Physicochemical Characterization of Moroccan Honey Varieties from the Fez-Meknes Region and Their Antioxidant and Antibacterial Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Sampling Physicochemical Analysis of Honey
2.2.1. Determining the Hydrogen Potential of Honey
2.2.2. Determination of Honey-Free Acidity
2.2.3. Determination of the Water Content and Brix Level of the Honey Studied
2.2.4. Determination of Honey Density
2.2.5. Determining the Ash Content of Honey
2.2.6. Determination of Electrical Conductivity
2.2.7. Determination of Hydroxymethylfurfural (HMF) Content
2.2.8. Determination of Honey Color
2.2.9. Determination of Glucose Content
2.2.10. Determination of Protein Content
2.3. Phytochemical Study of Honeys
2.3.1. Determination of Polyphenol Content
2.3.2. Determination of Flavonoid Content
2.4. Biological Study of Honeys
2.4.1. DPPH* Free-Radical-Scavenging Effect
2.4.2. Evaluation of Reducing Power (FRAP)
2.4.3. Determination of Antimicrobial Activity
2.5. Statistical Study
3. Results and Discussion
3.1. Physicochemical Analyses
3.1.1. Determination of Hydrogen Potential
3.1.2. Determining Free Acidity
3.1.3. Determining the Brix Value
3.1.4. Determination of Water Content
3.1.5. Determination of Honey Density
3.1.6. Determination of Ash Content
3.1.7. Determining Electrical Conductivity
3.1.8. Determination of Hydroxymethylfurfural (HMF) Content
3.1.9. Color Determination
3.2. Phytochemical Analysis
3.2.1. Determination of Polyphenol and Flavonoid Content
3.2.2. Determination of Glucose and Protein Content
3.3. Antioxidant Activity of Honey
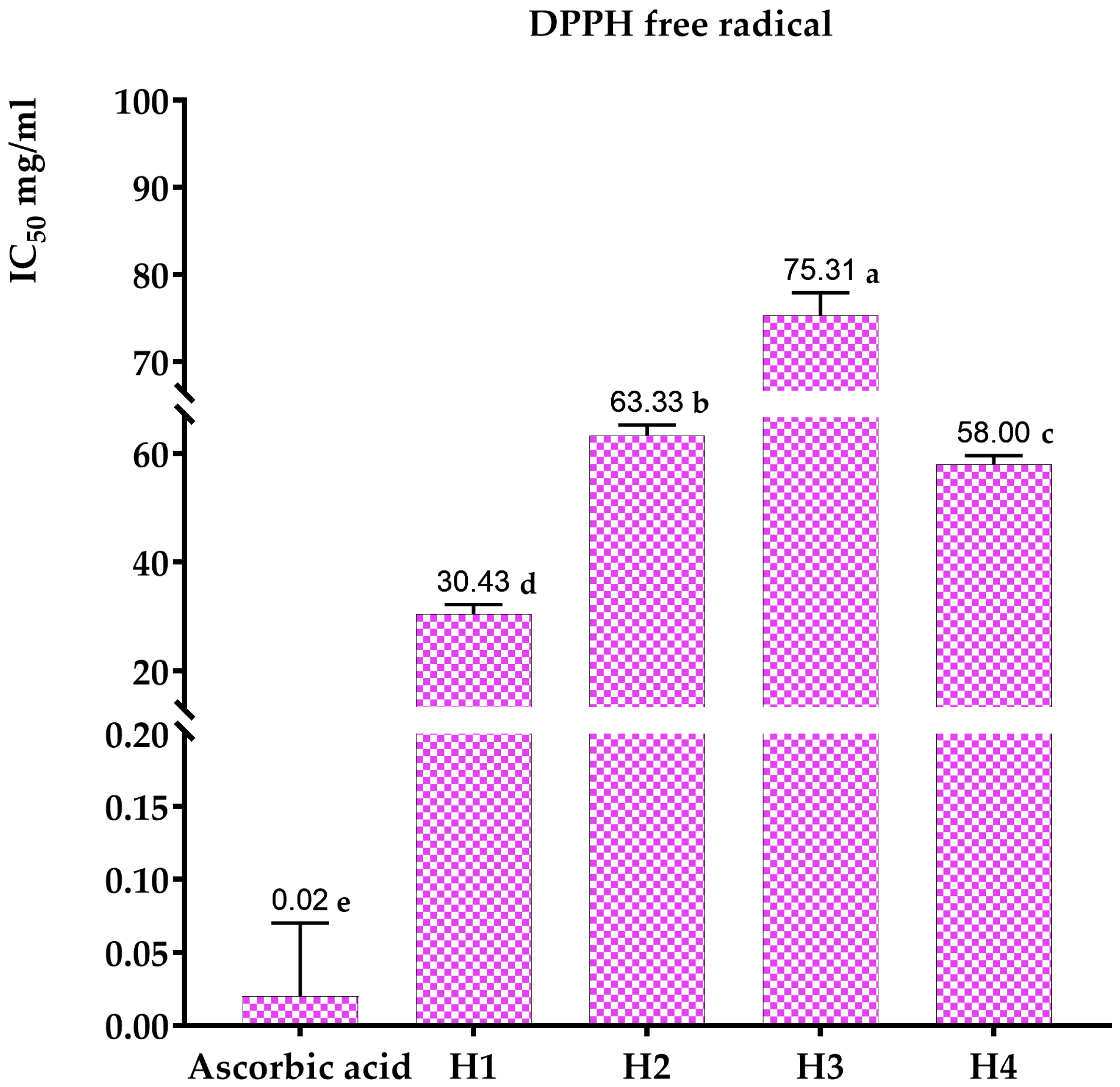
3.3.1. DPPH* Free-Radical-Scavenging Test
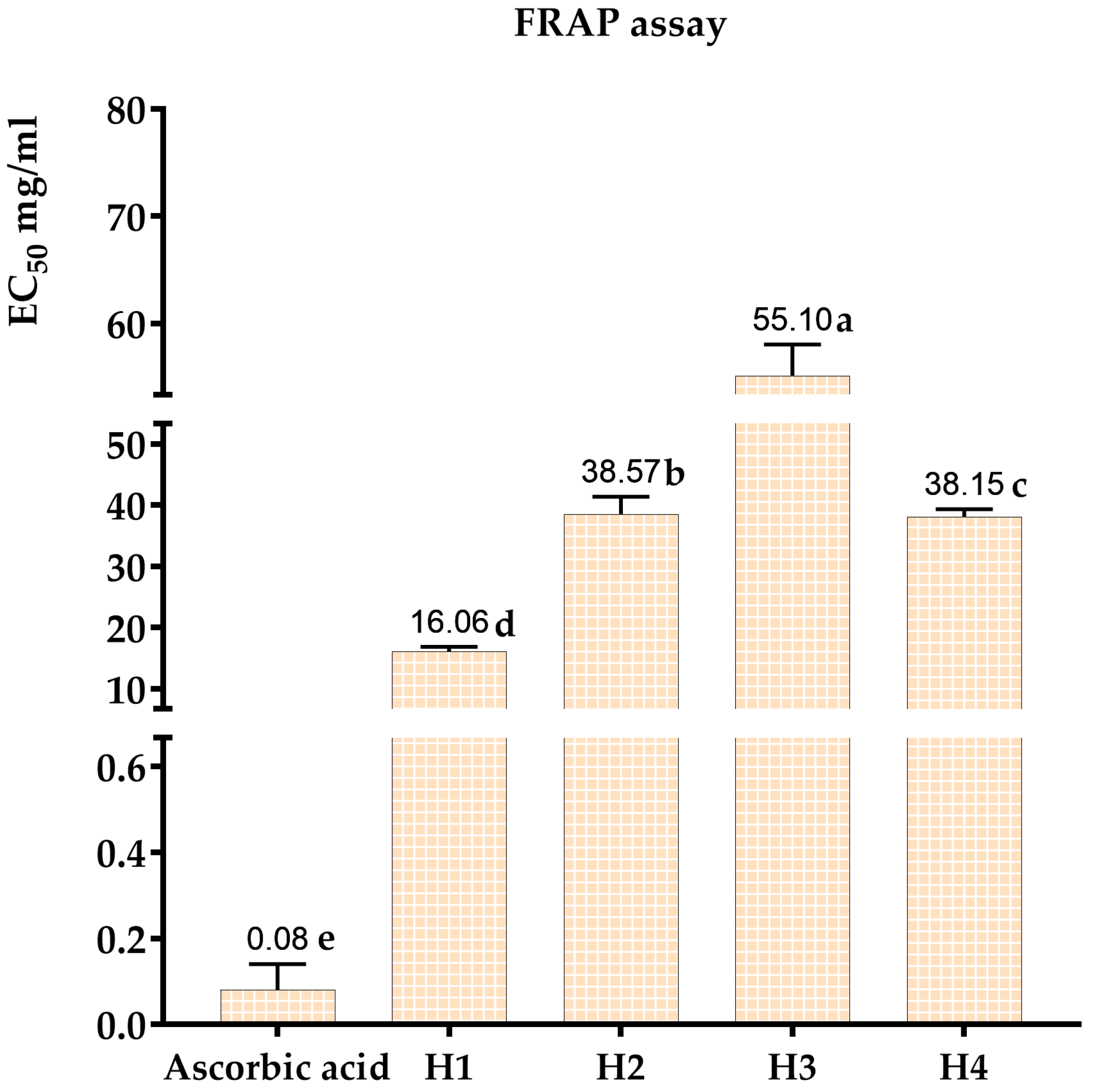
3.3.2. Evaluation of Antioxidant Activity by the FRAP Method
3.4. Study of Antimicrobial Activity
3.5. Pearson Correlation between Antioxidant Activity, Antimicrobial Activity, and the Polyphenol and Flavonoid Composition of Four Varieties of Honey
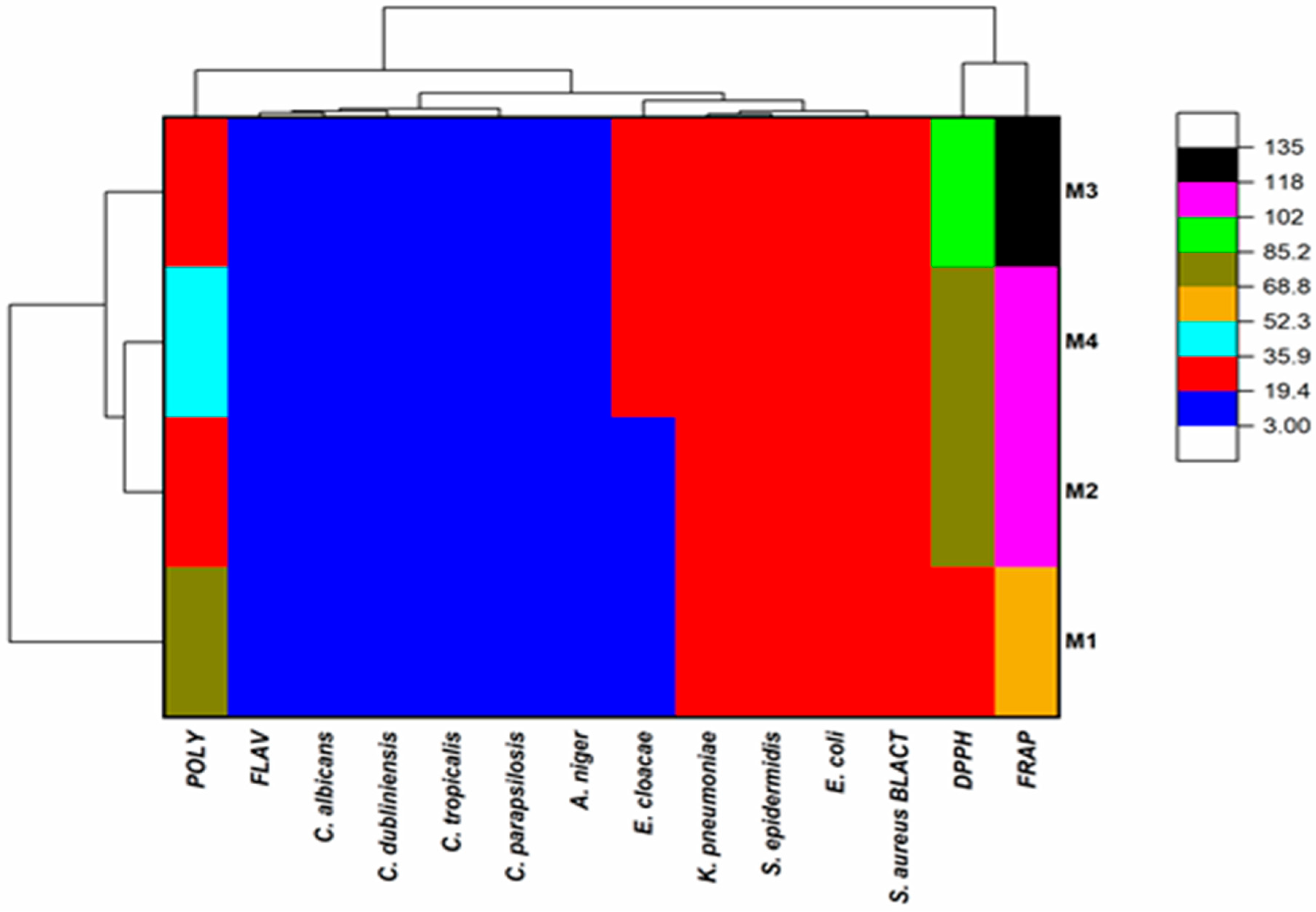
3.6. Heat Map with Clusters of the Honeys Studied According to Their Polyphenol and Flavonoid Composition and Their Biological Activities
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cianciosi, D.; Forbes-Hernández, T.Y.; Afrin, S.; Gasparrini, M.; Reboredo-Rodriguez, P.; Manna, P.P.; Zhang, J.; Bravo Lamas, L.; Martínez Flórez, S.; Agudo Toyos, P.; et al. Phenolic Compounds in Honey and Their Associated Health Benefits: A Review. Molecules 2018, 23, 2322. [Google Scholar] [CrossRef]
