Organic Trace Minerals Enhance the Gut Health of British Shorthair Cats by Regulating the Structure of Intestinal Microbiota
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Treatments
| Items | Content |
|---|---|
| Ingredient, % | |
| Chicken powder | 49.80 |
| Fish powder | 3.21 |
| Rice bran | 20.00 |
| Fish oil | 2.00 |
| Chicken oil | 3.00 |
| Potato starch | 1.00 |
| Tapioca flour | 0.60 |
| Taurine | 19.00 |
| Salt | 0.20 |
| Vitamin 1 | 0.49 |
| Mineral premix 2 | 0.40 |
| Total | 0.30 |
| Nutrient composition | |
| Metabolic energy, kcal/kg | 4031 |
| Phosphorus, % | 1.52 |
| Calcium, % | 1.72 |
| Ether extract, % | 17.66 |
| Crude protein, % | 41.01 |
| Treatments | The Composition and Content of Trace Minerals, mg/kg DM | ||||
|---|---|---|---|---|---|
| Zn | Fe | Cu | Mn | Se | |
| ITM | ZnSO4, 75 | Fe2(SO4)3, 80 | CuSO4, 5 | MnSO4, 7.6 | Na2SeO3, 0.3 |
| ITM + OTM | ZnSO4, 37.5 Bioplex 1 Zn, 37.5 | Fe2(SO4)3, 40 Bioplex Fe, 40 | CuSO4, 2.5 Bioplex Cu, 2.5 | MnSO4, 3.8 Bioplex Mn, 3.8 | Na2SeO3, 0.15 Sel-Plex 2 2000, 0.15 |
| OTM | Bioplex Zn, 75 | Bioplex Fe, 80 | Bioplex Cu, 5 | Bioplex Mn, 7.6 | Sel-Plex 2000, 0.3 |
2.2. Experimental Design and Sample Collection
2.3. Serum Parameters Measurement
2.4. Microbiota Analysis
2.5. Metabolomics Analysis
2.6. Statistical Analysis
3. Results
3.1. Growth Performance
3.2. Immune Indexes
3.3. Inflammatory Factors
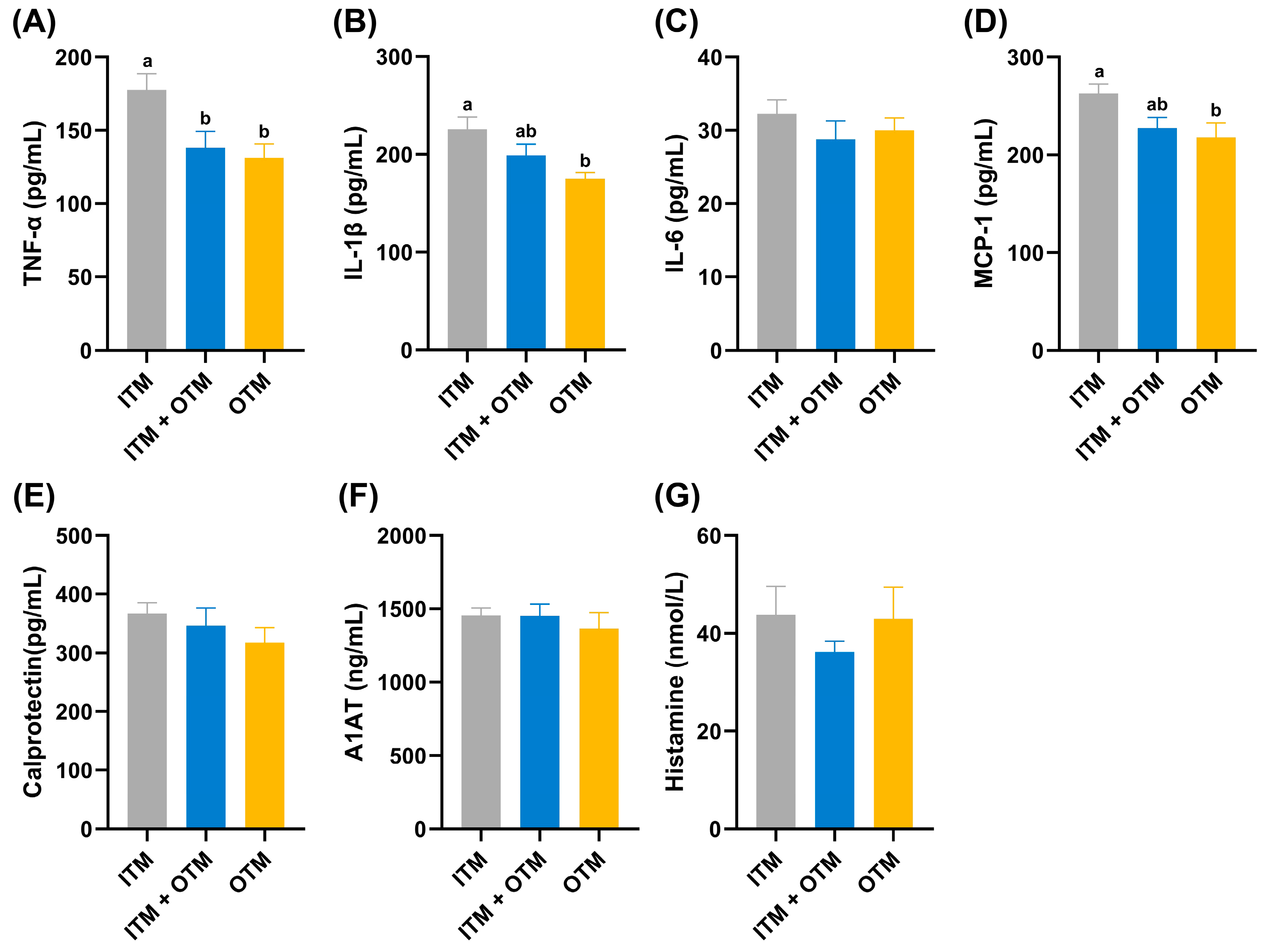
3.4. Intestinal Permeability Parameters
3.5. The Richness and Diversity of Fecal Microbiota
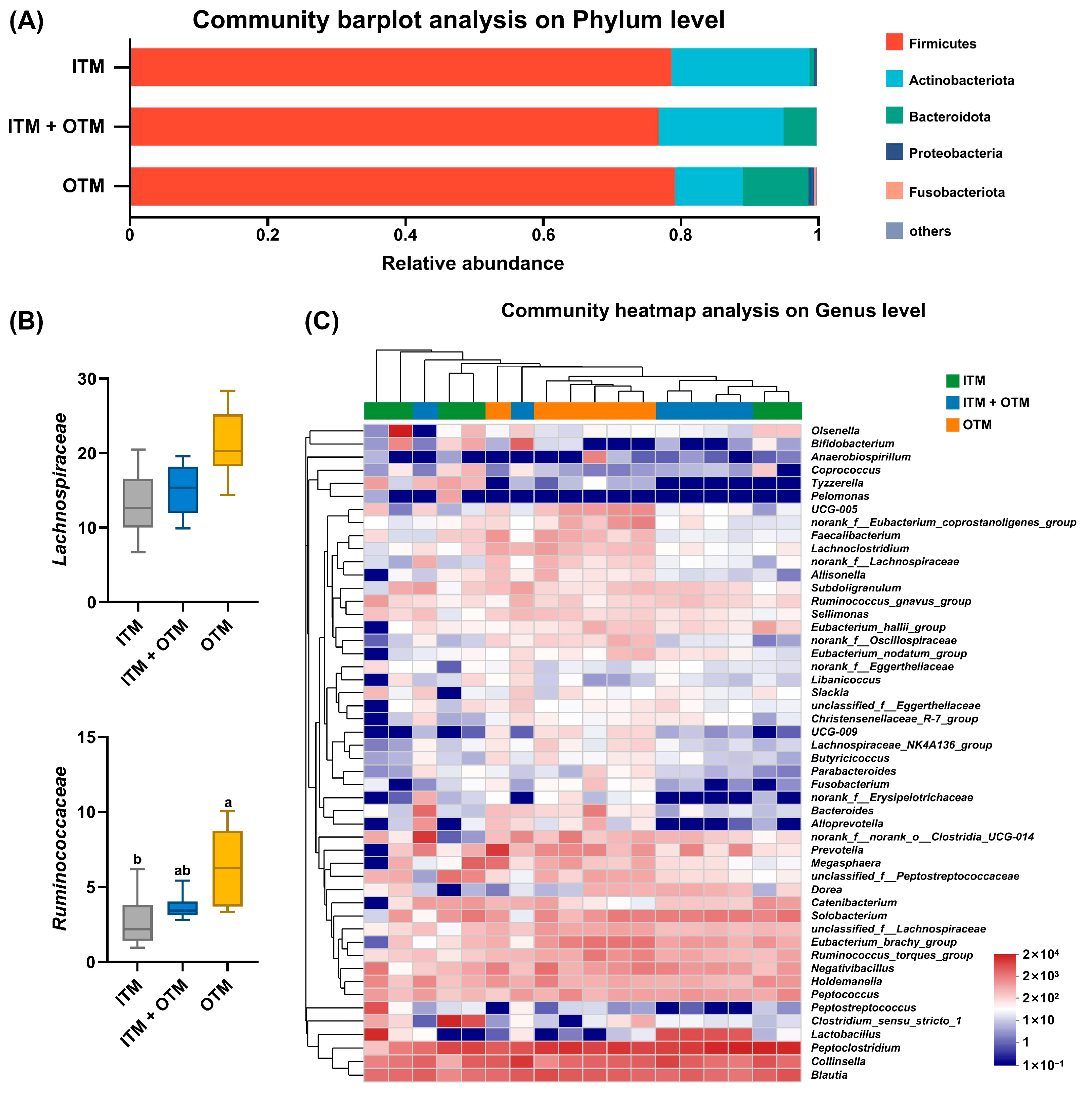
3.6. Fecal Microbiota Composition
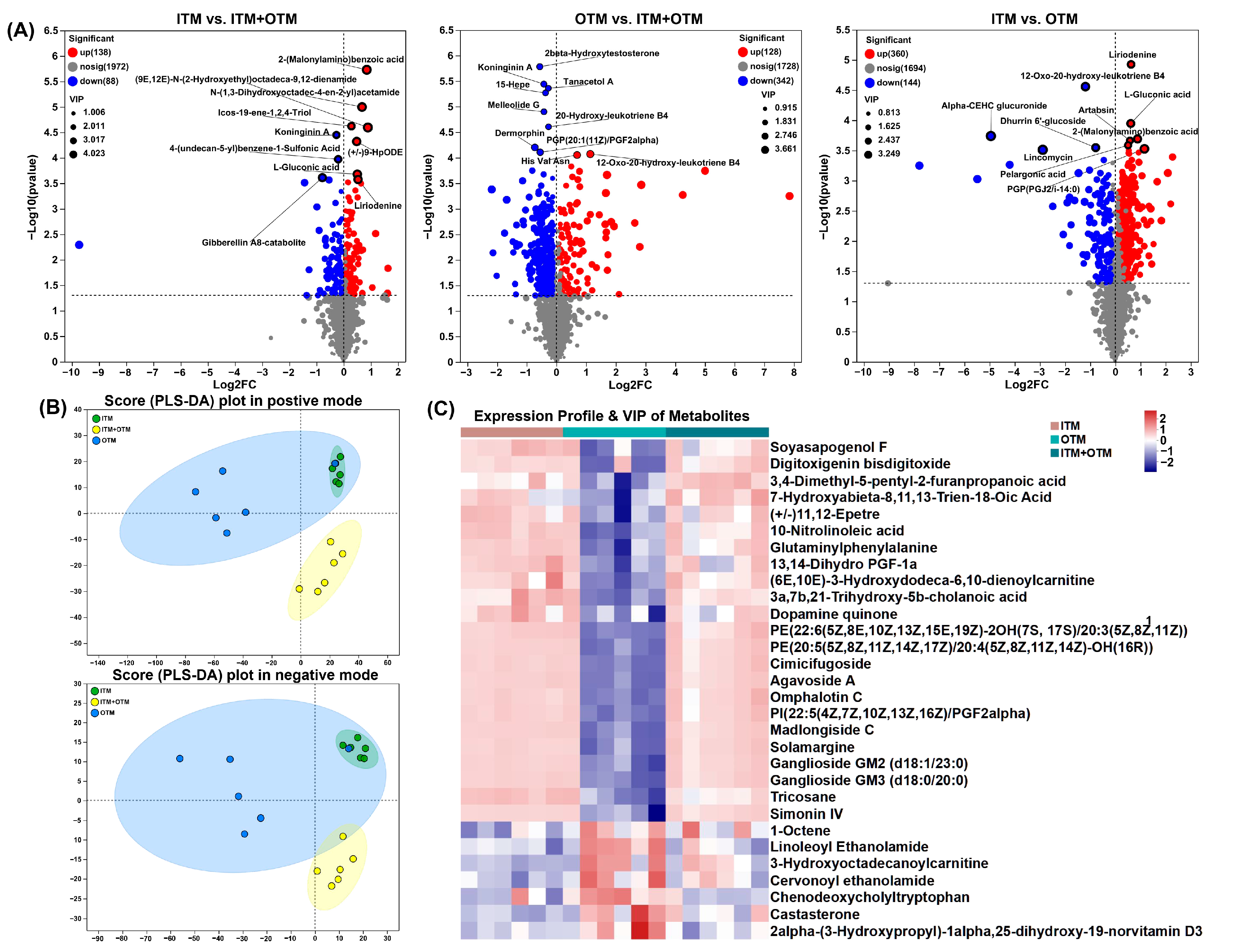
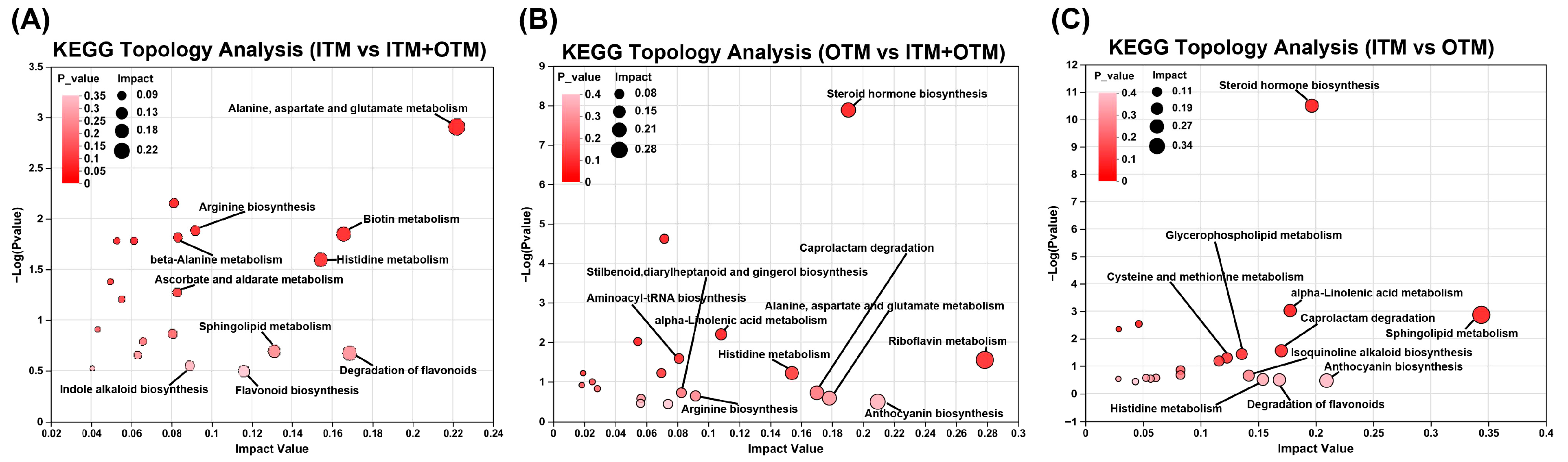
3.7. Fecal Metabolomics Composition
3.8. Correlation Analysis between Differential Metabolites and Genus Level Gut Microbiota
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yatoo, M.I.; Saxena, A.; Deepa, P.M.; Habeab, B.P.; Devi, S.; Jatav, R.S.; Dimri, U. Role of trace elements in animals: A review. Vet. World 2013, 6, 963. [Google Scholar] [CrossRef]
- Costa, M.I.; Sarmento-Ribeiro, A.B.; Gonçalves, A.C. Zinc: From biological functions to therapeutic potential. Int. J. Mol. Sci. 2023, 24, 4822. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Jiang, Y.; Shi, H.; Peng, Y.; Fan, X.; Li, C. The molecular mechanisms of copper metabolism and its roles in human diseases. Pflug. Arch. Eur. J. Physiol. 2020, 472, 1415–1429. [Google Scholar] [CrossRef] [PubMed]
