A Preliminary Study on the Whole-Plant Regulations of the Shrub Campylotropis polyantha in Response to Hostile Dryland Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Sites
2.2. Leaves and Roots Sampling
2.3. Biochemical Analyses
Biochemical Analyses of Plant Samples
2.4. Statistical Analysis
3. Results
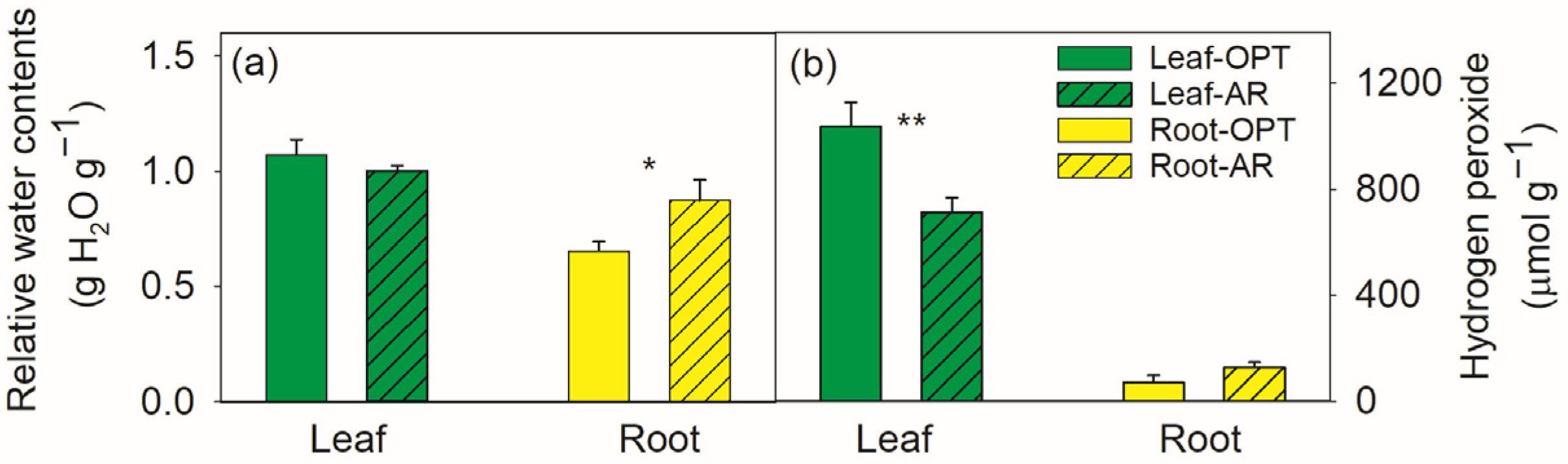
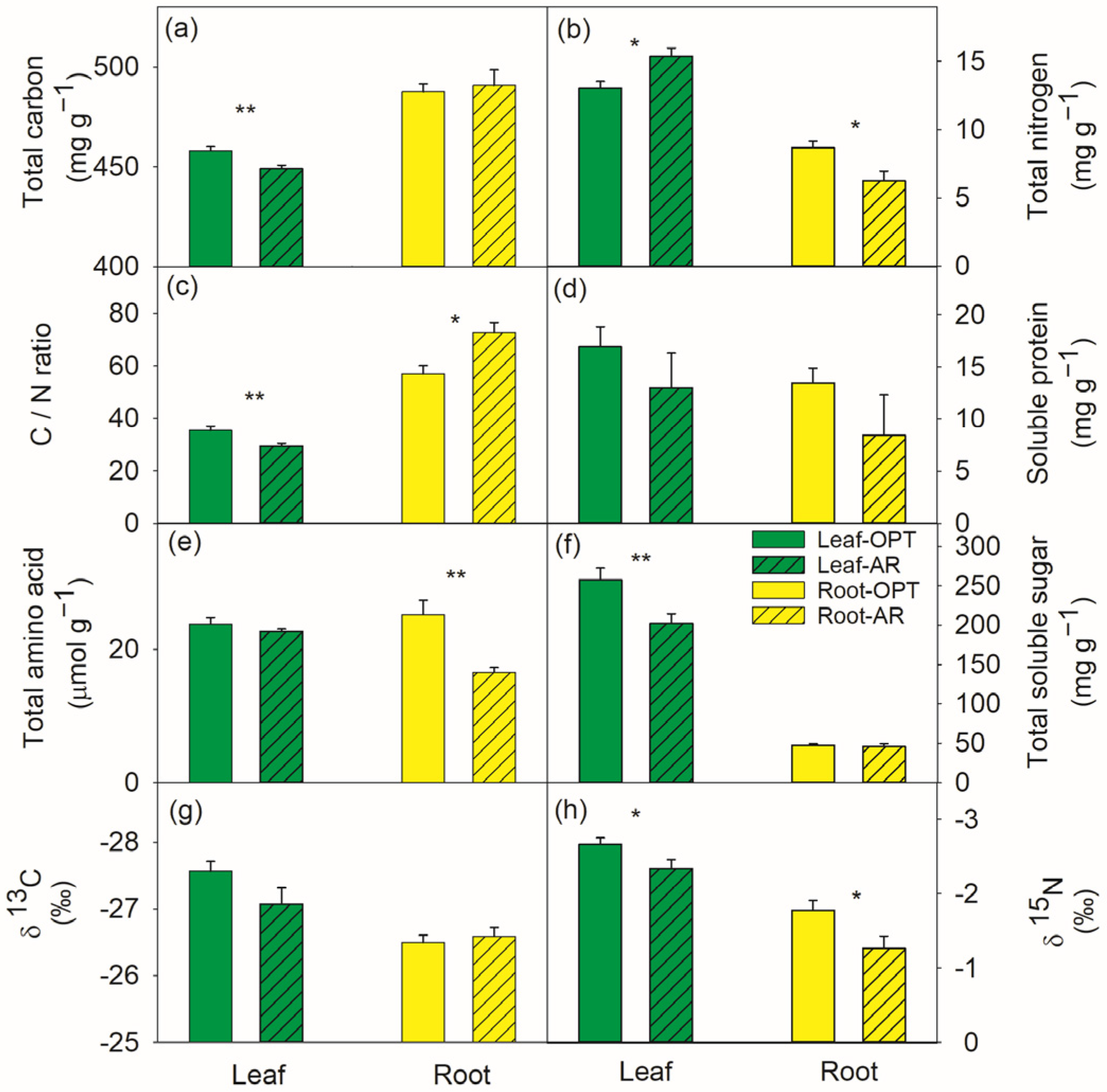
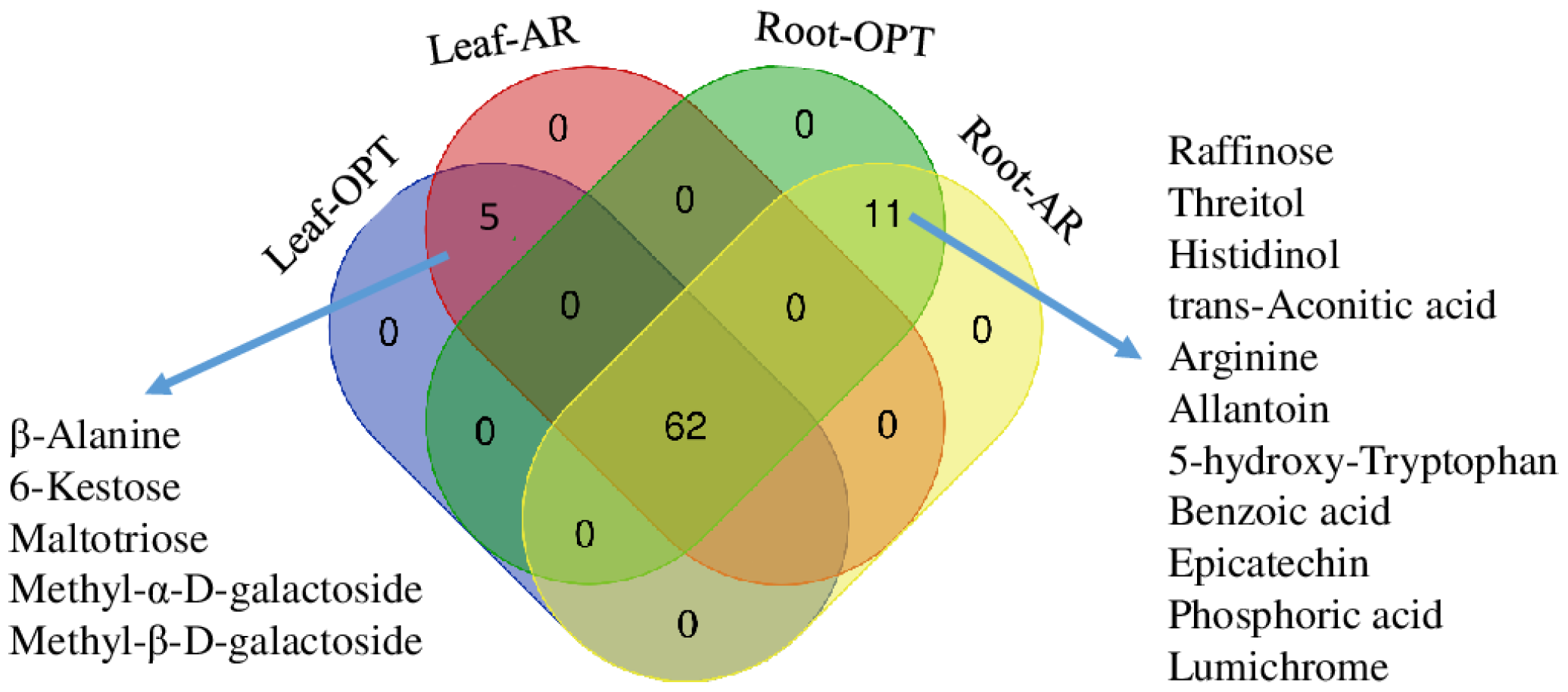
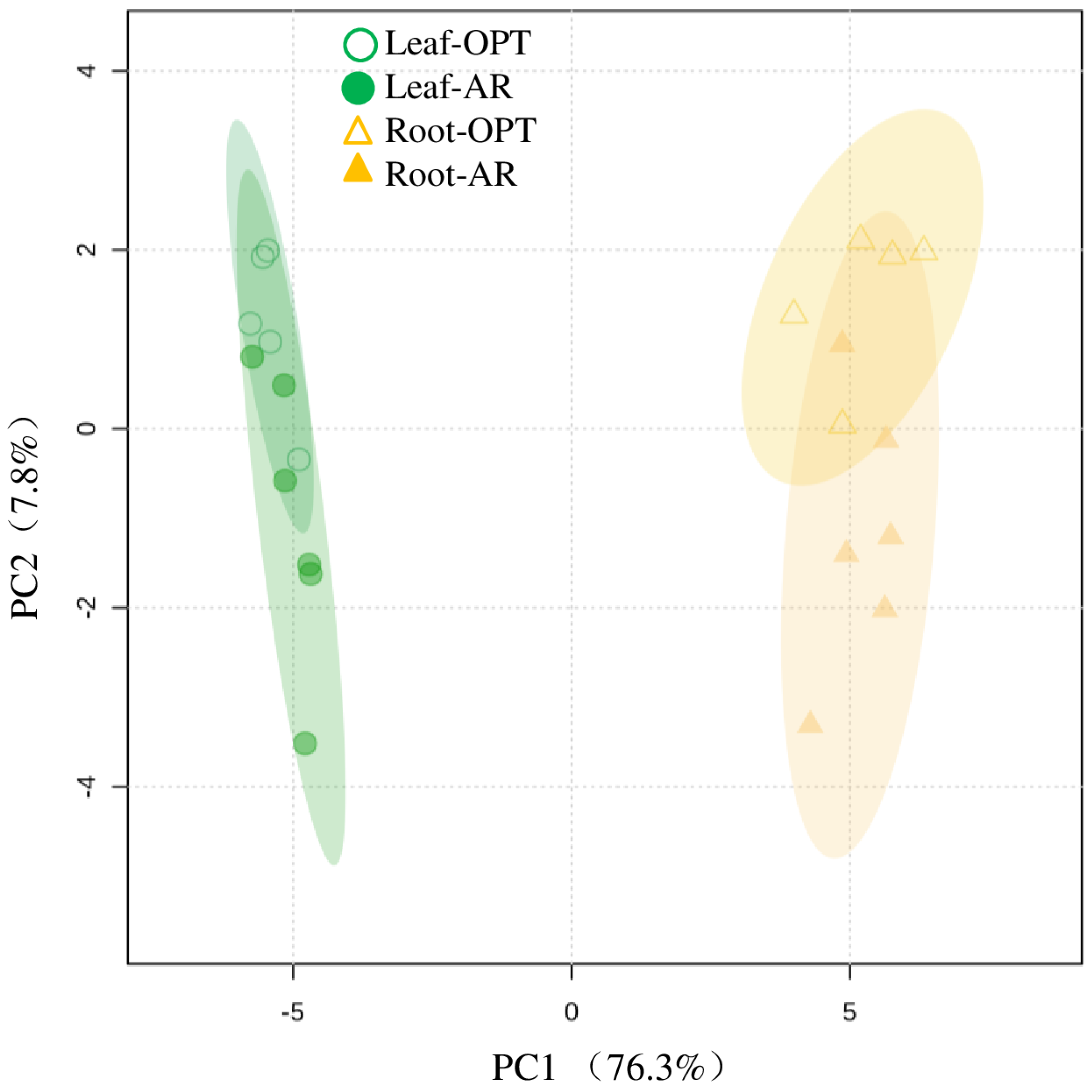
3.1. Differences in Leaves between the Two Sites
3.2. Differences in Roots between the Two Sites
4. Discussion
4.1. Water Relations, ROS Control and C, N Metabolic Regulations in Leaves
4.2. Water Relations, ROS Control and C, N Metabolic Regulations in Roots
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yu, R.; Zhai, P. More Frequent and Widespread Persistent Compound Drought and Heat Event Observed in China. Sci. Rep. 2020, 10, 14576. [Google Scholar] [CrossRef] [PubMed]
- Seneviratne, S.I.; Zhang, X.; Adnan, M.; Badi, W.; Dereczynski, C.; Luca, A.D.; Ghosh, S.; Iskandar, I.; Kossin, J.; Lewis, S.; et al. Weather and climate extreme events in a changing climate. In Climate Change 2021: The Physical Science Basis: Working Group I Contribution to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Masson-Delmotte, V.P., Zhai, A., Pirani, S.L., Connors, C., Eds.; Cambridge University Press: Cambridge, UK, 2021; pp. 1513–1766. [Google Scholar]
- Masson-Delmotte, V.P.; Zhai, P.; Pirani, S.L.; Connors, C.; Péan, S.; Berger, N.; Caud, Y.; Chen, L.; Goldfarb, M.I.; Scheel Monteiro, P.M. IPCC, 2021: Summary for Policymakers. In Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2021. [Google Scholar]
- Wang, D.C.; Zhang, X.; Huang, Y.; Wang, X.; Zhang, W.; Cao, Z.J.; Xin, Y.; Qu, M. Comparative Study on Temperature Response of Hydropower Development in the Dry-Hot Valley. GeoHealth 2021, 5, e2021GH000438. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yang, Z.; Wang, D.; Zhang, X. Climate Change and Causes in the Yuanmou Dry-Hot Valley of Yunnan, China. J. Arid Environ. 2002, 51, 153–162. [Google Scholar] [CrossRef]
- Li, F.L.; Bao, W.K. Elevational Trends in Leaf Size of Campylotropis Polyantha in the Arid Minjiang River Valley, SW China. J. Arid Environ. 2014, 108, 1–9. [Google Scholar] [CrossRef]
