Identification and Free Radical Scavenging Activity of Oligopeptides from Mixed-Distillate Fermented Baijiu Grains and Soy Sauce Residue
Abstract
1. Introduction
2. Material and Methods
2.1. Materials
2.1.1. Chemicals
2.1.2. Preparation of Mixed Distillation of Jiupei and Jiangzha
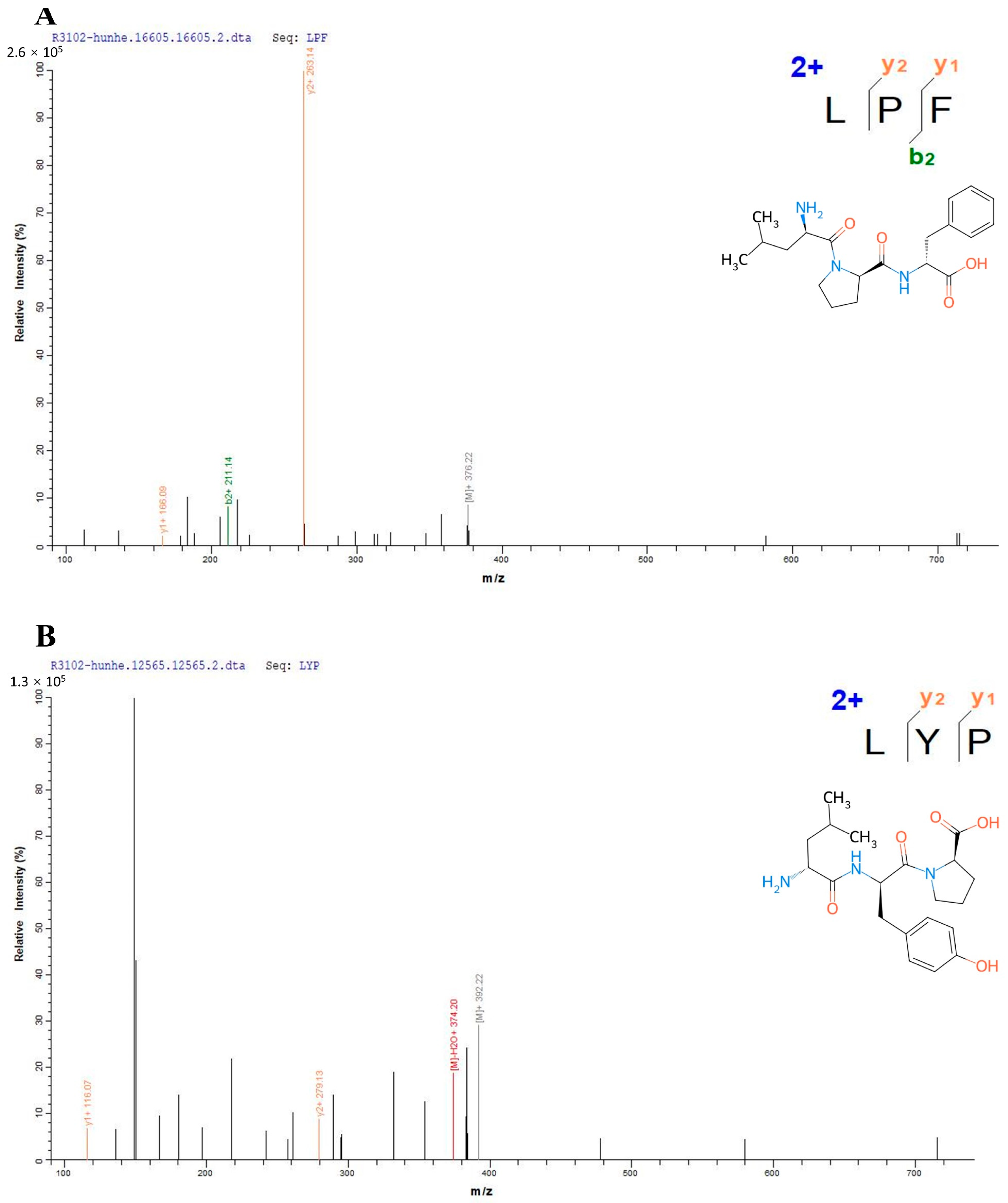
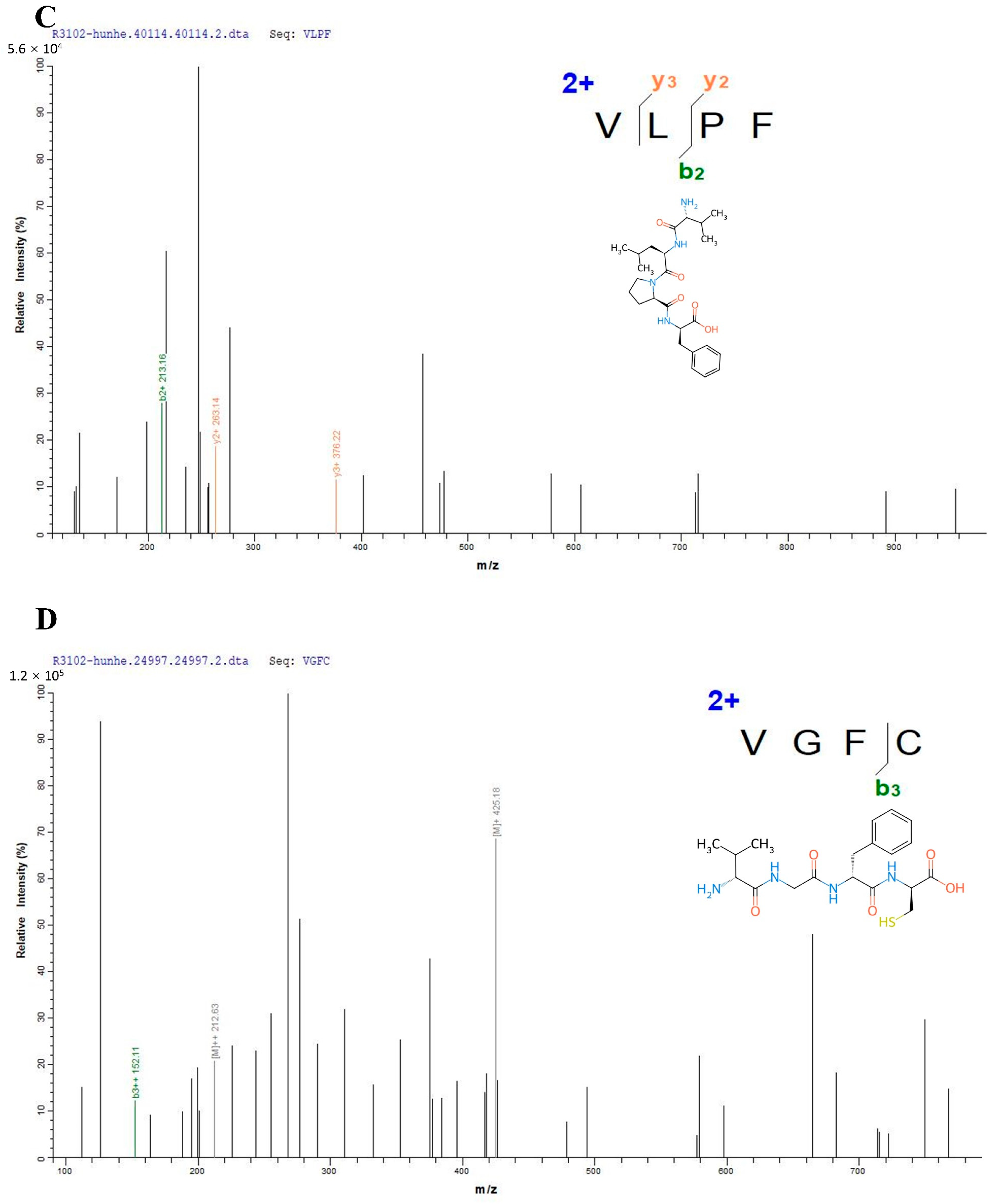
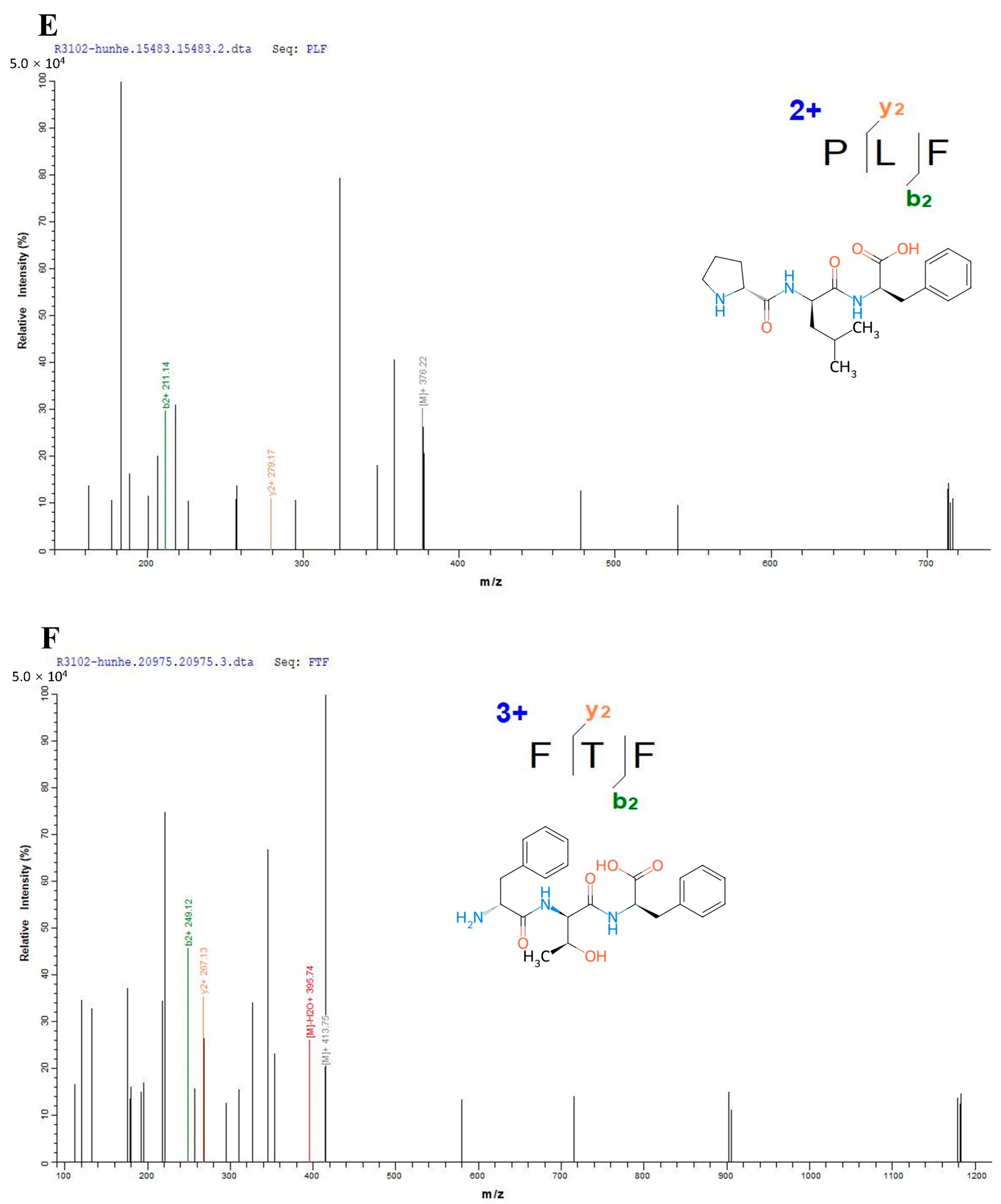
2.2. Structural Identification of the Peptides
2.3. Synthesis of Peptides
2.4. Determination of the Antioxidant Activity of the Peptides
2.4.1. DPPH Radical Scavenging Rate
2.4.2. ABTS•+ Scavenging Rate
2.4.3. Hydroxyl Radical Scavenging Rate
2.5. IC50 Calculation
2.6. Molecular Docking Simulation
2.7. Statistical Analysis
3. Results and Discussion
3.1. Structural Characterization of the Peptides from Mixed Distillation of Jiupei and Jiangzha
3.2. Antioxidant Activity of the Peptides from the Mixed Distillation of Jiupei and Jiangzha
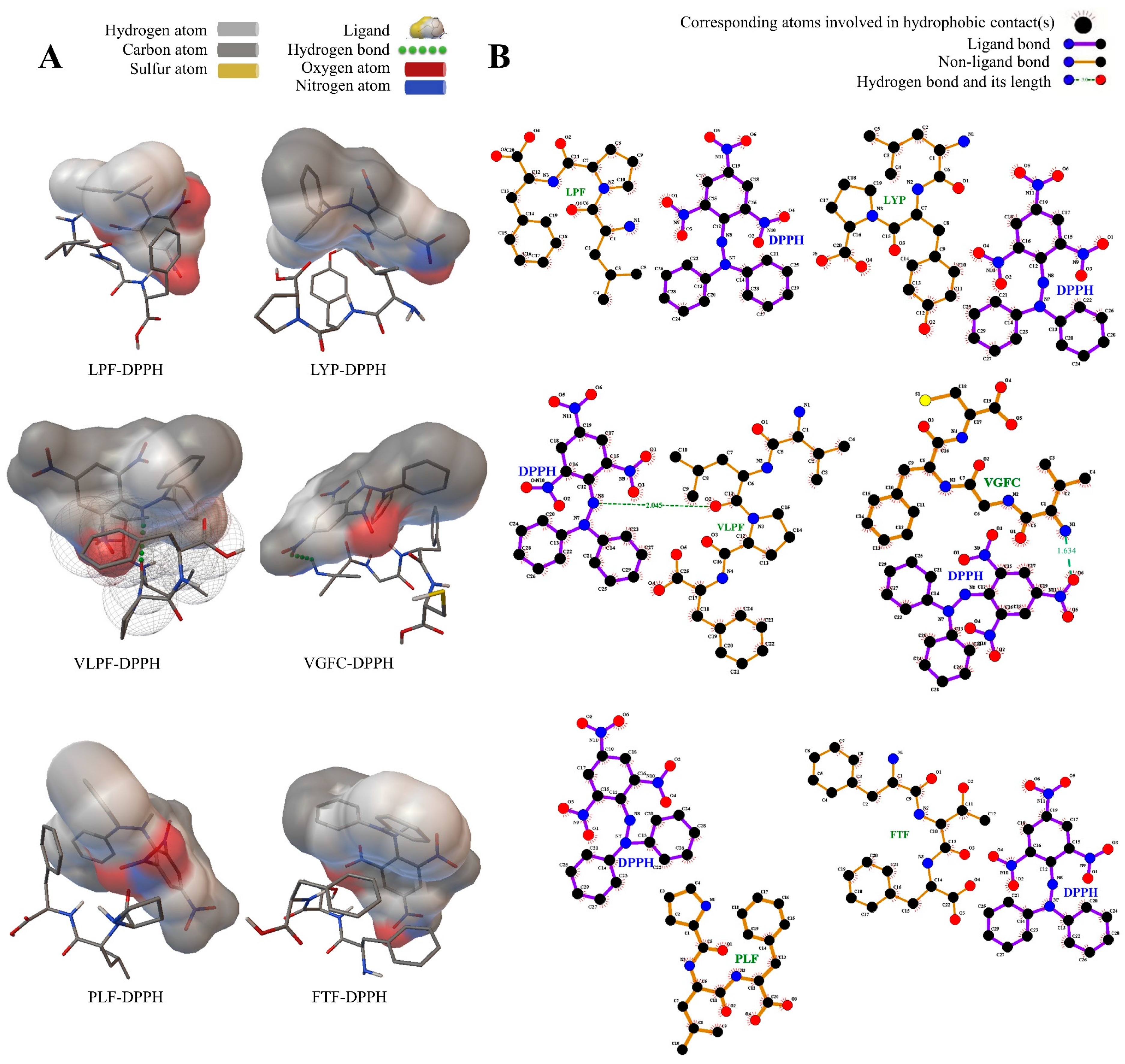
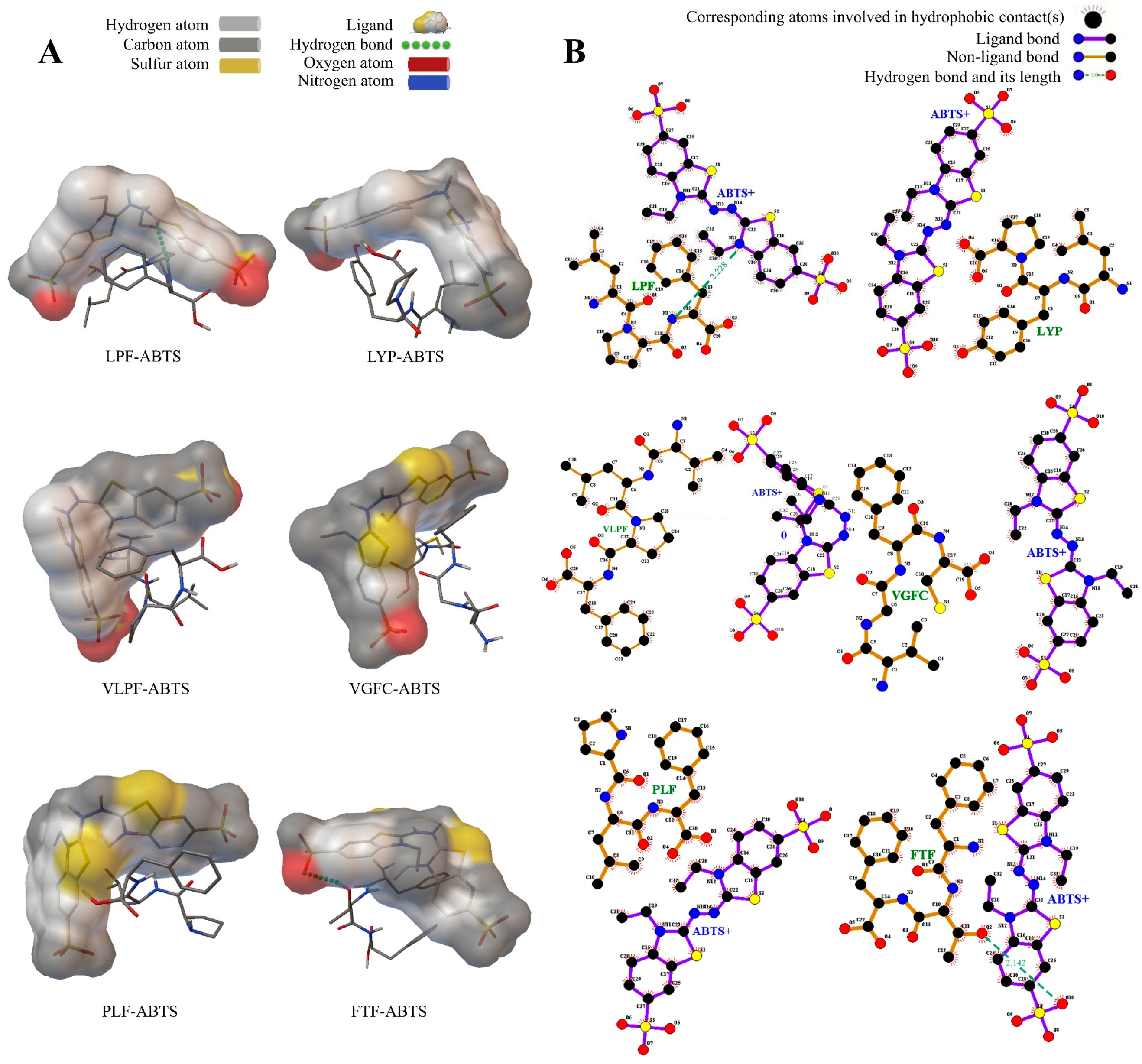
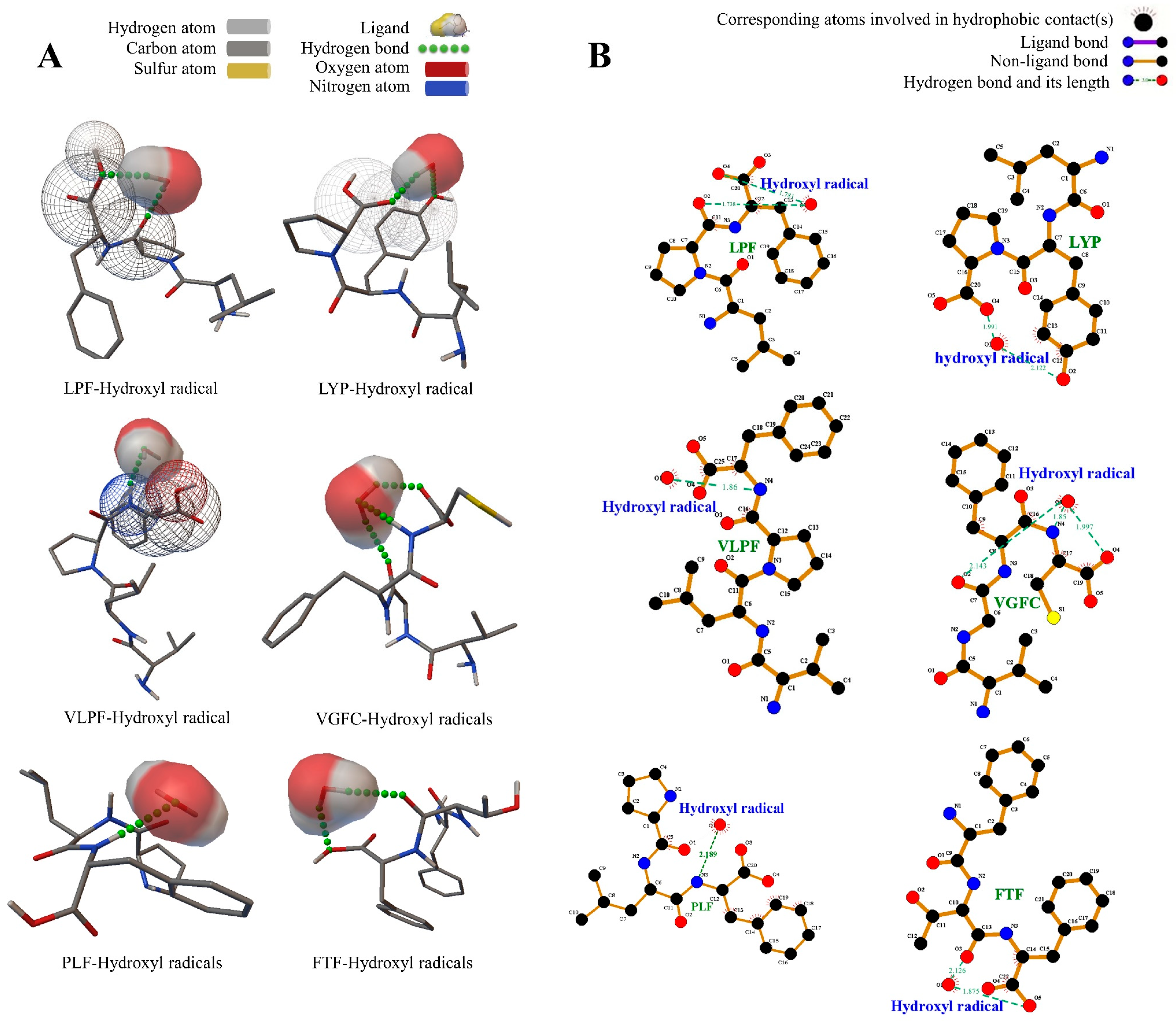
3.3. Intermolecular Interaction between the Peptides and Free Radicals
3.3.1. The Peptides and DPPH Free Radicals
3.3.2. The Peptides and ABTS+ Free Radicals
3.3.3. The Peptides and Hydroxide Free Radicals
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Østergaard, J.A.; Cooper, M.E.; Jandeleit-Dahm, K.A.M. Targeting Oxidative Stress and Anti-Oxidant Defence in Diabetic Kidney Disease. J. Nephrol. 2020, 33, 917–929. [Google Scholar] [CrossRef] [PubMed]
- Di Meo, S.; Venditti, P. Evolution of the Knowledge of Free Radicals and Other Oxidants. Oxid. Med. Cell Longev. 2020, 2020, 9829176. [Google Scholar] [CrossRef] [PubMed]