- Uttara, B.; Singh, A.V.; Zamboni, P.; Mahajan, R.T. Oxidative Stress and Neurodegenerative Diseases: A Review of Upstream and Downstream Antioxidant Therapeutic Options. Curr. Neuropharmacol. 2009, 7, 65–74. [Google Scholar] [CrossRef]
- Scalbert, A.; Manach, C.; Morand, C.; Rémésy, C.; Jiménez, L. Dietary Polyphenols and the Prevention of Diseases. Crit. Rev. Food Sci. Nutr. 2005, 45, 287–306. [Google Scholar] [CrossRef]
- Han, X.; Shen, T.; Lou, H. Dietary Polyphenols and Their Biological Significance. Int. J. Mol. Sci. 2007, 8, 950–988. [Google Scholar] [CrossRef]
- Bueno-Costa, F.M.; Zambiazi, R.C.; Bohmer, B.W.; Chaves, F.C.; da Silva, W.P.; Zanusso, J.T.; Dutra, I. Antibacterial and Antioxidant Activity of Honeys from the State of Rio Grande Do Sul, Brazil. LWT Food Sci. Technol. 2016, 65, 333–340. [Google Scholar] [CrossRef]
- Gül, A.; Pehlivan, T. Antioxidant Activities of Some Monofloral Honey Types Produced across Turkey. Saudi J. Biol. Sci. 2018, 25, 1056–1065. [Google Scholar] [CrossRef]
- Olas, B. Honey and Its Phenolic Compounds as an Effective Natural Medicine for Cardiovascular Diseases in Humans? Nutrients 2020, 12, 283. [Google Scholar] [CrossRef]
- Doukani, K.; Souhila, T.; Asma, D.; Zahira, H. Etude Physicochimique et Phytochimique de Quelques Types de Miels Algériens. Rev. Ecol.-Environ. 2014, 10, 1–13. [Google Scholar]
- Balas, F. Les propriétés thérapeutiques du miel et leurs domaines d’application en médecine générale: Revue de la littérature. 2015, 85. Available online: https://core.ac.uk/download/pdf/52773796.pdf (accessed on 11 December 2023).
- Imtara, H.; Elamine, Y.; Lyoussi, B. Honey Antibacterial Effect Boosting Using Origanum Vulgare L. Essential Oil. Evid.-Based Complement. Altern. Med. ECAM 2018, 2018, 7842583. [Google Scholar] [CrossRef]
- Kaznowski, A.; Szymas, B.; Jazdzinska, E.; Kazimierczak, M.; Paetz, H.; Mokracka, J. The Effects of Probiotic Supplementation on the Content of Intestinal Microflora and Chemical Composition of Worker Honey Bees (Apis mellifera). J. Apic. Res. 2005, 44, 10–14. [Google Scholar] [CrossRef]
- Saranraj, P.; Sivasakthi, S. Comprehensive Review on Honey: Biochemical and Medicinal Properties. J. Acad. Ind. Res. 2018, 6, 165–178. [Google Scholar]
- Gholizadeh, H.; Ghaffarifar, F.; Dalimi, A.; Dayer, M.S. In Vitro and in Vivo Effects of Natural Honey on Leishmania Major. Ann. Parasitol. 2022, 68, 71–76. [Google Scholar] [CrossRef]
- Zeina, B.; Othman, O.; al-Assad, S. Effect of Honey versus Thyme on Rubella Virus Survival in Vitro. J. Altern. Complement. Med. 1996, 2, 345–348. [Google Scholar] [CrossRef]
