1. Introduction
As the cheapest and widest energy source or ingredient, starch is also a good binder for animal feeds [
1,
2]. In most cases, adequate amount of dietary starch could enhance growth and feed efficiency, but it can also save protein by redirecting amino acids away from the oxidative process in humans, terrestrial animals, and fish species [
3,
4]. Meanwhile, optimal contents of dietary starch could improve metabolism and enhance antioxidant capacity and immunity in animals [
5,
6,
7]. Nevertheless, carnivorous fish exhibit reduced capacity to digest dietary starch compared to omnivorous and herbivorous fish, as indicated by various research studies involving grass carp (
Ctenopharyngodon idellus), Chinese longsnout catfish (
Leiocassis longirostris) [
8], jundiá catfish (
Rhamdia quelen), Nile tilapia (
Oreochromis niloticus) [
9], and largemouth bass (
Micropterus salmoides) [
10]. Several studies have proved that long-term intake of excessive dietary starch could induce metabolic dysfunction [
6,
11] and then impair the innate immunity in fish [
12,
13]. Therefore, the regulatory mechanisms of high starch have been a hot research area in carnivorous fish species and other animals.
Too much starch in the diet can cause an increase in serum glucose (GLU) levels, impacting glucose metabolism and lipid metabolic indices, like high-density lipoprotein cholesterol (HDL-C), triacylglycerol (TAG), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), etc. [
1,
7,
12]. Previous studies have found glucose and lipid metabolic processes can be regulated by different signaling pathways mediated by serum hormones, including glucagon (GC), insulin (INS), adiponectin (ADPN), and leptin (LEP) [
7,
14,
15]. In addition, the processes of glycogen synthesis and decomposition are also modulated by these serum hormones in animals in response to higher dietary starch [
14,
16,
17]. Moreover, several studies have reported that higher dietary starch could induce hepatic cholesterol and bile acid accumulation in humans and animals [
18]. These metabolic processes, such as glycolysis and gluconeogenesis, lipolysis and lipogenesis, glycogen synthesis, and cholesterol and bile acid accumulation, are mainly regulated by various signaling pathways, including AKT and SREBP signaling pathways in humans and animals [
19,
20]. While most research has concentrated on glycolysis, gluconeogenesis, lipolysis, and glycogen synthesis in carnivorous fish, there are limited data available regarding the synthesis and storage of bile acids through hepatic cholesterol in carnivorous fish species consuming higher dietary starch.
Throughout the breakdown of nutrients, such as starch, reactive oxygen species (ROS) were consistently formed in humans, terrestrial animals, and fish species [
3,
21]. Although optimal amounts of ROS could play essential functions in growth, metabolism, and immune defense, ROS overload could induce the damage of proteins, lipids, and DNA [
22,
23]. So, it is important to alleviate these oxidative stresses induced by ROS overload through different adequate antioxidant enzymes and molecules, including superoxide dismutase (SOD), catalase (CAT), thioredoxin 2 (
Trx2), thioredoxin reductase 2 (
TrxR2), peroxiredoxin 3 (
Prx3), and glutathione S-transferase (GST) [
13,
24,
25]. Past research has indicated that carnivorous fish fed diets high in starch exhibited low antioxidant capacity and strong oxidative stress [
13,
23,
26]. In addition, excessive ROS can cause cellular damage in these immune regulatory cells, including leukocytes, macrophages, and natural killer cells [
27]. Prasad and colleagues [
21] have shown that immune and inflammatory responses can be influenced by the release of various cytokines, such as tumor necrosis factor-α (TNF-α), interferons (IFNs), and interleukins (ILs), which was also supported by Herb and Schramm [
28] and Liu et al. [
29]. In animals, the leukocyte numbers and platelet (PLT) counts have appeared as biomarkers of inflammation or inflammatory response in clinical practices recently [
3,
30,
31,
32]. And the secretion and maturation of these pro-inflammatory cytokines could be mediated by the NLRP3 inflammasome in animal cells [
33]. Excessive production of ROS can activate the NLRP3 inflammasome [
34,
35,
36]. Yet, there were limited data regarding the connection among elevated starch-induced ROS, NLRP3 inflammasome, and inflammatory markers in carnivorous fish.
