Abstract
Since hop secondary metabolites have a direct correlation with the quality of beer and other hop-based beverages, and the volatile fraction of hop has a complex composition, requiring effective separation, here we explore the application of headspace solid-phase microextraction as a sample preparation method, coupled with comprehensive two-dimensional gas chromatography–mass spectrometry (GC×GC–MS) analysis. The methodology involved the use of a DVB/PDMS fibre with 500 mg of hop cone powder, extracted for 40 min at 50 °C, for both GC–MS and GC×GC–MS. The varieties Azacca, Cascade, Enigma, Loral, and Zappa were studied comprehensively. The results demonstrate that GC×GC–MS increases the number of peaks by over 300% compared to classical GC–MS. Overall, 137 compounds were identified or tentatively identified and categorised into 10 classes, representing between 87.6% and 96.9% of the total peak area. The composition revealed the highest concentration of sesquiterpene hydrocarbons for Enigma, whilst Zappa showed a relatively significant concentration of monoterpene hydrocarbons. Principal component analysis for all compounds and classes, along with hierarchical cluster analysis, indicated similarities between Zappa and Cascade, and Azacca and Loral. In conclusion, this method presents an optimistic advancement in hop metabolite studies with a simple and established sample preparation procedure in combination with an effective separation technique.
1. Introduction
Hop (Humulus lupulus L.) is a dioecious and diploid plant from the family Cannabaceae and is an extremely important raw material in drinks and beverages, such as sparkling teas and soft drinks and with major use in brewing. The use of hop as an ingredient was a significant turning point in the history of beer, increasing the beverage’s popularity and changing the physical/chemical properties of the beverage because of the secondary metabolites present in hop [1,2]. Nowadays, hop is the most expensive ingredient in brewing, with a market valued at USD 7.8 billion in 2022 and a growth prospect of more than USD 5.5 billion by 2030 [3]. Hop flavour is associated with the female flowers or strobili (termed cones) having a yellow powder named lupulin, which is the major source of hop aroma and is composed of acids, essential oils, and resins. Hop improves the aroma, stability properties, and bittering characteristics of the final product [2,4].
The composition of hop is complex and has an intrinsic characteristic based on properties such as variety/cultivar, geographic origin, environmental factors, and stability, among others [4]. The flavour, odour, and other physicochemical properties of hop are generally associated with five key categories of components: alpha acids, beta acids, polyphenols, terpenoids, and thiols. Alpha acids are responsible for the sensation of bitterness and foam stability, representing a mass fraction of 2 to 20% of the hop composition [5,6]. Beta acids influence the aromatic profile of the beer and are present in lower concentrations, due to their biochemical transformation during processing of the beverage. Polyphenols, such as tannins and flavonoids, act as stabilising agents in beer, influencing turbidity and flavour [6]. Finally, hydrocarbon and oxygenated terpenoids, as well as thiols, are some of the classes of components found in hop essential oil, which reportedly include more than 300 aroma compounds [4,7]. Essential oil compounds undergo biotransformation by yeast fermentation, such as geraniol generating beta-citronellol and, finally, the esterification product citronellyl acetate [8].
Numerous hop varieties are currently available in the market, with more than 100 cultivars being patented in the last decade and new products being continually developed and reported [9]. There are 363 results for Humulus lupulus species–metabolite relationships in the KNApSAcK Core System database [10], which aids in searching metabolomics and analytical studies. Metabolomics is a defined research field that has developed over the last 25 years, which involves the comprehensive analysis of small molecules (metabolites) present in complex biological matrices. Within this broad field, there are a number of different sub-disciplines that study the behaviour of matrices such as hop proteomics [11], genomics [12], beeromics [13], hopomics [14], and volatilomics [15].
Regarding the analysis of volatile organic compounds (VOCs) and volatilomics, gas chromatography (GC) is a standardised technique that has been applied to the determination of plant metabolites for more than 50 years [16]. Multiple analytical studies have targeted key aroma compounds in hop essential oil, such as myrcene (related to fresh odour), and linalool and geraniol (both related to floral notes) [17]. GC has also been applied in numerous analytical approaches for hop studies, such as chemical profiling [18,19,20], comparison of different types of sample preparation [21,22,23], and the study of the effect of hop in the brewing process [24,25].