- Ghapanchi, J.; Moattari, A.; Andisheh Tadbir, A.; Talatof, Z.; Shahidi, S.P.; Ebrahimi, H. The In Vitro Anti-Viral Activity of Honey on Type 1 Herpes Simplex Virus. Aust. J. Basic Appl. Sci. 2011, 5, 849–852. [Google Scholar]
- Grabek-Lejko, D.; Miłek, M.; Sidor, E.; Puchalski, C.; Dżugan, M. Antiviral and Antibacterial Effect of Honey Enriched with Rubus Spp. as a Functional Food with Enhanced Antioxidant Properties. Molecules 2022, 27, 4859. [Google Scholar] [CrossRef]
- Almeida-Muradian, L.; Barth, O.; Dietemann, V.; Eyer, M.; Freitas, A.; Martel, A.-C.; Marcazzan, G.; Marchese, C.; Mucignat-Caretta, C.; Pascual Maté, A.; et al. Standard Methods for Apis Mellifera Honey Research. J. Apic. Res. 2020, 59, 1–62. [Google Scholar] [CrossRef]
- Donkersley, P.; Rhodes, G.; Pickup, R.W.; Jones, K.C.; Power, E.F.; Wright, G.A.; Wilson, K. Nutritional Composition of Honey Bee Food Stores Vary with Floral Composition. Oecologia 2017, 185, 749–761. [Google Scholar] [CrossRef]
- da Silva, P.M.; Gauche, C.; Gonzaga, L.V.; Costa, A.C.O.; Fett, R. Honey: Chemical Composition, Stability and Authenticity. Food Chem. 2016, 196, 309–323. [Google Scholar] [CrossRef]
- Valverde, S.; Ares, A.M.; Stephen Elmore, J.; Bernal, J. Recent Trends in the Analysis of Honey Constituents. Food Chem. 2022, 387, 132920. [Google Scholar] [CrossRef]
- Liu, T.; Ming, K.; Wang, W.; Qiao, N.; Qiu, S.; Yi, S.; Huang, X.; Luo, L. Discrimination of Honey and Syrup-Based Adulteration by Mineral Element Chemometrics Profiling. Food Chem. 2021, 343, 128455. [Google Scholar] [CrossRef]
- Rossant, A. Le Miel: Un Composé Complexe Aux Propriétés Surprenantes = Honey, a Complex Compound with Surprising Properties. Ph.D. Thesis, Université de Limoges, Limoges, France, 2011. [Google Scholar]
- De-Melo, A.; Almeida-Muradian, L.; Sancho, M.; Pascual Maté, A. Composition and Properties of Apis Mellifera Honey: A Review. J. Apic. Res. 2017, 57, 1–33. [Google Scholar] [CrossRef]
- Chirife, J.; Zamora, M.C.; Motto, A. The Correlation between Water Activity and % Moisture in Honey: Fundamental Aspects and Application to Argentine Honeys. J. Food Eng. 2006, 72, 287–292. [Google Scholar] [CrossRef]
- Mijanur Rahman, M.; Gan, S.H.; Khalil, M.d.I. Neurological Effects of Honey: Current and Future Prospects. Evid.-Based Complement. Altern. Med. ECAM 2014, 2014, 958721. [Google Scholar] [CrossRef]
- El-Sohaimy, S.; Masry, S.; Shehata, M. Physicochemical Characteristics of Honey from Different Origins. Ann. Agric. Sci. 2015, 60, 279–287. [Google Scholar] [CrossRef]
- Bogdanov, S.; Martin, P.; Lüllmann, C. Harmonised Methods of the European Honey Com-Mission. Apidologie Extra 1997, 28, 53–55. [Google Scholar]