- Kuršvietienė, L.; Mongirdienė, A.; Bernatonienė, J.; Šulinskienė, J.; Stanevičienė, I. Selenium anticancer properties and impact on cellular redox status. Antioxidants 2020, 9, 80. [Google Scholar] [CrossRef]
- Islam, M.R.; Akash, S.; Jony, M.H.; Alam, M.N.; Nowrin, F.T.; Rahman, M.M.; Rauf, A.; Thiruvengadam, M. Exploring the potential function of trace elements in human health: A therapeutic perspective. Mol. Cell. Biochem. 2023, 478, 2141–2171. [Google Scholar] [CrossRef]
- Byrne, L.; Murphy, R.A. Relative bioavailability of trace minerals in production animal nutrition: A review. Animals 2022, 12, 1981. [Google Scholar] [CrossRef]
- Gong, J.; Xiao, M. Effect of organic selenium supplementation on selenium status, oxidative stress, and antioxidant status in selenium-adequate dairy cows during the periparturient period. Biol. Trace Elem. Res. 2018, 186, 430–440. [Google Scholar] [CrossRef]
- Fairweather-Tait, S.J.; Bao, Y.; Broadley, M.R.; Collings, R.; Ford, D.; Hesketh, J.E.; Hurst, R. Selenium in human health and disease. Antioxid. Redox Signal. 2011, 14, 1337–1383. [Google Scholar] [CrossRef]
- Caetano-Silva, M.E.; Netto, F.M.; Bertoldo-Pacheco, M.T.; Alegría, A.; Cilla, A. Peptide-metal complexes: Obtention and role in increasing bioavailability and decreasing the pro-oxidant effect of minerals. Crit. Rev. Food Sci. Nutr. 2021, 61, 1470–1489. [Google Scholar] [CrossRef]
- Zhang, K.; Han, M.; Dong, Y.; Miao, Z.; Zhang, J.; Song, X.; Feng, Y.; Li, H.; Zhang, L.; Wei, Q. Low levels of organic compound trace elements improve the eggshell quality, antioxidant capacity, immune function, and mineral deposition of aged laying hens. Animals 2021, 15, 100401. [Google Scholar] [CrossRef]
- Pereira, A.M.B. Improving Bioavailability of Trace Elements in Dog Feed: The Role of Organic Sources. Ph.D. Thesis, University of Porto (Portugal), Porto, Portugal, 2020. [Google Scholar]
- Cui, Y.; Li, D.; Zhang, M.; Liu, P.; Wang, H.; Li, Y.; Wu, Y. The Effects of Dietary Saccharomyces cerevisiae Supplementation on Gut Microbiota Composition and Gut Health in Aged Labrador Retrievers. Animals 2024, 14, 1713. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Mo, R.; Li, M.; Qu, Y.; Wang, H.; Liu, T.; Liu, P.; Wu, Y. Comparison of the effects of enzymolysis seaweed powder and saccharomyces boulardii on intestinal health and microbiota composition in kittens. Metabolites 2023, 13, 637. [Google Scholar] [CrossRef] [PubMed]