- Aboonabi, A.; Singh, I. The Effectiveness of Antioxidant Therapy in Aspirin Resistance, Diabetes Population for Prevention of Thrombosis. Biomed. Pharmacother. 2016, 83, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Ak, T.; Gülçin, İ. Antioxidant and Radical Scavenging Properties of Curcumin. Chem. Biol. Interact. 2008, 174, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Amorati, R.; Valgimigli, L. Advantages and Limitations of Common Testing Methods for Antioxidants. Free Radic. Res. 2015, 49, 633–649. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.W.; Han, B.Z. Baijiu (白酒), Chinese Liquor: History, Classification and Manufacture. J. Ethn. Foods 2016, 3, 19–25. [Google Scholar] [CrossRef]
- Zhao, H.; Li, H.; Sun, G.; Yang, B.; Zhao, M. Assessment of Endogenous Antioxidative Compounds and Antioxidant Activities of Lager Beers. J. Sci. Food Agric. 2013, 93, 910–917. [Google Scholar] [CrossRef] [PubMed]
- Boulton, R.B.; Singleton, V.L.; Bisson, L.F.; Kunkee, R.E. Principles and Practices of Winemaking; Springer: Boston, MA, USA, 1996. [Google Scholar]
- Iyengar, R.; McEvily, A.J. Anti-Browning Agents: Alternatives to the Use of Sulfites in Foods. Trends Food Sci. Tech. 1992, 3, 60–64. [Google Scholar] [CrossRef]
- Cho, Y.S.; Kim, J.J.; Jeon, G.; Chung, M.-S.; Joo, Y.; Lee, K.-W. Total SO2 Levels and Risk Assessment of Wine and Fruit Wine Consumed in South Korea. Food Control 2021, 127, 108124. [Google Scholar] [CrossRef]
- Garde-Cerdán, T.; Marsellés-Fontanet, A.R.; Arias-Gil, M.; Martín-Belloso, O.; Ancín-Azpilicueta, C. Influence of SO2 on the Consumption of Nitrogen Compounds through Alcoholic Fermentation of Must Sterilized by Pulsed Electric Fields. Food Chem. 2007, 103, 771–777. [Google Scholar] [CrossRef]