Sun et al. [26] reviewed research associated with different sample preparation methods, such as steam distillation [17,27], simultaneous distillation extraction [25], dynamic [18] and static [19] headspace, direct thermal desorption [21], direct microwave desorption [28], liquid–liquid extraction (LLE) [29], stir bar sorptive extraction (SBSE) [23], and headspace solid-phase microextraction (HS-SPME) applied to hop [22,30]. This last method attracts attention as being a “greener” approach since it is a solventless technique using sorptive polymeric fibres, is simple, versatile, and has a wide applications base, with SPME reported for samples in the environmental industry, food, drugs, and other areas, although the quantitative application of SPME is rather complex [22,31].
According to the level of complexity of the hop matrix, the full resolution and identification of compounds can be difficult to achieve. Comprehensive two-dimensional gas chromatography (GC×GC) is designed for such multi-component samples [22]. Advantages brought by GC×GC compared with conventional GC include enhancement of separation power, increased peak capacity, and increase in sensitivity [32].
The use of GC×GC in the metabolomics field with respect to performance attributes was shown by Wong and coworkers [33]. In that study, GC×GC coupled with high-resolution quadrupole time-of-flight mass spectrometry (qTOFMS) was applied to samples of Eucalyptus spp. leaf oils, characterising four different species and their untargeted metabolic profiles [33]. The expression of metabolomic profiles was also extended to hop for the study of new genotypes, chemotyped by Yan and coworkers [34].
The application of GC×GC to hop analysis (Table 1) has been reported by thirteen research studies between 2003 and 2019, according to the Web of Science database, and data are summarised here as illustrative of capabilities for untargeted metabolomics. The first study was published 20 years ago and shows the potential of the technique for the analysis of hop essential oil [35]. With the development of the GC×GC, different setups were described, such as the parallel comprehensive two-dimensional gas chromatography (2GC×2GC) [36], and a range of detectors and modulators. Greater resolution power was achieved more recently, with the largest number of compounds reported by Yan and collaborators [34], who separated 306 and identified 99 compounds in their samples. Table 1 shows sample preparation and the column set applied in each study. Not only were comprehensive analyses of hop presented, but also the detection of compounds such as 4-mercapto-4-methylpentan-2-one (4MMP) (IUPAC: 4-methyl-4-sulfanylpentan-2-one (4MSP)). 4MSP is a black-currant-like odorant with an odour detection threshold as low as 0.00055 µg L−1 in beers [37], and these studies explore the advantage of the improved sensitivity of the GC×GC system. Odorants in hop samples were also reported in other studies such as that of Eyres, Marriott, and Dufour [38], which were concerned with the detection and possible identification of the aroma-active compounds/regions present in the samples.

Table 1.
Review table for literature studies of hop by GC×GC.
The aim of the present study was the analysis of different commercial hop samples, applying HS-SPME and GC×GC techniques to obtain the chemical profile and chemotyping of five different hop varieties, to highlight the general value of GC×GC to generate valuable information for characterisation and metabolomics studies. Thus, the primary objective was to demonstrate the applicability of this new methodology for volatile metabolite profiling of hops and associated benefits. From the accompanying literature review (Table 1), we observed that comprehensive analyses combining SPME and GC×GC techniques for hop analysis were not previously reported in the literature. This paper presents the first report of HS-SPME-GC×GC–MS for hop samples, establishing its capacity and presenting new data on the volatile profile of different species of hop.
2. Materials and Methods
2.1. Chemicals and Samples
The chemical compounds and standards used in this work were hexane (HPLC grade), ethyl hexanoate (98%), and methanol (HPLC grade) from Merck (Darmstadt, Germany); 2-octanol (99%), undecane (99%), (+) camphene (80%), β-myrcene (90%), D-limonene (97%), linalool (95%), geraniol (98%), geranyl acetate (97%), humulene (96%), (−)-caryophyllene oxide (97%), and n-alkanes C8–C21 (99%) provided by Sigma Aldrich (Castle Hill, NSW, Australia); and α-pinene (95%), β-pinene (94%), and caryophyllene (90%) from TCI (Tokyo, Japan).