- Suchodolski, J.S. Companion animals symposium: Microbes and gastrointestinal health of dogs and cats. J. Anim. Sci. 2011, 89, 1520–1530. [Google Scholar] [CrossRef] [PubMed]
- Mion, B.; Van Winters, B.; King, K.; Spricigo, J.F.W.; Ogilvie, L.; Guan, L.; DeVries, T.J.; McBride, B.W.; LeBlanc, S.J.; Steele, M.A.; et al. Effects of replacing inorganic salts of trace minerals with organic trace minerals in pre- and postpartum diets on feeding behavior, rumen fermentation, and performance of dairy cows. J. Dairy Sci. 2022, 105, 6693–6709. [Google Scholar] [CrossRef]
- Ma, L.; He, J.; Hou, C.; Qiu, J.; Lu, X.; Liu, B.; Lin, G.; Xue, Y.; Koontz, A.; Yu, D. PSX-24 Effect of compound organic trace minerals on growth performance, serum indices and micromineral excretion in fattening pigs. J. Anim. Sci. 2018, 96, 485–486. [Google Scholar] [CrossRef]
- Ramos-Vidales, D.; Gómez-Verduzco, G.; Cortes-Cuevas, A.; del Río-García, J.C.; Fernández-Tinoco, S.; Chárraga-Aguilar, S.; Ávila-González, E. Organic trace minerals on productive performance, egg quality and immune response in Bovans White laying hens. J. Anim. Physiol. Anim. Nutr. 2019, 103, 1484–1491. [Google Scholar] [CrossRef]
- Pajarillo, E.A.B.; Lee, E.; Kang, D.-K. Trace metals and animal health: Interplay of the gut microbiota with iron, manganese, zinc, and copper. Anim. Nutr. 2021, 7, 750–761. [Google Scholar] [CrossRef]
- Augustsson, A.; Qvarforth, A.; Engström, E.; Paulukat, C.; Rodushkin, I. Trace and major elements in food supplements of different origin: Implications for daily intake levels and health risks. Toxicol. Rep. 2021, 8, 1067–1080. [Google Scholar] [CrossRef]
- López-Gálvez, G.; López-Alonso, M.; Pechova, A.; Mayo, B.; Dierick, N.; Gropp, J. Alternatives to antibiotics and trace elements (copper and zinc) to improve gut health and zootechnical parameters in piglets: A review. Anim. Feed Sci. Technol. 2021, 271, 114727. [Google Scholar] [CrossRef]
- Megha, K.; Mohanan, P. Role of immunoglobulin and antibodies in disease management. Int. J. Biol. Macromol. 2021, 169, 28–38. [Google Scholar] [CrossRef]
- Zemrani, B.; Bines, J.E. Recent insights into trace element deficiencies: Causes, recognition and correction. Curr. Opin. Gastroenterol. 2020, 36, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Schwab, I.; Nimmerjahn, F. Intravenous immunoglobulin therapy: How does IgG modulate the immune system? Nat. Rev. Immunol. 2013, 13, 176–189. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.-F.; Tian, M.; Song, J.-S.; Chen, F.; Lin, G.; Zhang, S.-H.; Guan, W.-T. Effect of replacing inorganic trace minerals at lower organic levels on growth performance, blood parameters, antioxidant status, immune indexes, and fecal mineral excretion in weaned piglets. Trop. Anim. Health Prod. 2021, 53, 121. [Google Scholar] [CrossRef]
