Functional Muffins Exert Bifidogenic Effects along with Highly Product-Specific Effects on the Human Gut Microbiota Ex Vivo
Abstract
1. Introduction
2. Materials and Methods
2.1. GoodBiome™ Foods
2.2. Experimental Design, Timeline, and Analysis
2.3. Key Fermentation Parameters
2.4. Microbial Community Composition
2.5. Metabolomics
2.6. Statistical Analysis
3. Results
3.1. The Study Cohort Covered Enterotypic Differences Described for Human Adult Gut Microbiota
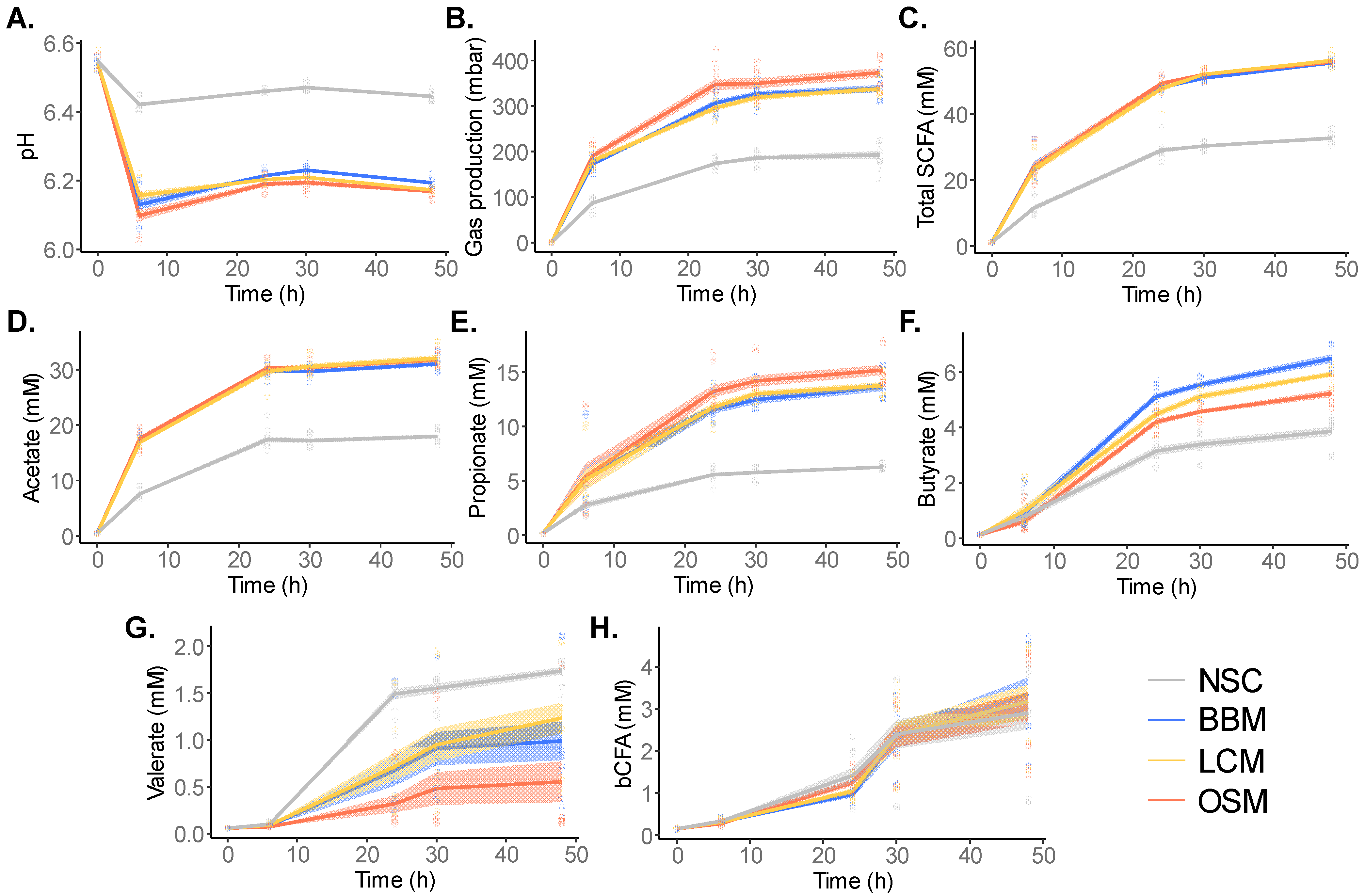
3.2. GoodBiome™ Foods Stimulated the Metabolic Activity of the Gut Microbiota and the Production of Short-Chain Fatty Acids
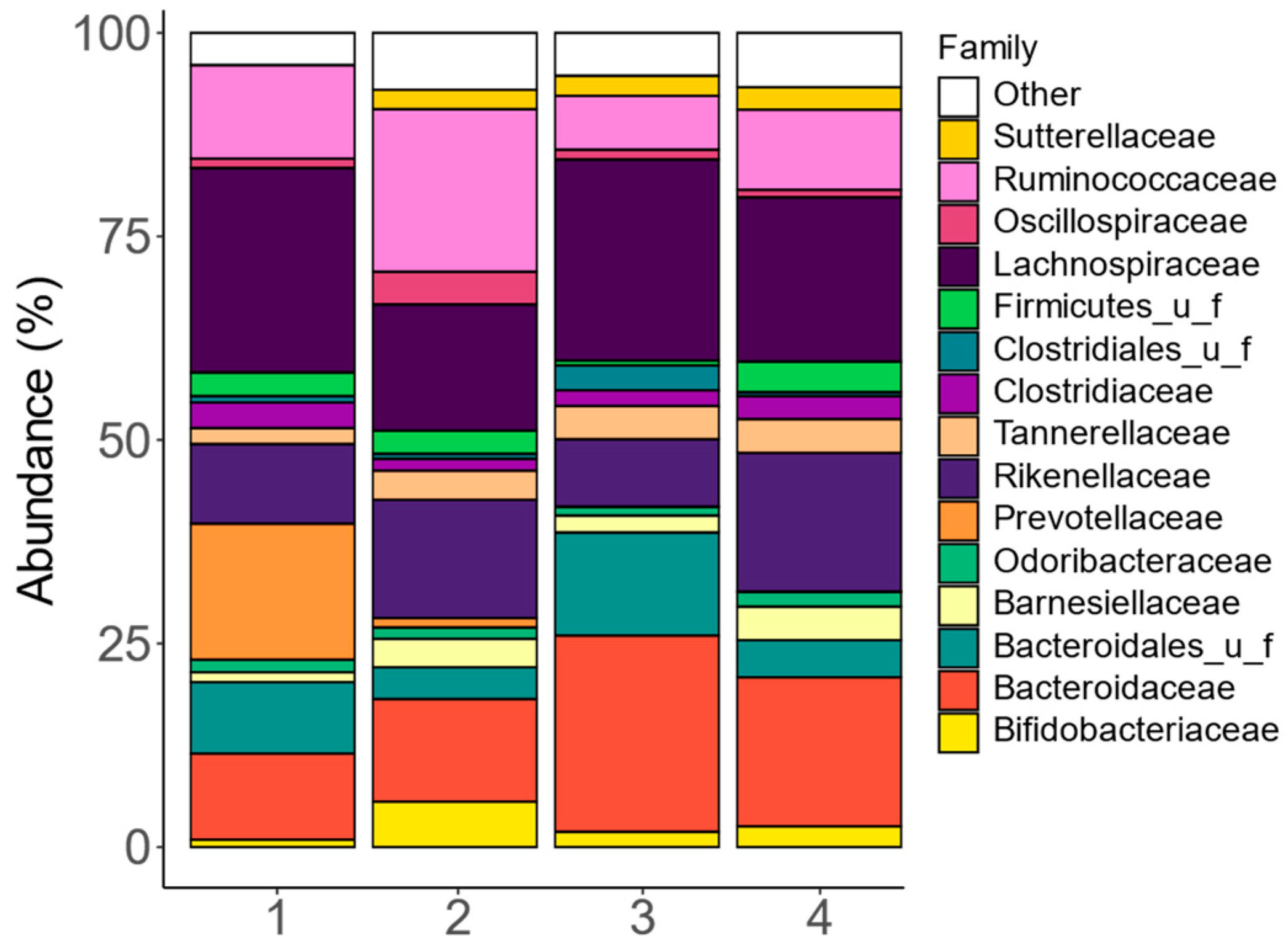
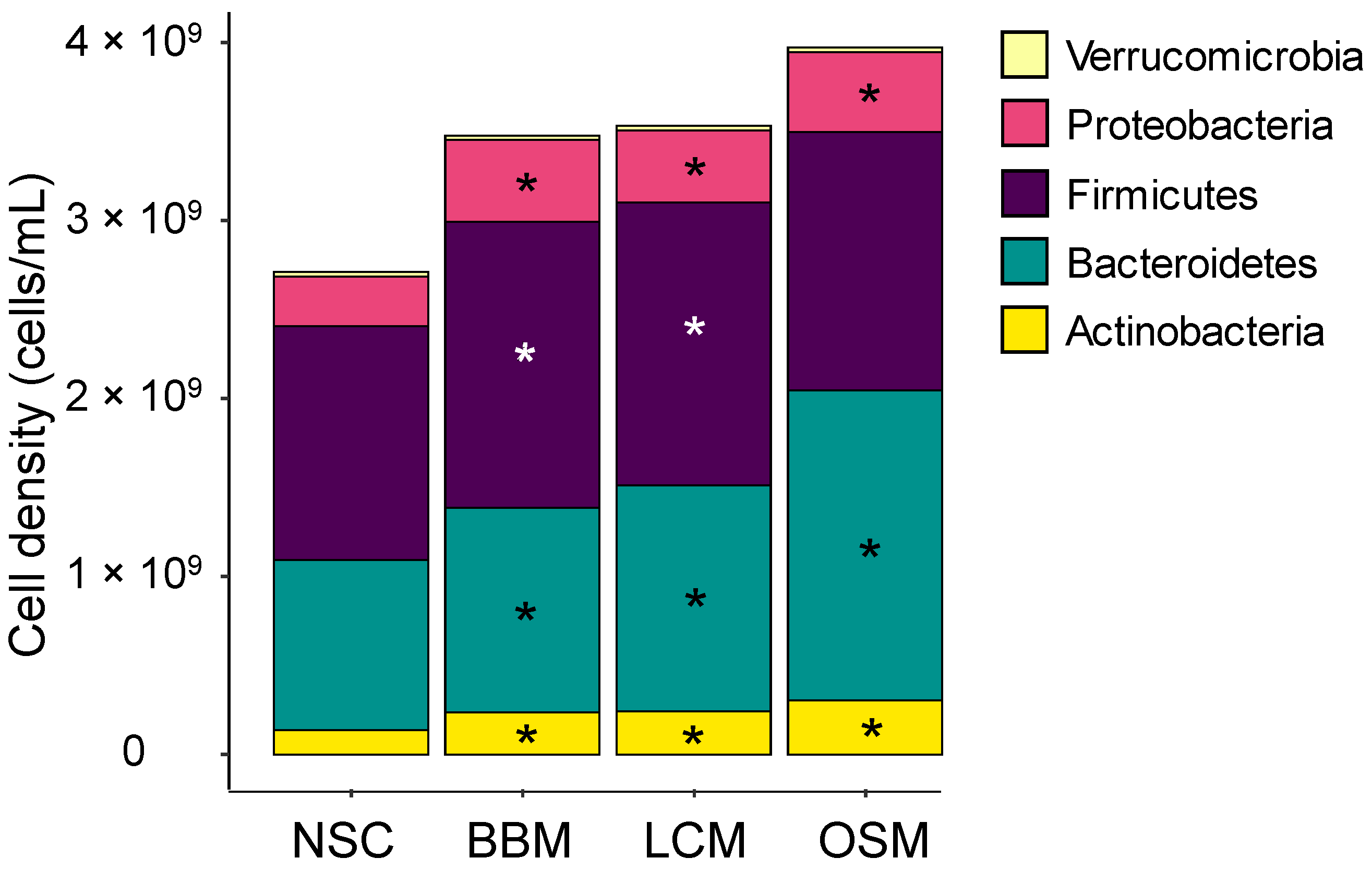
3.3. Fermentation of GoodBiome™ Foods Resulted in Product-Specific Changes in the Composition of Gut Microbial Community
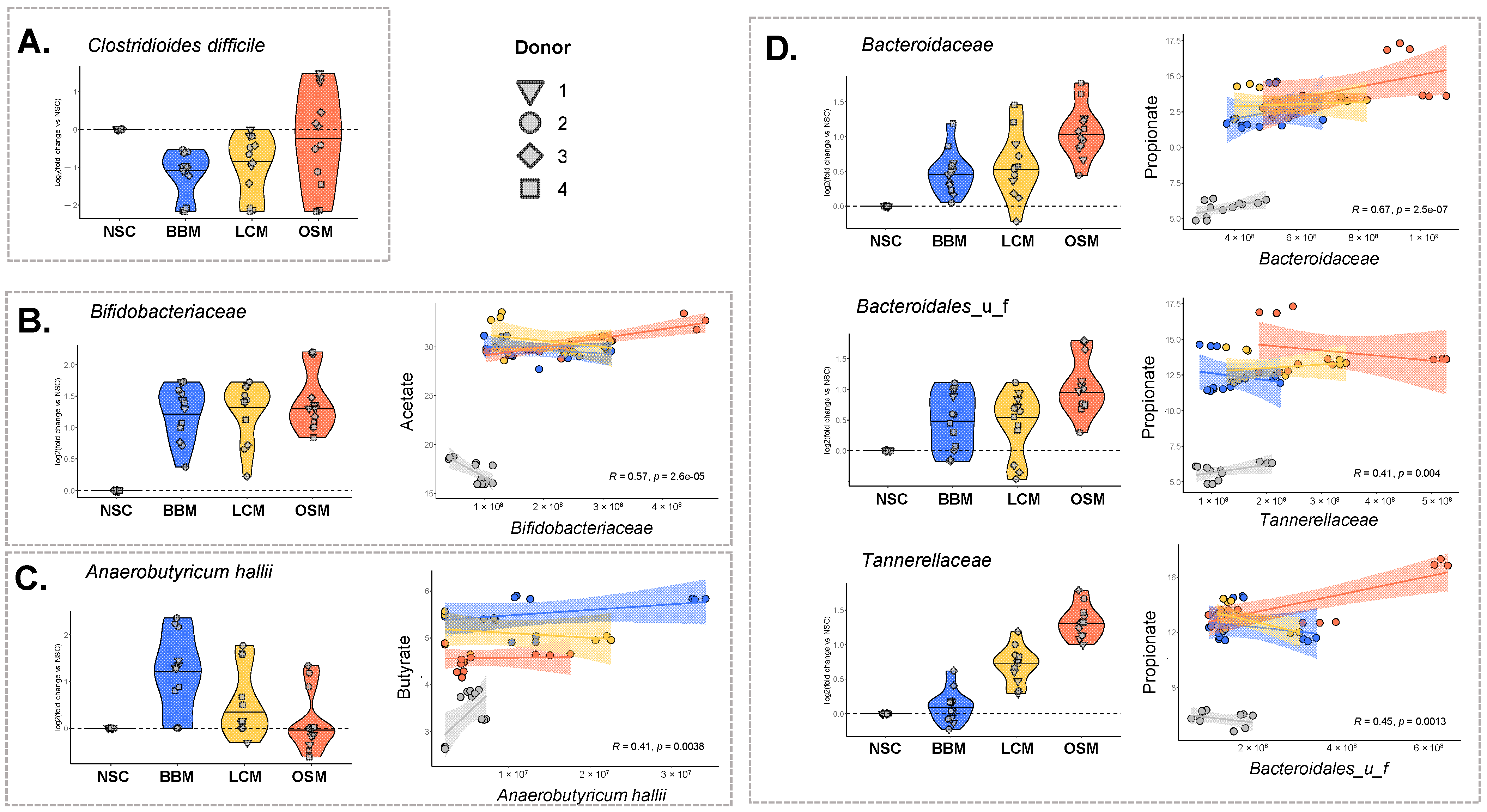
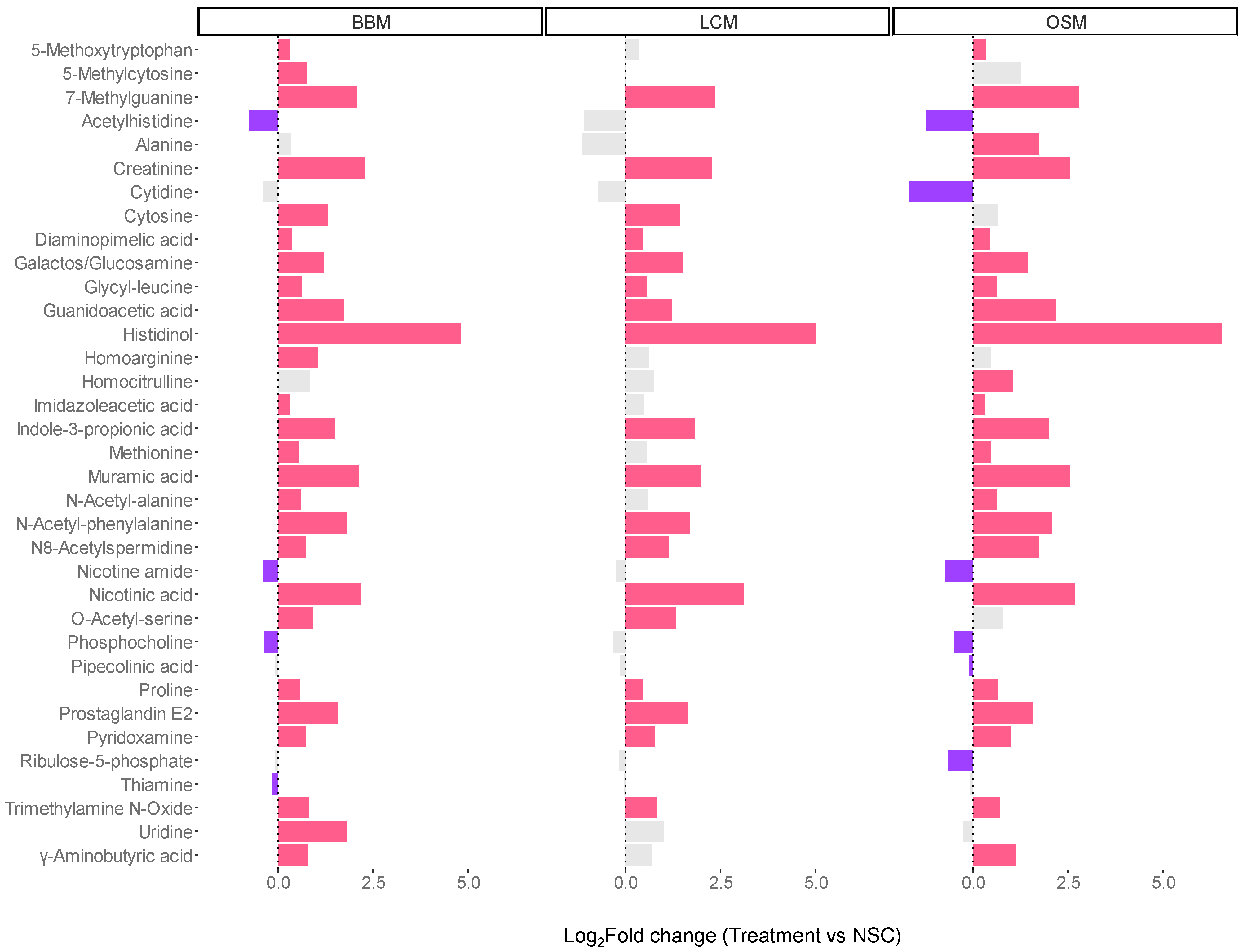
3.4. Fermentation of GoodBiome™ Foods Boosted the Production of Health-Promoting Microbial Metabolites
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
- Test product composition.
- GoodBiome™ Foods Lemon Chia Muffin (Microbiome Labs)
- Inulin powder, white sorghum flour, pea protein concentrate, flaxseed, soluble tapioca fiber, almond protein concentrated, black chia seeds, cane sugar, almond meal, sacha inchi protein concentrate; contains 2% or less of each of the following: leavening (monocalcium phosphate, sodium bicarbonate), xylooligosaccharides, lemon, tapioca starch, sea salt, orange powder, banana powder, papaya powder, shiitake mushroom powder, monk fruit extract, Bacillus subtilis HU58™, natural flavor.
- GoodBiome™ Foods Berry Blast Muffin (Microbiome Labs)
- Inulin powder, white sorghum flour, pea protein concentrate, flaxseed meal, erythritol, almond protein concentrate, almond meal, quinoa flour, coconut sugar, sacha inchi protein concentrate; contains 2% or less of each of the following: date powder, blueberry powder, strawberry powder, soluble tapioca fiber, cranberry powder, beet powder, leavening (monocalcium phosphate, sodium bicarbonate), xylooligosaccharides, monk fruit extract, orange powder, banana powder, papaya powder, shiitake mushroom powder, sea salt, tapioca starch, natural flavor, Bacillus subtilis HU58™.
- GoodBiome™ Foods Oat Spice Mookie (Microbiome Labs)
- Oats, almond meal, inulin powder, pea protein concentrate, flaxseed, soluble tapioca fiber, chocolate chips (cane sugar, cocoa liquor, cocoa butter), cane sugar; contains 2% or less of the following: leavening (monocalcium phosphate, sodium bicarbonate), xylooligosaccharides, fenugreek seed, anise, molasses, sea salt, orange powder, banana powder, papaya powder, shiitake mushroom powder, monk fruit extract, Bacillus subtilis HU58™.
References