- Zeng, F.; Zhang, B.; Lu, Y.; Li, C.; Liu, B.; An, G.; Gao, X. Morpho-Physiological Responses of Alhagi sparsifolia Shap. (Leguminosae) Seedlings to Progressive Drought Stress. Pak. J. Bot. 2016, 48, 429–438. [Google Scholar]
- Li, F.-L.; Bao, W.-K.; Wu, N. Morphological, Anatomical and Physiological Responses of Campylotropis polyantha (Franch.) Schindl. Seedlings to Progressive Water Stress. Sci. Hortic. 2011, 127, 436–443. [Google Scholar] [CrossRef]
- Mittler, R. Abiotic Stress, the Field Environment and Stress Combination. Trends Plant Sci. 2006, 11, 15–19. [Google Scholar] [CrossRef]
- Das, A.; Rushton, P.J.; Rohila, J.S. Metabolomic Profiling of Soybeans (Glycine max L.) Reveals the Importance of Sugar and Nitrogen Metabolism under Drought and Heat Stress. Plants 2017, 6, 21. [Google Scholar] [CrossRef]
- Du, Y.; Zhao, Q.; Chen, L.; Yao, X.; Xie, F. Effect of Drought Stress at Reproductive Stages on Growth and Nitrogen Metabolism in Soybean. Agronomy 2020, 10, 302. [Google Scholar] [CrossRef]
- Prasad, P.V.V.; Staggenborg, S.A.; Ristic, Z. Impacts of Drought and/or Heat Stress on Physiological, Developmental, Growth, and Yield Processes of Crop Plants. In Advances in Agricultural Systems Modeling; Ahuja, L.R., Reddy, V.R., Saseendran, S.A., Yu, Q., Eds.; American Society of Agronomy: Madison, WI, USA; Soil Science Society of America: Madison, WI, USA, 2015; pp. 301–355. [Google Scholar]
- Gloser, V.; Dvorackova, M.; Mota, D.H.; Petrovic, B.; Gonzalez, P.; Geilfus, C.M. Early Changes in Nitrate Uptake and Assimilation under Drought in Relation to Transpiration. Front. Plant Sci. 2020, 11, 602065. [Google Scholar] [CrossRef]
- Hussain, H.A.; Men, S.; Hussain, S.; Chen, Y.; Ali, S.; Zhang, S.; Zhang, K.; Li, Y.; Xu, Q.; Liao, C. Interactive Effects of Drought and Heat Stresses on Morpho-Physiological Attributes, Yield, Nutrient Uptake and Oxidative Status in Maize Hybrids. Sci. Rep. 2019, 9, 3890. [Google Scholar] [CrossRef] [PubMed]
- Sato, H.; Mizoi, J.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Complex Plant Responses to Drought and Heat Stress under Climate Change. Plant J. 2024, 117, 1873–1892. [Google Scholar] [CrossRef] [PubMed]