- Wang, S.-Q.; Peng, Z.; Sun, H.; Han, Y.-M.; Zhang, B.; Pineda, L.; Boerboom, G.; Sun, L.-h.; Liu, Y.; Deng, Z.-C. Evaluating the Impact of an Organic Trace Mineral mix on the Redox Homeostasis, Immunity, and Performance of Sows and their Offspring. Biol. Trace Elem. Res. 2024, 1–10. [Google Scholar] [CrossRef]
- Harley, R.; Gruffydd-Jones, T.J.; Day, M.J. Salivary and serum immunoglobulin levels in cats with chronic gingivostomatitis. Vet. Rec. 2003, 152, 125–129. [Google Scholar] [CrossRef]
- Sheinenzon, A.; Shehadeh, M.; Michelis, R.; Shaoul, E.; Ronen, O. Serum albumin levels and inflammation. Int. J. Biol. Macromol. 2021, 184, 857–862. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, Y.; Wang, J.; Xie, H.; Sun, X.; Zhu, X.; Wei, L.; Liu, Y. Protective effect of selenomethionine on T-2 toxin–induced rabbit immunotoxicity. Biol. Trace Elem. Res. 2022, 200, 172–182. [Google Scholar] [CrossRef]
- Wang, S.; Yin, J.; Liu, Y.; Jin, M.; Wang, Q.; Guo, J.; Gao, Z. An organic state trace element solution for rheumatoid arthritis treatment by modulating macrophage phenotypic from M1 to M2. Biomed. Pharmacother. 2024, 170, 116025. [Google Scholar] [CrossRef]
- Cheng, S.-C.; Huang, W.-C.; Pang, J.-H.S.; Wu, Y.-H.; Cheng, C.-Y. Quercetin inhibits the production of IL-1β-induced inflammatory cytokines and chemokines in ARPE-19 cells via the MAPK and NF-κB signaling pathways. Int. J. Mol. Sci. 2019, 20, 2957. [Google Scholar] [CrossRef]
- Zhao, H.; Wu, L.; Yan, G.; Chen, Y.; Zhou, M.; Wu, Y.; Li, Y. Inflammation and tumor progression: Signaling pathways and targeted intervention. Signal Transduct. Target. Ther. 2021, 6, 263. [Google Scholar] [CrossRef]
- Singh, S.; Anshita, D.; Ravichandiran, V. MCP-1: Function, regulation, and involvement in disease. Int. Immunopharmacol. 2021, 101, 107598. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, S.; Yan, L. Dietary supplementation with methylseleninic acid inhibits mammary tumorigenesis and metastasis in male MMTV-PyMT mice. Biol. Trace Elem. Res. 2018, 184, 186–195. [Google Scholar] [CrossRef]