Largemouth bass is a common fish species that is economically important and extensively cultivated in China due to its rapid growth, abundant harvest, soft flesh, lack of intermuscular spines, and other favorable characteristics [
37]. Numerous previous research projects have investigated the prolonged impacts of increased dietary starch on the development characteristics, metabolic capacities, and overall health of the liver and intestines in largemouth bass [
17,
18,
38]. Little information could be obtained on the short-time relationship between high starch-mediated hematological variations, metabolic changes, oxidative stress, inflammatory responses, and histopathology in largemouth bass. This research sought to investigate the processes that lead to the development of metabolic fatty liver in largemouth bass fed a diet high in starch. This study utilized a system dynamics approach, analyzing data from various body and blood measurements as well as metabolic, oxidative stress, antioxidant, inflammatory, and histopathological markers in largemouth bass fed with high starch.
4. Discussion
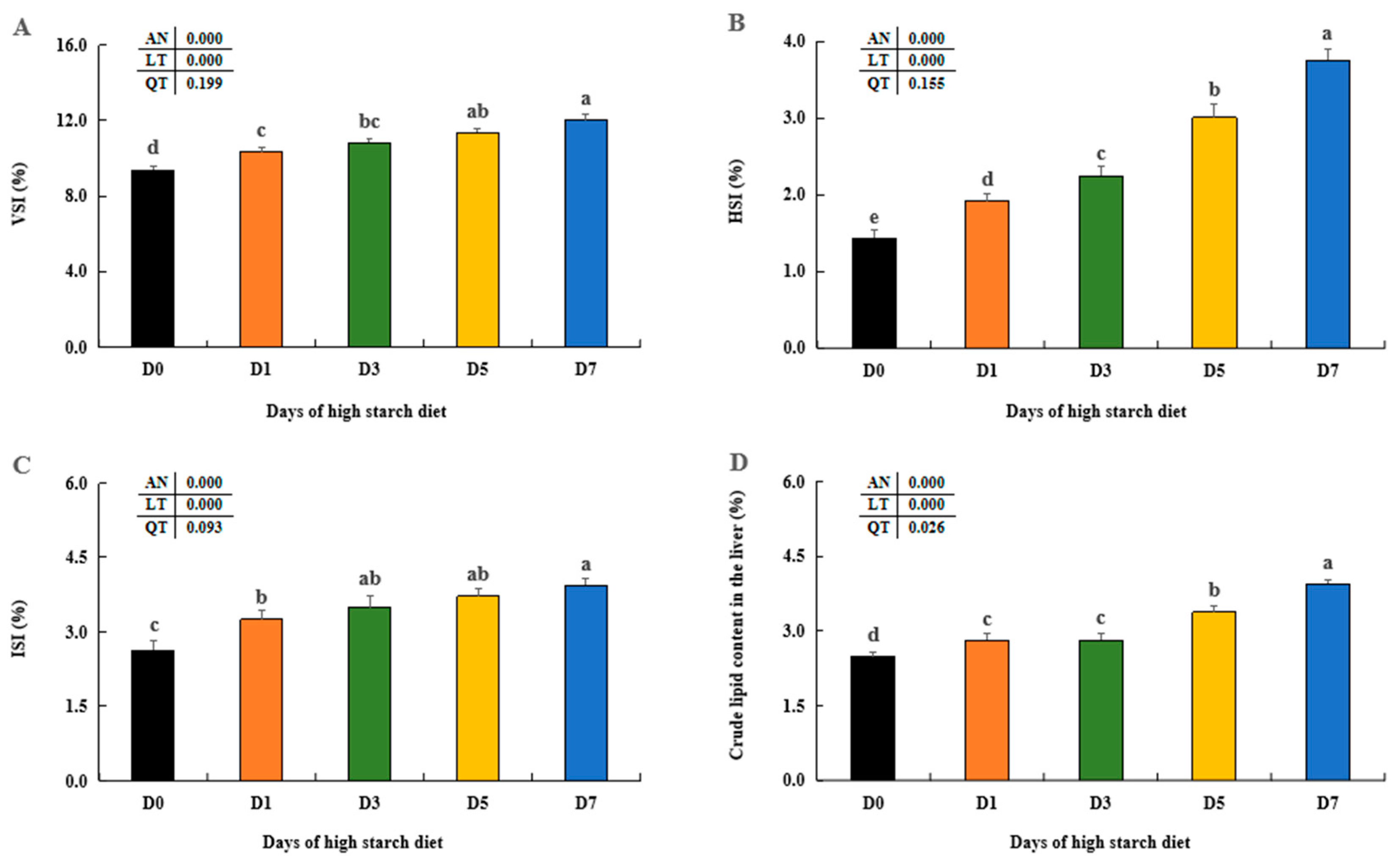
Previous studies have found dietary starch levels could modulate body indices (VSI, HSI, and ISI), which can also influence animal body compositions [
41,
42]. The current research found that high-starch diet (20%) led to a notable increase in VSI, HSI, and ISI in largemouth bass as the culturing days increased, similar with previous studies on giant gourami (
Osphronemus goramy) [
7], blunt snout bream (
Megalobrama amblycephala) [
43], and black carp (
Mylopharyngodon piceus) [
13]. Additionally, our findings indicated a significant rise in hepatic lipid levels over time due to high starch exposure, aligning with earlier studies on largemouth bass [
44,
45] and GIFT tilapia (
Oreochromis niloticus) [
5]. Higher dietary starch, when combined with these findings and our results, was found to induce hepatic lipid synthesis and accumulation in largemouth bass.
