossRef]
- El-Zaiat, H.M.; Ku-Vera, J.C.; Soltan, Y.A. Editorial: Natural phytochemicals to enhance animal productivity and health status with low greenhouse gas emissions. Front. Vet. Sci. 2023, 10, 1280611. [Google Scholar] [CrossRef]
- Romero, E.A.; Maldonado, M.A.; González, C.J.; Bahena, S.M.; Garduño, R.M.L.; Rodríguez, L.V.; Alvarez, L. Anti-inflammatory and antioxidative effects of six pentacyclic triterpenes isolated from the Mexican copal resin of Bursera copallifera. BMC Complement. Altern. Med. 2016, 16, 422. [Google Scholar] [CrossRef]
- Šojić, B.; Milošević, S.; Savanović, D.; Zeković, Z.; Tomović, V.; Pavlić, B. Isolation, bioactive potential, and application of essential oils and terpenoid-rich extracts as effective antioxidant and antimicrobial agents in meat and meat Products. Molecules 2023, 28, 2293. [Google Scholar] [CrossRef]
- Jolly, A.; Hour, Y.; Lee, Y.-C. An outlook on the versatility of plant saponins: A review. Fitoterapia 2024, 174, 105858. [Google Scholar] [CrossRef]
- Sana, T.; Khan, M.; Siddiqui, B.S.; Baig, T.A.; Jabeen, A.; Begum, S.; Hadda, T.B.; Shah, L. Anti-inflammatory and urease inhibitory iridoid glycosides from Nyctanthes arbor-tristis Linn. J Ethnopharmacol 2024, 319, 117368. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.H.; Bruguière, A.; Miyamoto, T.; Dias, A.M.M.; Bellaye, P.-S.; Collin, B.; Sautour, M.; Briand, L.; Mitaine-Offer, A.-C. Steroidal glycosides from Yucca rostrata and Dracaena braunii and their cytotoxic and antimicrobial evaluation. Biochem. Syst. Ecol. 2024, 113, 104791. [Google Scholar] [CrossRef]
- Yang, N.; Guo, J.; Zhang, J.; Gao, S.; Xiang, Q.; Wen, J.; Huang, Y.; Rao, C.; Chen, Y. A toxicological review of alkaloids. Drug Chem. Toxicol. 2024, 47, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Aalinezhad, S.; Dabaghian, F.; Namdari, A.; Akaberi, M.; Emami, S.A. Phytochemistry and pharmacology of alkaloids from Papaver spp.: A structure–activity based study. Phytochem. Rev. 2024, 1–73. [Google Scholar] [CrossRef]
- Chen, L.; Bagnicka, E.; Chen, H.; Guowei, S. Health potential of fermented goat dairy products: Composition comparison with fermented cow milk, probiotics selection, health benefits and mechanisms. Food Funct. 2023, 14, 3423–3436. [Google Scholar] [CrossRef]
- Delgadillo-Puga, C.; Torre-Villalvazo, I.; Noriega, L.G.; Rodríguez-López, L.A.; Alemán, G.; Torre-Anaya, E.A.; Cariño-Cervantes, Y.Y.; Palacios-Gonzalez, B.; Furuzawa-Carballeda, J.; Tovar, A.R.; et al. Pecans and its polyphenols prevent obesity, hepatic steatosis and diabetes by reducing dysbiosis, inflammation, and increasing energy expenditure in mice fed a high-fat diet. Nutrients 2023, 15, 2591. [Google Scholar] [CrossRef]
- Delgadillo, P.C.; Cuchillo, H.M.; Espinosa, M.J.G.; Medina, C.O.; Molina, J.E.; Díaz, M.M.; Álvarez, I.M.A.; Ledesma, S.J.A.; Pedraza-Chaverri, J. Antioxidant activity and protection against oxidative-induced damage of Acacia shaffneri and Acacia farnesiana pods extracts: In vitro and in vivo assays. BMC Comp. Altern. Med. 2015, 15, 435. [Google Scholar] [CrossRef]
- Puga, D.C.; Cuchillo, H.M.; Navarro, O.A.; Medina-Campos, O.N.; Nieto, C.A.; Lopez, T.Z.G.; Díaz, M.M.; Álvarez, I.M.A.; Cruz, M.Y.R.; Sánchez, Q.V.; et al. Phenolic compounds in organic and aqueous extracts from Acacia farnesiana pods analyzed by ULPS-ESI-Q-oa/TOF-MS. In vitro antioxidant activity and anti-inflammatory response in CD-1 mice. Molecules 2018, 23, 2386. [Google Scholar] [CrossRef]
- Cuchillo, H.M.; Puga, D.C.; Navarro, O.A.; Pérez-Gíl, R.F. Antioxidant activity, bioactive polyphenols in Mexican goats’ milk cheeses on summer grazing. J. Dairy Res. 2010, 77, 20–26. [Google Scholar] [CrossRef]
- Cuchillo, H.M.; Puga, D.C.; Wrage, N.; Pérez-Gíl, R.F. Feeding goats on scrubby Mexican rangeland and pasteurization: Influences on milk and artisan cheese quality. Trop. Anim. Health. Prod. 2010, 42, 1127–1134. [Google Scholar] [CrossRef]
- Baba, W.N.; Mudgil, P.; Kamal, H.; Kilari, B.P.; Gan, C.Y.; Maqsood, S. Identification and characterization of novel α-amylase and α-glucosidase inhibitory peptides from camel whey proteins. J. Dairy Sci. 2021, 104, 1364–1377. [Google Scholar] [CrossRef] [PubMed]
- Marshall, C.J.; Garrett, K.; Van Vliet, S.; Beck, M.R.; Gregorini, P. Dietary and animal strategies to reduce the environmental impact of pastoral dairy systems result in altered nutraceutical profiles in milk. Animals 2022, 12, 2994. [Google Scholar] [CrossRef] [PubMed]
- Stobiecka, M.; Król, J.; Brodziak, A. Antioxidant activity of milk and dairy products. Animals 2022, 12, 245. [Google Scholar] [CrossRef] [PubMed]
- Conboy, S.R.; Ross, R.P.; Stanton, C. Carotenoids in milk and the potential for dairy based functional foods. Foods 2021, 10, 1263. [Google Scholar] [CrossRef]
- Ali, M.A.; Kamal, M.M.; Rahman, M.H.; Siddiqui, M.N.; Haque, M.A.; Saha, K.K.; Rahman, M.A. Functional dairy products as a source of bioactive peptides and probiotics: Current trends and future prospectives. J. Food Sci. Technol. 2022, 59, 1263–1279. [Google Scholar] [CrossRef]
- Ianni, A.; Martino, G. Dietary grape pomace supplementation in dairy cows: Effect on nutritional quality of milk and its derived dairy products. Foods 2020, 9, 168. [Google Scholar] [CrossRef]
- Wrage, N.; Strodthoff, J.; Cuchillo, H.M.; Isselstein, J.; Kayser, M. Phytodiversity of temperate permanent grasslands: Ecosystem services for agriculture and livestock management for diversity conservation. Biodiv. Conserv. 2011, 20, 3317–3339. [Google Scholar] [CrossRef]
- Holguín, V.A.; Ortiz Grisalez, S.; Velasco Navia, A.; Mora-Delgado, J. Multi-criteria evaluation of 44 introductions of Tithonia diversifolia (Hemsl.) A. Gray in Candelaria, Valle del Cauca. Rev. Med. Vet. Zoot. 2015, 62, 57–72. [Google Scholar]
- Jerrentrup, J.S.; Komainda, M.; Seither, M.; Cuchillo-Hilario, M.; Wrage-Mönnig, N.; Isselstein, J. Diverse swards and mixed-grazing of cattle and sheep for improved productivity. Front. Sustain. Food Syst. 2020, 3, 125. [Google Scholar] [CrossRef]