- Vally, H.; Misso, N.L.A.; Madan, V. Clinical Effects of Sulphite Additives. Clin. Exp. Allergy 2009, 39, 1643–1651. [Google Scholar] [CrossRef] [PubMed]
- Winstel, D.; Marchal, A.; Nioi, C. Optimization of Extraction and Development of an LC-HRMS Method to Quantify Glutathione and Glutathione Disulfide in White Wine Lees and Yeast Derivatives. Food Chem. 2024, 439, 138121. [Google Scholar] [CrossRef] [PubMed]
- Huo, J.Y.; Sun, B.G.; Zeng, F.; Sun, J.Y.; SUN, X.T.; Li, H.H.; Luo, X.L.; Huang, M.Q. Identification of a Tripeptide Arg-Asn-His from Chinese Baijiu and Its Antioxidant Activity. Food Sci. 2018, 39, 126. [Google Scholar]
- Wu, J.; Sun, B.; Luo, X.; Zhao, M.; Zheng, F.; Sun, J.; Li, H.; Sun, X.; Huang, M. Cytoprotective Effects of a Tripeptide from Chinese Baijiu against AAPH-Induced Oxidative Stress in HepG2 Cells via Nrf2 Signaling. RSC Adv. 2018, 8, 10898–10906. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Huo, J.; Wu, J.; Zhao, M.; Zheng, F.; Sun, J.; Sun, X.; Li, H. Interactions between P-Cresol and Ala-Lys-Arg-Ala (AKRA) from Sesame-Flavor-Type Baijiu. Langmuir 2018, 34, 12549–12559. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yuan, S.; Yong, X.; Zhao, T.; Liu, J. Research Progress on Small Peptides in Chinese Baijiu. J. Funct. Foods 2020, 72, 104081. [Google Scholar] [CrossRef]
- Yamamoto, S.; Shiga, K.; Kodama, Y.; Imamura, M.; Uchida, R.; Obata, A.; Bamba, T.; Fukusaki, E. Analysis of the Correlation between Dipeptides and Taste Differences among Soy Sauces by Using Metabolomics-Based Component Profiling. J. Biosci. Bioeng. 