- Mundim, F.M.; Pringle, E.G. Whole-plant metabolic allocation under water stress. Front. Plant Sci. 2018, 9, 852. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Spaccarotella, K.; Gido, J.; Samanta, I.; Chowdhary, G. Effects of Heat Stress on Plant-Nutrient Relations: An Update on Nutrient Uptake, Transport, and Assimilation. Int. J. Mol. Sci. 2023, 24, 15670. [Google Scholar] [CrossRef]
- He, J.; Hu, W.; Li, Y.; Zhu, H.; Zou, J.; Wang, Y.; Meng, Y.; Chen, B.; Zhao, W.; Wang, S. Prolonged Drought Affects the Interaction of Carbon and Nitrogen Metabolism in Root and Shoot of Cotton. Environ. Exp. Bot. 2022, 197, 104839. [Google Scholar] [CrossRef]
- Clément, G.; Moison, M.; Soulay, F.; Reisdorf-Cren, M.; Masclaux-Daubresse, C. Metabolomics of Laminae and Midvein during Leaf Senescence and Source–Sink Metabolite Management in Brassica napus L. Leaves. J. Exp. Bot. 2018, 69, 891–903. [Google Scholar] [CrossRef]
- Vander Mijnsbrugge, K.; Vandepitte, J.; Moreels, S.; Mihaila, V.-V.; De Ligne, L.; Notivol, E.; Van Acker, J.; Van den Bulcke, J. Timing of Autumnal Leaf Senescence in a Common Shrub Species Depends on the Level of Preceding Summer Drought Symptoms. Environ. Exp. Bot. 2023, 216, 105539. [Google Scholar] [CrossRef]
- Liao, Z.; Zhu, L.; Liu, L.; Kreuzwieser, J.; Werner, C.; Du, B. Comparison of Growth and Metabolomic Profiles of Two Afforesta-Tion Cypress Species Cupressus chengiana and Platycladus orientalis Grown at Minjiang Valley in Southwest China. Metabolites 2024, 14, 453. [Google Scholar] [CrossRef]
- Li, F.; Bao, W.-K.; Pang, X.; Leng, L. Seedling emergence, survival and growth of five endemic species in the dry valley of Minjiang River. Acta Ecol. Sin. 2009, 29, 2219–2230. [Google Scholar]
- Ma, F.; Li, Z. Impacts of Extreme Climate on the Water Resource System in Sichuan Province. Water 2024, 16, 1217. [Google Scholar] [CrossRef]
- Du, B.; Kreuzwieser, J.; Winkler, J.B.; Ghirardo, A.; Schnitzler, J.-P.; Ache, P.; Alfarraj, S.; Hedrich, R.; White, P.; Rennenberg, H. Physiological Responses of Date Palm (Phoenix dactylifera) Seedlings to Acute Ozone Exposure at High Temperature. Environ. Pollut. 2018, 242, 905–913. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Peñuelas, J.; Li, T.; Liu, H.; Wu, H.; Zhang, Y.; Sardans, J.; Jiang, Y. Natural Abundance of 13C and 15N Provides Evidence for Plant–Soil Carbon and Nitrogen Dynamics in a N-Fertilized Meadow. Ecology 2021, 102, e03348. [Google Scholar] [CrossRef] [PubMed]