- Catalioto, R.-M.; A Maggi, C.; Giuliani, S. Intestinal epithelial barrier dysfunction in disease and possible therapeutical interventions. Curr. Med. Chem. 2011, 18, 398–426. [Google Scholar] [CrossRef]
- Wen, Z.; Du, M.; Tang, Z.; Zhou, T.; Zhang, Z.; Song, H.; Xiang, X.; Han, X. Low molecular seleno-aminopolysaccharides protect the intestinal mucosal barrier of rats under weaning stress. Int. J. Mol. Sci. 2019, 20, 5727. [Google Scholar] [CrossRef]
- Chang, Y.; Wang, K.; Wen, M.; Wu, B.; Liu, G.; Zhao, H.; Chen, X.; Cai, J.; Jia, G. Organic zinc glycine chelate is better than inorganic zinc in improving growth performance of cherry valley ducks by regulating intestinal morphology, barrier function, and the gut microbiome. J. Anim. Sci. 2023, 101, skad279. [Google Scholar] [CrossRef]
- Kim, H.s.; Kim, S.; Shin, S.J.; Park, Y.H.; Nam, Y.; Kim, C.w.; Lee, K.w.; Kim, S.-M.; Jung, I.D.; Yang, H.D. Gram-negative bacteria and their lipopolysaccharides in Alzheimer’s disease: Pathologic roles and therapeutic implications. Transl. Neurodegener. 2021, 10, 49. [Google Scholar] [CrossRef]
- Cucca, V.; Ramirez, G.A.; Pignatti, P.; Asperti, C.; Russo, M.; Della-Torre, E.; Breda, D.; Burastero, S.E.; Dagna, L.; Yacoub, M.-R. Basal serum diamine oxidase levels as a biomarker of histamine intolerance: A retrospective cohort study. Nutrients 2022, 14, 1513. [Google Scholar] [CrossRef]
- Rossi, G.; Cerquetella, M.; Scarpona, S.; Pengo, G.; Fettucciari, K.; Bassotti, G.; Jergens, A.; Suchodolski, J. Effects of probiotic bacteria on mucosal polyamines levels in dogs with IBD and colonic polyps: A preliminary study. Benef. Microbes 2018, 9, 247–255. [Google Scholar] [CrossRef]
- Barbara, G.; Barbaro, M.R.; Fuschi, D.; Palombo, M.; Falangone, F.; Cremon, C.; Marasco, G.; Stanghellini, V. Inflammatory and microbiota-related regulation of the intestinal epithelial barrier. Front. Nutr. 2021, 8, 718356. [Google Scholar] [CrossRef]
- Van Spaendonk, H.; Ceuleers, H.; Witters, L.; Patteet, E.; Joossens, J.; Augustyns, K.; Lambeir, A.-M.; De Meester, I.; De Man, J.G.; De Winter, B.Y. Regulation of intestinal permeability: The role of proteases. World. J. Gastroenterol. 2017, 23, 2106. [Google Scholar] [CrossRef]
- Veres-Székely, A.; Szász, C.; Pap, D.; Szebeni, B.; Bokrossy, P.; Vannay, Á. Zonulin as a potential therapeutic target in microbiota-gut-brain axis disorders: Encouraging results and emerging questions. Int. J. Mol. Sci. 2023, 24, 7548. [Google Scholar] [CrossRef] [PubMed]
