Long-Term Consumption of Purified Water Altered Amino Acid, Fatty Acid and Energy Metabolism in Livers of Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal and Experimental Design
2.2. Measurement of Blood and Urine Samples
2.3. Metabolomics Analysis
2.4. Statistics
3. Results
3.1. Data of Water Quality
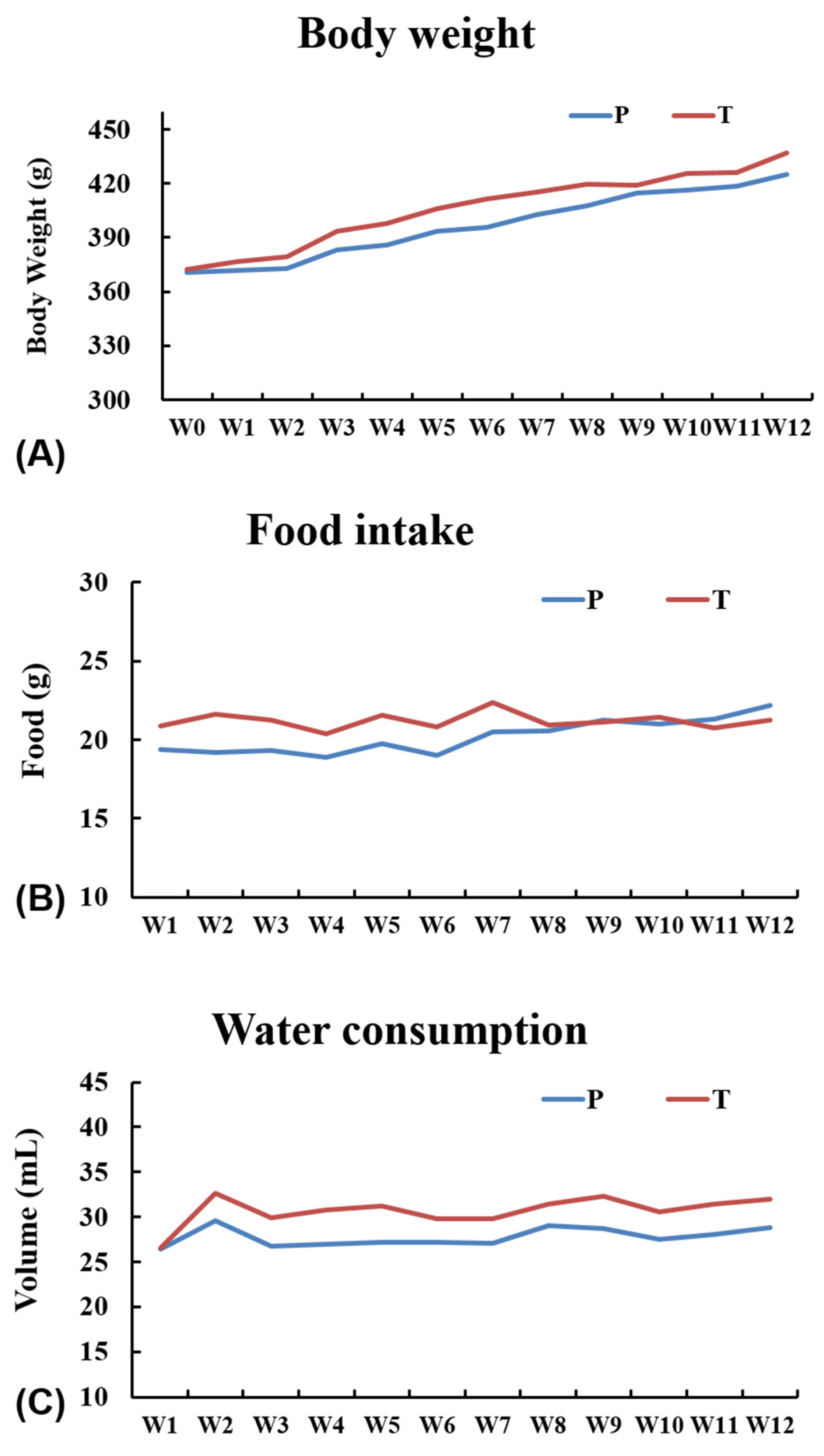
3.2. General Observations
3.3. Serum and Urine Biochemistry
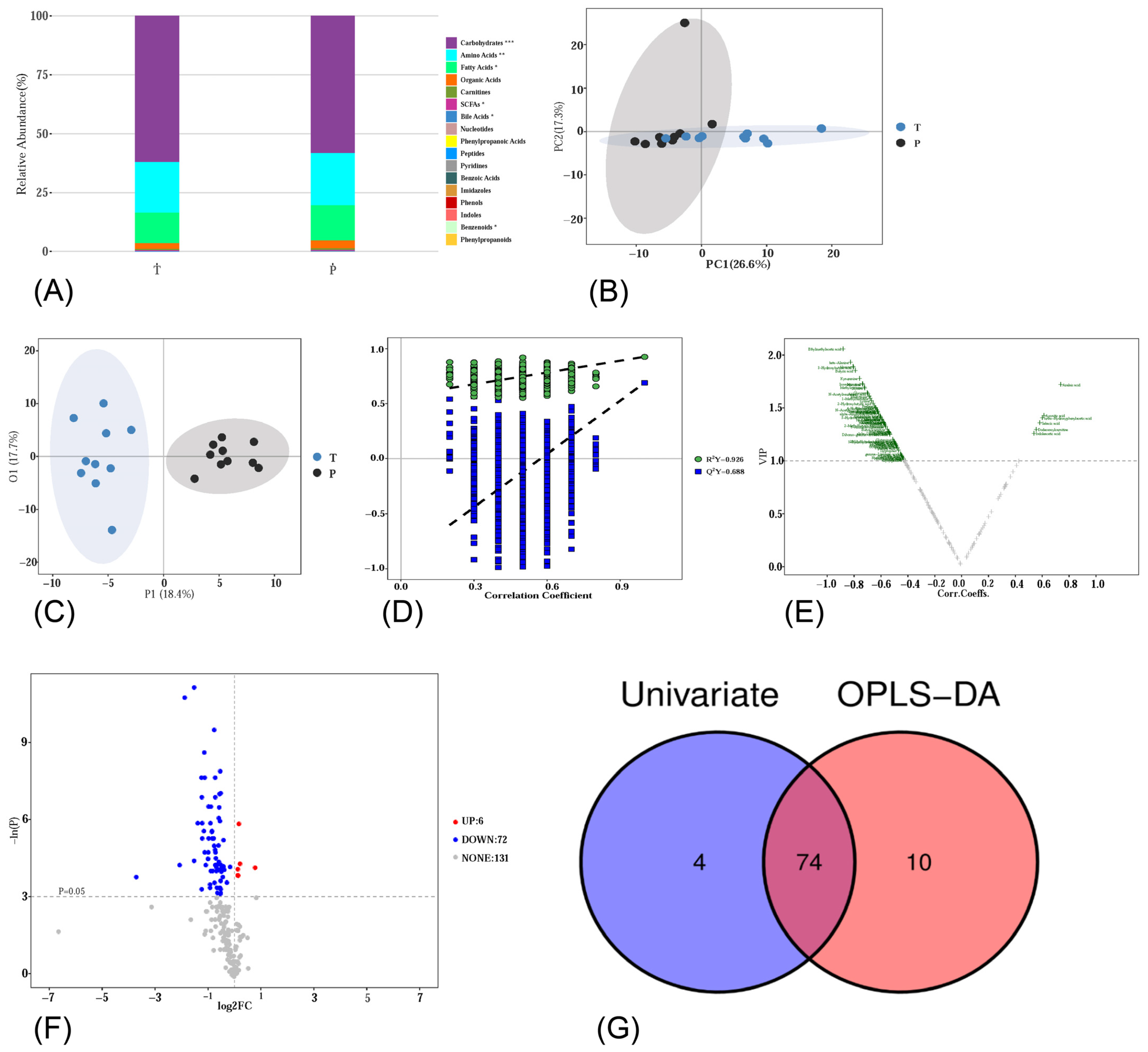
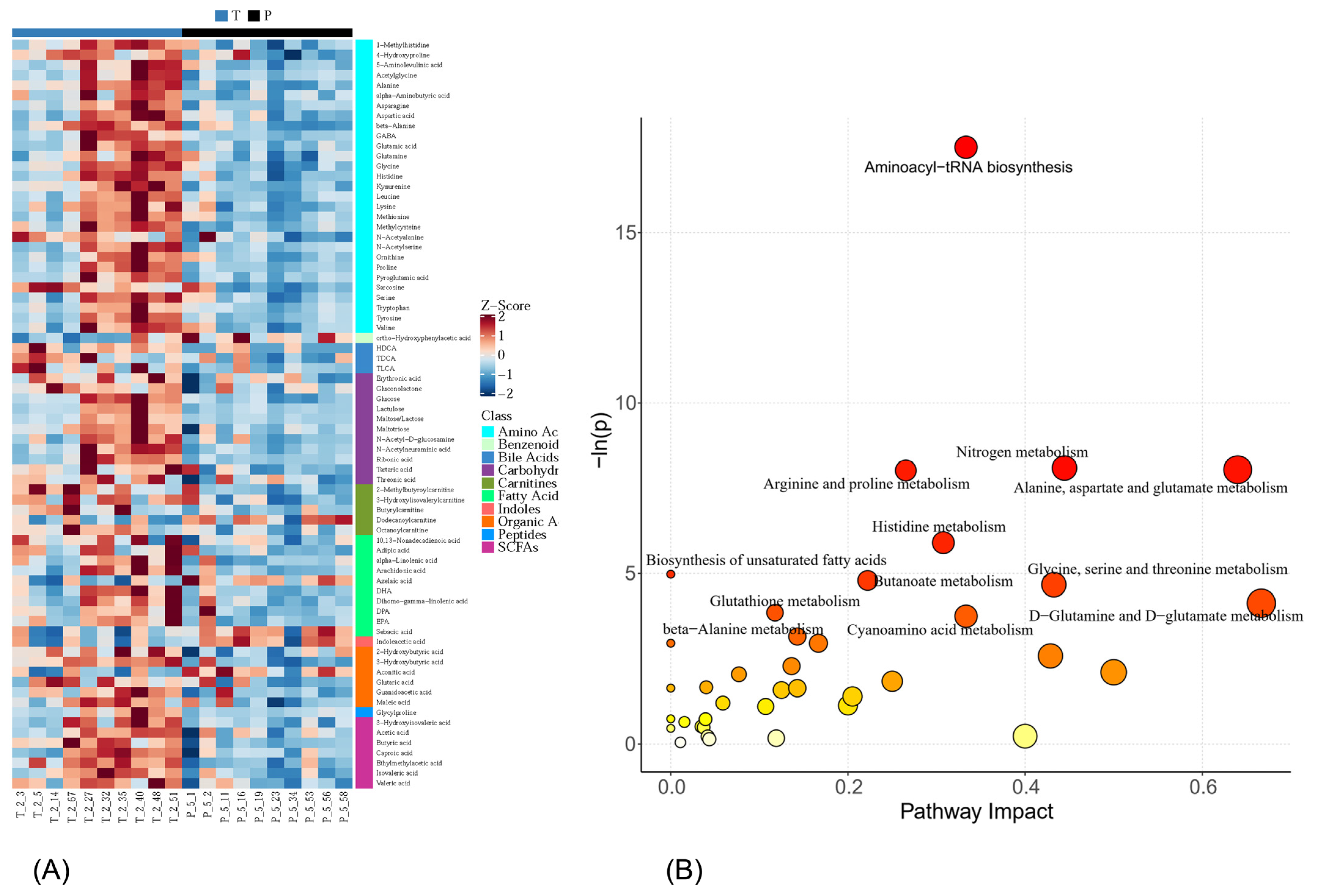
3.4. Metabolic Profiling
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mekonnen, M.M.; Hoekstra, A.Y. Four billion people facing severe water scarcity. Sci. Adv. 2016, 2, e1500323. [Google Scholar] [CrossRef] [PubMed]
- Ruan, G.; Wang, M.; An, Z.; Xu, G.; Ge, Y.; Zhao, H. Progress and Perspectives of Desalination in China. Membranes 2021, 11, 206. [Google Scholar] [CrossRef] [PubMed]
- Sedlak, D.L. The Unintended Consequences of the Reverse Osmosis Revolution. Environ. Sci. Technol. 2019, 53, 3999–4000. [Google Scholar] [CrossRef] [PubMed]
- Cotruvo, J.B.J. (Ed.) Calcium and Magnesium in Drinking-Water; WHO: Geneva, Switzerland, 2009. [Google Scholar]
- Kamalapriya, V.; Mani, R.; Venkatesh, V.; Kunhikannan, S. The Role of Low Mineral Water Consumption in Reducing the Mineral Density of Bones and Teeth: A Narrative Review. Cureus 2023, 15, e49119. [Google Scholar] [CrossRef] [PubMed]
- Kozisek, F. Health Risk from Drinking Demineralized Water. In Rolling Revision of the WHO Guideline for Drinking-Water; WHO: Geneva, Switzerland, 2004. [Google Scholar]
- Catling, L.A.; Abubakar, I.; Lake, I.R.; Swift, L.; Hunter, P.R. A systematic review of analytical observational studies investigating the association between cardiovascular disease and drinking water hardness. J. Water Health 2008, 6, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Lake, I.R.; Swift, L.; Catling, L.A.; Abubakar, I.; Sabel, C.E.; Hunter, P.R. Effect of water hardness on cardiovascular mortality: An ecological time series approach. J. Public Health 2010, 32, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Dahl, C.; Sogaard, A.J.; Tell, G.S.; Forsen, L.; Flaten, T.P.; Hongve, D.; Omsland, T.K.; Holvik, K.; Meyer, H.E.; Aamodt, G. Population data on calcium in drinking water and hip fracture: An association may depend on other minerals in water. A NOREPOS study. Bone 2015, 81, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Ma, X.; Tan, Y.; Wang, L.; Wang, J.; Lan, L.; Qiu, Z.; Luo, J.; Zeng, H.; Shu, W. Consumption of Very Low Mineral Water Is Associated with Lower Bone Mineral Content in Children. J. Nutr. 2019, 149, 1994–2000. [Google Scholar] [CrossRef] [PubMed]
- Jacqmin, H.; Commenges, D.; Letenneur, L.; Barberger-Gateau, P.; Dartigues, J.F. Components of drinking water and risk of cognitive impairment in the elderly. Am. J. Epidemiol. 1994, 139, 48–57. [Google Scholar] [CrossRef]