- Delgadillo-Puga, C.; Cuchillo-Hilario, M.; León-Ortiz, L.; Ramírez-Rodríguez, A.; Cabiddu, A.; Navarro-Ocaña, A.; Morales-Romero, A.M.; Medina-Campos, O.N.; Pedraza-Chaverri, J. Goats’ feeding supplementation with Acacia farnesiana pods and their relationship with milk composition: Fatty acids, polyphenols, and antioxidant activity. Animals 2019, 9, 515. [Google Scholar] [CrossRef]
- Alothman, M.; Hogan, S.A.; Hennessy, D.; Dillon, P.; Kilcawley, K.N.; O’Donovan, M.; Tobin, J.; Fenelon, M.A.; O’Callaghan, T.F. The “grass-fed” milk story: Understanding the impact of pasture feeding on the composition and quality of bovine milk. Foods 2019, 8, 350. [Google Scholar] [CrossRef] [PubMed]
- Galina, M.A.; Osnaya, F.; Cuchillo, H.M.; Haenlein, G.F.W. Cheese quality from milk of grazing or indoor fed Zebu cows and Alpine crossbred goats. Small Rumin. Res. 2007, 71, 264–272. [Google Scholar] [CrossRef]
- Delgadillo-Puga, C.; Noriega, L.G.; Morales-Romero, A.M.; Nieto-Camacho, A.; Granados-Portillo, O.; Rodríguez-López, L.A.; Alemán, G.; Furuzawa-Carballeda, J.; Tovar, A.R.; Cisneros-Zevallos, L.; et al. Goat’s milk intake prevents obesity, hepatic steatosis and insulin resistance in mice fed a high-fat diet by reducing inflammatory markers and increasing energy expenditure and mitochondrial content in skeletal muscle. Int. J. Mol. Sci. 2020, 21, 5530. [Google Scholar] [CrossRef] [PubMed]
- Rocchetti, G.; Gallo, A.; Nocetti, M.; Lucini, L.; Masoero, F. Milk metabolomics based on ultra-high-performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry to discriminate different cows feeding regimens. Food Res. Int. 2020, 134, 109279. [Google Scholar] [CrossRef] [PubMed]
- Kilari, B.P.; Mudgil, P.; Azimullah, S.; Bansal, N.; Ojha, S.; Maqsood, S. Effect of camel milk protein hydrolysates against hyperglycemia, hyperlipidemia, and associated oxidative stress in streptozotocin (STZ)-induced diabetic rats. J. Dairy Sci. 2021, 104, 1304–1317. [Google Scholar] [CrossRef]
- Ashraf, A.; Mudgil, P.; Palakkott, A.; Iratni, R.; Gan, C.Y.; Maqsood, S.; Ayoub, M.A. Molecular basis of the anti-diabetic properties of camel milk through profiling of its bioactive peptides on dipeptidyl peptidase IV (DPP-IV) and insulin receptor activity. J. Dairy Sci. 2021, 104, 61–77. [Google Scholar] [CrossRef]
- Prommachart, R.; Uriyapongson, J.; Cherdthong, A.; Uriyapongson, S. Feed intake, nutrient digestibility, antioxidant activity in plasma, and growth performance of male dairy cattle fed black rice and purple corn extracted residue. Trop. Anim. Sci. J. 2021, 44, 307–315. [Google Scholar] [CrossRef]
- He, L.; Zhou, W.; Wang, C.; Yang, F.; Chen, X.; Zhang, Q. Effect of cellulase and Lactobacillus casei on ensiling characteristics, chemical composition, antioxidant activity, and digestibility of mulberry leaf silage. J. Dairy Sci. 2019, 102, 9919–9931. [Google Scholar] [CrossRef]
- Gessner, D.K.; Koch, C.; Romberg, F.J.; Winkler, A.; Dusel, G.; Herzog, E.; Most, E.; Eder, K. The effect of grape seed and grape marc meal extract on milk performance and the expression of genes of endoplasmic reticulum stress and inflammation in the liver of dairy cows in early lactation. J. Dairy Sci. 2015, 98, 8856–8868. [Google Scholar] [CrossRef]
- Santos, N.W.; Santos, G.T.D.; Silva-Kazama, D.C.; Grande, P.A.; Pintro, P.M.; de Marchi, F.E.; Jobim, C.C.; Petit, H.V. Production, composition and antioxidants in milk of dairy cows fed diets containing soybean oil and grape residue silage. Livest. Sci. 2014, 159, 37–45. [Google Scholar] [CrossRef]
- Diaz-Medina, L.K.; Colín-Navarro, V.; Arriaga-Jordán, C.M.; Brunett-Pérez, L.; Vázquez-de-Aldana, B.R.; Estrada-Flores, J.G. In vitro nutritional quality and antioxidant activity of three weed species as feed additives for sheep in the Central Highlands of Mexico. Trop. Anim. Health Prod. 2021, 53, 394. [Google Scholar] [CrossRef] [PubMed]
- Buffa, G.; Tsiplakou, E.; Mitsiopoulou, C.; Pulina, G.; Nudda, A. Supplementation of by-products from grape, tomato and myrtle affects antioxidant status of dairy ewes and milk fatty acid profile. J. Anim Physiol. Anim. Nutr. 2020, 104, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Kotsampasi, B.; Tsiplakou, E.; Christodoulou, C.; Mavrommatis, A.; Mitsiopoulou, C.; Karaiskou, C.; Sossidou, E.; Fragioudakis, N.; Kapsomenos, I.; Bampidis, V.A.; et al. Effects of dietary orange peel essential oil supplementation on milk yield and composition, and blood and milk antioxidant status of dairy ewes. Anim. Feed Sci. Technol. 2018, 245, 20–31. [Google Scholar] [CrossRef]
- Tian, X.Z.; Paengkoum, P.; Paengkoum, S.; Chumpawadee, S.; Ban, C.; Thongpea, S. Short communication: Purple corn (Zea mays L.) stover silage with abundant anthocyanins transferring anthocyanin composition to the milk and increasing antioxidant status of lactating dairy goats. J. Dairy Sci. 2019, 102, 413–418. [Google Scholar] [CrossRef]
- Sharifi, M.; Bashtani, M.; Naserian, A.A.; Farhangfar, H. The Effect of increasing levels of date palm (Phoenix dactylifera L.) seed on the performance, ruminal fermentation, antioxidant status and milk fatty acid profile of Saanen dairy goats. J. Anim. Physiol. Anim. Nutr. 2017, 101, e332–e341. [Google Scholar] [CrossRef]
- Paraskevakis, N. Effects of dietary dried greek oregano (Origanum vulgare ssp. hirtum) supplementation on blood and milk enzymatic antioxidant indices, on milk total antioxidant capacity and on productivity in goats. Anim. Feed Sci. Technol. 2015, 209, 90–97. [Google Scholar] [CrossRef]
- Beriain, M.J.; Gómez, I.; Ibáñez, F.C.; Sarriés, M.V.; Ordóñez, A.I. Chapter 1—Improvement of the functional and healthy properties of meat products. In Food Quality: Balancing Health and Disease; Holban, A.M., Grumezescu, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 1–74. [Google Scholar]
- USDA. Department of Agriculture. Food Safety and Inspection Service. Available online: https://www.fsis.usda.gov/food-safety (accessed on 26 June 2024).
- USDA. Department of Agriculture and U.S. Department of Health and Human Services. Dietary Guidelines for Americans, 2020–2025. 9th Edition. December 2020. Available at DietaryGuidelines.gov; 2020. Available online: https://www.dietaryguidelines.gov/resources/2020-2025-dietary-guidelines-online-materials (accessed on 15 July 2024).