- Anklam, E. A Review of the Analytical Methods to Determine the Geographical and Botanical Origin of Honey. Food Chem. 1998, 63, 549–562. [Google Scholar] [CrossRef]
- Bouhlali, E.D.T.; Bammou, M.; Sellam, K.; El Midaoui, A.; Bourkhis, B.; Ennassir, J.; Alem, C.; Filali-Zegzouti, Y. Physicochemical Properties of Eleven Monofloral Honey Samples Produced in Morocco. Arab J. Basic Appl. Sci. 2019, 26, 476–487. [Google Scholar] [CrossRef]
- White, J.W. Honey. In Advances in Food Research; Chichester, C.O., Ed.; Academic Press: Cambridge, MA, USA, 1978; Volume 24, pp. 287–374. [Google Scholar]
- Kocyigit, A.; Aydogdu, G.; Balkan, E.; Yenigun, V.B.; Guler, E.M.; Bulut, H.; Koktasoglu, F.; Gören, A.C.; Atayoglu, A.T. Quercus Pyrenaica Honeydew Honey With High Phenolic Contents Cause DNA Damage, Apoptosis, and Cell Death Through Generation of Reactive Oxygen Species in Gastric Adenocarcinoma Cells. Integr. Cancer Ther. 2019, 18, 1534735419876334. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. [14] Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. In Methods in Enzymology; Oxidants and Antioxidants Part A; Academic Press: Cambridge, MA, USA, 1999; Volume 299, pp. 152–178. [Google Scholar]
- Hung, P.V.; Maeda, T.; Miyatake, K.; Morita, N. Total Phenolic Compounds and Antioxidant Capacity of Wheat Graded Flours by Polishing Method. Food Res. Int. 2009, 42, 185–190. [Google Scholar] [CrossRef]
- Djeridane, A.; Yousfi, M.; Nadjemi, B.; Boutassouna, D.; Stocker, P.; Vidal, N. Antioxidant Activity of Some Algerian Medicinal Plants Extracts Containing Phenolic Compounds. Food Chem. 2006, 97, 654–660. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Zovko Končić, M.; Kremer, D.; Karlović, K.; Kosalec, I. Evaluation of Antioxidant Activities and Phenolic Content of Berberis Vulgaris L. and Berberis Croatica Horvat. Food Chem. Toxicol. 2010, 48, 2176–2180. [Google Scholar] [CrossRef] [PubMed]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in Vitro Evaluating Antimicrobial Activity: A Review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Julika, W.N.; Ajit, A.; Sulaiman, A.Z.; Naila, A. Physicochemical and Microbiological Analysis of Stingless Bees Honey Collected from Local Market in Malaysia. Indones. J. Chem. 2019, 19, 522–530. [Google Scholar] [CrossRef]
- EUCAST EUCAST. Breakpoint Tables for Interpretation of MICs and Zone Diameters, Ver. 13.0; European Committee on Antimicrobial Susceptibility Testing: Växjö, Sweden, 2023; Available online: https://www.eucast.org/fileadmin/src/media/pdfs/eucast_files/breakpoint_tables/v_13.0_breakpoint_tables.pdf (accessed on 11 December 2023).