The hop samples Azacca (AZAC) and Loral (LORA) were kindly donated by Carlton & United Breweries (Asahi-CUB, Abbotsford, Australia) and the samples Cascade (CASC), Enigma (ENIG), and Zappa (ZAPP) were obtained from various other local suppliers. All samples were obtained in the format of pellet type 90, stored in the presence of nitrogen, and kept in a freezer until ready for analysis. More information about the hop samples is presented in Table S1 of the Supplementary Materials.
2.2. Sample Preparation
The sample preparation and analysis procedure (Figure 1) was adapted and optimised based on previous studies [20,22,46]. The data regarding this initial examination of various experimental parameters, including the choice of the SPME fibres, among 65 µm DVB/PDMS (pink), 50/30 µm DVB/CAR/PDMS (grey), 100 µm PDMS (red), and 85 µm CAR/PDMS (blue), will be reported elsewhere. The following conditions were selected for the present study. HS-SPME was performed with a manual holder and a 65 µm DVB/PDMS (pink) fibre from Supelco (Castle Hill, Australia), conditioned in accordance with the manufacturer’s guidelines. In the first step, the pellets were ground in a mortar and pestle with 500 mg taken into a 20 mL screw top vial with clear glass. The vials were heated at 50 °C using a hot plate, with an equilibrium time of 10 min and 30 min for sorption. After extraction, the DVB/PDMS (pink) fibre was introduced into the GC injector and desorbed for 3 min.
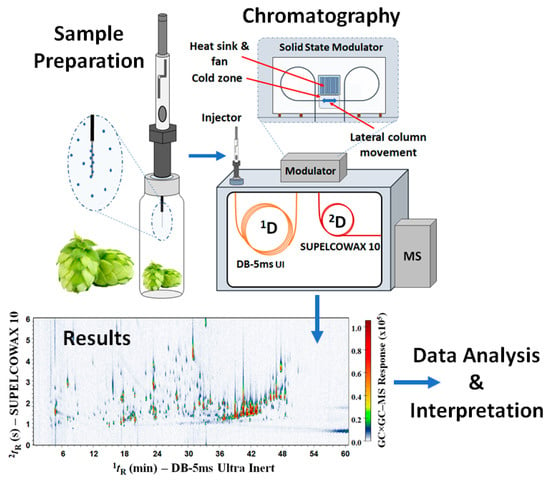
Figure 1.
Schematic representation of the HS–SPME and GC×GC–MS analyses of hop.
The retention index (R.I.) was calculated by the van den Dool and Kratz equation (Equation (1)) with a series of alkanes (C8–C21) analysed using 20 µL of 100 mg L−1 C8–C15 in hexane, 5 µL of 100 mg L−1 C16–C21 in hexane, and extracted by HS-SPME (same conditions as for the samples). The aliquots were introduced directly into a 20 mL clear glass vial with a screw top for extraction as above.
where “Ix” is the retention index, “n” is the number of carbons in the alkane prior to the analyte being determined, “tRx” is the retention time of the analyte, “tRn” is the retention time of the alkane prior to the analyte, and “tRn+1” is the retention time of the alkane after the analyte.
2.3. Gas Chromatography (GC–MS and GC×GC–MS)
Chromatographic analyses were performed using two different systems. The GC–MS system was an Agilent 7890A GC with a 7000 triple quadrupole MS (Agilent Technologies, Mulgrave, Australia), with the first quadrupole operated with total ion transfer. The GC×GC–MS system was an Agilent 7890A GC with a 5975C single quadrupole MS (Agilent Technologies) and included an SSM1800 solid-state modulator (SSM) system from J&X (J&X Technologies, Nanjing, China).
The GC–MS method was adapted from hop studies in the literature [22] and included a DB-5ms UI column (30 m × 0.25 mm I.D. × 0.25 µm df) connected to the MS transfer line by a deactivated fused silica (DFS, 1.0 m × 0.18 mm I.D.) and a glass press fit. Helium, grade 99.99%, was used as a carrier gas in constant flow (1.0 mL min−1). The injector was set in split mode (50:1) at 250 °C. The oven program was 50 °C (3 min), 3 °C min−1 to 200 °C, and 10 °C min−1 to 240 °C (3 min) (60 min analysis time). The MS settings were as follows: transfer line 240 °C, source 250 °C, quadrupole 150 °C, electron ionisation energy 70 eV, scan mode 35–350 m/z, and scan time of 300 ms.