- Stanton, C.; Mills, S.; Ryan, A.; Di Gioia, D.; Ross, R.P. Influence of pasture feeding on milk and meat products in terms of human health and product quality. Irish J. Agr. Food Res. 2021, 59, 292–302. [Google Scholar] [CrossRef]
- Gilmore, L.A.; Walzem, R.L.; Crouse, S.F.; Smith, D.R.; Adams, T.H.; Vaidyanathan, V.; Cao, X.; Smith, S.B. Consumption of high-oleic acid ground beef increases HDL-cholesterol concentration but both high- and low-oleic acid ground beef decrease HDL particle diameter in normocholesterolemic men. J. Nutr. 2011, 141, 1188–1194. [Google Scholar] [CrossRef]
- Adams, T.H.; Walzem, R.L.; Smith, D.R.; Tseng, S.; Smith, S.B. Hamburger high in total, saturated and trans-fatty acids decreases HDL cholesterol and LDL particle diameter, and increases TAG, in mildly hypercholesterolaemic men. Br. J. Nutr. 2010, 103, 91–98. [Google Scholar] [CrossRef]
- Provenza, F.D.; Kronberg, S.L.; Gregorini, P. Is grassfed meat and dairy better for human and environmental health? Front. Nutr. 2019, 6, 26. [Google Scholar] [CrossRef]
- Manessis, G.; Kalogianni, A.I.; Lazou, T.; Moschovas, M.; Bossis, I.; Gelasakis, A.I. Plant-derived natural antioxidants in meat and meat products. Antioxidants 2020, 9, 1215. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, D.; Xiong, Y.L.; Castillo, M.; Payne, F.A.; Garrido, M.D. Textural and viscoelastic properties of pork frankfurters containing canola-olive oils, rice bran, and walnut. Meat Sci. 2012, 92, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Zamora, G.; García-Macías, J.A.; Santellano-Estrada, E.; Chávez-Martínez, A.; Durán-Meléndez, L.A.; Silva-Vázquez, R.; Quintero-Ramos, A. Fat reduction in the formulation of frankfurter sausages using inulin and pectin. Food Sci Technol. 2015, 35, 25–31. [Google Scholar] [CrossRef]
- Namir, M.; Siliha, H.; Ramadan, M.F. Fiber pectin from tomato pomace: Characteristics, functional properties and application in low-fat beef burger. J. Food Meals. Charact. 2015, 9, 305–312. [Google Scholar] [CrossRef]
- Odiase, O.M.; Igene, J.O.; Evivie, S.E.; Ebabhamiegbebho, P. Determination and sensory evaluation of soy flour-meat combinations in the production of meatballs. J. Appl. Nat. Sci. 2013, 5, 482–487. [Google Scholar] [CrossRef]
- Petersson, K.; Godard, O.; Eliasson, A.C.; Tornberg, E. The effects of cereal additives in low-fat sausages and meatballs. Part 1: Untreated and enzyme-treated rye bran. Meat Sci. 2014, 96, 423–428. [Google Scholar] [CrossRef]
- Wouters, A.G.; Rombouts, I.; Lagrain, B.; Delcour, J.A. Impact of casein and egg white proteins on the structure of wheat gluten-based protein-rich food. J. Sci. Food. Agric. 2016, 96, 757–763. [Google Scholar] [CrossRef]
- Youssef, M.K.; Barbut, S. Effects of two types of soy protein isolates, native and preheated whey protein isolates on emulsified meat batters prepared at different protein levels. Meat Sci. 2011, 87, 54–60. [Google Scholar] [CrossRef]
- Galli, G.M.; Gerbet, R.R.; Griss, L.G.; Fortuoso, B.F.; Petrolli, T.G.; Boiago, M.M.; Souza, C.F.; Baldissera, M.D.; Mesadri, J.; Wagner, R.; et al. Combination of herbal components (curcumin, carvacrol, thymol, cinnamaldehyde) in broiler chicken feed: Impacts on response parameters, performance, fatty acid profiles, meat quality and control of coccidia and bacteria. Microb. Pathog. 2020, 139, 103916. [Google Scholar] [CrossRef]
- Hassan, F.A.; Mahrose, K.M.; Basyony, M.M. Effects of grape seed extract as a natural antioxidant on growth performance, carcass characteristics and antioxidant status of rabbits during heat stress. Arch. Anim. Nutr. 2016, 70, 141–154. [Google Scholar] [CrossRef]
- Liu, H.W.; Gai, F.; Gasco, L.; Brugiapaglia, A.; Lussiana, C.; Guo, K.J.; Tong, J.M.; Zoccarato, I. Effects of chestnut tannins on carcass characteristics, meat quality, lipid oxidation and fatty acid composition of rabbits. Meat Sci. 2009, 83, 678–683. [Google Scholar] [CrossRef] [PubMed]
- Simitzis, P.E.; Deligeorgis, S.G.; Bizelis, J.A.; Dardamani, A.; Theodosiou, I.; Fegeros, K. Effect of dietary oregano oil supplementation on lamb meat characteristics. Meat Sci. 2008, 79, 217–223. [Google Scholar] [CrossRef]
- Ranucci, D.; Beghelli, D.; Trabalza-Marinucci, M.; Branciari, R.; Forte, C.; Olivieri, O.; Badillo Pazmay, G.V.; Cavallucci, C.; Acuti, G. Dietary effects of a mix derived from oregano (Origanum vulgare L.) essential oil and sweet chestnut (Castanea sativa Mill.) wood extract on pig performance, oxidative status and pork quality traits. Meat Sci. 2015, 100, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Fasseas, M.K.; Mountzouris, K.C.; Tarantilis, P.A.; Polissiou, M.; Zervas, G. Antioxidant activity in meat treated with oregano and sage essential oils. 2008, 106, 1188–1194. [CrossRef]
- Fernandes, R.P.P.; Trindade, M.A.; Tonin, F.G.; Pugine, S.M.P.; Lima, C.G.; Lorenzo, J.M.; de Melo, M.P. Evaluation of oxidative stability of lamb burger with Origanum vulgare extract. Food Chem. 2017, 233, 101–109. [Google Scholar] [CrossRef]
- de Alencar, M.G.; de Quadros, C.P.; Luna, A.; Neto, A.F.; da Costa, M.M.; Queiroz, M.; de Carvalho, F.A.L.; da Silva Araújo, D.H.; Gois, G.C.; Dos Anjos Santos, V.L.; et al. Grape skin flour obtained from wine processing as an antioxidant in beef burgers. Meat. Sci. 2022, 194, 108963. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.R.; Gokulakrishnan, P.; Giriprasad, R.; Yatoo, M.A. Fruit-based natural antioxidants in meat and meat products: A review. Crit. Rev. Food Sci. Nutr. 2015, 55, 1503–1513. [Google Scholar] [CrossRef] [PubMed]
- Nuñez de Gonzalez, M.T.; Boleman, R.M.; Miller, R.K.; Keeton, J.T.; Rhee, K.S. Antioxidant properties of dried plum ingredients in raw and precooked pork sausage. J. Food Sci. 2008, 73, H63–H71. [Google Scholar] [CrossRef]
- Díaz-Vela, J.; Totosaus, A.; Escalona-Buendía, H.B.; Pérez-Chabela, M.L. Influence of the fiber from agro-industrial co-products as functional food ingredient on the acceptance, neophobia and sensory characteristics of cooked sausages. J. Food Sci. Technol. 2017, 54, 379–385. [Google Scholar] [CrossRef]
- Escalona-Buendia, H.B.; Escutia, R.P.C.; Castillejos-Gómez, B.I.; Chaparro-Hernández, J.; Perez-Chabela, M.L. Sensory evaluation of sausages with orange peel flour and maguey leaf. Nacameh 2013, 7, 23–40. [Google Scholar]
- Maillard-Berdeja, K.V.; Ponce-Alquicira, E.; Schettinobermúdez, B.S.; Perez-Chabela, M.L. Pomegranate (L.) peel flour as functional ingredient for chorizo: Effect physicochemical and sensory characteristics of functional meat products. Acta Univ. Cibiniensis Ser. E Food Technol. 2022, 26, 33–42. [Google Scholar] [CrossRef]
- Hernández-Alcántara, A.M.; Wacher, C.; Llamas, M.G.; López, P.; Pérez-Chabela, M.L. Probiotic properties and stress response of thermotolerant lactic acid bacteria isolated from cooked meat products. LWT 2018, 91, 249–257. [Google Scholar] [CrossRef]
- Sánchez, A.; Vázquez, A. Bioactive peptides: A review. Food Qual. Saf. 2017, 1, 29–46. [Google Scholar] [CrossRef]
- Abd-Talib, N.; Yaji, E.L.A.; Wahab, N.S.A.; Razali, N.; Len, K.Y.T.; Roslan, J.; Saari, N.; Pa’ee, K.F. Bioactive peptides and its alternative processes: A review. Biotechnol. Bioprocess Eng. 2022, 27, 306–335. [Google Scholar] [CrossRef]
- Besharati, M.; Lackner, M. Bioactive peptides: A review. EuroBiotech J. 2023, 7, 176–188. [Google Scholar] [CrossRef]
- López-Pedrouso, M.; Lorenzo, J.M.; Bou, R.; Vazquez, J.A.; Valcarcel, J.; Toldrà, M.; Franco, D. Valorisation of pork by-products to obtain antioxidant and antihypertensive peptides. Food Chem. 2023, 423, 136351. [Google Scholar] [CrossRef]
- Mora, L.; González-Rogel, D.; Heres, A.; Toldrá, F. Iberian dry-cured ham as a potential source of α-glucosidase-inhibitory peptides. J. Funct. Foods 2020, 67, 103840. [Google Scholar] [CrossRef]