- EUCAST Guidelines for Detection of Resistance Mechanisms and Specific Resistances of Clinical and/or Epidemiological Importance, Ver. 2.0; European Committee on Antimicrobial Susceptibility Testing: Växjö, Sweden, 2017; Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Resistance_mechanisms/EUCAST_detection_of_resistance_mechanisms_170711.pdf (accessed on 11 December 2023).
- Food and Agriculture Organization of the United Nations; World Health Organization. Joint FAO/WHO Food Standard Programme Codex Alimentarius Commission. In Proceedings of the Twenty-Fourth Session, Geneva, Switzerland, 2–7 July 2001. Codex 2001. [Google Scholar]
- Naman Malika, N.M.; Faid Mohamed, F.M.; El-Adlouni Chakib, E.-A.C. Microbiological and Physico-Chemical Properties of Moroccan Honey. Int. J. Agric. Biol. 2005, 7, 773–776. [Google Scholar]
- Iritie, B.M.; Wandan, E.N.; Yapo, M.Y.; Fantodji, A.; Bodji, N.C. Comparative Analysis of Physico-Chemical Characteristics of Honeys Produced in the Multi-Floral Arboretum of the National School of Agronomy of Yamoussoukro. Int. J. Agric. Policy Res. 2014, 2, 379–382. [Google Scholar]
- Kouamé, K.F.; Gbouhoury, E.K.B.; Fofié, N.B.Y.; Kassi, N.J. Caractéristiques Physicochimiques Récoltés Des Miels De La Sous-Préfecture De Cechi (Dans Le Département D’agboville, Côte D’ivoire). Eur. Sci. J. ESJ 2021, 17, 286–300. [Google Scholar]
- Bakchiche, B.; Habati, M.; Benmebarek, A.; Gherib, A. Caractéristiques Physico-Chimiques, Concentrations des Composés Phénoliques et Pouvoir Antioxydant de Quatre Variétés de Miels Locales (Algérie). Rev. Marocaine Sci. Agron. Vét. 2018, 6, 118–123. [Google Scholar]
- Chen, C. Relationship between Water Activity and Moisture Content in Floral Honey. Foods 2019, 8, 30. [Google Scholar] [CrossRef]
- Ouchemoukh, S.; Louaileche, H.; Schweitzer, P. Physicochemical Characteristics and Pollen Spectrum of Some Algerian Honeys. Food Control 2007, 18, 52–58. [Google Scholar] [CrossRef]
- Jean-Prost, P. Apiculture; Connaitre l’abeille, Conduire Le Rucher; Paris (France) Technique et Documentation (Lavoisier): Paris, France, 1987. [Google Scholar]
- Fechner, D.C.; Moresi, A.L.; Díaz, J.D.R.; Pellerano, R.G.; Vazquez, F.A. Multivariate Classification of Honeys from Corrientes (Argentina) According to Geographical Origin Based on Physicochemical Properties. Food Biosci. 2016, 15, 49–54. [Google Scholar] [CrossRef]
- Imtara, H.; Elamine, Y.; Lyoussi, B. Physicochemical Characterization and Antioxidant Activity of Palestinian Honey Samples. Food Sci. Nutr. 2018, 6, 2056–2065. [Google Scholar] [CrossRef] [PubMed]
- Al-Mamary, M.A. Antioxidant Activity of Commonly Consumed Vegetables in Yemen. Malays. J. Nutr. 2002, 8, 179–189. [Google Scholar] [PubMed]
- Perna, A.; Simonetti, A.; Intaglietta, I.; Sofo, A.; Gambacorta, E. Metal Content of Southern Italy Honey of Different Botanical Origins and Its Correlation with Polyphenol Content and Antioxidant Activity. Int. J. Food Sci. Technol. 2012, 47, 1909–1917. [Google Scholar] [CrossRef]
- Bouyahya, A.; Dakka, N.; Talbaoui, A.; Moussaoui, N.E.; Abrini, J.; Bakri, Y. Phenolic Contents and Antiradical Capacity of Vegetable Oil from Pistacia lentiscus (L). J. Mater. Environ. Sci. 2018, 9, 1518–1524. [Google Scholar]
- Šarić, G.; Marković, K.; Major, N.; Krpan, M.; Uršulin-Trstenjak, N.; Hruškar, M.; Vahčić, N. Changes of Antioxidant Activity and Phenolic Content in Acacia and Multifloral Honey During Storage. Food Technol. Biotechnol. 2012, 50, 434–441. [Google Scholar]
- Kadri, S.M.; Zaluski, R.; Lima, G.P.P.; Mazzafera, P.; de Oliveira Orsi, R. Characterization of Coffea Arabica Monofloral Honey from Espírito Santo, Brazil. Food Chem. 2016, 203, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Habib, H.M.; Al Meqbali, F.T.; Kamal, H.; Souka, U.D.; Ibrahim, W.H. Physicochemical and Biochemical Properties of Honeys from Arid Regions. Food Chem. 2014, 153, 35–43. [Google Scholar] [CrossRef]
- Benhamou, N.; Rey, P. Stimulateurs Des Défenses Naturelles Des Plantes: Une Nouvelle Stratégie Phytosanitaire Dans Un Contexte d’écoproduction Durable. I. Principes de La Résistance Induite. Phytoprotection 2012, 92, 1–23. [Google Scholar] [CrossRef]
- Gonnet, M. Le Miel. Composition Propriétés et Conservation, 2nd ed.; Opida: Montigny-Le-Bretonneux, France, 1982; 31p. [Google Scholar]
- White, J.W., Jr.; Riethof, M.L.; Subers, M.H.; Kushnir, I. Composition of American Honeys; Technical Bulletin 1261; Agricultural Research Service (ARS): Washington, DC, USA, 1962.
- Somerville, D. Fat Bees Skinny Bees; A Manual on Honey Bee Nutrition for Beekeepers; Australian Government Rural Industries Research and Development Corporation: Goulburn, Australia, 2005; pp. 1–142.