- Du, B.; Jansen, K.; Junker, L.V.; Eiblmeier, M.; Kreuzwieser, J.; Gessler, A.; Ensminger, I.; Rennenberg, H. Elevated Temperature Differently Affects Foliar Nitrogen Partitioning in Seedlings of Diverse Douglas Fir Provenances. Tree Physiol. 2014, 34, 1090–1101. [Google Scholar] [CrossRef] [PubMed]
- Du, B.; Rennenberg, H. Physiological Responses of Lavender (Lavandula angustifolia Mill.) to water Deficit and Recovery. S. Afr. J. Bot. 2018, 119, 212–218. [Google Scholar] [CrossRef]
- Pang, Z.; Lu, Y.; Zhou, G.; Hui, F.; Xu, L.; Viau, C.; Spigelman, A.F.; MacDonald, P.E.; Wishart, D.S.; Li, S.; et al. MetaboAnalyst 6.0: Towards a Unified Platform for Metabolomics Data Processing, Analysis and Interpretation. Nucleic Acids Res. 2024, 52, W398–W406. [Google Scholar] [CrossRef]
- Stein, S. Coping with the “World’s Biggest Dust Bowl”. Towards a History of China’s Forest Shelterbelts, 1950s-Present. Glob. Environ. 2015, 8, 320–348. [Google Scholar] [CrossRef]
- Winkler, D.E.; Belnap, J.; Hoover, D.; Reed, S.C.; Duniway, M.C. Shrub Persistence and Increased Grass Mortality in Response to Drought in Dryland Systems. Glob. Chang. Biol. 2019, 25, 3121–3135. [Google Scholar] [CrossRef]
- Li, F.; Zhu, L.-H.; Bao, W. Effects of Environmental Stress on Seedlings Root Growth and Nodulation of Leguminous Shrubs in the Dry Valley of Minjiang River. J. Appl. Ecol. 2009, 20, 1825–1831. [Google Scholar]
- Smirnoff, N.; Arnaud, D. Hydrogen Peroxide Metabolism and Functions in Plants. New Phytol. 2019, 221, 1197–1214. [Google Scholar] [CrossRef]
- Du, B.; Haensch, R.; Alfarraj, S.; Rennenberg, H. Strategies of Plants to Overcome Abiotic and Biotic Stresses. Biol. Rev. 2024, 99, 1524–1536. [Google Scholar] [CrossRef]
- Yildizli, A.; Çevik, S.; Ünyayar, S. Effects of Exogenous Myo-Inositol on Leaf Water Status and Oxidative Stress of Capsicum annuum under Drought Stress. Acta Physiol. Plant 2018, 40, 122. [Google Scholar] [CrossRef]
- Li, Z.; Fu, J.; Shi, D.; Peng, Y. Myo-inositol Enhances Drought Tolerance in Creeping Bentgrass through Alteration of Osmotic Adjustment, Photosynthesis, and Antioxidant Defense. Crop Sci. 2020, 60, 2149–2158. [Google Scholar] [CrossRef]
- Ahn, C.; Park, U.; Park, P.B. Increased Salt and Drought Tolerance by D-Ononitol Production in Transgenic Arabidopsis thaliana. Biochem. Biophys. Res. Commun. 2011, 415, 669–674. [Google Scholar] [CrossRef] [PubMed]