Hematological parameters have been important physiological assays for analyzing pathological variations in human and fish species [
31,
46,
47]. Our results showed that high starch increased RBC counts and HGB levels, especially in the D1, D3 and D5 groups, which was in line with former results in Siberian sturgeon (
Acipenser baerii) [
48]. It is well-known that RBC and HGB undertake key roles in increasing oxygen-carrying, metabolic capacity and redox regulation in humans and animals [
49,
50,
51]. Therefore, combining these findings and our results, it could be inferred that the largemouth bass needs more oxygen to carry out various physiological activities during the absorbing and metabolic processes of higher dietary starch. In addition, our results found high starch significantly increased counts of WBC, NEU, LYM, MON, EOS, and BAS with increasing culturing times, which agrees with previous results in
Labeo rohita [
52] and gilthead sea bream (
Sparus aurata) [
53]. It is well-known that adequate counts of WBC, NEU, LYM, MON, EOS, and BAS could improve immunity and enhance the defense effects when the animal is infected by some kinds of pathogens or fed with adequate nutrients [
54]. However, excessive counts of WBC, NEU, MON, EOS, and BAS could induce inflammation and/or inflammatory responses in dairy goat [
55], rat [
56], and humans with type 2 diabetes mellitus [
57]. The combination of these discoveries and our study outcomes indicates that increased consumption of dietary starch may lead to inflammation or inflammatory reactions in largemouth bass.
Serum biochemical indices are widely recognized as crucial indicators of health and metabolic status in both humans and animals. ALT and AST variations reflect hepatic metabolism and environmental abnormalities in animals [
58]. Increased serum ALT and AST levels in the D3, D5, and D7 groups indicated that hepatic metabolism might be disturbed or damaged mediated by high dietary starch in largemouth bass [
17]. Meanwhile, serum BUN variations could correlate with nitrogen utilization and reflect the homeostasis of exogenous amino acids [
59]. So, higher BUN contents presented in D1–D7 groups indicated high dietary starch might impair exogenous nitrogen utilization in largemouth bass. Although ALP could act as an immune defense biomarker [
60,
61], excessive levels of ALP could couple degeneration, necrosis, and destruction of the liver due to cellular damage [
62]. Moreover, higher levels of serum ALP are also correlated with the bile ducts obstructed in metabolic liver diseases [
60]. Combined with higher serum ALP activities in fish fed high-starch diets in this study, it is suggested high dietary starch could cause cellular damage in largemouth bass. In addition to being an important immune index, serum ALB also plays a key role in the antioxidant function [
63]. Considering the lower levels of serum ALB in D7 groups, it indicated that high dietary starch could impair immunity and increase the danger of oxidative stress in largemouth bass. Previous studies have found higher levels of serum TBA could be correlated with early-stage metabolic liver disease mediated by higher contents of cholesterol in animals [
37,
64]. Therefore, elevated levels of serum TBA suggest that increased dietary starch not only worsens liver damage but also triggers cholestatic disease in largemouth bass [
65,
66]. In typical physiological conditions, HDL-C moves total cholesterol from peripheral tissues to the liver for additional processing and bile acid production, whereas LDL-C transports hepatic cholesterol to other tissues [
67]. Our findings showed contrasting trends in the HDL-C and LDL-C levels in D7 groups when compared with those in D0 groups (
Table 4), which is consistent with previous findings in mice [
68] and juvenile blunt snout bream [
43]. Moreover, some researches have shown the level of TG and TC is positively proportional to the severity of fatty liver [
67,
69]. Combined with higher contents of serum TAG and TC, it is suggested that high dietary starch could not only prevent TC and TAG from being absorbed and utilized by the body, but also induce their accumulation in the body and then result in dyslipidemia and metabolic fatty liver [
70].