- Kanoun, K. Contribution à l’étude Phytochimique et Activité Antioxydante Des Extraits de Myrtus communis L. (Rayhane) de La Région de Tlemcen (Honaine). Mém. Magister Univ. Aboubekr Belkaid–Tlemcen 2011, 2, 1–118. [Google Scholar]
- Can, Z.; Yildiz, O.; Sahin, H.; Turumtay, E.A.; Silici, S.; Kolayli, S. An Investigation of Turkish Honeys: Their Physico-Chemical Properties, Antioxidant Capacities and Phenolic Profiles. Food Chem. 2015, 180, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Abdellah, F. Antimicrobial Properties of Natural Honey. In Melittology-New Advances; IntechOpen: London, UK, 2023; ISBN 978-1-83769-370-2. [Google Scholar]
- Al-Kafaween, M.A.; Alwahsh, M.; Mohd Hilmi, A.B.; Abulebdah, D.H. Physicochemical Characteristics and Bioactive Compounds of Different Types of Honey and Their Biological and Therapeutic Properties: A Comprehensive Review. Antibiotics 2023, 12, 337. [Google Scholar] [CrossRef]
- Halawani, E.; Shohayeb, M. Survey of the Antibacterial Activity of Saudi and Some International Honeys. J. Microbiol. Antimicrob. 2011, 3, 94–101. [Google Scholar]
- Moussa, A.; Noureddine, D.; Saad, A.; Abdelmelek, M.; Abdelkader, B. Antifungal Activity of Four Honeys of Different Types from Algeria against Pathogenic Yeast: Candida albicans and Rhodotorula sp. Asian Pac. J. Trop. Biomed. 2012, 2, 554–557. [Google Scholar] [CrossRef] [PubMed]
- Estevinho, M.L.; Afonso, S.E.; Feás, X. Antifungal Effect of Lavender Honey against Candida Albicans, Candida Krusei and Cryptococcus Neoformans. J. Food Sci. Technol. 2011, 48, 640–643. [Google Scholar] [CrossRef]
- Candiracci, M.; Citterio, B.; Diamantini, G.; Blasa, M.; Accorsi, A.; Piatti, E. Honey Flavonoids, Natural Antifungal Agents Against Candida Albicans. Int. J. Food Prop. 2011, 14, 799–808. [Google Scholar] [CrossRef]
- Anyanwu, C. Investigation of in Vitro Antifungal Activity of Honey. J. Med. Plants Res. 2012, 6, 3512–3516. [Google Scholar] [CrossRef]
- Henriques, A.; Jackson, S.; Cooper, R.; Burton, N. Free Radical Production and Quenching in Honeys with Wound Healing Potential. J. Antimicrob. Chemother. 2006, 58, 773–777. [Google Scholar] [CrossRef]
- Sherlock, O.; Dolan, A.; Athman, R.; Power, A.; Gethin, G.; Cowman, S.; Humphreys, H. Comparison of the Antimicrobial Activity of Ulmo Honey from Chile and Manuka Honey against Methicillin-Resistant Staphylococcus aureus, Escherichia coli and Pseudomonas aeruginosa. BMC Complement. Altern. Med. 2010, 10, 47. [Google Scholar] [CrossRef]
- İzol, E. Phytochemicals in Honey and Health Effects. 2023; pp. 85–96. ISBN 9786256598034. 2024. Available online: https://www.researchgate.net/profile/Ebubekir-Izol/publication/374873469_Phytochemicals_in_Honey_and_Health_Effects/links/653369691d6e8a70703fef7a/Phytochemicals-in-Honey-and-Health-Effects.pdf (accessed on 11 December 2023).
- Bernklau, E.; Bjostad, L.; Hogeboom, A.; Carlisle, A.; Seshadri, A. Dietary Phytochemicals, Honey Bee Longevity and Pathogen Tolerance. Insects 2019, 10, 14. [Google Scholar] [CrossRef] [PubMed]
- Zammit Young, G.-W.; Blundell, R. A Review on the Phytochemical Composition and Health Applications of Honey. Heliyon 2023, 9, e12507. [Google Scholar] [CrossRef] [PubMed]
- Khalil, M.L.; Sulaiman, S.A. The Potential Role of Honey and Its Polyphenols in Preventing Heart Disease: A Review. Afr. J. Tradit. Complement. Altern. Med. 2010, 7, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Schramm, D.D.; Karim, M.; Schrader, H.R.; Holt, R.R.; Cardetti, M.; Keen, C.L. Honey with High Levels of Antioxidants Can Provide Protection to Healthy Human Subjects. J. Agric. Food Chem. 2003, 51, 1732–1735. [Google Scholar] [CrossRef] [PubMed]
- Ayoub, W.S.; Ritu; Zahoor, I.; Dar, A.H.; Farooq, S.; Mir, T.A.; Ganaie, T.A.; Srivastava, S.; Pandey, V.K.; Altaf, A. Exploiting the Polyphenolic Potential of Honey in the Prevention of Chronic Diseases. Food Chem. Adv. 2023, 3, 100373. [Google Scholar] [CrossRef]
- Uthurry, C.; Hevia, D.; Gomez-Cordoves, C. Role of Honey Polyphenols in Health. J. ApiProduct ApiMedical Sci. 2011, 3, 141–159. [Google Scholar] [CrossRef]
- Combarros-Fuertes, P.; Fresno, J.M.; Estevinho, M.M.; Sousa-Pimenta, M.; Tornadijo, M.E.; Estevinho, L.M. Honey: Another Alternative in the Fight against Antibiotic-Resistant Bacteria? Antibiotics 2020, 9, 774. [Google Scholar] [CrossRef] [PubMed]
- Almasaudi, S. The Antibacterial Activities of Honey. Saudi J. Biol. Sci. 2021, 28, 2188–2196. [Google Scholar] [CrossRef]