- Gensollen, T.; Iyer, S.S.; Kasper, D.L.; Blumberg, R.S. How Colonization by Microbiota in Early Life Shapes the Immune System. Science 2016, 352, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Keeney, K.M.; Yurist-Doutsch, S.; Arrieta, M.-C.; Finlay, B.B. Effects of Antibiotics on Human Microbiota and Subsequent Disease. Annu. Rev. Microbiol. 2014, 68, 217–235. [Google Scholar] [CrossRef] [PubMed]
- Levin, D.; Raab, N.; Pinto, Y.; Rothschild, D.; Zanir, G.; Godneva, A.; Mellul, N.; Futorian, D.; Gal, D.; Leviatan, S.; et al. Diversity and Functional Landscapes in the Microbiota of Animals in the Wild. Science 2021, 372, 254. [Google Scholar] [CrossRef] [PubMed]
- Tamburini, S.; Shen, N.; Wu, H.C.; Clemente, J.C. The Microbiome in Early Life: Implications for Health Outcomes. Nat. Med. 2016, 22, 713–722. [Google Scholar] [CrossRef]
- Vatanen, T.; Kostic, A.D.; d’Hennezel, E.; Siljander, H.; Franzosa, E.A.; Yassour, M.; Kolde, R.; Vlamakis, H.; Arthur, T.D.; Hämäläinen, A.-M.; et al. Variation in Microbiome LPS Immunogenicity Contributes to Autoimmunity in Humans. Cell 2016, 165, 1551. [Google Scholar] [CrossRef] [PubMed]
- Rajilić-Stojanović, M.; de Vos, W.M. The First 1000 Cultured Species of the Human Gastrointestinal Microbiota. FEMS Microbiol. Rev. 2014, 38, 996–1047. [Google Scholar] [CrossRef]
- Carding, S.; Verbeke, K.; Vipond, D.T.; Corfe, B.M.; Owen, L.J. Dysbiosis of the Gut Microbiota in Disease. Microb. Ecol. Health Dis. 2015, 26, 26191. [Google Scholar] [CrossRef]
- Martinez, J.E.; Kahana, D.D.; Ghuman, S.; Wilson, H.P.; Wilson, J.; Kim, S.C.J.; Lagishetty, V.; Jacobs, J.P.; Sinha-Hikim, A.P.; Friedman, T.C. Unhealthy Lifestyle and Gut Dysbiosis: A Better Understanding of the Effects of Poor Diet and Nicotine on the Intestinal Microbiome. Front. Endocrinol. 2021, 12, 667066. [Google Scholar] [CrossRef]
- Sonnenburg, E.D.; Sonnenburg, J.L. Starving Our Microbial Self: The Deleterious Consequences of a Diet Deficient in Microbiota-Accessible Carbohydrates. Cell Metab. 2014, 20, 779–786. [Google Scholar] [CrossRef]
- Koropatkin, N.M.; Cameron, E.A.; Martens, E.C. How Glycan Metabolism Shapes the Human Gut Microbiota. Nat. Rev. Microbiol. 2012, 10, 323–335. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, Y.; Wang, P.; Huang, Y.; Wang, F. Short-Chain Fatty Acids Manifest Stimulative and Protective Effects on Intestinal Barrier Function Through the Inhibition of NLRP3 Inflammasome and Autophagy. Cell Physiol. Biochem. 2018, 49, 190–205. [Google Scholar] [CrossRef] [PubMed]
- Topping, D.L.; Clifton, P.M. Short-Chain Fatty Acids and Human Colonic Function: Roles of Resistant Starch and Nonstarch Polysaccharides. Physiol. Rev. 2001, 81, 1031–1064. [Google Scholar] [CrossRef] [PubMed]
- Hassler, C.M.; Brown, A.C. American Dietetic Association Position of the American Dietetic Association: Functional Foods. J. Am. Diet. Assoc. 2009, 109, 735–746. [Google Scholar] [CrossRef]