Carnivorous fish are recognized as having weak capabilities for processing glucose and often present persistent hyperglycemia after feeding on a diet with high starch [
2]. INS, ADPN, and GC play a crucial role as endocrine hormones in maintaining the glucose balance by managing fluctuations in serum glucose levels in both mammals and animals, as highlighted in studies by del sol Novoa et al. [
71], Xu et al. [
72], and Zhong et al. [
17]. Elevated levels of serum glucose can trigger INS and ADPN release or decrease glucagon production to alleviate stress from hyperglycemia and maintain the glucose balance [
73]. Our research discovered contrasting trends in the levels of serum INS, ADPN, and GC, despite a consistent increase in serum glucose levels in largemouth bass fed a high-starch diet. Likewise, earlier research has demonstrated that a 20% wheat starch diet led to a notable increase in blood glucose and INS levels in largemouth bass [
6] and over a 4-week period in rainbow trout [
74]. Conversely, it was observed that a high-starch diet resulted in significantly higher blood glucose levels after 3 h of feeding, but had no impact on INS levels in largemouth bass fed a diet with high starch (13.56%) [
18]. As for these differences on the INS contents, it might be due to the different sample time in these studies. Furthermore, the levels of INS and ADPN were both decreased in the D7 groups, indicating that inadequate secretion of these hormones was the primary cause of glucose intolerance in carnivorous fish species [
2]. In addition to reducing appetite signal, LEP also plays a crucial role in controlling fat or lipid reserves in both humans and animals [
75,
76,
77]. Considering these findings and higher contents of serum LEP in this study, it indicated that higher starch could induce LEP secretion, which could then initiate the self-regulatory mechanism in response to higher serum glucose in largemouth bass [
78]. Moreover, higher LEP levels were also presented in these obese individuals with a higher percentage of body fat, which was mainly mediated by typical LEP resistance in obesity [
79]. The combination of these discoveries and our own results indicates that elevated starch levels may lead to LEP resistance, ultimately disrupting the regulation of lipid metabolism in largemouth bass, although further research about this resistance mechanism is needed to fully understand this.
As a major metabolic organ, the liver tissue plays important roles regulating glucose and lipid metabolic processes, include glycolysis, gluconeogenesis, glycogen synthesis, lipogenesis and lipolysis [
10]. The important role of GLUT2 in regulating glucose transfer between the liver and serum through the PI3K/AKT signaling pathway has been widely recognized [
80,
81]. Our research found that elevated levels of dietary starch significantly increased GLUT2 levels at both transcription and protein levels, consistent with findings in rainbow trout (
Oncorhynchus mykiss) [
82], blunt snout bream [
83], and Gibel carp (
Carassius gibelio) [
84]. This indicates that high starch intake may enhance the transport of plasma glucose into hepatic cells by stimulating GLUT2 in largemouth bass. Numerous past research has indicated that elevated levels of starch can trigger glycolysis pathways through the INS signal pathway in mice [
85], blunt snout bream [
86], common carp (
Cyprinus carpio) [
87], and largemouth bass [
45]. Levels of GCK, PFK, PK,
ISRa,
ISRS2b,
PI3Kc, and
AKT1 were significantly increased in the D5–D7 groups that were fed the high-starch diet, as well as with higher PA contents in our findings. Our results, along with these discoveries, suggest that elevated levels of starch may enhance glucose metabolism by stimulating glycolysis through the activation of the typical PI3K/AKT signaling pathway in largemouth bass. Additionally, gluconeogenesis was also stimulated with elevated levels of FBP and G6Pase in the D5–D7 groups that were given high-starch diet, which is similar with former results in largemouth bass fed 13.56% dietary starch [
18], suggesting there was a competition between dietary glucose and endogenous productive glucose. Based on these findings, it was suggested that high levels of starch could lead to glucose metabolic disorders and hinder glucose utilization in largemouth bass.