- Yang, C.Y.; Chiu, H.F.; Chang, C.C.; Wu, T.N.; Sung, F.C. Association of very low birth weight with calcium levels in drinking water. Environ. Res. 2002, 89, 189–194. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, J.; Tan, Y.; Wang, L.; Lin, H.; Lan, L.; Xiong, Y.; Huang, W.; Shu, W. Low-mineral direct drinking water in school may retard height growth and increase dental caries in schoolchildren in China. Environ. Int. 2018, 115, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Chiu, H.F.; Chang, C.C.; Chen, C.C.; Yang, C.Y. Calcium and magnesium in drinking water and risk of death from kidney cancer. J. Toxicol. Environ. Health A 2011, 74, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.Y.; Chiu, H.F.; Cheng, M.F.; Hsu, T.Y.; Cheng, M.F.; Wu, T.N. Calcium and magnesium in drinking water and the risk of death from breast cancer. J. Toxicol. Environ. Health A 2000, 60, 231–241. [Google Scholar] [PubMed]
- Luo, J.; Zhao, Q.; Zhang, L.; Qiu, Z.; Liu, L.; Chen, J.; Zeng, H.; Huang, Y.; Tan, Y.; Yang, L.; et al. The consumption of low-mineral bottled water increases the risk of cardiovascular disease: An experimental study of rabbits and young men. Int. J. Cardiol. 2013, 168, 4454–4456. [Google Scholar] [CrossRef] [PubMed]
- Pop, M.S.; Cheregi, D.C.; Onose, G.; Munteanu, C.; Popescu, C.; Rotariu, M.; Turnea, M.-A.; Dograru, G.; Ionescu, E.V.; Oprea, D.; et al. Exploring the Potential Benefits of Natural Calcium-Rich Mineral Waters for Health and Wellness: A Systematic Review. Nutrients 2023, 15, 3126. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.; Tan, Y.; Zeng, H.; Wang, L.; Wang, D.; Luo, J.; Zhang, L.; Huang, Y.; Chen, J.A.; Shu, W. Multi-generational drinking of bottled low mineral water impairs bone quality in female rats. PLoS ONE 2015, 10, e0121995. [Google Scholar] [CrossRef] [PubMed]
- Narciso, L.; Martinelli, A.; Torriani, F.; Frassanito, P.; Bernardini, R.; Chiarotti, F.; Marianelli, C. Natural Mineral Waters and Metabolic Syndrome: Insights from Obese Male and Female C57BL/6 Mice on Caloric Restriction. Front. Nutr. 2022, 9, 886078. [Google Scholar] [CrossRef] [PubMed]
- Murakami, S.; Goto, Y.; Ito, K.; Hayasaka, S.; Kurihara, S.; Soga, T.; Tomita, M.; Fukuda, S. The Consumption of Bicarbonate-Rich Mineral Water Improves Glycemic Control. Evid.-Based Complement. Altern. Med. 2015, 2015, 824395. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Xu, A.; Qiu, Z.; Wang, L.; Wang, J.; Luo, J.; Zeng, H.; Jin, H.; Wang, Y.; Xue, J.; et al. Drinking Natural Mineral Water Maintains Bone Health in Young Rats with Metabolic Acidosis. Front. Nutr. 2022, 9, 813202. [Google Scholar] [CrossRef]