- Luo, D.; Li, Y.; Xu, B.; Ren, G.; Li, P.; Li, X.; Han, S.; Liu, J. Effects of Inulin with Different Degree of Polymerization on Gelatinization and Retrogradation of Wheat Starch. Food Chem. 2017, 229, 35–43. [Google Scholar] [CrossRef]
- Le Bastard, Q.; Chapelet, G.; Javaudin, F.; Lepelletier, D.; Batard, E.; Montassier, E. The Effects of Inulin on Gut Microbial Composition: A Systematic Review of Evidence from Human Studies. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Cutting, S.M. Bacillus Probiotics. Food Microbiol. 2011, 28, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Horosheva, T.V.; Vodyanoy, V.; Sorokulova, I. Efficacy of Bacillus Probiotics in Prevention of Antibiotic-associated Diarrhoea: A Randomized, Double-blind, Placebo-controlled Clinical Trial. JMM Case Rep. 2014, 1, e004036. [Google Scholar] [CrossRef]
- Sudha, M.R.; Jayanthi, N.; Aasin, M.; Dhanashri, R.D.; Anirudh, T. Efficacy of Bacillus coagulans Unique IS2 in Treatment of Irritable Bowel Syndrome in Children: A Double Blind, Randomised Placebo Controlled Study. Benef. Microbes 2018, 9, 563–572. [Google Scholar] [CrossRef]
- Nyangale, E.P.; Farmer, S.; Cash, H.A.; Keller, D.; Chernoff, D.; Gibson, G.R. Bacillus coagulans GBI-30, 6086 Modulates Faecalibacterium Prausnitzii in Older Men and Women. J. Nutr. 2015, 145, 1446–1452. [Google Scholar] [CrossRef]
- Madempudi, R.S.; Ahire, J.J.; Neelamraju, J.; Tripathi, A.; Nanal, S. Randomized Clinical Trial: The Effect of Probiotic Bacillus coagulans Unique IS2 vs. Placebo on the Symptoms Management of Irritable Bowel Syndrome in Adults. Sci. Rep. 2019, 9, 12210. [Google Scholar] [CrossRef]
- Majeed, M.; Majeed, S.; Nagabhushanam, K.; Natarajan, S.; Sivakumar, A.; Ali, F. Evaluation of the Stability of Bacillus coagulans MTCC 5856 during Processing and Storage of Functional Foods. Int. J. Food. Sci. Technol. 2016, 51, 894–901. [Google Scholar] [CrossRef]
- Urgesi, R.; Casale, C.; Pistelli, R.; Rapaccini, G.L.; de Vitis, I. A Randomized Double-Blind Placebo-Controlled Clinical Trial on Efficacy and Safety of Association of Simethicone and Bacillus coagulans (Colinox®) in Patients with Irritable Bowel Syndrome. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 1344–1353. [Google Scholar] [PubMed]
- Catinean, A.; Neag, A.M.; Nita, A.; Buzea, M.; Buzoianu, A.D. Bacillus Spp. Spores—A Promising Treatment Option for Patients with Irritable Bowel Syndrome. Nutrients 2019, 11, 1968. [Google Scholar] [CrossRef] [PubMed]
- Catinean, A.; Neag, M.A.; Krishnan, K.; Muntean, D.M.; Bocsan, C.I.; Pop, R.M.; Mitre, A.O.; Melincovici, C.S.; Buzoianu, A.D. Probiotic Bacillus Spores Together with Amino Acids and Immunoglobulins Exert Protective Effects on a Rat Model of Ulcerative Colitis. Nutrients 2020, 12, 3607. [Google Scholar] [CrossRef]