Furthermore, the hepatic production of glycogen in animals may be controlled by the PI3K/AKT signaling pathway, which acts on its downstream proteins and enzymes (
GYG2, GCS, GBE, and GDE) as indicated by Yang et al. [
37] and Zhang et al. [
88]. Typically, increased levels of
GYG2, GCS, and GBE may play important functions in enhancing glycogen production, whereas elevated GDE levels could facilitate the breakdown of hepatic glycogen [
2]. Past research has shown that the amounts of glycogen in the liver increased as the levels of starch in the diet rose for red spotted grouper (
Epinephelus akaara) [
89] and blunt snout bream [
90]. Furthermore, by utilizing [U-14C] glucose as a marker, it showed a significant rise in glycogen synthesis from glucose in largemouth bass when the dietary starch level was raised from 5% to 15% [
91]. Combined with these findings, it indicated that high starch may induce hepatic glycogen synthesis in largemouth bass through the PI3K/AKT signal transduction pathway, as evidenced by the variations of these key molecules, LAG contents, and PAS-stained histological sections in our results. Nevertheless, levels of hepatic LA and LDH activities in serum and liver showed significant increases in the D1–D7 groups, mirroring findings in goats [
92] and cattle [
93] that were given a high-starch diet. Past research has shown that increased LA accumulation can lead to risks such as lactic acidosis, nonalcoholic fatty liver disease, and tumors in humans and animals [
94,
95,
96,
97]. Based on these discoveries and our findings, it appears that excessive LA levels could lead to lactic acidosis and metabolic disorders in largemouth bass fed a diet high in starch.
Typically, Ac-CoA is converted by ACC1 into Mal-CoA, which is then transformed by FAS into fatty acids (FAs) within the liver cells [
18,
98]. The citrate-pyruvate pathway, facilitated by ME, and the pentose-citrate pathway, facilitated by
G6PD, supply the necessary energy for this process [
37]. Consistent with prior findings in largemouth bass that were given a high-starch diet [
18], we also observed elevated levels of ACC, FAS, Mal-CoA,
SCD,
ME1,
G6PD, and
PGD in fish fed a high-starch diet. This suggests that high starch intake can stimulate fatty acid production by up-regulating the expression of these genes associated with lipid synthesis in the liver of largemouth bass. Moreover,
FATP1 can facilitate the transfer of FA to the endoplasmic reticulum within liver cells for the production of LTAG, as demonstrated by Ipsen et al. [
99] and López [
100]. And
GK,
GPAT3,
GPAT4, and
DGAT2 are the rate-limiting enzymes during LTAG synthesizing processes [
101]. Then, these synthesized LTAG could bind apolipoprotein B100 (
APOB100) and enter the bloodstream by free diffusion [
102]. Meanwhile,
Plin2, as a key lipid droplet coated protein, is tightly related with lipid droplet biogenesis and storage in tissues [
103]. Elevated levels of
Plin2 are often associated with various metabolic disorders, including insulin resistance and type 2 diabetes in human and animals [
103,
104]. The study found that
FABP10,
GPAT3,
GPAT4,
DGAT2,
APOB100, and
Plin2 levels were notably higher in groups consuming high-starch diets, consistent with earlier findings in largemouth bass [