2014, 118, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.L.; Watanabe, K.; Shiraishi, K.; Ueki, T.; Noda, Y.; Matsui, T.; Matsumoto, K. Identification of ACE-Inhibitory Peptides in Salt-Free Soy Sauce That Are Transportable across Caco-2 Cell Monolayers. Peptides 2008, 29, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Luo, Y.; Qi, B.; Luo, J.; Wan, Y. Improving the Hydrolysis Efficiency of Soy Sauce Residue Using Ultrasonic Probe-Assisted Enzymolysis Technology. Ultrason. Sonochem. 2017, 35, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Sanjukta, S.; Padhi, S.; Sarkar, P.; Singh, S.P.; Sahoo, D.; Rai, A.K. Production, Characterization and Molecular Docking of Antioxidant Peptides from Peptidome of Kinema Fermented with Proteolytic bacillus spp. Food Res. Int. 2021, 141, 110161. [Google Scholar] [CrossRef] [PubMed]
- Shimada, K.; Fujikawa, K.; Yahara, K.; Nakamura, T. Antioxidative Properties of Xanthan on the Autoxidation of Soybean Oil in Cyclodextrin Emulsion. J. Agric. Food Chem. 1992, 40, 945–948. [Google Scholar] [CrossRef]
- Mejri, L.; Vásquez-Villanueva, R.; Hassouna, M.; Marina, M.L.; García, M.C. Identification of Peptides with Antioxidant and Antihypertensive Capacities by RP-HPLC-Q-TOF-MS in Dry Fermented Camel Sausages Inoculated with Different Starter Cultures and Ripening Times. Food Res. Int. 2017, 100, 708–716. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jiang, B.; Zhang, T.; Mu, W.; Liu, J. Antioxidant and Free Radical-Scavenging Activities of Chickpea Protein Hydrolysate (CPH). Food Chem. 2008, 106, 444–450. [Google Scholar] [CrossRef]
- Zhang, X.; Li, X.; Zhao, Y.; Wu, Q.; Wan, Y.; Yu, Y. Endogenous Peptides Identified in Soy Sauce Aroma Style Baijiu Which Interacts with the Main Flavor Compounds during the Distillation Process. Foods 2022, 11, 3339. [Google Scholar] [CrossRef] [PubMed]