- McFarlin, B.K.; Henning, A.L.; Bowman, E.M.; Gary, M.A.; Carbajal, K.M. Oral Spore-Based Probiotic Supplementation Was Associated with Reduced Incidence of Post-Prandial Dietary Endotoxin, Triglycerides, and Disease Risk Biomarkers. World J. Gastrointest. Pathophysiol. 2017, 8, 117. [Google Scholar] [CrossRef]
- Neag, M.A.; Catinean, A.; Muntean, D.M.; Pop, M.R.; Bocsan, C.I.; Botan, E.C.; Buzoianu, A.D. Probiotic Bacillus Spores Protect Against Acetaminophen Induced Acute Liver Injury in Rats. Nutrients 2020, 12, 632. [Google Scholar] [CrossRef]
- Van den Abbeele, P.; Deyaert, S.; Thabuis, C.; Perreau, C.; Bajic, D.; Wintergerst, E.; Joossens, M.; Firrman, J.; Walsh, D.; Baudot, A. Bridging Preclinical and Clinical Gut Microbiota Research Using the Ex Vivo SIFR® Technology. Front. Microbiol. 2023, 14, 1131662. [Google Scholar] [CrossRef] [PubMed]
- Van den Abbeele, P.; Kunkler, C.N.; Poppe, J.; Rose, A.; van Hengel, I.A.J.; Baudot, A.; Warner, C.D. Serum-Derived Bovine Immunoglobulin Promotes Barrier Integrity and Lowers Inflammation for 24 Human Adults Ex Vivo. Nutrients 2024, 16, 1585. [Google Scholar] [CrossRef]
- Van den Abbeele, P.; Goggans, M.; Deyaert, S.; Baudot, A.; Van de Vliet, M.; Calatayud, M.; Lelah, M. Lacticaseibacillus Rhamnosus ATCC 53103 and Limosilactobacillus Reuteri ATCC 53608 Synergistically Boost Butyrate Levels upon Tributyrin Administration Ex Vivo. Int. J. Mol. Sci. 2023, 24, 5859. [Google Scholar] [CrossRef]
- Van den Abbeele, P.; Poppe, J.; Deyaert, S.; Laurie, I.; Otto Gravert, T.K.; Abrahamsson, A.; Baudot, A.; Karnik, K.; Risso, D. Low-No-Calorie Sweeteners Exert Marked Compound-Specific Impact on the Human Gut Microbiota Ex Vivo. Int. J. Food Sci. Nutr. 2023, 74, 630–644. [Google Scholar] [CrossRef]
- Bajic, D.; Wiens, F.; Wintergerst, E.; Deyaert, S.; Baudot, A.; Van den Abbeele, P. HMOs Exert Marked Bifidogenic Effects on Children’s Gut Microbiota Ex Vivo, Due to Age-Related Bifidobacterium Species Composition. Nutrients 2023, 15, 1701. [Google Scholar] [CrossRef] [PubMed]
- Van den Abbeele, P.; Deyaert, S.; Albers, R.; Baudot, A.; Mercenier, A. Carrot RG-I Reduces Interindividual Differences between 24 Adults through Consistent Effects on Gut Microbiota Composition and Function Ex Vivo. Nutrients 2023, 15, 2090. [Google Scholar] [CrossRef] [PubMed]
- Tintoré, M.; Cuñé, J.; Vu, L.D.; Poppe, J.; Van den Abbeele, P.; Baudot, A.; de Lecea, C. A Long-Chain Dextran Produced by Weissella Cibaria Boosts the Diversity of Health-Related Gut Microbes Ex Vivo. Biology 2024, 13, 51. [Google Scholar] [CrossRef] [PubMed]