18] and gilthead sea bream [
105]. Previous research has shown that the AKT/SREBP1c signaling pathway can stimulate LTG synthesis in both animals and fish, as documented by Wang et al. [
106] and Ferre et al. [
107]. In addition to increased levels of these important molecules, this study also showed that high starch in largemouth bass can activate the AKT/SREBP1c signal pathway, leading to enhanced FA and LTAG synthesis, as well as lipid droplet formation, as demonstrated by LTAG levels and histological sections stained with HE and ORO in this study.
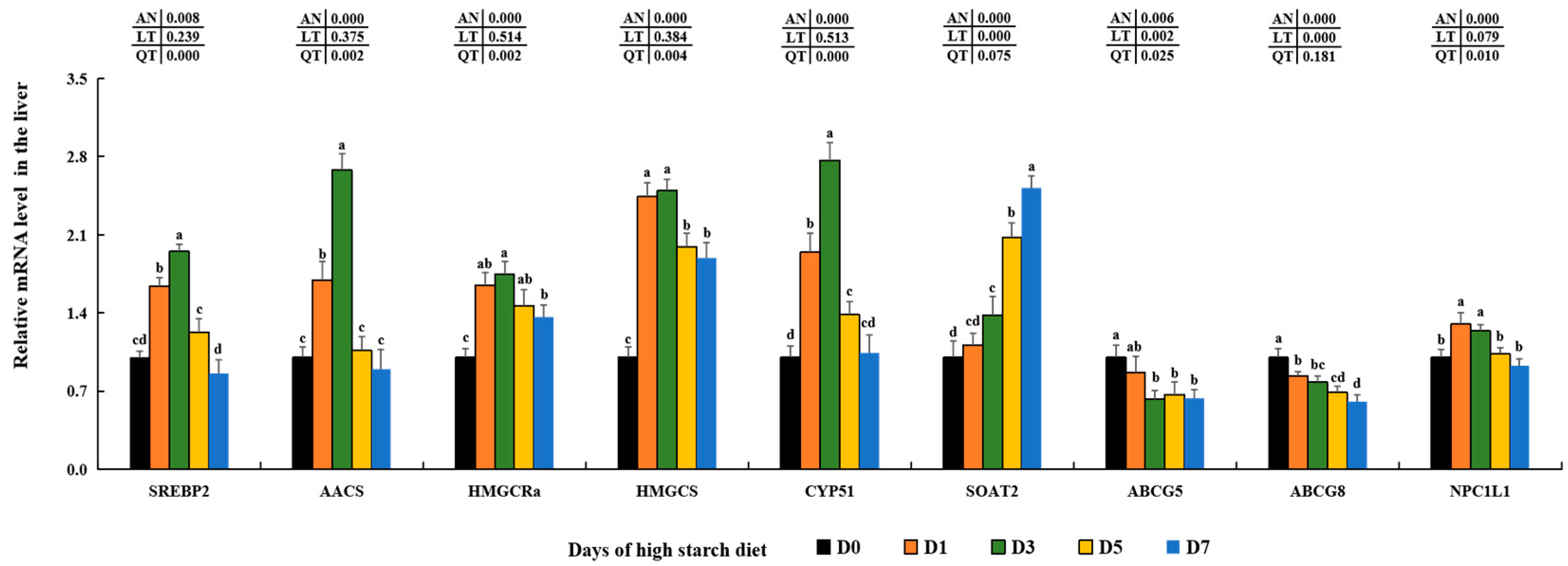
SREBP2, a crucial transcription factor, has the ability to regulate the metabolism and balance of cholesterol in the liver by influencing the transcription of genes that are regulated by sterols, such as
AACS, HMGCS,
HMGCRa, and
CYP51 [
18,
19].
AACS has the ability to use ketone bodies for the creation of cholesterol and fatty acids, converting acetoacetate into its CoA ester to generate acetoacetyl-CoA in the cytosol [
108]. This process is facilitated by
HMGCS, which transforms acetoacetyl-CoA into 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) [
109,
110]. Subsequently,
HMGCRa is able to facilitate the transformation of HMG-CoA into mevalonic acid in order to generate cholesterol [
111].
CYP51 plays an essential role in the sterols’ production and is a significant focus of cholesterol-lowering medications according to recent studies [
112,
113,
114]. Our results showed that a diet with high-starch significantly increased the levels of
SREBP2 and its target genes related to cholesterol synthesis (
AACS,
HMGCS,
HMGCRa, and
CYP51) in groups D1, D3, and D5. This effect was consistent with findings in largemouth bass [
18] and male C57BL/6J mice [
115], suggesting that high starch intake may stimulate cholesterol production through the
SREBP2 signaling pathway in the hepatic cells of largemouth bass. The expression of these genes was significantly reduced in the D7 groups compared to that in the D3 groups, which might be due to the negative feedback regulatory mechanism mediated by higher contents of TC in the liver of largemouth bass [
19,
20], although further research is needed to fully understand this regulatory process. In addition,
SOAT2 plays an important function in lipid droplet biogenesis and storage by converting cholesterol to cholesteryl ester in cells [
104,
116]. Prior research has shown that an increase in lipid droplets can be triggered by elevated glucose levels in normal colon cells and colorectal cancer stem cells [
117], as well as in mice with type 2 diabetes [
118]. Based on these results, it is proposed that increased levels of
SOAT2 may be responsible for the accumulation of lipid droplets in largemouth bass fed a high-starch diet. Meanwhile,
ABCG5 and
ABCG8 act as key proteins transporting endogenous cholesterol out of the liver into other tissues, while
NPC1L1 plays key roles in up-taking intestine cholesterol into hepatic cells [
119,
120]. Combined with these findings and lower levels of
ABCG5,
ABCG8, and
NPC1L1 in this study, it indicated these three key reduced transporters might mediate the hepatic cholesterol decomposition in largemouth bass [
116].