- Gallego, M.; Aristoy, M.C.; Toldrá, F. Dipeptidyl peptidase IV inhibitory peptides generated in Spanish dry-cured ham. Meat Sci. 2014, 96, 757–761. [Google Scholar] [CrossRef]
- Martini, S.; Conte, A.; Tagliazucchi, D. Comparative peptidomic profile and bioactivities of cooked beef, pork, chicken and turkey meat after in vitro gastro-intestinal digestion. J. Proteomics 2019, 208, 103500. [Google Scholar] [CrossRef]
- Kęska, P.; Stadnik, J. Dipeptidyl peptidase iv inhibitory peptides generated in dry-cured pork loin during aging and gastrointestinal digestion. Nutrients 2022, 14, 770. [Google Scholar] [CrossRef]
- Abou-Diab, M.; Thibodeau, J.; Deracinois, B.; Flahaut, C.; Fliss, I.; Dhulster, P.; Bazinet, L.; Nedjar, N. Bovine hemoglobin enzymatic hydrolysis by a new eco-efficient process-part ii: Production of bioactive peptides. Membranes 2020, 10, 268. [Google Scholar] [CrossRef]
- Bravo, F.I.; Calvo, E.; López-Villalba, R.A.; Torres-Fuentes, C.; Muguerza, B.; García-Ruiz, A.; Morales, D. Valorization of chicken slaughterhouse byproducts to obtain antihypertensive peptides. Nutrients 2023, 15, 457. [Google Scholar] [CrossRef] [PubMed]
- Ibarz-Blanch, N.; Alcaide-Hidalgo, J.M.; Cortés-Espinar, A.J.; Albi-Puig, J.; Suárez, M.; Mulero, M.; Morales, D.; Bravo, F.I. Chicken slaughterhouse by-products: A source of protein hydrolysates to manage non-communicable diseases. Trends Food Sci. Technol. 2023, 139, 104125. [Google Scholar] [CrossRef]
- Boldyrev, A.A. Does carnosine possess direct antioxidant activity? Int. J. Biochem. 1993, 25, 1101–1107. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Decker, E.A. Ability of carnosine and other skeletal muscle components to quench unsaturated aldehydic lipid oxidation products. J. Agric. Food. Chem. 1999, 47, 51–55. [Google Scholar] [CrossRef]
- Creighton, J.V.; de Souza Gonçalves, L.; Artioli, G.G.; Tan, D.; Elliott-Sale, K.J.; Turner, M.D.; Doig, C.L.; Sale, C. Physiological roles of carnosine in myocardial function and health. Adv. Nutr. 2022, 13, 1914–1929. [Google Scholar] [CrossRef]
- Cheung, W.; Keski-Rahkonen, P.; Assi, N.; Ferrari, P.; Freisling, H.; Rinaldi, S.; Slimani, N.; Zamora-Ros, R.; Rundle, M.; Frost, G.; et al. A metabolomic study of biomarkers of meat and fish intake. Am. J. Clin. Nutr. 2017, 105, 600–608. [Google Scholar] [CrossRef]
- Li, S.; Gao, D.; Jiang, Y. Function, detection and alteration of acylcarnitine metabolism in hepatocellular carcinoma. Metabolites 2019, 9, 36. [Google Scholar] [CrossRef]
- McCann, M.R.; George De la Rosa, M.V.; Rosania, G.R.; Stringer, K.A. L-carnitine and acylcarnitines: Mitochondrial biomarkers for precision medicine. Metabolites 2021, 11, 51. [Google Scholar] [CrossRef]
- Altorf-van der Kuil, W.; Brink, E.J.; Boetje, M.; Siebelink, E.; Bijlsma, S.; Engberink, M.F.; van’t Veer, P.; Tomé, D.; Bakker, S.J.; van Baak, M.A.; et al. Identification of biomarkers for intake of protein from meat, dairy products and grains: A controlled dietary intervention study. Br. J. Nutr. 2013, 110, 810–822. [Google Scholar] [CrossRef]
- Jara-Palacios, M.J.; Gonçalves, S.; Heredia, F.J.; Hernanz, D.; Romano, A. Extraction of antioxidants from winemaking byproducts: Effect of the solvent on phenolic composition, antioxidant and anti-cholinesterase activities, and electrochemical behaviour. Antioxidants 2020, 9, 675. [Google Scholar] [CrossRef]
- Uribe-Martínez, S.; Rendón-Huerta, J.A.; Hernández-Briones, V.G.; Grajales-Lagunes, A.; Morales-Rueda, J.; Álvarez-Fuentes, G.; García-López, J.C. Effects of chia seeds on growth performance, carcass traits and fatty acid profile of lamb meat. Animals 2023, 13, 1005. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, R.S.B.; Farias, I.M.S.C.; Francisco, C.L.; Moreno, G.M.B. Physicochemical quality and fatty acid profile in the meat of goats fed forage cactus as a substitute for tifton 85 hay. Animals 2023, 13, 957. [Google Scholar] [CrossRef] [PubMed]
- Orzuna-Orzuna, J.F.; Hernández-García, P.A.; Chay-Canul, A.J.; Díaz Galván, C.; Razo Ortíz, P.B. Microalgae as a dietary additive for lambs: A meta-analysis on growth performance, meat quality, and meat fatty acid profile. Small Rumin. Res. 2023, 227, 107072. [Google Scholar] [CrossRef]
- Alghonaim, A.A.; Alqahtani, M.F.; Al-Garadi, M.A.; Suliman, G.M.; Al-Baadani, H.H.; Al-Badwi, M.A.; Abdelrahman, M.M.; Alowaimer, A.N.; Khan, R.U.; Alhidary, I.A. Effects of different levels of spirulina (Arthrospira platensis) supplementation on productive performance, nutrient digestibility, blood metabolites, and meat quality of growing Najdi lambs. Trop. Anim. Health Prod. 2022, 54, 124. [Google Scholar] [CrossRef]
- Kaneko, J.; Enya, A.; Enomoto, K.; Ding, Q.; Hisatsune, T. Anserine (beta-alanyl-3-methyl-L-histidine) improves neurovascular-unit dysfunction and spatial memory in aged AβPPswe/PSEN1dE9 Alzheimer’s-model mice. Sci. Rep. 2017, 7, 12571. [Google Scholar] [CrossRef]
- Wang, Z.; Klipfell, E.; Bennett, B.J.; Koeth, R.; Levison, B.S.; DuGar, B.; Feldstein, A.E.; Britt, E.B.; Fu, X.; Chung, Y.-M.; et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011, 472, 57–63. [Google Scholar] [CrossRef]
- Aparicio, A.; Salas, G.M.D.; Cuadrado, S.E.; Ortega, R.M.; López, S.A.M. El huevo como fuente de antioxidantes y componentes protectores frente a procesos crónicos. Nutr. Hosp. 2018, 35, 36–40. [Google Scholar] [CrossRef]
- Wu, J. Eggs as Functional Foods and Nutraceuticals for Human Health; The Royal Society of Chemistry: London, UK, 2019; 406p. [Google Scholar]
- Nimalaratne, C.; Wu, J. Hen egg as an antioxidant food commodity: A review. Nutrients 2015, 7, 8274–8293. [Google Scholar] [CrossRef]
- Halliwell, B. Biochemistry of oxidative stress. Biochem. Soc. Trans. 2007, 35, 1147–1150. [Google Scholar] [CrossRef]
- Khlebnikov, A.I.; Schepetkin, I.A.; Domina, N.G.; Kirpotina, L.N.; Quinn, M.T. Improved quantitative structure-activity relationship models to predict antioxidant activity of flavonoids in chemical, enzymatic, and cellular systems. Bioorg. Med. Chem. 2007, 15, 1749–1770. [Google Scholar] [CrossRef]
- Valenzuela, B.R.; Tapia, O.G.; González, E.M.; Valenzuela, B.A. Ácidos grasos omega-3 (EPA y DHA) y su aplicación en diversas situaciones clínicas. Rev. Chil. Nutr. 2011, 38, 356–367. [Google Scholar] [CrossRef][Green Version]
- Carranco, M.E.; Calvo, C.C.; Carrillo, D.S.; Ramírez, C.R.; Morales, B.E.; Sanginés, G.L.; Fuente, M.B.; Ávila, G.E.; Pérez-Gil, R.F. Crustacean meal in laying hen rations. Effect on productive variables and sensory evaluation of eggs stored in different conditions. Cuban J. Agric. Sci. 2011, 45, 171–175. [Google Scholar]
- Carranco, M.E.; Calvo, C.; Arellano, L.; Pérez-Gil, F.; Ávila, E.; Fuente, B. Inclusión de la harina de cabezas de camarón Penaeus sp. en raciones para gallinas ponedoras. Efecto sobre la concentración de pigmento rojo de yema y calidad de huevo. Interciencia 2003, 28, 328–333. [Google Scholar]
- Carranco-Jáuregui, M.E.; Sanginés-García, L.; Morales-Barrera, E.; Carrillo-Domínguez, S.; Ávila, G.E.; Fuente-Martínez, B.; Ramírez, P.M.; Pérez-Gil, R.F. Shrimp head meal in laying hen rations and its effects on fresh and stored egg quality. Interciencia 2006, 31, 822–827. [Google Scholar]
- Carranco-Jáuregui, M.E.; Fuente-Martínez, B.; Calvo-Carrillo, M.C.; Carrillo-Domínguez, S.; Castillo-Domínguez, R.M.; Ávila-González, E. Effect on lipid fraction of egg stored at different times and temperatures of hens fed with shrimp meal Litopenaeus spp. Int. J. Sci. Res. Sci. Technol. 2018, 4, 58–70. [Google Scholar]
- Carrillo, D.S.; Pérez-Gil, R.F.; Castro, G.M.I. Crustáceo marino que alimenta y pigmenta. La langostilla, como alimento de ponedoras. In Acontecer Avícola; Ediciones Pecuarias de Mexico, S.A.: Col del Valle, Mexico City, Mexico, 1993. [Google Scholar]