- Alseekh, S.; Aharoni, A.; Brotman, Y.; Contrepois, K.; D’auria, J.; Ewald, J.; Ewald, J.C.; Fraser, P.D.; Giavalisco, P.; Hall, R.D.; et al. Mass spectrometry-based metabolomics: A guide for annotation, quantification and best reporting practices. Nat. Methods 2021, 18, 747–756. [Google Scholar] [CrossRef]
- GB 14924.3-2010; Laboratory Animals—Nutrients for Formula Feeds. China Standard Press: Beijing, China, 2011.
- Xie, G.; Wang, L.; Chen, T.; Zhou, K.; Zhang, Z.; Li, J.; Sun, B.; Guo, Y.; Wang, X.; Wang, Y.; et al. A Metabolite Array Technology for Precision Medicine. Anal. Chem. 2021, 93, 5709–5717. [Google Scholar] [CrossRef]
- Qiu, S.; Cai, Y.; Yao, H.; Lin, C.; Xie, Y.; Tang, S.; Zhang, A. Small molecule metabolites: Discovery of biomarkers and therapeutic targets. Signal Transduct. Target. Ther. 2023, 8, 132. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wu, B.; Zhang, X.; Li, A.; Cheng, S. Metabolic profiles in serum of mouse after chronic exposure to drinking water. Hum. Exp. Toxicol. 2011, 30, 1088–1095. [Google Scholar] [CrossRef]
- Wu, G. Dietary protein intake and human health. Food Funct. 2016, 17, 1251–1265. [Google Scholar] [CrossRef]
- Cao, Y.; Liu, S.; Liu, K.; Abbasi, I.H.R.; Cai, C.; Yao, J. Molecular mechanisms relating to amino acid regulation of protein synthesis. Nutr. Res. Rev. 2019, 32, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Berg, M.D.; Brandl, C.J. Transfer RNAs: Diversity in form and function. RNA Biol. 2020, 18, 316–339. [Google Scholar] [CrossRef]
- Kurmi, K.; Haigis, M.C. Nitrogen Metabolism in Cancer and Immunity. Trends Cell Biol. 2020, 30, 408–424. [Google Scholar] [CrossRef]
- Tsumoto, T. Excitatory amino acid transmitters and their receptors in neural circuits of the cerebral neocortex. Neurosci. Res. 1990, 9, 79–102. [Google Scholar] [CrossRef] [PubMed]
- Leinekugel, X.; Khalilov, I.; McLean, H.; Caillard, O.; Gaiarsa, J.L.; Ben-Ari, Y.; Khazipov, R. GABA is the principal fast-acting excitatory transmitter in the neonatal brain. Adv. Neurol. 1999, 79, 189–201. [Google Scholar]
- Finkelman, T.; Furman-Haran, E.; Paz, R.; Tal, A. Quantifying the excitatory-inhibitory balance: A comparison of SemiLASER and MEGA-SemiLASER for simultaneously measuring GABA and glutamate at 7T. Neuroimage 2022, 247, 118810. [Google Scholar] [CrossRef]
- Schur, R.R.; Draisma, L.W.; Wijnen, J.P.; Boks, M.P.; Koevoets, M.G.; Joels, M.; Klomp, D.W.; Kahn, R.S.; Vinkers, C.H. Brain GABA levels across psychiatric disorders: A systematic literature review and meta-analysis of (1) H-MRS studies. Hum. Brain Mapp. 2016, 37, 3337–3352. [Google Scholar] [CrossRef]
- Gillette-Guyonnet, S.; Andrieu, S.; Nourhashemi, F.; de La Gueronniere, V.; Grandjean, H.; Vellas, B. Cognitive impairment and composition of drinking water in women: Findings of the EPIDOS Study. Am. J. Clin. Nutr. 2005, 81, 897–902. [Google Scholar] [CrossRef] [PubMed]