The GC×GC–MS method including the solid-state modulator operation was also based on previous research [40,47] and included a DB-5ms UI 1D column (30 m × 0.25 mm I.D. × 0.25 µm df) and a SUPELCOWAX 10 2D column (1.0 m × 0.10 mm I.D. × 0.10 µm df). Deactivated fused silica capillaries were used as the modulator column (1.0 m × 0.15 mm I.D.) and the transfer line (0.43 m × 0.10 mm I.D.), and were connected to the main columns using glass press fit connectors. The GC temperature programme was the same as used in GC–MS analyses, except for the MS source 230 °C, quadrupole 150 °C, threshold 80, 12500 scans (u/s), and 22.8 scan/s. The solid-state modulator configuration was adapted from the literature [47] and included the same temperature program of the GC oven for the modulator entry oven and a 20 °C offset above the GC oven for the exit oven. The trap temperature program was −50 °C (8 min) and 2 °C min−1 to −20 °C (37 min).
2.4. Data Analysis
The GC–MS and GC×GC–MS data processing and chromatographic analyses were performed using Agilent MassHunter workstation Qualitative Analysis version 10.0 (Agilent Technologies, Santa Clara, CA, USA) and J&X Canvas W1.5.14.30115 (J&X Technologies, Shanghai, China) software. The MS identification was performed using the NIST 11 mass spectrometry library.
Statistical analysis was performed by Excel® (Microsoft, Redmond, WA, USA), MATLAB® software, version 7.13 (MathWorks, Natick, MA, USA), including the PLS Toolbox, version 6.5 (Eigenvector Technologies, Manson, WA, USA). The multivariate statistics analysis included principal component analysis (PCA) and hierarchical cluster analysis (HCA). This analysis included pre-processing autoscaling and removal of outliers in the graphs and Hotelling’s T2 versus Q residuals. Two types of PCA were performed, viz. by the areas (%) of compounds found in the samples, and by the sum of areas (%) of the classes of compounds. For HCA for the classes of compounds, Ward’s method was applied and PCA with 3 components.
3. Results and Discussion
One of the crucial steps in the analysis of hop varieties and their volatile compounds is to achieve an acceptable separation of peaks, leading to the increase in selectivity and improvement in the identification of substances due to reduced overlapping interferences. The complexity of hop samples can be represented by the single separation dimension for the Cascade hop GC–MS result shown in Figure 2A, for which the given GC column is apparently of conventional separation quality with a peak capacity for the 32 min analysis of approximately 300; this indicates responses that have considerable peak overlap. For example, “d” (15.4 min) is a linalool with a cluster of poorly resolved peaks, and peak “e” at 22.0 min (geraniol) has an evident shoulder before a peak on the geraniol tail. The molecular composition of such regions will be indeterminate. The complexity becomes immediately clear when GC–MS (Figure 2A) is compared with GC×GC–MS (Figure 2B) with retention times on the DB-5ms UI columns arranged to show the same peaks aligned vertically in each panel. The existence of significantly more compounds is confirmed in the latter GC×GC result, simply through the addition of the second dimension (2D) separation. Being a more polar column (wax-type), the 2D retention proceeds from less to more polar along this axis, and so this indicates the relative polarity of compounds that coeluted on the 1D column, and can serve as a check for possible chemical class. This leads to the notion of molecular structure–retention relationships in GC×GC [48]. It is evident that the 2D presentation of GC×GC with most peaks resolved covers a large proportion of the volatile (headspace) composition of the sample, leading to the suggestion that this embodies the requirements for comprehensive metabolite profiling [49] and is a quintessential untargeted method for volatile compounds [50].
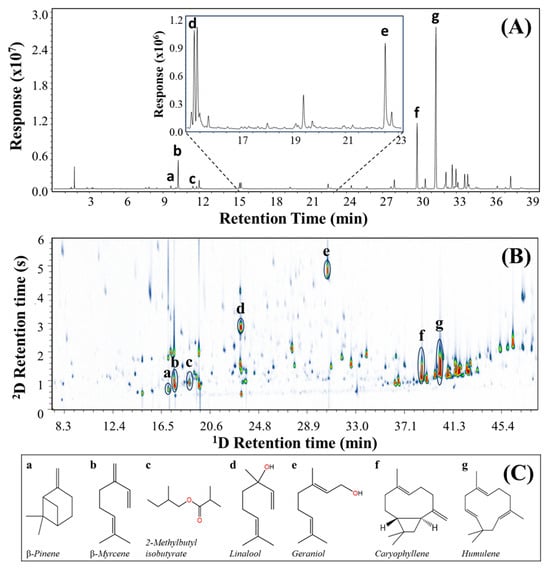
Figure 2.