- De la Concenpción Calvo, M.; Carranco, M.E.; Carrillo, S.; Sanginés, L.; Fuente, B.; Ávila, E. Physical and chemical changes in the soluble fraction of eggs from hens fed with Pleuroncodes planipes (Red Crab), stored for different lengths of time at different temperatures. Int. J. Sci. Res. Sci. Technol. 2016, 2, 64–71. [Google Scholar]
- Carranco-Jauregui, M.E.; Calvo-Carrillo, M.C.; Carrillo-Domínguez, S.; Fuente-Martínez, B.; Avila-González, E. Red crab (Pleuroncodes planipes) meal in laying hen rations and their effect on lipid fraction and oxidation of egg stored at different times and temperatures. Int. J. Sci. Res. Sci. Technol. 2016, 2, 296–304. [Google Scholar]
- Carrillo-Domínguez, S.; Carranco-Jauregui, M.E.; Castillo-Domínguez, R.M.; Castro-González, M.I.; Avila-González, E.; Pérez-Gil, F. Cholesterol and n-3 and n-6 fatty acid content in eggs from laying hens fed with red crab meal (Pleuroncodes planipes). Poult. Sci. 2005, 84, 167–172. [Google Scholar] [CrossRef]
- Rodríguez-Michel, A.; Morales-Barrera, E.; García-Márquez, L.; QuezadaTristán, T.; Carrillo-Domínguez, S.; Prado-Rebolledo, O. Harina de atún negra en dietas de gallina para incrementar los ácidos eicosapentanoico y docosahexaenoico. Abanico Vet. 2018, 8, 75–85. [Google Scholar] [CrossRef]
- Carrillo, D.S.; Casas, V.M.; Ramos, R.F.; Pérez-Gil, F.; Sánchez, R.I. Algas marinas de Baja California Sur, México: Valor nutrimental. Arch. Latinoam. Nutr. 2002, 52, 400–405. [Google Scholar]
- Carrillo, D.S.; Castro, G.M.I.; Pérez-Gil, R.F.; Rosales, E.; Manzano, R.E. El alga marina (Sargassum sinicola Setchel & Gardner) como alternativa en la alimentación animal. Rev. Cub. Cienc. Agri. 1992, 26, 177–184. [Google Scholar]
- Carrillo, D.S. Las Algas Marinas Como Alternativa para Reducir las Concentraciones de Colesterol en el Huevo y Carne de Pollo; Sociedad Mexicana de Geografía y Estadística: Mexico City, Mexico, 1995; Volune 7, pp. 19–23. [Google Scholar]
- Pérez, M.J.; Cuca, G.J.M.; Ramírez, V.G.; Carrillo, D.S.; Pro, M.A.; Ávila, G.E.; Sosa, M.E. Evaluación de dos aceites acidulados de soya en la producción y calidad de huevo en gallinas Bovans. Rev. Mex. Cienc. Pecu. 2019, 10, 283–297. [Google Scholar] [CrossRef]
- Ahmad, S.; Khalique, A.; Mehmood, S.; Hussain, K.; Naeem, M.; Shafiq, M.; Pasha, T. Effect of Moringa oleifera (Lam.) pods as feed additive on egg antioxidants, chemical composition and performance of commercial layers. S. Afr. J. Anim. Sci. 2017, 47, 864–874. [Google Scholar] [CrossRef]
- de Souza, Z.L.A.; Lima, D.H.J.; Martins, A.R.; Assunção, A.A.S.; Junior, N.D.A.; Silva, F.W.; da Silva, G.F. Egg yolk colour and retinol concentration of eggs from laying hens fed diets containing carrot and beetroot meal. Czech J. Anim. Sci. 2019, 64, 395–403. [Google Scholar] [CrossRef]
- Ortiz, R.E.; Afanador, G.; Vásquezt, D.R.; Ariza-Nieto, C. Efecto del aceite esencial de orégano sobre el desempeño productivo de ponedoras y la estabilidad oxidativa de huevos enriquecidos con ácidos grasos poliinsaturados. Rev. Med. Vet. Zoot. 2017, 64, 61–70. [Google Scholar] [CrossRef]
- Carranco- Jáuregui, M.E.; Barrita-Ramírez, V.; Fuente-Martínez, B.; Ávila González, E.; Sanginés-García, L. Inclusión de harina de Tithonia diversifolia en raciones para gallinas ponedoras de primer ciclo y su efecto sobre la pigmentación de yema de huevo. Rev. Mex. Cienc. Pecu. 2020, 11, 355–368. [Google Scholar] [CrossRef]
- Botsoglou, N.A.; Florou-Paneri, P.; Nikolakakis, I.; Giannenas, I.; Dotas, V.; Botsoglou, E.N.; Aggelopoulos, S. Effect of dietary saffron (Crocus sativus L.) on the oxidative stability of egg yolk. Br. Poult. Sci. 2005, 46, 701–707. [Google Scholar] [CrossRef]
- Akdemir, F.; Orhan, C.; Sahin, N.; Sahin, K.; Hayirli, A. Tomato powder in laying hen diets: Effects on concentrations of yolk carotenoids and lipid peroxidation. Br. Poult. Sci. 2012, 53, 675–680. [Google Scholar] [CrossRef]
- Kara, K.; Kocaoğlu Güçlü, B.; Baytok, E.; Şentürk, M. Effects of grape pomace supplementation to laying hen diet on performance, egg quality, egg lipid peroxidation and some biochemical parameters. J. Appl. Anim. Res. 2016, 44, 303–310. [Google Scholar] [CrossRef]
- Imbaquingo, N.N.P. Evaluación de Tres Niveles de Harina de Bledo (Amaranthus retroflexus) en Dietas Para Codornices (Coturnix japónica) en la Etapa de Postura en la Granja Experimental La Pradera, Chaltura; Universidad Técnia del Norte Ibarra: Ibarra, Ecuador, 2019. [Google Scholar]
- Núñez-Torres, O.P.; Delgado-Álvarez, V.E.; Almeida-Secará, R.I.; Cruz, Q.S.M. Suplementación de jengibre en codornices como alternativa nutricional en la producción y calidad de huevo. J. Selva Andin. Anim. Sci. 2021, 8, 90–101. [Google Scholar] [CrossRef]
- Degollado, A.K.M. Efecto de la Inclusión de Moringa oleífera Lam. en Dietas de Codorniz, Sobre Postura, Utilización de Energía, Proteína Metabolizable y Calidad de Huevo. Doctoral Dissertation, Universidad Autónoma de Nuevo León México, San Nicolás de los Garza, Mexico, 2018. [Google Scholar]
- Carranco-Jáuregui, M.E.; Fuente-Martínez, B.; Ramírez-Poblano, M.; Calvo-Carrillo, M.C.; Ávila-González, E. Inclusión de harina de calamar gigante Dosidicus gigas como fuente de proteína en dietas para gallinas ponedoras. Abanico Vet. 2020, 10, e109. [Google Scholar] [CrossRef]
- Carrillo, S.; Bahena, A.; Casas, M.; Carranco, M.E.; Calvo, C.C.; Ávila, E.; Pérez-Gil, F. El alga Sargassum spp. como alternativa para reducir el contenido de colesterol en el huevo. Rev. Cub. Cienc. Agríc. 2012, 46, 181–186. [Google Scholar]
- Buenaño, B.J.P. Producción de huevos de codorniz (Coturnix coturnix japónica) utilizando dietas alimenticias enriquecidas con azolla (Azolla anabaena). Investigación. Bachelor’s Thesis, Universidad Técnica de Ambato Tungurahua, Ambato, Ecuador, 2016. [Google Scholar]
- Coronado, I.A.R. Performance Productiva, Calidad Interna y Externa del Huevo de Codornices Alimentadas con Inulina en Dietas Normales y Bajas en Calcio. Investigación; Universidad Nacional Agraria la Molina: Lima, Perú, 2022. [Google Scholar]
- Boran, G.; Karaçam, H.; Boran, M. Changes in the quality of fish oils due to storage temperature and time. Food Chem. 2006, 98, 693–698. [Google Scholar] [CrossRef]
- Simopoulos, A. An increase in the omega-6/omega-3 fatty acid ratio increases the risk for obesity. Nutrients 2016, 8, 128. [Google Scholar] [CrossRef]
- Schwartz, S.G.; Wang, X.; Chavis, P.; Kuriyan, A.E.; Abariga, S.A. Vitamin A and fish oils for preventing the progression of retinitis pigmentosa. Cochrane Database Syst. Rev. 2020, 6, CD008428. [Google Scholar] [CrossRef]
- Alvarez-Campano, C.G.; Macleod, M.J.; Aucott, L.; Thies, F. Marine-derived n-3 fatty acids therapy for stroke. Cochrane Database Syst. Rev. 2022, 6, CD012815. [Google Scholar] [CrossRef] [PubMed]
- Abdelhamid, A.S.; Martin, N.; Bridges, C.; Brainard, J.S.; Wang, X.; Brown, T.J.; Hanson, S.; Jimoh, O.F.; Ajabnoor, S.M.; Deane, K.H.; et al. Polyunsaturated fatty acids for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2018, 7, CD012345. [Google Scholar] [CrossRef]
- Czernichow, S.; Thomas, D.; Bruckert, E. n-6 fatty acids and cardiovascular health: A review of the evidence for dietary intake recommendations. Br. J. Nutr. 2010, 104, 788–796. [Google Scholar] [CrossRef]
- Yang, W.S.; Chen, Y.Y.; Chen, P.C.; Hsu, H.C.; Su, T.C.; Lin, H.J.; Chen, M.F.; Lee, Y.T.; Chien, K.L. Association between plasma n-6 polyunsaturated fatty acids levels and the risk of cardiovascular disease in a community-based cohort study. Sci. Rep. 2019, 9, 19298. [Google Scholar] [CrossRef]
- Tam, K.W.; Wu, M.Y.; Siddiqui, F.J.; Chan, E.S.; Zhu, Y.; Jafar, T.H. Omega-3 fatty acids for dialysis vascular access outcomes in patients with chronic kidney disease. Cochrane Database Syst. Rev. 2018, 11, CD011353. [Google Scholar] [CrossRef]
- Ramírez, A.L.C.; Espinoza, M.G.G.; Ramos, A.R.P. Use of fish hydrolysate in aquaculture: A review of some beneficial results in aquafeeds. Manglar 2021, 18, 215–222. [Google Scholar] [CrossRef]