- Rondeau, V.; Jacqmin-Gadda, H.; Commenges, D.; Helmer, C.; Dartigues, J.F. Aluminum and silica in drinking water and the risk of Alzheimer’s disease or cognitive decline: Findings from 15-year follow-up of the PAQUID cohort. Am. J. Epidemiol. 2009, 169, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Shu, W.Q.; Zhao, Q.; Chen, Q. Reproductive and neurobehavioral outcome of drinking purified water under magnesium deficiency in the rat’s diet. Food Chem. Toxicol. 2008, 46, 1495–1502. [Google Scholar] [CrossRef]
- Zeng, H.; Chen, J.A.; Liu, L.; Wang, D.H.; Fu, W.J.; Wang, L.Q.; Luo, J.H.; Zhang, L.; Tan, Y.; Qiu, Z.Q.; et al. Experimental comparison of the reproductive outcomes and early development of the offspring of rats given five common types of drinking water. PLoS ONE 2014, 9, e108955. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yao, Y.; Han, W. Proline Metabolism in Neurological and Psychiatric Disorders. Mol. Cells 2022, 45, 781–788. [Google Scholar] [CrossRef]
- Liang, X.; Zhang, L.; Natarajan, S.K.; Becker, D.F. Proline Mechanisms of Stress Survival. Antioxid. Redox Signal. 2013, 19, 998–1011. [Google Scholar] [CrossRef]
- Vettore, L.A.; Westbrook, R.L.; Tennant, D.A. Proline metabolism and redox; maintaining a balance in health and disease. Amino Acids 2021, 53, 1779–1788. [Google Scholar] [CrossRef]
- Holeček, M. Histidine in Health and Disease: Metabolism, Physiological Importance, and Use as a Supplement. Nutrients 2020, 12, 848. [Google Scholar] [CrossRef]
- Moro, J.; Tomé, D.; Schmidely, P.; Demersay, T.-C.; Azzout-Marniche, D. Histidine: A Systematic Review on Metabolism and Physiological Effects in Human and Different Animal Species. Nutrients 2020, 12, 1414. [Google Scholar] [CrossRef]
- Samovski, D.; Jacome-Sosa, M.; Abumrad, N.A. Fatty Acid Transport and Signaling: Mechanisms and Physiological Implications. Annu. Rev. Physiol. 2023, 85, 317–337. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, C.; Caramujo, M. The Various Roles of Fatty Acids. Molecules 2018, 23, 2583. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Hussain, M.; Jiang, B.; Zheng, L.; Pan, Y.; Hu, J.; Khan, A.; Ashraf, A.; Zou, X. Omega-3 long-chain polyunsaturated fatty acids: Metabolism and health implications. Prog. Lipid Res. 2023, 92, 101255. [Google Scholar] [CrossRef]
- Nilsen, D.W.T.; Aarsetoey, H.; Pönitz, V.; Brugger-Andersen, T.; Staines, H.; Harris, W.S.; Grundt, H. The prognostic utility of dihomo-gamma-linolenic acid (DGLA) in patients with acute coronary heart disease. Int. J. Cardiol. 2017, 249, 12–17. [Google Scholar] [CrossRef]
- Wang, D.D. Dietary n-6 polyunsaturated fatty acids and cardiovascular disease: Epidemiologic evidence. Prostaglandins Leukot. Essent. Fat. Acids 2018, 135, 5–9. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Sun, J.; Zhang, W.; Guo, Z.; Ma, Q. Arachidonic acid metabolism in health and disease. MedComm 2023, 4, e363. [Google Scholar] [CrossRef]