- Peoc’h, K.; Nuzzo, A.; Guedj, K.; Paugam, C.; Corcos, O. Diagnosis biomarkers in acute intestinal ischemic injury: So close, yet so far. Clin. Chem. Lab. Med. 2018, 56, 373–385. [Google Scholar] [CrossRef] [PubMed]
- Tyszko, M.; Lemańska-Perek, A.; Śmiechowicz, J.; Tomaszewska, P.; Biecek, P.; Gozdzik, W.; Adamik, B. Citrulline, intestinal fatty acid-binding protein and the acute gastrointestinal injury score as predictors of gastrointestinal failure in patients with sepsis and septic shock. Nutrients 2023, 15, 2100. [Google Scholar] [CrossRef] [PubMed]
- Mo, R.; Zhang, M.; Wang, H.; Liu, T.; Liu, P.; Wu, Y. Chitosan enhances intestinal health in cats by altering the composition of gut microbiota and metabolites. Metabolites 2023, 13, 529. [Google Scholar] [CrossRef]
- Ritchie, L.E.; Burke, K.F.; Garcia-Mazcorro, J.F.; Steiner, J.M.; Suchodolski, J.S. Characterization of fecal microbiota in cats using universal 16S rRNA gene and group-specific primers for Lactobacillus and Bifidobacterium spp. Vet. Microbiol. 2010, 144, 140–146. [Google Scholar] [CrossRef]
- Suchodolski, J.S. Intestinal microbiota of dogs and cats: A bigger world than we thought. Vet. Clin. Small Anim. Pract. 2011, 41, 261–272. [Google Scholar] [CrossRef]
- Portincasa, P.; Bonfrate, L.; Vacca, M.; De Angelis, M.; Farella, I.; Lanza, E.; Khalil, M.; Wang, D.Q.-H.; Sperandio, M.; Di Ciaula, A. Gut microbiota and short chain fatty acids: Implications in glucose homeostasis. Int. J. Mol. Sci. 2022, 23, 1105. [Google Scholar] [CrossRef]
- Gieryńska, M.; Szulc-Dąbrowska, L.; Struzik, J.; Mielcarska, M.B.; Gregorczyk-Zboroch, K.P. Integrity of the intestinal barrier: The involvement of epithelial cells and microbiota—A mutual relationship. Animals 2022, 12, 145. [Google Scholar] [CrossRef]
- Rabee, A.E.; Khalil, M.M.; Khadiga, G.A.; Elmahdy, A.; Sabra, E.A.; Zommara, M.A.; Khattab, I.M. Response of rumen fermentation and microbiota to dietary supplementation of sodium selenite and bio-nanostructured selenium in lactating Barki sheep. BMC Vet. Res. 2023, 19, 247. [Google Scholar] [CrossRef]
- Flint, H.J.; Duncan, S.H.; Scott, K.P.; Louis, P. Links between diet, gut microbiota composition and gut metabolism. Proc. Nutr. Soc. 2015, 74, 13–22. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, S.; Gan, R.; Zhou, T.; Xu, D.; Li, H. Impacts of gut bacteria on human health and diseases. Int. J. Mol. Sci. 2015, 16, 7493–7519. [Google Scholar] [CrossRef] [PubMed]