- Pamuru, R.R.; Puli, C.O.R.; Pandita, D.; Wani, S.H. Sugar Alcohols and Osmotic Stress Adaptation in Plants. In Compatible Solutes Engineering for Crop Plants Facing Climate Change; Wani, S.H., Gangola, M.P., Ramadoss, B.R., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 189–203. [Google Scholar]
- Foyer, C.H.; Noctor, G. Ascorbate and Glutathione: The Heart of the Redox Hub. Plant Physiol. 2011, 155, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Du, B.; Jansen, K.; Kleiber, A.; Eiblmeier, M.; Kammerer, B.; Ensminger, I.; Gessler, A.; Rennenberg, H.; Kreuzwieser, J. A Coastal and an Interior Douglas Fir Provenance Exhibit Different Metabolic Strategies to Deal with Drought Stress. Tree Physiol. 2016, 36, 148–163. [Google Scholar] [CrossRef]
- Du, B.; Kruse, J.; Winkler, J.B.; Alfarray, S.; Schnitzler, J.-P.; Ache, P.; Hedrich, R.; Rennenberg, H. Climate and Development Modulate the Metabolome and Antioxidative System of Date Palm Leaves. J. Exp. Bot. 2019, 70, 5959–5969. [Google Scholar] [CrossRef]
- Parvin, K.; Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Mohsin, S.M.; Fujita, M. Quercetin Mediated Salt Tolerance in Tomato through the Enhancement of Plant Antioxidant Defense and Glyoxalase Systems. Plants 2019, 8, 247. [Google Scholar] [CrossRef]
- Parvin, K.; Nahar, K.; Mohsin, S.M.; Al Mahmud, J.; Fujita, M.; Hasanuzzaman, M. Plant Phenolic Compounds for Abiotic Stress Tolerance. In Managing Plant Production under Changing Environment; Hasanuzzaman, M., Ahammed, G.J., Nahar, K., Eds.; Springer Nature: Singapore, 2022; pp. 193–237. [Google Scholar]
- Du, B.; Kruse, J.; Winkler, J.B.; Alfarraj, S.; Albasher, G.; Schnitzler, J.-P.; Ache, P.; Hedrich, R.; Rennenberg, H. Metabolic Responses of Date Palm (Phoenix dactylifera L.) Leaves to Drought Differ in Summer and Winter Climate. Tree Physiol. 2021, 41, 1685–1700. [Google Scholar] [CrossRef]
- Liao, Z.; Liu, L.; Rennenberg, H.; Du, B. Water Deprivation Modifies the Metabolic Profile of Lavender (Lavandula angustifolia Mill.) Leaves. Physiol. Plant. 2024, 176, e14365. [Google Scholar] [CrossRef]
- Neilson, E.H.; Goodger, J.Q.D.; Woodrow, I.E.; Møller, B.L. Plant Chemical Defense: At What Cost? Trends Plant Sci. 2013, 18, 250–258. [Google Scholar] [CrossRef]