- Nilsen, D.W.T.; Myhre, P.L.; Kalstad, A.; Schmidt, E.B.; Arnesen, H.; Seljeflot, I. Serum Levels of Dihomo-Gamma (γ)-Linolenic Acid (DGLA) Are Inversely Associated with Linoleic Acid and Total Death in Elderly Patients with a Recent Myocardial Infarction. Nutrients 2021, 13, 3475. [Google Scholar] [CrossRef] [PubMed]
- Dalile, B.; Van Oudenhove, L.; Vervliet, B.; Verbeke, K. The role of short-chain fatty acids in microbiota–gut–brain communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478. [Google Scholar] [CrossRef] [PubMed]
- Macfarlane, S.; Macfarlane, G.T. Regulation of short-chain fatty acid production. Proc. Nutr. Soc. 2007, 62, 67–72. [Google Scholar] [CrossRef]
- Zhang, D.; Jian, Y.-P.; Zhang, Y.-N.; Li, Y.; Gu, L.-T.; Sun, H.-H.; Liu, M.-D.; Zhou, H.-L.; Wang, Y.-S.; Xu, Z.-X. Short-chain fatty acids in diseases. Cell Commun. Signal. 2023, 21, 212. [Google Scholar] [CrossRef]
- Hu, T.; Wu, Q.; Yao, Q.; Jiang, K.; Yu, J.; Tang, Q. Short-chain fatty acid metabolism and multiple effects on cardiovascular diseases. Ageing Res. Rev. 2022, 81, 101706. [Google Scholar] [CrossRef]
- Chandel, N.S. Carbohydrate Metabolism. Cold Spring Harb. Perspect. Biol. 2021, 13, a040568. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Shao, Y.; Yan, K.; Yao, T.; Liu, L.; Sun, F.; Wu, J.; Huang, Y. The Link between Trace Metal Elements and Glucose Metabolism: Evidence from Zinc, Copper, Iron, and Manganese-Mediated Metabolic Regulation. Metabolites 2023, 13, 1048. [Google Scholar] [CrossRef] [PubMed]
- Day, R.E.; Rogers, P.J.; Dawes, I.W.; Higgins, V.J. Molecular Analysis of Maltotriose Transport and Utilization by Saccharomycescerevisiae. Appl. Environ. Microbiol. 2002, 68, 5326–5335. [Google Scholar] [CrossRef] [PubMed]
- Morales, V.; Olano, A.; Corzo, N. Ratio of maltose to maltulose and furosine as quality parameters for infant formula. J. Agric. Food Chem. 2004, 52, 6732–6736. [Google Scholar] [CrossRef] [PubMed]
- Gröber, U.; Schmidt, J.; Kisters, K. Magnesium in Prevention and Therapy. Nutrients 2015, 7, 8199–8226. [Google Scholar] [CrossRef] [PubMed]
- Hei, Z.; Fang, P. Sequential magnesium binding facilitates lysyl-tRNA synthetase to recognize ATP. Biochem. Biophys. Rep. 2023, 33, 101426. [Google Scholar] [CrossRef]
- Yu, Y.; Cai, Z.; Zheng, J.; Chen, J.; Zhang, X.; Huang, X.-F.; Li, D. Serum levels of polyunsaturated fatty acids are low in Chinese men with metabolic syndrome, whereas serum levels of saturated fatty acids, zinc, and magnesium are high. Nutr. Res. 2012, 32, 71–77. [Google Scholar] [CrossRef]
| Index | pH | TDS g/m3 | TH g/m3 | HCO3− g/m3 | Ca2+ g/m3 | Mg2+ g/m3 | K+ g/m3 | Na+ g/m3 | H2SiO3 g/m3 |
|---|---|---|---|---|---|---|---|---|---|