In general, bile acid synthesis is mainly regulated by two key rate-limiting enzymes (
CYP7A1 and
CYP27A1) mediating the oxidation of cholesterol in hepatocytes [
120,
121]. Higher
CYP7A1 and
CYP27A1 levels in D1–D3 groups indicated high starch could induce bile acid synthesis via the oxidation of cholesterol in hepatocytes of largemouth bass. The reduced levels of
CYP7A1 and
CYP27A1 in D5–D7 groups might be caused by the negative feedback regulatory mechanism mediated by higher TBA contents in hepatocytes [
121,
122]. Furthermore, liver bile acids may be moved into the bile canaliculus using the bile salt transporter known as the canalicular bile salt export pump (
BSEP) [
123,
124]. Although there were no marked differences among D0–D5 groups, lower
BSEP levels in D7 groups indicated high starch might block the transportation of hepatic bile acids into bile canaliculus and then result in possible cholestasis [
120,
121]. Two important transporting polypeptides,
NTCP and
OATP1, facilitate the absorption of bile acids from the portal vein into hepatocytes [
125]. Higher
NTCP and
OATP1 levels in D1–D5 groups indicated that bile acids could be taken up into hepatocytes induced by high starch, which might further aggravate cholestasis in largemouth bass. In humans and rodents, studies have found higher levels of hepatic
OST-α could help bile acid excretion into the circulating system [
122,
126]. Combined with higher
OST-α mRNA levels and serum TBA contents, it indicated that high starch could drive bile acid excretion into the circulating system via a heightening expression of hepatic
OST-α in largemouth bass, although this driving mechanism needed to be studied in fish species [
127].
It is well known that cellular ROS is mainly generated by NADPH oxidases (
NOX) and the respiratory electron transport chain (ETC) of mitochondria during glucose metabolic processes in animals [
28,
128,
129]. Our study revealed that the expression of certain genes, such as
G6PD,
NOX1, NADPH oxidase activator 1 (
NOXA1), and cytochrome c oxidase 4 (
COX4), along with the levels of H
2O
2 significantly increased with high-starch diets. This is consistent with findings in humans with diabetes [
23,
25,
130], suggesting that high starch intake may enhance oxidative phosphorylation, leading to elevated ROS levels in largemouth bass liver. In general, SOD and CAT in antioxidant systems could catalyze ROS and reduce oxidative stress in animals [
5,
21]. Meanwhile, increased GST and MDA amounts can symbolize physiological biomarkers to oxidative or inflammatory stress in cultured animals [
1,
3]. Past research has shown that the antioxidant abilities were diminished by elevated starch levels in black carp [
131], black sea bream (
Acanthopagrus schlegelii) [
12], GIFT tilapia [
5], and largemouth bass [
132]. Similarly, our results also found a notable reduction in the amounts of SOD, CAT and T-AOC along with a significant increase in the amounts of GST, MDA, and H
2O