- Burrello, C.; Giuffrè, M.R.; Macandog, A.D.; Diaz-Basabe, A.; Cribiù, F.M.; Lopez, G.; Borgo, F.; Nezi, L.; Caprioli, F.; Vecchi, M. Fecal microbiota transplantation controls murine chronic intestinal inflammation by modulating immune cell functions and gut microbiota composition. Cells 2019, 8, 517. [Google Scholar] [CrossRef] [PubMed]
- Peterson, C.T.; Sharma, V.; Elmén, L.; Peterson, S.N. Immune homeostasis, dysbiosis and therapeutic modulation of the gut microbiota. Clin. Exp. Immunol. 2015, 179, 363–377. [Google Scholar] [CrossRef]
- Cheng, W.; Lu, J.; Li, B.; Lin, W.; Zhang, Z.; Wei, X.; Sun, C.; Chi, M.; Bi, W.; Yang, B. Effect of functional oligosaccharides and ordinary dietary fiber on intestinal microbiota diversity. Front. Microbiol. 2017, 8, 1750. [Google Scholar] [CrossRef]
- Coyte, K.Z.; Schluter, J.; Foster, K.R. The ecology of the microbiome: Networks, competition, and stability. Science 2015, 350, 663–666. [Google Scholar] [CrossRef]
- Leser, T.D.; Mølbak, L. Better living through microbial action: The benefits of the mammalian gastrointestinal microbiota on the host. Env. Microbiol. 2009, 11, 2194–2206. [Google Scholar] [CrossRef]
- An, J.; Liu, Y.; Wang, Y.; Fan, R.; Hu, X.; Zhang, F.; Yang, J.; Chen, J. The role of intestinal mucosal barrier in autoimmune disease: A potential target. Front. Immunol. 2022, 13, 871713. [Google Scholar] [CrossRef]
- Yoo, J.Y.; Groer, M.; Dutra, S.V.O.; Sarkar, A.; McSkimming, D.I. Gut microbiota and immune system interactions. Microorganisms 2020, 8, 1587. [Google Scholar] [CrossRef]
- Chuang, W.-H.; Arundhathi, A.; Lu, C.; Chen, C.-C.; Wu, W.-C.; Susanto, H.; Purnomo, J.D.; Wang, C.-H. Altered plasma acylcarnitine and amino acid profiles in type 2 diabetic kidney disease. Metabolomics 2016, 12, 108. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, W.; Cui, X.; Zhu, P.; Li, S.; Yuan, S.; Peng, D.; Peng, C. Ganoderma lucidum ethanol extracts ameliorate hepatic fibrosis and promote the communication between metabolites and gut microbiota g_Ruminococcus through the NF-κB and TGF-β1/Smads pathways. J. Ethnopharmacol. 2024, 322, 117656. [Google Scholar] [CrossRef]
- Chang, M.-L.; Yang, S.-S. Metabolic signature of hepatic fibrosis: From individual pathways to systems biology. Cells 2019, 8, 1423. [Google Scholar] [CrossRef] [PubMed]
- Syed, S.K.; Bui, H.H.; Beavers, L.S.; Farb, T.B.; Ficorilli, J.; Chesterfield, A.K.; Kuo, M.-S.; Bokvist, K.; Barrett, D.G.; Efanov, A.M. Regulation of GPR119 receptor activity with endocannabinoid-like lipids. Am. J. Physiol. Endocrinol. Metab. 2012, 303, E1469–E1478. [Google Scholar] [CrossRef] [PubMed]