| Tap water | 8.32 | 333.87 | 184.64 | 166.99 | 50.4 | 14.9 | 5.12 | 14.16 | 5.44 |
| Purified water | 6.33 | 3.63 | 0.43 | 0 | 0.17 | 0.08 | 0.04 | 0.33 | 0 |
| Class | HMDB | KEGG | Metabolite | p-Value | FDR | Fold Change | VIP | Trend |
|---|---|---|---|---|---|---|---|---|
| Amino Acids | HMDB0000182 | C00047 | Lysine | 2.652 × 10−3 | 0.029 | 0.687 | 1.56 | ↓ |
| Amino Acids | HMDB0000177 | C00135 | Histidine | 9.229 × 10−4 | 0.018 | 0.679 | 1.72 | ↓ |
| Amino Acids | HMDB0000214 | C00077 | Ornithine | 3.546 × 10−2 | 0.102 | 0.636 | 1.14 | ↓ |
| Amino Acids | HMDB0000641 | C00064 | Glutamine | 1.768 × 10−2 | 0.065 | 0.768 | 1.20 | ↓ |
| Amino Acids | HMDB0000148 | C00025 | Glutamic acid | 1.469 × 10−2 | 0.064 | 0.616 | 1.46 | ↓ |
| Amino Acids | HMDB0000271 | C00213 | Sarcosine | 5.196 × 10−3 | 0.039 | 0.563 | 1.39 | ↓ |
| Amino Acids | HMDB0000056 | C00099 | beta-Alanine | 1.050 × 10−3 | 0.018 | 0.428 | 1.93 | ↓ |
| Amino Acids | HMDB0000161 | C00041 | Alanine | 7.578 × 10−5 | 0.005 | 0.591 | 1.89 | ↓ |
| Amino Acids | HMDB0000112 | C00334 | GABA | 8.931 × 10−3 | 0.053 | 0.461 | 1.45 | ↓ |
| Amino Acids | HMDB0000187 | C00065 | Serine | 1.295 × 10−2 | 0.064 | 0.670 | 1.35 | ↓ |
| Amino Acids | HMDB0002108 | NA | Methylcysteine | 4.871 × 10−4 | 0.013 | 0.461 | 1.69 | ↓ |
| Amino Acids | HMDB0000158 | C00082 | Tyrosine | 1.150 × 10−2 | 0.063 | 0.502 | 1.34 | ↓ |
| Amino Acids | HMDB0000168 | C00152 | Asparagine | 3.886 × 10−3 | 0.035 | 0.558 | 1.45 | ↓ |
| Amino Acids | HMDB0000684 | C00328 | Kynurenine | 2.165 × 10−5 | 0.002 | 0.273 | 1.77 | ↓ |
| Amino Acids | HMDB0000191 | C00049 | Aspartic acid | 4.326 × 10−2 | 0.117 | 0.697 | 1.36 | ↓ |
| Amino Acids | HMDB0000766 | NA | N-Acetyalanine | 5.196 × 10−3 | 0.039 | 0.581 | 1.39 | ↓ |
| Amino Acids | HMDB0000123 | C00037 | Glycine | 3.805 × 10−4 | 0.013 | 0.691 | 1.68 | ↓ |
| Amino Acids | HMDB0000162 | C00148 | Proline | 2.879 × 10−3 | 0.029 | 0.432 | 1.51 | ↓ |
| Amino Acids | HMDB0000532 | NA | Acetylglycine | 1.553 × 10−2 | 0.064 | 0.753 | 1.36 | ↓ |
| Amino Acids | HMDB0002931 | NA | N-Acetylserine | 3.546 × 10−2 | 0.102 | 0.529 | 1.36 | ↓ |
| Amino Acids | HMDB0000883 | C00183 | Valine | 1.615 × 10−2 | 0.064 | 0.719 | 1.27 | ↓ |
| Amino Acids | HMDB0000267 | C01879 | Pyroglutamic acid | 6.841 × 10−3 | 0.047 | 0.662 | 1.46 | ↓ |
| Amino Acids | HMDB0000696 | C00073 | Methionine | 1.854 × 10−2 | 0.065 | 0.568 | 1.27 | ↓ |
| Amino Acids | HMDB0000687 | C00123 | Leucine | 8.931 × 10−3 | 0.053 | 0.610 | 1.32 | ↓ |
| Amino Acids | HMDB0000929 | C00078 | Tryptophan | 1.469 × 10−2 | 0.064 | 0.633 | 1.09 | ↓ |
| Amino Acids | HMDB0000001 | C01152 | 1-Methylhistidine | 8.977 × 10−4 | 0.018 | 0.701 | 1.59 | ↓ |
| Amino Acids | HMDB0000452 | C02356 | alpha-Aminobutyric acid | 3.886 × 10−3 | 0.035 | 0.450 | 1.44 | ↓ |