- Quinto, B.P.T.; Albuquerque, J.; Bezerra, R.S.; Peixoto, S.; Soares, R. Replacement of fishmeal by two types of fish protein hydrolysate in feed for postlarval shrimplitopenaeus vannamei. Aquacult. Nutr. 2017, 24, 768–776. [Google Scholar] [CrossRef]
- Lizárraga-Velázquez, C.E.; Hernández, C.; González-Aguilar, G.A.; Basilio-Heredia, J. Propiedades antioxidantes e inmunoestimulantes de polifenoles en peces carnívoros de cultivo. Ciencia UAT 2018, 12, 127. [Google Scholar] [CrossRef][Green Version]
- Alanes Oña, L.E. Alimentación y nutrición en peces de agua dulce. Rev. Estud. AGRO-VET 2021, 42, 604–608. [Google Scholar]
- Watson, H.; Stackhouse, C. Omega-3 fatty acid supplementation for cystic fibrosis. Cochrane Database Syst. Rev. 2020, 4, CD002201. [Google Scholar] [CrossRef]
- Howes, N.; Atkinson, C.; Thomas, S.; Lewis, S.J. Immunonutrition for patients undergoing surgery for head and neck cancer. Cochrane Database Syst. Rev. 2018, 8, CD010954. [Google Scholar] [CrossRef]
- Dushianthan, A.; Cusack, R.; Burgess, V.A.; Grocott, M.P.; Calder, P.C. Immunonutrition for acute respiratory distress syndrome (ARDS) in adults. Cochrane Database Syst. Rev. 2019, 1, CD012041. [Google Scholar] [CrossRef]
- Middleton, P.; Gomersall, J.C.; Gould, J.F.; Shepherd, E.; Olsen, S.F.; Makrides, M. Omega-3 fatty acid addition during pregnancy. Cochrane Database Syst. Rev. 2018, 15, CD003402. [Google Scholar] [CrossRef]
- Woods, R.K.; Thien, F.C.; Abramson, M.J. Dietary marine fatty acids (fish oil) for asthma in adults and children. Cochrane Database Syst. Rev. 2002, 3, CD001283. [Google Scholar] [CrossRef]
- Valenzuela, B.A.; Sanhueza, C.J. Aceites de origen marino; su importancia en la nutrición y en la ciencia de alimentos. Rev. Chil. Nutr. 2009, 36, 246–257. [Google Scholar] [CrossRef][Green Version]
- Russo, G.L. Dietary n-6 and n-3 polyunsaturated fatty acids: From biochemistry to clinical implications in cardiovascular prevention. Biochem. Pharmacol. 2009, 77, 937–946. [Google Scholar] [CrossRef]
- Miles, E.A.; Childs, C.E.; Calder, P.C. Long-chain polyunsaturated fatty acids (LCPUFAs) and the developing immune system: A narrative review. Nutrients 2021, 13, 247. [Google Scholar] [CrossRef] [PubMed]
- Nasiff-Hadad, A.; Meriño-Ibarra, E. Ácidos grasos omega-3: Pescados de carne azul y concentrados de aceites de pescado. Lo bueno y lo malo. Rev. Cubana Med. 2003, 42, 128–133. [Google Scholar]
- Dal Bosco, A.; Mugnai, C.; Roscini, V.; Castellini, C. Fillet fatty acid composition, estimated indexes of lipid metabolism and oxidative status of wild and farmed brown trout (Salmo trutta L.). Ital. J. Food Sci. 2013, 25, 83. [Google Scholar]
- Cai, Z.; Li, W.; Mai, K.; Xu, W.; Zhang, Y.; Ai, Q. Effects of dietary size-fractionated fish hydrolysates on growth, activities of digestive enzymes and aminotransferases and expression of some protein metabolism related genes in large yellow croaker (Larimichthys crocea) larvae. Aquaculture 2015, 440, 40–47. [Google Scholar] [CrossRef]
- Goosen, N.J.; de Wet, L.F.; Görgens, J.F. The effects of protein hydrolysates on the immunity and growth of the abalone Haliotis midae. Aquaculture 2014, 428–429, 243–248. [Google Scholar] [CrossRef]
- Bringas-Alvarado, L.; Zamorano-Ochoa, A.; Rojo-Rodríguez, J.B.; González- Félix, M.L.; Pérez-Velázquez, M.; Cárdenas-López, J.L.; Navarro-García, G. Evaluación del ensilado fermentado de subproductos de tilapia y su utilización como ingrediente en dietas para bagre de canal. Biotecnia 2018, 20, 85–94. [Google Scholar] [CrossRef]


| NRC Feed Classification | Description | Main Characteristic | Example of Feedstuff | Main Metabolites | Author |
|---|---|---|---|---|---|
| 1 | Dry forages and roughages | ≥18% fiber | Browse/range plants | Tannins and Saponins | Okunade et al., 2014 [24] |
| 2 | Pasture, range plants and forages fed fresh | ≥18% fiber | Sainfoin Trifolium | Tannins Isoflavones | Mueller-Harvey et al., 2019 [20] Hloucalova, et al., 2016 [25] |
| 3 | Silages | ≥18% fiber | |||
| 4 | Energetic sources | ≤20% protein and ≤18% fiber | Citrus pulp Corn | Hesperidin, narangin Anthocyanins | Simitzis, and Deligeorgis, 2018 [6] Antunović et al., 2022 [26] |
| 5 | Protein sources | ≥20% protein and ≤18% fiber | Alfalfa Clover | Flavonoids Isoflavones | Dabbou et al., 2018 [27] Dadáková et al., 2020 [28] |
| 6 | Minerals | Guaranteed analysis | Selenium | Selenites with tetravalent (Se4+), and Selenates with hexavalent (Se6+) cations | Pecoraro, et al., 2022 [29] Gu and Gao, 2022 [30] |
| 7 | Vitamins | Guaranteed analysis | Retinyl acetate | Vitamin A | Shask and Pelletier, 2023 [31] |
| 8 | Additives | Specific | Prebiotics | Inulin | Pandey et al., 2019 [32] Juárez et al., 2019 [33] |
| Metabolite Category | Metabolite | By-Product Added to Feedstuff | Dose, Concentration, or Treatment | Biological Function of Metabolites, Biochemistry, and Biotransformation | Reference |
|---|---|---|---|---|---|
| Polyunsaturated fatty acids and astaxanthin | EPA, DHA, ALA and AA, and astaxanthin | Red crab meal | 3%, 6%, and 9% | The results were that total lipids, EPA, DHA, ALA EPA, DHA, ALA, and AA fatty acids increased with respect to the control. | Carrillo et al., 2005 [167] |
| Polyunsaturated fatty acids and astaxanthin | Polyunsaturated fatty acids and antioxidant | Red crab meal | 4% and 6% | Inclusions of 4% and 6% red crab meal in diets for laying hens resulted in an increased fatty acid profile and egg yolk pigmentation. | Calvo et al., 2016 [165] |
| Polyunsaturated fatty acids and astaxanthin | Polyunsaturated fatty acids and antioxidant | Red crab meal | 4% | The inclusion of 4% lobster meal in diets for laying hens allows obtaining eggs enriched with n-3 fatty acids and astaxanthin. Also, astaxanthin has an antioxidant function, protecting fatty acids. | Carranco et al., 2016 [166] |
| Pigments | Astaxanthin | Shrimp meal | 4–25% | The results of this work showed that the color of the yolk was lower when compared to the control and decreased as the storage time passed (30 days/20° and 4 °C). | Carranco et al., 2003, 2006, 2011 [160,161,162] |
| Polyunsaturated fatty acids and astaxanthin | Polyunsaturated fatty acids and antioxidant | Shrimp meal | 20% | This study evaluated the concentration of astaxanthin, fatty acids, and peroxidation of egg yolk stored for 15 and 30 days at room temperature and refrigeration. The differences observed were due to the normal deterioration that all perishable foods undergo during normal deterioration and prolonged storage. | Carranco et al., 2018 [163] |
| Carotene | Lutein, canthaxanthin | Tithonia diversifolia meal | 1.8, 5, 10, and 15% | Leaf meal with petioles of Tithonia diversifolia can be considered as an alternative for poultry feed up to a level of 10% without affecting productive parameters and providing pigmentation to egg yolk. | Carranco et al., 2020 [176] |
| Protein | Protein | Giant squid meal | 10 and 20% | This meal can be used in laying hen diets in no more than 10% so as not to affect production parameters and egg flavor. It also showed a slight increase in protein content. | Carranco et al., 2020 [183] |
| Polyunsaturated fatty acids and cholesterol | DHA, ALA, AA, LA, cholesterol | Black tuna meal (BTM) | 1, 2, and 3% | Black tuna meal (3%) can be used to increase the fatty acids in eggs (DHA, ALA). | Rodríguez-Michel et al., 2018 [168] |
| Cholesterol | Cholesterol | Sargassum spp. algae | 2, 4, 6, and 8% | Sargassum spp. was used in diets for laying hens, resulting in a significant decrease in egg cholesterol concentration. | Carrillo et al., 2012 [184] |
| Myristic and palmitic acid | Myristic and palmitic acid | Acidulated soybean oil | 2 and 4% | Myristic and palmitic fatty acid concentration increased in concentration without affecting the value of stearic acid in eggs. | Pérez et al., 2019 [172] |