- Ferrell, J.M.; Dilts, M.; Stahl, Z.; Boehme, S.; Pokhrel, S.; Wang, X.; Chiang, J.Y. Altered serotonin metabolism in Takeda G protein-coupled receptor 5 knockout mice protects against diet-induced hepatic fibrosis. Liver Res. 2022, 6, 214–226. [Google Scholar] [CrossRef]




| Score | Criteria |
|---|---|
| 1 | “Bullet like”, disintegrates with minimal pressure The stool is hard and dry, and it fractures when compressed |
| 2 | Properly shaped, it does not leave any residue when lifted Well-formed with slightly damp surface, leaves a trace when lifted |
| 3 | Moist and starting to lose its shape, it leaves a clear mark when lifted Very moist, retaining some definite form |
| 4 | Most or all structure is lost, lacking any definite shape Liquid stool with minimal consistency |
| 5 | Completely liquid stool |
| Items | ITM | ITM + OTM | OTM | p-Valves |
|---|---|---|---|---|
| BW at d 0, kg | 4.02 ± 0.08 | 3.88 ± 0.20 | 3.39 ± 0.32 | 0.14 |
| BW at d 28, kg | 3.92 ± 0.13 | 3.80 ± 0.22 | 3.41 ± 0.35 | 0.34 |
| ADG, g/d | −3.75 ± 4.08 | −3.10 ± 2.30 | 0.42 ± 4.81 | 0.72 |
| Fecal score | 1.95 ± 0.08 | 2.05 ± 0.10 | 2.08 ± 0.06 | 0.50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, Y.; Zhang, M.; Wang, H.; Yu, T.; Zhang, A.; Lin, G.; Guo, Y.; Wu, Y. Organic Trace Minerals Enhance the Gut Health of British Shorthair Cats by Regulating the Structure of Intestinal Microbiota. Metabolites 2024, 14, 494. https://doi.org/10.3390/metabo14090494
Cui Y, Zhang M, Wang H, Yu T, Zhang A, Lin G, Guo Y, Wu Y. Organic Trace Minerals Enhance the Gut Health of British Shorthair Cats by Regulating the Structure of Intestinal Microbiota. Metabolites. 2024; 14(9):494. https://doi.org/10.3390/metabo14090494
Chicago/Turabian StyleCui, Yingyue, Mingrui Zhang, Haotian Wang, Tong Yu, Anxuan Zhang, Gang Lin, Yuhan Guo, and Yi Wu. 2024. "Organic Trace Minerals Enhance the Gut Health of British Shorthair Cats by Regulating the Structure of Intestinal Microbiota" Metabolites 14, no. 9: 494. https://doi.org/10.3390/metabo14090494
APA StyleCui, Y., Zhang, M., Wang, H., Yu, T., Zhang, A., Lin, G., Guo, Y., & Wu, Y. (2024). Organic Trace Minerals Enhance the Gut Health of British Shorthair Cats by Regulating the Structure of Intestinal Microbiota. Metabolites, 14(9), 494. https://doi.org/10.3390/metabo14090494