- Cebrero, G.; Sanhueza, O.; Pezoa, M.; Báez, M.; Martínez, J.; Báez, M.; Fuentes, E. Relationship among the Minor Constituents, Antibacterial Activity and Geographical Origin of Honey: A Multifactor Perspective. Food Chem. 2020, 315, 126296. [Google Scholar] [CrossRef]
- Pyrzynska, K.; Biesaga, M. Analysis of Phenolic Acids and Flavonoids in Honey. TrAC Trends Anal. Chem. 2009, 28, 893–902. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Yung An, C.; Rao, P.V.; Hawlader, M.N.I.; Azlan, S.A.B.M.; Sulaiman, S.A.; Gan, S.H. Identification of Phenolic Acids and Flavonoids in Monofloral Honey from Bangladesh by High Performance Liquid Chromatography: Determination of Antioxidant Capacity. BioMed Res. Int. 2014, 2014, e737490. [Google Scholar] [CrossRef]
- Beretta, G.; Granata, P.; Ferrero, M.; Orioli, M.; Maffei Facino, R. Standardization of Antioxidant Properties of Honey by a Combination of Spectrophotometric/Fluorimetric Assays and Chemometrics. Anal. Chim. Acta 2005, 533, 185–191. [Google Scholar] [CrossRef]
- Gheldof, N.; Wang, X.-H.; Engeseth, N.J. Identification and Quantification of Antioxidant Components of Honeys from Various Floral Sources. J. Agric. Food Chem. 2002, 50, 5870–5877. [Google Scholar] [CrossRef]
- Stefanis, C.; Stavropoulou, E.; Giorgi, E.; Voidarou, C.; Constantinidis, T.; Vrioni, G.; Tsakris, A. Honey’s Antioxidant and Antimicrobial Properties: A Bibliometric Study. Antioxidants 2023, 12, 414. [Google Scholar] [CrossRef]
- Alvarez-Suarez, J.M.; Gasparrini, M.; Forbes-Hernández, T.Y.; Mazzoni, L.; Giampieri, F. The Composition and Biological Activity of Honey: A Focus on Manuka Honey. Foods 2014, 3, 420–432. [Google Scholar] [CrossRef]
- Viuda-Martos, M.; Ruiz-Navajas, Y.; Fernández-López, J.; Pérez-Álvarez, J.A. Antibacterial Activity of Different Essential Oils Obtained from Spices Widely Used in Mediterranean Diet. Int. J. Food Sci. Technol. 2008, 43, 526–531. [Google Scholar] [CrossRef]
- Alvarez-Suarez, J.M.; Tulipani, S.; Díaz, D.; Estevez, Y.; Romandini, S.; Giampieri, F.; Damiani, E.; Astolfi, P.; Bompadre, S.; Battino, M. Antioxidant and Antimicrobial Capacity of Several Monofloral Cuban Honeys and Their Correlation with Color, Polyphenol Content and Other Chemical Compounds. Food Chem. Toxicol. 2010, 48, 2490–2499. [Google Scholar] [CrossRef]
- Boukraâ, L. Honey in Traditional and Modern Medicine; CRC Press: Boca Raton, FL, USA, 2023. [Google Scholar]
- Küçük, M.; Kolaylı, S.; Karaoğlu, Ş.; Ulusoy, E.; Baltacı, C.; Candan, F. Biological Activities and Chemical Composition of Three Honeys of Different Types from Anatolia. Food Chem. 2007, 100, 526–534. [Google Scholar] [CrossRef]
- Al-Mamary, M.; Al-Meeri, A.; Al-Habori, M. Antioxidant Activities and Total Phenolics of Different Types of Honey. Nutr. Res. 2002, 22, 1041–1047. [Google Scholar] [CrossRef]
- Patouna, A.; Vardakas, P.; Skaperda, Z.; Spandidos, D.A.; Kouretas, D. Evaluation of the Antioxidant Potency of Greek Honey from the Taygetos and Pindos Mountains Using a Combination of Cellular and Molecular Methods. Mol. Med. Rep. 2023, 27, 54. [Google Scholar] [CrossRef]
- Derwich, E.; Benziane, Z.; Boukir, A. Chemical Compositions and Insectisidal Activity of Essential Oils of Three Plants Artemisia Sp: Artemisia Herba-Alba, Artemisia Absinthium and Artemisia Pontica (Morocco). Electron. J. Environ. Agric. Food Chem. 2009, 8, 1202. [Google Scholar]
- François, E.A.; Bertin, G.; Armand, P.; Durand, M.D.-N.; Elvire, G.; Farid, B.-M.; Madjid, A.; Latifou, L.; Victorien, D.; Lamine, B.-M. Polyphenolic Profile, and Antioxidant and Antifungal Activities of Honey Products in Benin. Afr. J. Microbiol. Res. 2018, 12, 9–18. [Google Scholar] [CrossRef]
- Mohammed, S.E.A.; El-Niweiri, M.; AL-Shehri, B.; Ghramh, H.; Khan, K.A.; Elimam, M.; Kabbashi, A.; Koko, W. Antimicrobial, Antioxidant and Cytotoxic Properties of Four Types of Honey as Related to Their Phenolic and Flavonoid Contents. Pharmacol. Clin. Pharm. Res. 2024, 2024012236. [Google Scholar] [CrossRef]






| Sample Coding | Botanical Origin | Localization | Coordinates | Harvest Date |
|---|---|---|---|---|
| H1 | Multi flower | Zaouia Ifrane | 33°31′60″ North 5°7′0″ West | July 2021 |
| H2 | Jujube | Bouderbala Meknes | 33°49′0″ North 5°16′33″ West | July 2021 |
| H3 | Carob | Moulay Driss Zerhoun | 34°3′15.00″ North 5°31′38.00″ West | July 2021 |