- Van den Abbeele, P.; Detzel, C.; Rose, A.; Deyaert, S.; Baudot, A.; Warner, C. Serum-Derived Bovine Immunoglobulin Stimulates SCFA Production by Specific Microbes in the Ex Vivo SIFR® Technology. Microorganisms 2023, 11, 659. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST Static in Vitro Simulation of Gastrointestinal Food Digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Agarwal, K.; Maki, K.A.; Vizioli, C.; Carnell, S.; Goodman, E.; Hurley, M.; Harris, C.; Colwell, R.; Steele, K.; Joseph, P.V. The Neuro-Endo-Microbio-Ome Study: A Pilot Study of Neurobiological Alterations Pre- Versus Post-Bariatric Surgery. Biol. Res. Nurs. 2022, 24, 362–378. [Google Scholar] [CrossRef]
- Hasan, N.A.; Young, B.A.; Minard-Smith, A.T.; Saeed, K.; Li, H.; Heizer, E.M.; McMillan, N.J.; Isom, R.; Abdullah, A.S.; Bornman, D.M.; et al. Microbial Community Profiling of Human Saliva Using Shotgun Metagenomic Sequencing. PLoS ONE 2014, 9, e97699. [Google Scholar] [CrossRef]
- Doneanu, C.E. UPLC/MS Monitoring of Water-Soluble Vitamin Bs in Cell Culture Media in Minutes. Water Appl. Note 2011, 2011, 7. [Google Scholar]
- Adams, K.J.; Pratt, B.; Bose, N.; Dubois, L.G.; St John-Williams, L.; Perrott, K.M.; Ky, K.; Kapahi, P.; Sharma, V.; MacCoss, M.J.; et al. Skyline for Small Molecules: A Unifying Software Package for Quantitative Metabolomics. J. Proteome Res. 2020, 19, 1447–1458. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Kassambara, A. Rstatix: Pipe-Friendly Framework for Basic Statistical Tests; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Wickham, H.; Chang, W.; Henry, L.; Pedersen, T.L.; Takahashi, K.; Wilke, C.; Woo, K.; Yutani, H.; Dunnington, D. Ggplot2: Create Elegant Data Visualisations Using the Grammar of Graphics; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Costea, P.I.; Hildebrand, F.; Arumugam, M.; Bäckhed, F.; Blaser, M.J.; Bushman, F.D.; de Vos, W.M.; Ehrlich, S.D.; Fraser, C.M.; Hattori, M.; et al. Enterotypes in the Landscape of Gut Microbial Community Composition. Nat. Microbiol. 2018, 3, 8–16. [Google Scholar] [CrossRef] [PubMed]
- De Vuyst, L.; Moens, F.; Selak, M.; Rivière, A.; Leroy, F. Summer Meeting 2013: Growth and Physiology of Bifidobacteria. J. Appl. Microbiol. 2014, 116, 477–491. [Google Scholar] [CrossRef] [PubMed]
- Duranti, S.; Longhi, G.; Ventura, M.; van Sinderen, D.; Turroni, F. Exploring the Ecology of Bifidobacteria and Their Genetic Adaptation to the Mammalian Gut. Microorganisms 2020, 9, 8. [Google Scholar] [CrossRef] [PubMed]
- Vandeputte, D.; Falony, G.; Vieira-Silva, S.; Wang, J.; Sailer, M.; Theis, S.; Verbeke, K.; Raes, J. Prebiotic Inulin-Type Fructans Induce Specific Changes in the Human Gut Microbiota. Gut 2017, 66, 1968–1974. [Google Scholar] [CrossRef] [PubMed]
- Vacca, M.; Celano, G.; Calabrese, F.M.; Portincasa, P.; Gobbetti, M.; De Angelis, M. The Controversial Role of Human Gut Lachnospiraceae. Microorganisms 2020, 8, E573. [Google Scholar] [CrossRef]