| Chitin, protein, amino acids, astaxanthin | Chitin, protein, amino acids, astaxanthin | Red crab meal | 3, 6, and 9% | Astaxanthin contributes to pigment egg yolk. | Carrillo, 1993 [164] |
| Monounsaturated fatty acids Polyunsaturated fatty acids | Fatty acids and antioxidants | Oregano oil, palm oil, and fish oil | Oregano oil (100 g/ton) + palm oil (2%) Oil of oregano (100 g/ton) + fish oil (2%) | Oregano oil in the feed of laying hens is a natural alternative to increase PUFA fatty acids and to replace synthetic antioxidants used in the feed industry. | Ortíz et al., 2017 [175] |
| Carotene, vitamins and minerals | Lutein, vitamin E, selenium, zeaxanthin, and iodine | NR | NR | The use of certain carotenoids as poultry feed additives improves the color of egg yolk. They also help neutralize singlet oxygen and free radicals and protect against oxidative damage. Lutein and zeaxanthin present in the egg are also found in human serum, skin, and the ocular macula and will play protective roles against oxidative stress. | Aparicio et al., 2018 Nimalarante and Wu, 2015 [154,156] |
| Carotene | β-carotene, betalain | Freeze-dried carrot and sorghum Freeze-dried beet and sorghum Carrot, beet, and sorghum | 0.4 and 0.8% | The use of 0.8% carrot and beet meal increased the retinol concentration and egg yolk color compared to the corn and soybean diets. | Souza et al., 2019 [174] |
| Carotene, quercetin, mineral, and serum biochemical markers | β-carotene, quercetin, selenium, and serum biochemical markers | Moringa meal Oleifera (Lam.) | 0.5, 1.0 and 1.5% | Moringa oleifera pods could be used as alternative growth promoters, which improve antioxidant activity and the performance of laying birds. | Ahmad et al., 2017 [173] |
| Antioxidants | NR | Rosemary, oregano, saffron, and α-tocopheryl acetate | Basal an additional 200 mg α-tocopheryl acetate/kg, or rosemary at 5 g/kg diet, oregano at 5 g/kg diet, or saffron at 20 mg/kg diet. | Considering that egg yolks from the dietary supplemented groups exhibited increased resistance to lipid oxidation compared to control, one could say that antioxidant constituents of rosemary, oregano, and saffron passed from the feed into the developing yolk, providing eggs with increased antioxidant properties. | Botsoglou et al., 2005 [177] |
| Carotenes and vitamins | Lycopene, beta-carotene, lutein, vitamin A | Tomato powder | 5 and 10 g/kg | Concentrations of lycopene in serum and egg yolk beta-carotene, lutein, and vitamin A increased powder, while MDA decreased linearly at both concentrations of tomato with increasing tomato powder. Tomato powder supplementation increased the concentration of carotenoids and vitamin A and a reduction of peroxidation. | Akdemir et al., 2012 [178] |
| Antioxidants | Serum cholesterol, total protein, glucose, triglycerides, and MDA | Raisin pomace | 4 and 6% | The addition of raisin pomace significantly decreased plasma levels of MDA and serum glucose. Egg yield, egg quality, and serum levels of total cholesterol, total protein, and triglycerides were not negatively affected. Plasma and yolk MDA and serum glucose levels were reduced by 4% and 6% supplementation. By raising pomace, supplementation has the potential to extend shelf life. | Kara et al., 2016 [179] |
| Fatty acids | Pentadecanoic acid | Azolla anabaena | 5, 10, and 15% | The inclusion of 5% Azolla showed better results on productive behavior, voluntary consumption, and apparent nutrient digestibility with respect to the control diet. | Buenaño, 2016 [185] |
| Fructooligosaccharides | Inulin | Inulin | Control diet with Ca without inulin (1) Diet with Ca and inulin (2) Low Ca diet with inulin (3) Low Ca diet without inulin (4) | Incorporating inulin in the feed had an effect on the shape index, and the yolk diameter was lower with the incorporation of inulin in the feed yolk diameter with diet 4. | Coronado, 2022 [186] |
| Antioxidants | productive parameters and egg quality | Ginger flour (Zingiber officinale) | 0.2, 0.4, and 0.6% | The inclusion of ginger flour improves the productive parameters and the quality of the eggs. | Núñez et al., 2021 [181] |
| Category | Metabolite | Feedstuff | Dose, Concentration or Treatment | Function | Reference |
|---|---|---|---|---|---|
| Hydrolyzed fish | Low-molecular-weight peptides | Fish food | 60% protein, less than 5% lipids, and less than 10% moisture. | Hydrolyzed proteins encompass essential and non-essential amino acids, with notable levels of aspartic and glutamic acid derived from muscle, head, skin, and viscera. These hydrolyzates contain a significant proportion of peptides ranging from 500 to 2500 Da, followed by 200 to 500 Da. Fish exhibit heightened dipeptide and tripeptide absorption instead of free amino acids. | Cardoza-Ramírez, 2021 [195] |
| Hydrolyzed fish | Free amino acids and nucleotides | Fish food | Hydrolyzed fish contain free amino acids, such as glutamic acid, aspartic acid, glycine, arginine, alanine, proline, leucine, and isoleucine, along with specific nucleotides, which impart an attractive aroma to the feed, appealing to fish and shrimp. The inclusion of lysine, methionine, nucleotides, anserine, and taurine is proposed to elicit the secretion of insulin-like growth hormones (IGF-I and IGF-II). | Quinto et al., 2018 [196] | |
| Polyphenols | Catechins, flavonoids, and anthocyanins | Green tea; mango; corn | 0.5 g/kg for tilapia: 50 g/kg; 5 g/kg; 2 g/kg for grass carp | Plants can enhance fish species’ immune defense and antioxidant systems as a source of polyphenols. Green tea is widely used due to its high polyphenolic content. Evaluating purified polyphenols from vegetable sources is necessary to identify the components responsible for the immune and antioxidant responses in different species, aiding in the development of functional foods for aquaculture. Determining optimal doses for each species and analyzing the feed matrix’s influence on response variables is also crucial. | Lizárraga-Velázquez et al., 2018 [197] |
| Pigments | Astaxanthin | Crab meal, shrimp meal, oil, and seaweed. | This carotenoid helps fish’s good health and rapid growth, together with the color it provides, especially to the salmon family. It is an excellent antioxidant that potentiates its function, combined with vitamins A and E. It is mainly found bound to specific proteins. | Alanes-Oña, 2020 [198] | |
| Pigments | Astaxanthin | Microalgae and foods that consume it, such as red trout, salmon, or crustaceans | Astaxanthin is the most potent antioxidant carotenoid for free radical scavenging: 65 times more potent than vitamin C. This compound can inhibit certain cancers and positively impact degenerative diseases. It protects membrane phospholipids and other lipids against peroxidation and contributes to terminating the induction of inflammation in biological systems. Additionally, it may have therapeutic effects against cardiovascular disease and is reported to protect against LDL cholesterol oxidation and oxidative stress. | Alanes-Oña, 2020 [198] | |
| Fatty acids | Fatty acids n-3 | PUFA from marine origin | 400–3300 mg/día | In animal research studies, EPA and DHA appear to protect brain cells after stroke, especially if given very early. However, their effects as a treatment for stroke in humans have yet to be apparent. | Alvarez-Campano et al., 2020 [190] |
| Fatty acids | n-3 FA and n-6 FA | n-6 as part of the diet | 3.9–8% of total energy intake | Consuming polyunsaturated fatty acids (PUFA) may lower blood cholesterol and reduce the risk of cardiovascular disease, but it could also lead to weight gain and inflammation. Current evidence is inconclusive, and further research is needed to understand the full health effects of increased PUFA intake. | Adbelhamid et al., 2018 [191] |