| H4 | Rosemary | Oulad Ali Youssef Boulemane | 33°23′43″ North 3°34′53″ West | July 2021 |
| Bacterial Strains | References | Fungal Strains | References |
|---|---|---|---|
| Enterobacter cloacae | 02EV317 | Candida albicans | Ca |
| Klebsiella pneumoniae | 3DT1823 | Candida dubliniensis | Cd |
| Escherichia coli sauvage | 3DT1938 | Candida tropicalis | Ct |
| Staphylococcus aureus BLACT | 4IH2510 | Candida parapsilosis | Cpa |
| Staphylococcus epidermidis | 5994 | Aspergillus niger | AspN |
| Ref. | pH | Free Acidity (meq/kg) | Brix Degree (%) | Water Content (%) | Density at 20 °C (g/mL) | Ash Content (%) | Conductivity (mS/cm) | Hydroxymethylfurfurale HMF (mg/Kg) | Color Intensity, ABS450 (mAU, 50 w/v) |
|---|---|---|---|---|---|---|---|---|---|
| H1 | 3.34 ± 0.02 | 13 ± 0.10 | 80.2 ± 0.38 | 19.8 ± 0.38 | 1.412 ± 0.01 | 0.29 ± 0.01 | 0.199 ± 0.01 | 37.5 ± 0.24 | 1202 ± 8 |
| H2 | 3.09 ± 0.01 | 11 ± 0.30 | 81.2 ± 0.46 | 18.8 ± 0.31 | 1.421 ± 0.01 | 0.6 ± 0.01 | 0.473 ± 0.01 | 35.5 ± 0.26 | 622 ± 6 |
| H3 | 3.51 ± 0.02 | 8 ± 0.10 | 82.1 ± 0.41 | 17.9 ± 0.42 | 1.437 ± 0.01 | 0.59 ± 0.01 | 0.372 ± 0.01 | 30.34 ± 0.44 | 558 ± 4 |
| H4 | 2.91 ± 0.01 | 9 ± 0.20 | 81.2 ± 0.31 | 18.8 ± 0.36 | 1.426 ± 0.01 | 0.31 ± 0.01 | 0.256 ± 0.01 | 48.65 ± 0.51 | 313 ± 3 |
| Codex Alimentarius standards 2001siem [42] | Nectar honey: [3.5–4.5]. Honeydew honey: [5–5.5] | ˂50 | - | ≤20 | [1.39–1.44] | Nectar honey: ≤0.6 | Nectar honey: ≤0.8. Honeydew honey: >0.8 | ˂40 | - |
| Strains | Antibiotics * | Antifungals # | ||||
|---|---|---|---|---|---|---|
| Gentamycin | Amoxicillin–Clavulanate | Vancomycin | Trimethoprim-Sulfamethoxazole | Terbinafine | ||
| Bacteria | S. epidermidis | 2 | >8 | >4/76 | ||
| S. aureus BLACT | <0.5 | 2 | <10 | |||
| E. coli | 2 | 8/2 | ≤1/19 | |||
| E. cloacae | >4 | >8/2 | >4/76 | |||
| K. pneumoniae | ≤1 | ≤2/2 | ≤1/19 | |||
| Yeasts | C. albicans | 12.500 | ||||
| C. parapsilosis | 6.250 | |||||
| C. tropicalis | 12.500 | |||||
| C. dubliniensis | 3.125 | |||||
| Molds | A. niger | 3.125 | ||||
| Strains | H1 | H2 | H3 | H4 | |||||
|---|---|---|---|---|---|---|---|---|---|
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | ||
| Bacteria | Enterobactercloacae | 6.25 | 6.25 | 12.5 | 12.5 | 25 | 25 | 25 | 25 |
| Klebsiellapneumoniae | 25 | 25 | 25 | 25 | 25 | 25 | 25 | 25 | |
| Escherichia coli sauvage | 25 | 25 | 25 | 25 | 25 | 25 | 25 | 25 | |
| Staphylococcus aureus BLACT | 25 | 25 | 25 | 25 | 25 | 25 | 25 | 25 | |
| Staphylococcus epidermidis | 25 | 25 | 25 | 25 | 25 | 25 | 25 | 25 | |
| Fungi | Candida albicans | 3.125 | 3.125 | 6.25 | 6.25 | 6.25 | 6.25 | 6.25 | 6.25 |
| Candida dubliniensis | 6.25 | 6.25 | 6.25 | 6.25 | 6.25 | 6.25 | 12.5 | 12.5 | |
| Candida tropicalis | 6.25 | 6.25 | 12.5 | 12.5 | 12.5 | 12.5 | 12.5 | 12.5 | |
| Candida parapsilosis | 6.25 | 6.25 | 12.5 | 12.5 | 12.5 | 12.5 | 12.5 | 12.5 | |
| Aspergillus niger | 6.25 | 6.25 | 12.5 | 12.5 | 12.5 | 12.5 | 12.5 | 12.5 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ailli, A.; Zibouh, K.; Eddamsyry, B.; Drioiche, A.; Fetjah, D.; Ayyad, F.Z.; Mothana, R.A.; Hawwal, M.F.; Radi, M.; Tarik, R.; et al. Physicochemical Characterization of Moroccan Honey Varieties from the Fez-Meknes Region and Their Antioxidant and Antibacterial Properties. Metabolites 2024, 14, 364. https://doi.org/10.3390/metabo14070364
Ailli A, Zibouh K, Eddamsyry B, Drioiche A, Fetjah D, Ayyad FZ, Mothana RA, Hawwal MF, Radi M, Tarik R, et al. Physicochemical Characterization of Moroccan Honey Varieties from the Fez-Meknes Region and Their Antioxidant and Antibacterial Properties. Metabolites. 2024; 14(7):364. https://doi.org/10.3390/metabo14070364
Chicago/Turabian StyleAilli, Atika, Khalid Zibouh, Brahim Eddamsyry, Aziz Drioiche, Dounia Fetjah, Fatima Zahra Ayyad, Ramzi A. Mothana, Mohammed F. Hawwal, Mohamed Radi, Redouane Tarik, and et al. 2024. "Physicochemical Characterization of Moroccan Honey Varieties from the Fez-Meknes Region and Their Antioxidant and Antibacterial Properties" Metabolites 14, no. 7: 364. https://doi.org/10.3390/metabo14070364
APA StyleAilli, A., Zibouh, K., Eddamsyry, B., Drioiche, A., Fetjah, D., Ayyad, F. Z., Mothana, R. A., Hawwal, M. F., Radi, M., Tarik, R., Elomri, A., Mouradi, A., & Zair, T. (2024). Physicochemical Characterization of Moroccan Honey Varieties from the Fez-Meknes Region and Their Antioxidant and Antibacterial Properties. Metabolites, 14(7), 364. https://doi.org/10.3390/metabo14070364