- García-López, M.; Meier-Kolthoff, J.P.; Tindall, B.J.; Gronow, S.; Woyke, T.; Kyrpides, N.C.; Hahnke, R.L.; Göker, M. Analysis of 1,000 Type-Strain Genomes Improves Taxonomic Classification of Bacteroidetes. Front. Microbiol. 2019, 10, 2083. [Google Scholar] [CrossRef]
- Louis, P.; Flint, H.J. Formation of Propionate and Butyrate by the Human Colonic Microbiota. Environ. Microbiol. 2017, 19, 29–41. [Google Scholar] [CrossRef]
- Adolphus, K.; Van den Abbeele, P.; Poppe, J.; Deyaert, S.; Gravert, T.K.O.; Abrahamsson, A.; Baudot, A.; Laurie, I.; Karnik, K.; Risso, D. D-Allulose and Erythritol Increase Butyrate Production Due to a Selective Increase in Specific Gut Microbiota in Healthy Adults and Adults with Type 2 Diabetes Ex Vivo. Benef. Microbes, 2024; submitted. [Google Scholar]
- Fabiano, G.A.; Shinn, L.M.; Antunes, A.E.C. Relationship between Oat Consumption, Gut Microbiota Modulation, and Short-Chain Fatty Acid Synthesis: An Integrative Review. Nutrients 2023, 15, 3534. [Google Scholar] [CrossRef]
- Wang, K.; Liao, M.; Zhou, N.; Bao, L.; Ma, K.; Zheng, Z.; Wang, Y.; Liu, C.; Wang, W.; Wang, J.; et al. Parabacteroides Distasonis Alleviates Obesity and Metabolic Dysfunctions via Production of Succinate and Secondary Bile Acids. Cell Rep. 2019, 26, 222–235.e5. [Google Scholar] [CrossRef]
- Roberti, M.P.; Yonekura, S.; Duong, C.P.M.; Picard, M.; Ferrere, G.; Tidjani Alou, M.; Rauber, C.; Iebba, V.; Lehmann, C.H.K.; Amon, L.; et al. Chemotherapy-Induced Ileal Crypt Apoptosis and the Ileal Microbiome Shape Immunosurveillance and Prognosis of Proximal Colon Cancer. Nat. Med. 2020, 26, 919–931. [Google Scholar] [CrossRef]
- Cox, L.M.; Maghzi, A.H.; Liu, S.; Tankou, S.K.; Dhang, F.H.; Willocq, V.; Song, A.; Wasén, C.; Tauhid, S.; Chu, R.; et al. Gut Microbiome in Progressive Multiple Sclerosis. Ann. Neurol. 2021, 89, 1195–1211. [Google Scholar] [CrossRef] [PubMed]
- Gevers, D.; Kugathasan, S.; Denson, L.A.; Vázquez-Baeza, Y.; Van Treuren, W.; Ren, B.; Schwager, E.; Knights, D.; Song, S.J.; Yassour, M.; et al. The Treatment-Naive Microbiome in New-Onset Crohn’s Disease. Cell Host Microbe 2014, 15, 382–392. [Google Scholar] [CrossRef] [PubMed]
- Dey, N.; Soergel, D.A.; Repo, S.; Brenner, S.E. Association of Gut Microbiota with Post-Operative Clinical Course in Crohn’s Disease. BMC Gastroenterol. 2013, 13, 131. [Google Scholar] [CrossRef] [PubMed]
- Labbé, A.; Ganopolsky, J.G.; Martoni, C.J.; Prakash, S.; Jones, M.L. Bacterial Bile Metabolising Gene Abundance in Crohn’s, Ulcerative Colitis and Type 2 Diabetes Metagenomes. PLoS ONE 2014, 9, e115175. [Google Scholar] [CrossRef]
- Liu, S.; Li, E.; Sun, Z.; Fu, D.; Duan, G.; Jiang, M.; Yu, Y.; Mei, L.; Yang, P.; Tang, Y.; et al. Altered Gut Microbiota and Short Chain Fatty Acids in Chinese Children with Autism Spectrum Disorder. Sci. Rep. 2019, 9, 287. [Google Scholar] [CrossRef]