| Fatty acids | Fatty acids n-3 | Fish oil (supplementation) | Oral omega-3 fatty acid supplementation may help prevent vascular access blockage by reducing the risk of thrombosis and stenosis. | Tam et al., 2018 [194] | |
| Fatty acids | Fatty acids n-3 | Fish oil (supplementation) | Recurrent cycles of infection and inflammation are thought to worsen lung function in patients with cystic fibrosis. Using n-3 FA and fish oil derivatives may counteract inflammation and benefit chronic inflammatory diseases, including cystic fibrosis. A 12-month study reported reduced pulmonary exacerbations and antibiotic use when taking omega-3 supplements compared to placebo. | Watson and Stackhouse, 2020 [199] | |
| Fatty acids | Fatty acids n-3 | Fish oil | Head and neck cancer can affect the oral cavity, throat, or larynx. Complications such as infections and pneumonia are common. The possibility of adding amino acids, n-3 fatty acids, and nucleotides to the diet has been analyzed to determine their potential for improving postoperative recovery. This nutritional strategy would aid recovery and reduce the number of days of hospitalization compared to a control diet. | Howes et al., 2018 [200] | |
| Fatty acids | Fatty acids n-3 (DHA y EPA) | Obtained from fish and, in some cases, combined with antioxidants. | Ten studies involving 1015 adults with acute respiratory distress syndrome (ARDS) to compare the effects of immunonutrition with standard feeding. The studies compared standard nutrition with supplemental nutrition containing omega-3 FA or a placebo and no antioxidants. The study found uncertainty regarding the long-term survival benefits, impact on intensive care unit stay duration and ventilator dependency, and potential harm associated with this type of nutrition. | Dushianthan et al., 2019 [201] | |
| Fatty acids | Fatty acids n-3 (DHA y EPA) | As a supplement or addition to food | Intake of n-3 during pregnancy may reduce the risk of preterm and newborns with low weight. It is essential to explore different ways of increasing n-3 intake during pregnancy. | Middleton et al., 2018 [202] | |
| Fatty acids | Fatty acids n-3 | Fish oil and fatty fish diet | A trial showed that adding fish oil (n-3 marine fatty acid) to asthmatic patients’ diets did not improve asthma symptoms. | Woods et al., 2000 [203] | |
| Fatty acids | Linolenic acid (LA) (n-6), α-linolenic ac. (ALA) (n-3) | 500 mg/day of EPA + DHA in adults, not less than 300 mg in mothers and wet-nurses, and 150 mg/day in lactating and schoolchildren. | Linolenic acid (LA) (n-6) and α-linolenic acid (ALA) (n-3) are essential fatty acids, as humans or other higher animals cannot synthesize them. In the human body, these fatty acids give rise to arachidonic acid (ARA n-6), eicosapentaenoic acid (EPA, n-3), and docosahexaenoic acid (DHA n-3). Locally acting bioactive signaling lipids called eicosanoids derived from these fatty acids also regulate various homeostatic processes. Generally, ARA gives rise to pro-inflammatory eicosanoids, while EPA and DHA give rise to anti-inflammatory eicosanoids. | Valenzuela and Samhueza, 2009; Russo, 2009; Abdelhamid, 2018; Miles, 2021 [191,204,205,206] | |
| Fatty acids | Omega-3 fatty acids: eicosapentaenoic Acid (EPA) and Docosahexaenoic acid (DHA) | Sardine, Mackerel, Herring, Anchovy, Salmon, Sablefish, Salmon, Cod Liver, Herring Oils. | g (EPA/DHA)/100 g of raw fish: 3.3, 2.5, 1.7, 1.4, 1.4, 1.4, and 1.4. g (EPA/DHA)/100 g of oil: 44.2, 19.9, 18.5, and 11.4. | Lipoproteins have been reduced in patients with a diet rich in n-3 fatty acids. This reduction in hypertriglyceridemia is due to decreased hepatic triglyceride synthesis, increased plasma clearance, and activation of peroxisome proliferator-activated receptors (PPAR). The slight elevation of LDL with n-3 fatty acids is associated with the rapid conversion of VLDLc to LDLc, although this has only been tested in pigs and not humans. Omega-3 results in the production of smaller VLDL particles that are more susceptible to conversion to LDLc. For hypertensive patients, doses between 3 and 4 g daily of EPA/DHA have been used for periods ranging from 4 weeks to 1 year. However, in some patients, it increased the risk of stroke due to arterial hypertension. Dietary supplementation with n-3 has a hypotensive effect in hypertensive patients. | Nasif-Hadad and Meriño-Ibarra 2003 [207] |
| Fatty acids | n-3 FA and n-6 FA | The captive trout’s diet was commercialized in Perugia, Italy. The wild trout was caught in the Nero River, Italy. | SFA: 761.5, MUFA 433.9, PUFA 1560.6, n-3 n1234.9. n-6 157.8, w-3/w-6 7.8 | The fatty acid profile in fish reflects the composition of fatty acids in their diet. Some variables indicate that the incorporation of FA into the tissue is carried out under certain metabolic effects. | Dal Bosco et al., 2013 [208] |
| Fatty acids | Omega-3 fatty acids: Docosahexaenoic Acid (DHA) | Fish oils (DHA) | DHA = 2000–3600 mg/d | Retinitis pigmentosa is one of several inherited eye diseases characterized by progressive degeneration of the photoreceptors located in the retina, causing severe vision loss and leading to blindness. So far, there is no treatment for this health problem. Vitamin A, fish oils, or both may help slow the progression of this group’s vision loss. Two trials evaluated the effect of DHA. | Schwartz et al., 2000 [189] |
| Bioactive peptides | Peptides | Fish by-products | The waste from the fishing industry contains fatty acids and proteins, which are very unstable (rancidity), so hydrolysis has been chosen to separate the fatty acids and proteins. In such a way that active peptides have been obtained as an energy source, nitrogen has physiological activity such as antioxidants, anticoagulants, antimicrobials, antidiabetics, and anticancer. These have been used to elaborate fish, poultry, and swine feeding concentrates. | Cai et al., 2015; Goosen et al., 2014; Bringas-Alvarado et al., 2018 [209,210,211] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cuchillo-Hilario, M.; Fournier-Ramírez, M.-I.; Díaz Martínez, M.; Montaño Benavides, S.; Calvo-Carrillo, M.-C.; Carrillo Domínguez, S.; Carranco-Jáuregui, M.-E.; Hernández-Rodríguez, E.; Mora-Pérez, P.; Cruz-Martínez, Y.R.; et al. Animal Food Products to Support Human Nutrition and to Boost Human Health: The Potential of Feedstuffs Resources and Their Metabolites as Health-Promoters. Metabolites 2024, 14, 496. https://doi.org/10.3390/metabo14090496
Cuchillo-Hilario M, Fournier-Ramírez M-I, Díaz Martínez M, Montaño Benavides S, Calvo-Carrillo M-C, Carrillo Domínguez S, Carranco-Jáuregui M-E, Hernández-Rodríguez E, Mora-Pérez P, Cruz-Martínez YR, et al. Animal Food Products to Support Human Nutrition and to Boost Human Health: The Potential of Feedstuffs Resources and Their Metabolites as Health-Promoters. Metabolites. 2024; 14(9):496. https://doi.org/10.3390/metabo14090496
Chicago/Turabian StyleCuchillo-Hilario, Mario, Mareli-Itzel Fournier-Ramírez, Margarita Díaz Martínez, Sara Montaño Benavides, María-Concepción Calvo-Carrillo, Silvia Carrillo Domínguez, María-Elena Carranco-Jáuregui, Elizabeth Hernández-Rodríguez, Patricia Mora-Pérez, Yesica R. Cruz-Martínez, and et al. 2024. "Animal Food Products to Support Human Nutrition and to Boost Human Health: The Potential of Feedstuffs Resources and Their Metabolites as Health-Promoters" Metabolites 14, no. 9: 496. https://doi.org/10.3390/metabo14090496
APA StyleCuchillo-Hilario, M., Fournier-Ramírez, M.-I., Díaz Martínez, M., Montaño Benavides, S., Calvo-Carrillo, M.-C., Carrillo Domínguez, S., Carranco-Jáuregui, M.-E., Hernández-Rodríguez, E., Mora-Pérez, P., Cruz-Martínez, Y. R., & Delgadillo-Puga, C. (2024). Animal Food Products to Support Human Nutrition and to Boost Human Health: The Potential of Feedstuffs Resources and Their Metabolites as Health-Promoters. Metabolites, 14(9), 496. https://doi.org/10.3390/metabo14090496