| Amino Acids | HMDB0001149 | C00430 | 5-Aminolevulinic acid | 6.915 × 10−3 | 0.047 | 0.590 | 1.32 | ↓ |
| Amino Acids | HMDB0000725 | C01157 | 4-Hydroxyproline | 5.550 × 10−3 | 0.040 | 0.752 | 1.49 | ↓ |
| Benzenoids | HMDB0000669 | C05852 | ortho-Hydroxyphenylacetic acid | 1.628 × 10−2 | 0.064 | 1.722 | 1.40 | ↑ |
| Bile Acids | HMDB0000896 | C05463 | TDCA | 4.498 × 10−2 | 0.121 | 0.696 | 1.27 | ↓ |
| Bile Acids | HMDB0000722 | C02592 | TLCA | 1.130 × 10−2 | 0.063 | 0.616 | 1.26 | ↓ |
| Bile Acids | HMDB0000733 | NA | HDCA | 2.352 × 10−2 | 0.076 | 0.077 | 1.46 | ↓ |
| Carbohydrates | HMDB0000943 | C01620 | Threonic acid | 3.723 × 10−2 | 0.105 | 0.702 | 1.15 | ↓ |
| Carbohydrates | HMDB0000150 | C00198 | Gluconolactone | 1.902 × 10−2 | 0.065 | 0.712 | 1.42 | ↓ |
| Carbohydrates | HMDB0000613 | NA | Erythronic acid | 1.774 × 10−2 | 0.064 | 0.661 | 1.37 | ↓ |
| Carbohydrates | HMDB0000230 | C00270 | N-Acetylneuraminic acid | 2.879 × 10−3 | 0.029 | 0.534 | 1.63 | ↓ |
| Carbohydrates | HMDB0000122 | C00221 | Glucose | 4.871 × 10−4 | 0.013 | 0.606 | 1.64 | ↓ |
| Carbohydrates | HMDB0000740 | C07064 | Lactulose | 2.879 × 10−3 | 0.029 | 0.383 | 1.25 | ↓ |
| Carbohydrates | NA | NA | Maltose/Lactose | 4.871 × 10−4 | 0.013 | 0.424 | 1.15 | ↓ |
| Carbohydrates | HMDB0001262 | C01835 | Maltotriose | 2.364 × 10−3 | 0.029 | 0.664 | 1.61 | ↓ |
| Carbohydrates | HMDB0000215 | C00140 | N-Acetyl-D-glucosamine | 8.931 × 10−3 | 0.053 | 0.504 | 1.48 | ↓ |
| Carbohydrates | HMDB0000956 | C00898 | Tartaric acid | 8.127 × 10−3 | 0.053 | 0.604 | 1.16 | ↓ |
| Carbohydrates | HMDB0000867 | C01685 | Ribonic acid | 1.469 × 10−2 | 0.064 | 0.239 | 1.50 | ↓ |
| Carnitines | HMDB0002013 | C02862 | Butyrylcarnitine | 1.245 × 10−2 | 0.064 | 0.349 | 1.29 | ↓ |
| Carnitines | HMDB0000378 | NA | 2-Methylbutyroylcarnitine | 1.505 × 10−3 | 0.022 | 0.509 | 1.33 | ↓ |
| Carnitines | NA | NA | 3-Hydroxylisovalerylcarnitine | 1.616 × 10−2 | 0.064 | 0.739 | 1.17 | ↓ |
| Carnitines | HMDB0000791 | C02838 | Octanoylcarnitine | 1.578 × 10−2 | 0.064 | 0.897 | 1.13 | ↓ |
| Carnitines | HMDB0002250 | NA | Dodecanoylcarnitine | 2.223 × 10−2 | 0.074 | 1.107 | 1.29 | ↑ |
| Fatty Acids | HMDB0000784 | C08261 | Azelaic acid | 2.959 × 10−3 | 0.029 | 1.130 | 1.72 | ↑ |
| Fatty Acids | HMDB0000792 | C08277 | Sebacic acid | 1.727 × 10−2 | 0.065 | 1.099 | 1.35 | ↑ |
| Fatty Acids | HMDB0000448 | C06104 | Adipic acid | 1.469 × 10−2 | 0.064 | 0.708 | 1.12 | ↓ |
| Fatty Acids | HMDB0001388 | C06427 | alpha-Linolenic acid | 1.469 × 10−2 | 0.064 | 0.609 | 1.25 | ↓ |
| Fatty Acids | NA | NA | 10,13-Nonadecadienoic acid | 2.920 × 10−2 | 0.090 | 0.597 | 1.18 | ↓ |
| Fatty Acids | HMDB0001999 | C06428 | EPA | 5.196 × 10−3 | 0.039 | 0.433 | 1.40 | ↓ |
| Fatty Acids | HMDB0001043 | C00219 | Arachidonic acid | 1.150 × 10−2 | 0.063 | 0.504 | 1.42 | ↓ |