- Laskowski, R.A.; Swindells, M.B. LigPlot+: Multiple Ligand-Protein Interaction Diagrams for Drug Discovery. J. Chem. Inf. Model. 2011, 51, 2778–2786. [Google Scholar] [CrossRef] [PubMed]
- Wong, F.C.; Xiao, J.; Wang, S.; Ee, K.Y.; Chai, T.T. Advances on the Antioxidant Peptides from Edible Plant Sources. Trends Food Sci. Techn. 2020, 99, 44–57. [Google Scholar] [CrossRef]
- Benslima, A.; Sellimi, S.; Hamdi, M.; Nasri, R.; Jridi, M.; Cot, D.; Li, S.; Nasri, M.; Zouari, N. The Brown Seaweed Cystoseira Schiffneri as a Source of Sodium Alginate: Chemical and Structural Characterization, and Antioxidant Activities. Food Biosci. 2021, 40, 100873. [Google Scholar] [CrossRef]
- Ding, Y.; Ko, S.-C.; Moon, S.-H.; Lee, S.-H. Protective Effects of Novel Antioxidant Peptide Purified from Alcalase Hydrolysate of Velvet Antler Against Oxidative Stress in Chang Liver Cells in Vitro and in a Zebrafish Model In Vivo. Int. J. Mol. Sci. 2019, 20, 5187. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Jabalera, A.; Cortés-Giraldo, I.; Dávila-Ortíz, G.; Vioque, J.; Alaiz, M.; Girón-Calle, J.; Megías, C.; Jiménez-Martínez, C. Influence of Peptides–Phenolics Interaction on the Antioxidant Profile of Protein Hydrolysates from Brassica Napus. Food Chem. 2015, 178, 346–357. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Lu, A.; Sun, Y.; Liu, B.; Zhang, J.; Zhang, L.; Huang, P.; Yang, A.; Li, Z.; Cao, Y.; et al. Purification and Identification of Antioxidant and Angiotensin Converting Enzyme-Inhibitory Peptides from Guangdong Glutinous Rice Wine. LWT 2022, 169, 113953. [Google Scholar] [CrossRef]