- Keunen, E.; Peshev, D.; Vangronsveld, J.; Van Den Ende, W.; Cuypers, A. Plant Sugars Are Crucial Players in the Oxidative Challenge during Abiotic Stress: Extending the Traditional Concept. Plant Cell Environ. 2013, 36, 1242–1255. [Google Scholar] [CrossRef]
- Loescher, W.H.; McCamant, T.; Keller, J.D. Carbohydrate Reserves, Translocation, and Storage in Woody Plant Roots. HortScience 1990, 25, 274–281. [Google Scholar] [CrossRef]
- Thompson, R.A.; Adams, H.D.; Breshears, D.D.; Collins, A.D.; Dickman, L.T.; Grossiord, C.; Manrique-Alba, À.; Peltier, D.M.; Ryan, M.G.; Trowbridge, A.M.; et al. No Carbon Storage in Growth-Limited Trees in a Semi-Arid Woodland. Nat. Commun. 2023, 14, 1959. [Google Scholar] [CrossRef] [PubMed]
- Farquhar, G.D.; Ehleringer, J.R.; Hubick, K.T. Carbon Isotope Discrimination and Photosynthesis. Annu. Rev. Plant Biol. 1989, 40, 503–537. [Google Scholar] [CrossRef]
- Warren, C.R.; McGrath, J.F.; Adams, M.A. Water Availability and Carbon Isotope Discrimination in Conifers. Oecologia 2001, 127, 476–486. [Google Scholar] [CrossRef]
- Yoon, J.; Cho, L.-H.; Tun, W.; Jeon, J.-S.; An, G. Sucrose Signaling in Higher Plants. Plant Sci. 2021, 302, 110703. [Google Scholar] [CrossRef]
- Du, Y.; Zhao, Q.; Chen, L.; Yao, X.; Zhang, W.; Zhang, B.; Xie, F. Effect of Drought Stress on Sugar Metabolism in Leaves and Roots of Soybean Seedlings. Plant Physiol. Biochem. 2020, 146, 1–12. [Google Scholar] [CrossRef]
- Kumar, P.A.; Polisetty, R.; Abrol, Y.P. Interaction between Carbon and Nitrogen Metabolism. In Photosynthesis: Photoreactions to Plant Productivity; Abrol, Y.P., Mohanty, P., Govindjee, Eds.; Springer: Dordrecht, The Netherlands, 1993; pp. 339–350. [Google Scholar]
- Ågren, G.I.; Wetterstedt, J.Å.M.; Billberger, M.F.K. Nutrient Limitation on Terrestrial Plant Growth—Modeling the Interaction between Nitrogen and Phosphorus. New Phytol. 2012, 194, 953–960. [Google Scholar] [CrossRef]
- Wang, B.; Qu, L.Y.; Ma, K.M.; Zhang, X.Y.; Song, C.J. Patterns of Ecoenzymatic Stoichiometry in the Dominant Shrubs in the Semi-Arid Upper Minjiang River Valley. Acta Ecol. Sin. 2015, 35, 6078–6088. [Google Scholar]