| Fatty Acids | HMDB0002925 | C03242 | Dihomo-gamma-linolenic acid | 3.546 × 10−2 | 0.102 | 0.635 | 1.25 | ↓ |
| Fatty Acids | HMDB0002183 | C06429 | DHA | 1.469 × 10−2 | 0.064 | 0.475 | 1.50 | ↓ |
| Fatty Acids | HMDB0006528 | C16513 | DPA | 1.854 × 10−2 | 0.065 | 0.626 | 1.02 | ↓ |
| Indoles | HMDB0000197 | C00954 | Indoleacetic acid | 2.191 × 10−2 | 0.074 | 1.097 | 1.25 | ↑ |
| Organic Acids | HMDB0000661 | C00489 | Glutaric acid | 2.890 × 10−2 | 0.090 | 0.824 | 1.26 | ↓ |
| Organic Acids | HMDB0000072 | C02341 | Aconitic acid | 1.398 × 10−2 | 0.064 | 1.164 | 1.42 | ↑ |
| Organic Acids | HMDB0000128 | C00581 | Guanidoacetic acid | 5.196 × 10−3 | 0.039 | 0.510 | 1.47 | ↓ |
| Organic Acids | HMDB0000357 | C01089 | 3-Hydroxybutyric acid | 1.050 × 10−3 | 0.018 | 0.609 | 1.88 | ↓ |
| Organic Acids | HMDB0000008 | C05984 | 2-Hydroxybutyric acid | 4.014 × 10−3 | 0.035 | 0.554 | 1.54 | ↓ |
| Organic Acids | HMDB0000176 | C01384 | Maleic acid | 2.349 × 10−2 | 0.076 | 0.740 | 1.17 | ↓ |
| Peptides | HMDB0000721 | NA | Glycylproline | 1.854 × 10−2 | 0.065 | 0.542 | 1.00 | ↓ |
| SCFAs | HMDB0000042 | C00033 | Acetic acid | 2.711 × 10−2 | 0.086 | 0.699 | 1.39 | ↓ |
| SCFAs | HMDB0000754 | NA | 3-Hydroxyisovaleric acid | 3.115 × 10−2 | 0.094 | 0.528 | 1.41 | ↓ |
| SCFAs | HMDB0000039 | C00246 | Butyric acid | 1.835 × 10−4 | 0.010 | 0.456 | 1.85 | ↓ |
| SCFAs | HMDB0000535 | C01585 | Caproic acid | 1.609 × 10−2 | 0.064 | 0.625 | 1.39 | ↓ |
| SCFAs | HMDB0002176 | C18319 | Ethylmethylacetic acid | 1.462 × 10−5 | 0.002 | 0.350 | 2.06 | ↓ |
| SCFAs | HMDB0000718 | C08262 | Isovaleric acid | 1.508 × 10−3 | 0.022 | 0.542 | 1.72 | ↓ |
| SCFAs | HMDB0000892 | C00803 | Valeric acid | 1.556 × 10−3 | 0.022 | 0.674 | 1.63 | ↓ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Qiu, Z.; Zeng, H.; Tan, Y.; Huang, Y.; Luo, J.; Shu, W. Long-Term Consumption of Purified Water Altered Amino Acid, Fatty Acid and Energy Metabolism in Livers of Rats. Metabolites 2024, 14, 289. https://doi.org/10.3390/metabo14050289
Wang J, Qiu Z, Zeng H, Tan Y, Huang Y, Luo J, Shu W. Long-Term Consumption of Purified Water Altered Amino Acid, Fatty Acid and Energy Metabolism in Livers of Rats. Metabolites. 2024; 14(5):289. https://doi.org/10.3390/metabo14050289
Chicago/Turabian StyleWang, Jia, Zhiqun Qiu, Hui Zeng, Yao Tan, Yujing Huang, Jiaohua Luo, and Weiqun Shu. 2024. "Long-Term Consumption of Purified Water Altered Amino Acid, Fatty Acid and Energy Metabolism in Livers of Rats" Metabolites 14, no. 5: 289. https://doi.org/10.3390/metabo14050289
APA StyleWang, J., Qiu, Z., Zeng, H., Tan, Y., Huang, Y., Luo, J., & Shu, W. (2024). Long-Term Consumption of Purified Water Altered Amino Acid, Fatty Acid and Energy Metabolism in Livers of Rats. Metabolites, 14(5), 289. https://doi.org/10.3390/metabo14050289