2 in the D5 and D7 groups that were given a high-starch diet. Moreover, a specialized thioredoxin system within mitochondria, consisting of
Trx2,
TrxR2, and
Prx3, has been identified as crucial for protecting against oxidative stress induced by H
2O
2 through disulfide reductase functions [
133,
134]. Our results show that a high-starch diet could inhibit the genetic expressions of
Trx2,
TrxR2, and
Prx3 in the liver in largemouth bass. Considering these findings and our data, it indicated that high starch could aggravate ROS damage and disturb mitochondrial redox homeostasis by decreasing antioxidant capabilities in largemouth bass [
128].
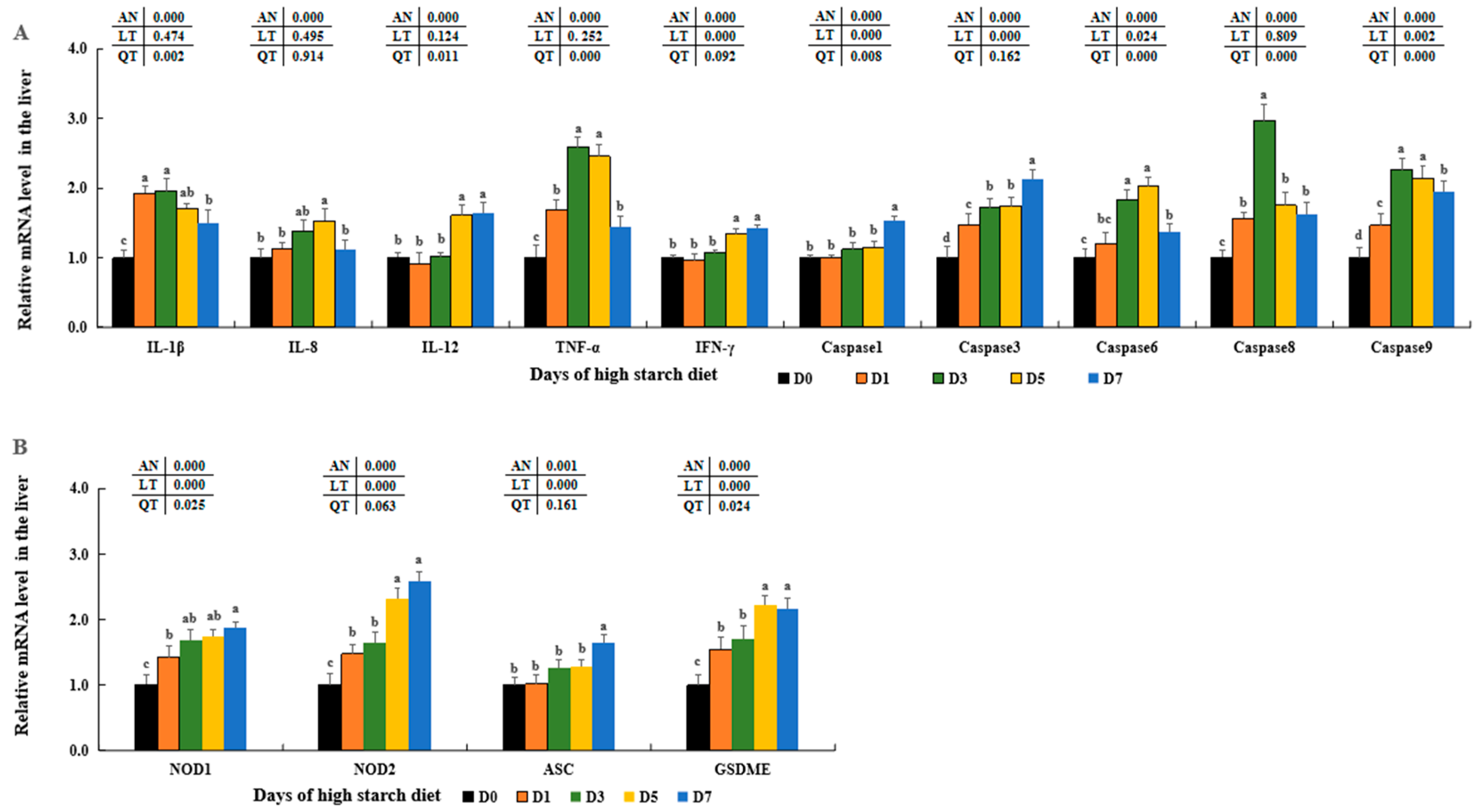
Numerous research studies have shown that an overabundance of
NOX-derived ROS can lead to inflammation by boosting the generation of pro-inflammatory cytokines in the liver, such as IL-1β, TNF-α, IFN-γ, IL-6, IL-8, IL-12, IL-17, and others [
24,
29]. Prior studies have discovered elevated amounts of inflammatory markers such as IL-1β, TNF-α, and IFN-γ, which were presented in human with diabetes [
135,
136,
137], pig with malnutrition [
138], and mice fed high-carbohydrate diets [
139]. Likewise, our results showed elevated concentrations of IL-1β, IL-6, IL-8, IL-12, IL-17, TNF-α, and IFN-γ in the hepatic cells of largemouth bass with increasing time of feeding high-starch diet in this study. Combined with previous results and our findings, it further proved that inflammatory responses could be caused by ROS excessively generated during the metabolism of higher dietary starch in largemouth bass. In addition, the secretion and maturation of these pro-inflammatory cytokines were mediated by the typical NLRP3 inflammasome in human and animal cells [
24,
33]. The NLRP3 inflammasome consists of the NLRP3 sensor molecule, the
ASC adaptor protein, and pro-caspase-1 [
35]. Various molecular and cellular events, including ROS overload, can lead to the stimulation of the NLRP3 inflammasome [
27,
140]. In the formation of NLRP3 inflammasomes, pro-caspase-1 can transform into cleaved-caspase-1, triggering the processing of pro-IL-1β precursors into their active states [
135,
141]. Recent research has shown that elevated glucose levels can stimulate the generation of ROS, boost the triggering of the NLRP3 inflammasome, and increase IL-1β release in macrophages [
34,
142] as well as in RSC96 (rat Schwann cell line) cells [
27]. Moreover, additional research has shown that elevated glucose levels can lead to the depletion of retinal pericytes through a process of inflammatory cell death involving NLRP3 and caspase-1 [
141]. The research also revealed increased
ASC,
NOD1,
NOD2,
GSDME, and caspase-1 expression in fish groups exposed to high starch, mirroring findings in human and rat cells [
27,
34]. By combining these discoveries with our own results, we can conclude that inflammatory reactions may be initiated by the activation of the NLRP3 inflammasome induced by ROS overload in largemouth bass fed with high starch (
Figure 11).