- He, D.; Wu, H.; Song, Y.; Wang, C.; Wang, D.; Wei, G. Se/GSH-Enriched Candida utilis Ameliorates DSS-Induced Colitis in Mice by Preventing Oxidative Stress and Improving Immune Responses. Food Biosci. 2024, 58, 103656. [Google Scholar] [CrossRef]
- Yang, H.; Wang, S.; Chen, M.; Lu, J. The Saccharomyces cerevisiae through Increasing the Expression of Membrane Transporter to Produce More GSH Inhibit the Browning of Pear Wine. Food Biosci. 2024, 58, 103689. [Google Scholar] [CrossRef]
- Samaranayaka, A.G.P.; Li-Chan, E.C.Y. Food-Derived Peptidic Antioxidants: A Review of Their Production, Assessment, and Potential Applications. J. Funct. Foods 2011, 3, 229–254. [Google Scholar] [CrossRef]
- Liu, D.; Chen, X.; Huang, J.; Huang, M.; Zhou, G. Generation of Bioactive Peptides from Duck Meat during Post-Mortem Aging. Food Chem. 2017, 237, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, M.; Mirdamadi, S.; Ehsani, M.R.; Aminlari, M. Production of Antioxidant and ACE-Inhibitory Peptides from Kluyveromyces Marxianus Protein Hydrolysates: Purification and Molecular Docking. J. Food Drug Anal. 2018, 26, 696–705. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Chi, H.; Ma, S.; Zhao, L.; Cai, S. Identification of Novel α-Glucosidase Inhibitory Peptides in Rice Wine and Their Antioxidant Activities Using in Silico and in Vitro Analyses. LWT 2023, 178, 114629. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, D.; Liu, M.; Li, Y.; Lv, R.; Li, X.; Wang, Q.; Ren, D.; Wu, L.; Zhou, H. Identification of Antioxidant Peptides Derived from Tilapia (Oreochromis niloticus) Skin and Their Mechanism of Action by Molecular Docking. Foods 2022, 11, 2576. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Mora, L.; Toldrá, F. Structure-Function Relationship of Small Peptides Generated during the Ripening of Spanish Dry-Cured Ham: Peptidome, Molecular Stability and Computational Modelling. Food Chem. 2022, 375, 131673. [Google Scholar] [CrossRef]