- Marzorati, M.; Bubeck, S.; Bayne, T.; Krishnan, K.; Young, A. Evaluation of the Effect of Food Products Containing Prebiotics and Bacillus Subtilis HU58 on the Gut Microbial Community Activity and Community Composition Using an In Vitro M-SHIME® Model. Appl. Sci. 2021, 11, 11963. [Google Scholar] [CrossRef]
- Van den Abbeele, P.; Belzer, C.; Goossens, M.; Kleerebezem, M.; De Vos, W.M.; Thas, O.; De Weirdt, R.; Kerckhof, F.-M.; Van de Wiele, T. Butyrate-Producing Clostridium Cluster XIVa Species Specifically Colonize Mucins in an in Vitro Gut Model. ISME J. 2013, 7, 949–961. [Google Scholar] [CrossRef]
- Chanin, R.B.; Winter, M.G.; Spiga, L.; Hughes, E.R.; Zhu, W.; Taylor, S.J.; Arenales, A.; Gillis, C.C.; Büttner, L.; Jimenez, A.G.; et al. Epithelial-Derived Reactive Oxygen Species Enable AppBCX-Mediated Aerobic Respiration of Escherichia Coli during Intestinal Inflammation. Cell Host Microbe 2020, 28, 780–788.e5. [Google Scholar] [CrossRef]
- Thanassi, D.G.; Cheng, L.W.; Nikaido, H. Active Efflux of Bile Salts by Escherichia Coli. J. Bacteriol. 1997, 179, 2512–2518. [Google Scholar] [CrossRef]
- Chalova, V.I.; Sirsat, S.A.; O’Bryan, C.A.; Crandall, P.G.; Ricke, S.C. Escherichia Coli, an Intestinal Microorganism, as a Biosensor for Quantification of Amino Acid Bioavailability. Sensors 2009, 9, 7038–7057. [Google Scholar] [CrossRef]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert Consensus Document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.D.; Ellermeier, C.D. Ferric Uptake Regulator Fur Control of Putative Iron Acquisition Systems in Clostridium Difficile. J. Bacteriol. 2015, 197, 2930–2940. [Google Scholar] [CrossRef]
- Ramezani Kapourchali, F.; Glueck, B.; Han, Y.; Shapiro, D.; Fulmer, C.G.; Cresci, G.A.M. A Spore-Forming Probiotic Supplement Improves the Intestinal Immune Response and Protects the Intestinal Health During Recurrent Clostridioides Difficile Colonization in Mice. J. Parenter. Enter. Nutr. 2020, 44, 1428–1438. [Google Scholar] [CrossRef] [PubMed]
- Fajardo-Cavazos, P.; Nicholson, W.L. Shelf Life and Simulated Gastrointestinal Tract Survival of Selected Commercial Probiotics During a Simulated Round-Trip Journey to Mars. Front. Microbiol. 2021, 12, 748950. [Google Scholar] [CrossRef]
- Hinnebusch, B.F.; Meng, S.; Wu, J.T.; Archer, S.Y.; Hodin, R.A. The Effects of Short-Chain Fatty Acids on Human Colon Cancer Cell Phenotype Are Associated with Histone Hyperacetylation. J. Nutr. 2002, 132, 1012–1017. [Google Scholar] [CrossRef]
- McDonald, J.A.K.; Mullish, B.H.; Pechlivanis, A.; Liu, Z.; Brignardello, J.; Kao, D.; Holmes, E.; Li, J.V.; Clarke, T.B.; Thursz, M.R.; et al. Inhibiting Growth of Clostridioides Difficile by Restoring Valerate, Produced by the Intestinal Microbiota. Gastroenterology 2018, 155, 1495–1507.e15. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.-H.; Xin, F.-Z.; Xue, Y.; Hu, Z.; Han, Y.; Ma, F.; Zhou, D.; Liu, X.-L.; Cui, A.; Liu, Z.; et al. Indole-3-Propionic Acid Inhibits Gut Dysbiosis and Endotoxin Leakage to Attenuate Steatohepatitis in Rats. Exp. Mol. Med. 2019, 51, 1–14. [Google Scholar] [CrossRef]