- Xiong, J.; Dong, L.; Lu, J.; Hu, W.; Gong, H.; Xie, S.; Zhao, D.; Zhang, Y.; Wang, X.; Deng, Y.; et al. Variation in Plant Carbon, Nitrogen and Phosphorus Contents across the Drylands of China. Funct. Ecol. 2022, 36, 174–186. [Google Scholar] [CrossRef]
- Elser, J.J.; Fagan, W.F.; Denno, R.F.; Dobberfuhl, D.R.; Folarin, A.; Huberty, A.; Interlandi, S.; Kilham, S.S.; McCauley, E.; Schulz, K.L.; et al. Nutritional Constraints in Terrestrial and Freshwater Food Webs. Nature 2000, 408, 578–580. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Gao, J.; Zhang, S.Q.; Wang, G.X. Variations in Leaf and Root Stoichiometry of Nitraria Tangutorum along Aridity Gradients in the Hexi Corridor, Northwest China. Contemp. Probl. Ecol. 2014, 7, 308–314. [Google Scholar] [CrossRef]
- Cavender-Bares, J.; Bazzaz, F.A. Changes in Drought Response Strategies with Ontogeny in Quercus Rubra: Implications for Scaling from Seedlings to Mature Trees. Oecologia 2000, 124, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Bar-On, Y.M.; Milo, R. The Global Mass and Average Rate of Rubisco. Proc. Natl. Acad. Sci. USA 2019, 116, 4738–4743. [Google Scholar] [CrossRef]
- Robinson, D. δ15N as an Integrator of the Nitrogen Cycle. Trends Ecol. Evol. 2001, 16, 153–162. [Google Scholar] [CrossRef]
- Kalcsits, L.A.; Buschhaus, H.A.; Guy, R.D. Nitrogen Isotope Discrimination as an Integrated Measure of Nitrogen Fluxes, Assimilation and Allocation in Plants. Physiol. Plant. 2014, 151, 293–304. [Google Scholar] [CrossRef]
- Hobbie, E.A.; Högberg, P. Nitrogen Isotopes Link Mycorrhizal Fungi and Plants to Nitrogen Dynamics. New Phytol. 2012, 196, 367–382. [Google Scholar] [CrossRef]
- Handley, L.L.; Austin, A.T.; Stewart, G.R.; Robinson, D.; Scrimgeour, C.M.; Raven, J.A.; Heaton, T.H.E.; Schmidt, S. The 15N Natural Abundance (δ15N) of Ecosystem Samples Reflects Measures of Water Availability. Funct. Plant Biol. 1999, 26, 185–199. [Google Scholar] [CrossRef]
- Song, C.J.; Ma, K.M.; Fu, B.J.; Qu, L.Y.; Xu, X.L.; Liu, Y.; Zhong, J.F. Distribution Patterns of Shrubby N-Fixers and Non-N Fixers in an Arid Valley in Southwest China: Implications for Ecological Restoration. Ecol. Res. 2010, 25, 553–564. [Google Scholar] [CrossRef]
- Erktan, A.; McCormack, M.L.; Roumet, C. Frontiers in Root Ecology: Recent Advances and Future Challenges. Plant Soil 2018, 424, 1–9. [Google Scholar] [CrossRef]