- Wei, G.; Zhao, Q.; Wang, D.; Fan, Y.; Shi, Y.; Huang, A. Novel ACE Inhibitory, Antioxidant and α-Glucosidase Inhibitory Peptides Identified from Fermented Rubing Cheese through Peptidomic and Molecular Docking. LWT 2022, 159, 113196. [Google Scholar] [CrossRef]
- Nikoo, M.; Benjakul, S.; Ehsani, A.; Li, J.; Wu, F.; Yang, N.; Xu, B.; Jin, Z.; Xu, X. Antioxidant and Cryoprotective Effects of a Tetrapeptide Isolated from Amur Sturgeon Skin Gelatin. J. Funct. Foods 2014, 7, 609–620. [Google Scholar] [CrossRef]
| Peptide | Radical Scavenging Ability (IC50, mg/mL) | ||
|---|---|---|---|
| DPPH• | ABTS•+ | Hydroxyl• | |
| Control (GSH) | 0.51 ± 0.10 a | 0.52 ± 0.10 a | 0.81 ± 0.36 a |
| LPF | 2.68 ± 1.78 c | 3.90 ± 2.72 c | 4.05 ± 0.37 c |
| LYP | 5.91 ± 3.08 e | 8.56 ± 2.50 d | 3.85 ± 0.37 c |
| VLPF | 3.94 ± 1.35 d | 0.53 ± 0.05 a | 5.45 ± 0.57 e |
| VGFC | 0.51 ± 0.01 a | 0.53 ± 0.11 a | 0.89 ± 0.18 b |
| PLF | 9.48 ± 3.15 e | 8.50 ± 0.19 d | 3.99 ± 0.10 c |
| FTF | 1.32 ± 2.45 b | 3.17 ± 0.33 b | 4.41 ± 0.10 d |
| Sequence | Radical | Electrostatic Energy (kcal/mol) | Binding Energy (kcal/mol) | Number of Hydrogen Bonds | Hydrogen Bond Distance | Residues Formed Hydrogen Bonds with the Ligand |
|---|---|---|---|---|---|---|
| LPF | DPPH | −0.16 | 1.88 | 0 | - | - |
| ABTS | 0.75 | 61.63 | 1 | 2.228 | Phe (HN) | |
| Hydroxyl | −0.34 | −1.48 | 2 | 1.738, 1.781 | Phe (O), Pro (O) | |
| LYP | DPPH | −0.59 | 6.15 | 0 | - | - |
| ABTS | 0.57 | 36.91 | 0 | - | - | |
| Hydroxyl | −0.22 | −1.46 | 2 | 1.991, 2.122 | Pro (O), Tyr (O) | |
| VLPF | DPPH | 0.22 | 4.96 | 1 | 2.045 | Leu (O) |