- Foster, A.C.; Kemp, J.A. Glutamate- and GABA-Based CNS Therapeutics. Curr. Opin. Pharmacol. 2006, 6, 7–17. [Google Scholar] [CrossRef]
- Strandwitz, P.; Kim, K.H.; Terekhova, D.; Liu, J.K.; Sharma, A.; Levering, J.; McDonald, D.; Dietrich, D.; Ramadhar, T.R.; Lekbua, A.; et al. GABA-Modulating Bacteria of the Human Gut Microbiota. Nat. Microbiol. 2019, 4, 396–403. [Google Scholar] [CrossRef]
- Wu, K.K.; Kuo, C.-C.; Yet, S.-F.; Lee, C.-M.; Liou, J.-Y. 5-Methoxytryptophan: An Arsenal against Vascular Injury and Inflammation. J. Biomed. Sci. 2020, 27, 79. [Google Scholar] [CrossRef]
- Schoefer, L.; Mohan, R.; Schwiertz, A.; Braune, A.; Blaut, M. Anaerobic Degradation of Flavonoids by Clostridium Orbiscindens. Appl. Environ. Microbiol. 2003, 69, 5849–5854. [Google Scholar] [CrossRef] [PubMed]
- Sheng, W.; Ji, G.; Zhang, L. Immunomodulatory Effects of Inulin and Its Intestinal Metabolites. Front. Immunol. 2023, 14, 1224092. [Google Scholar] [CrossRef] [PubMed]
- Wilson, B.; Rossi, M.; Dimidi, E.; Whelan, K. Prebiotics in Irritable Bowel Syndrome and Other Functional Bowel Disorders in Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Am. J. Clin. Nutr. 2019, 109, 1098–1111. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deyaert, S.; Poppe, J.; Dai Vu, L.; Baudot, A.; Bubeck, S.; Bayne, T.; Krishnan, K.; Giusto, M.; Moltz, S.; Van den Abbeele, P. Functional Muffins Exert Bifidogenic Effects along with Highly Product-Specific Effects on the Human Gut Microbiota Ex Vivo. Metabolites 2024, 14, 497. https://doi.org/10.3390/metabo14090497
Deyaert S, Poppe J, Dai Vu L, Baudot A, Bubeck S, Bayne T, Krishnan K, Giusto M, Moltz S, Van den Abbeele P. Functional Muffins Exert Bifidogenic Effects along with Highly Product-Specific Effects on the Human Gut Microbiota Ex Vivo. Metabolites. 2024; 14(9):497. https://doi.org/10.3390/metabo14090497
Chicago/Turabian StyleDeyaert, Stef, Jonas Poppe, Lam Dai Vu, Aurélien Baudot, Sarah Bubeck, Thomas Bayne, Kiran Krishnan, Morgan Giusto, Samuel Moltz, and Pieter Van den Abbeele. 2024. "Functional Muffins Exert Bifidogenic Effects along with Highly Product-Specific Effects on the Human Gut Microbiota Ex Vivo" Metabolites 14, no. 9: 497. https://doi.org/10.3390/metabo14090497
APA StyleDeyaert, S., Poppe, J., Dai Vu, L., Baudot, A., Bubeck, S., Bayne, T., Krishnan, K., Giusto, M., Moltz, S., & Van den Abbeele, P. (2024). Functional Muffins Exert Bifidogenic Effects along with Highly Product-Specific Effects on the Human Gut Microbiota Ex Vivo. Metabolites, 14(9), 497. https://doi.org/10.3390/metabo14090497