- Brunner, I.; Herzog, C.; Dawes, M.A.; Arend, M.; Sperisen, C. How Tree Roots Respond to Drought. Front. Plant Sci. 2015, 6, 547. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Urdaneta, A.B.; Peña-Valdivia, C.B.; Trejo, C.; Aguirre R., J.R.; Cárdenas, E.S. Root Growth and Proline Content in Drought Sensitive and Tolerant Maize (Zea mays L.) Seedlings under Different Water Potentials. Cereal Res. Commun. 2005, 33, 697–704. [Google Scholar] [CrossRef]
- Feller, U.; Fischer, A. Nitrogen Metabolism in Senescing Leaves. Crit. Rev. Plant Sci. 1994, 13, 241–273. [Google Scholar] [CrossRef]
- Gargallo-Garriga, A.; Sardans, J.; Pérez-Trujillo, M.; Rivas-Ubach, A.; Oravec, M.; Vecerova, K.; Urban, O.; Jentsch, A.; Kreyling, J.; Beierkuhnlein, C.; et al. Opposite Metabolic Responses of Shoots and Roots to Drought. Sci. Rep. 2014, 4, 6829. [Google Scholar] [CrossRef] [PubMed]
- Akram, M. Citric Acid Cycle and Role of Its Intermediates in Metabolism. Cell Biochem. Biophys. 2014, 68, 475–478. [Google Scholar] [CrossRef]
- Lihavainen, J.; Edlund, E.; Björkén, L.; Bag, P.; Robinson, K.M.; Jansson, S. Stem Girdling Affects the Onset of Autumn Senescence in Aspen in Interaction with Metabolic Signals. Physiol. Plant. 2021, 172, 201–217. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Jiang, X.; Zhu, L.; Liu, L.; Liao, Z.; Du, B. A Preliminary Study on the Whole-Plant Regulations of the Shrub Campylotropis polyantha in Response to Hostile Dryland Conditions. Metabolites 2024, 14, 495. https://doi.org/10.3390/metabo14090495
Zhang H, Jiang X, Zhu L, Liu L, Liao Z, Du B. A Preliminary Study on the Whole-Plant Regulations of the Shrub Campylotropis polyantha in Response to Hostile Dryland Conditions. Metabolites. 2024; 14(9):495. https://doi.org/10.3390/metabo14090495
Chicago/Turabian StyleZhang, Hua, Xue Jiang, Lijun Zhu, Lei Liu, Zhengqiao Liao, and Baoguo Du. 2024. "A Preliminary Study on the Whole-Plant Regulations of the Shrub Campylotropis polyantha in Response to Hostile Dryland Conditions" Metabolites 14, no. 9: 495. https://doi.org/10.3390/metabo14090495
APA StyleZhang, H., Jiang, X., Zhu, L., Liu, L., Liao, Z., & Du, B. (2024). A Preliminary Study on the Whole-Plant Regulations of the Shrub Campylotropis polyantha in Response to Hostile Dryland Conditions. Metabolites, 14(9), 495. https://doi.org/10.3390/metabo14090495