| ABTS | 0.62 | 29.18 | 0 | - | - | |
| Hydroxyl | −0.24 | −1.78 | 1 | 1.86 | Phe (HN) | |
| VGFC | DPPH | −1.29 | −1.26 | 1 | 1.634 | Val (HN) |
| ABTS | 0.03 | −1.33 | 0 | - | - | |
| Hydroxyl | −0.26 | −1.93 | 3 | 1.997, 2.143, 1.85 | Cys (O), Gly (O), Cys (HN) | |
| PLF | DPPH | −1.09 | 14.86 | 0 | - | - |
| ABTS | 0.43 | 20.83 | 0 | - | - | |
| Hydroxyl | −0.02 | −1.75 | 1 | 2.189 | Phe (HN) | |
| FTF | DPPH | −0.8 | −0.97 | 0 | - | - |
| ABTS | −0.23 | 62 | 1 | 2.142 | Thr (H) | |
| Hydroxyl | −0.3 | −1.47 | 2 | 1.875, 2.126 | Phe (O), Thr (O) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Liu, X.; Zhang, S.; Wang, Z.; Tian, S.; Wu, Q. Identification and Free Radical Scavenging Activity of Oligopeptides from Mixed-Distillate Fermented Baijiu Grains and Soy Sauce Residue. Metabolites 2024, 14, 298. https://doi.org/10.3390/metabo14060298
Zhao Y, Liu X, Zhang S, Wang Z, Tian S, Wu Q. Identification and Free Radical Scavenging Activity of Oligopeptides from Mixed-Distillate Fermented Baijiu Grains and Soy Sauce Residue. Metabolites. 2024; 14(6):298. https://doi.org/10.3390/metabo14060298
Chicago/Turabian StyleZhao, Yunhao, Xiangyue Liu, Sijie Zhang, Zhengwei Wang, Shanlin Tian, and Qiang Wu. 2024. "Identification and Free Radical Scavenging Activity of Oligopeptides from Mixed-Distillate Fermented Baijiu Grains and Soy Sauce Residue" Metabolites 14, no. 6: 298. https://doi.org/10.3390/metabo14060298
APA StyleZhao, Y., Liu, X., Zhang, S., Wang, Z., Tian, S., & Wu, Q. (2024). Identification and Free Radical Scavenging Activity of Oligopeptides from Mixed-Distillate Fermented Baijiu Grains and Soy Sauce Residue. Metabolites, 14(6), 298. https://doi.org/10.3390/metabo14060298