A comparison between two chromatograms of (A) GC–MS and (B) GC×GC–MS applying HS–SPME for Cascade hop, where the GC–MS and 1D column of GC×GC-MS is a DB-5ms UI (30 m × 0.25 mm I.D. × 0.25 µm df), the 2D column is SUPELCOWAX 10 (1.0 m × 0.10 mm I.D. × 0.10 µm df), and the structure of the selected compounds (C) is represented by a–g indicated in (A,B).
The GC×GC–MS configuration scenario for metabolite analysis, generating a greater number of peaks, should also include better identification by the MS through more intense peaks that improve minor constituent detectability, fewer matrix interferences, and less phase bleeding. Here GC–MS analysis was similar to that in the literature [22]. For GC×GC–MS, 1D was a nonpolar DB-5ms UI phase column (5% phenyl methylpolysiloxane), and two 2D phase columns were tested: an intermediate polarity BPX50 phase (50% phenyl polysilphenylene-siloxane) and a polar phase (SUPELCOWAX 10; polyethylene glycol), both of the same dimensions. The results (Figure S1) displayed an enhanced separation with the polar 2D phase, better resolution of peaks, and a larger number of recorded metabolites. The modulation period (PM) used for GC×GC corresponds to the time available for completion of the 2D analysis before the wrap-around might occur. Times of 4, 5, 6, and 7 s were tested, with the best separation for 6 s, and a little wrap-around. As for the choice of the SPME fibre, the higher number of peaks, which we correlate with better coverage of total metabolites, was the pink fibre (Figure S2).
The SPME method presented in this work is advantageous for the study of volatiles in hop. Compared to other methods previously applied (Table 1), SPME has the advantages of being easier, requiring fewer resources, being solventless, and using mild temperatures and small amounts of sample. This limits compound degradation, while being environmentally friendly with less energy requirements and generating less waste. This sample preparation method is established in the literature, the fibres are commercially available, and automation is possible, which can facilitate and reduce costs in operation.
The intra-day and inter-day precision were evaluated using Cascade hop with HS-SPME-GC×GC–MS selected for 22 compounds and tabulated in Table S2. The intra-day precision (n = 5 injections on the same day) displayed RSD % of retention time of 0.00–0.23% for 1tR, 0.05–4.30% for 2tR, and between 1.51% and 9.63% for peak areas. The inter-day precision was analysed with n = 9, where three replicates were injected daily over 3 different days with results of 0.22–0.58% for 1tR, 1.21–8.77% for 2tR, and 1.65–12.81% for the area. These results indicate an acceptable repeatability for the proposed method, as compared with the values of Yan et al. [34], which means a low value of RSD even when considering the SPME reproducibility for peak area.
Compared to previous studies (Table 1), the present methodology resulted in the highest number of peaks tentatively identified in the hop by using GC×GC–MS. The profile of the metabolites present in the five different hops identified numerous peaks by GC×GC–MS, totalling 205 for AZAC, 258 for CASC, 421 for LORA, 472 for ZAPP, and 413 for ENIG. Comparing the number of compounds reported with the GC–MS for the samples (Table 2) proved the improvement in metabolic coverage by GC×GC–MS with increases of 140.2% for CASC, 273.5% for ENIG, and 316.8% for ZAPP. Figure 2 (see Figures S6 and S7 for the other samples) represents aligned peaks with seven metabolites that have significant characteristics for the flavour and odour of hop: (a) β-pinene, (b) β-myrcene, (c) 2-methylbutyl isobutyrate, (d) linalool, (e) geraniol, (f) caryophyllene, and (g) humulene. All these components are now apparently free from interference, although the suite of non-polar compounds could potentially lead to overlapping compounds. Better separation should correspond to improved MS matching with databases. The overall results demonstrate evidence of a better separation, clear distinction of compounds and, consequently, an increase in identified peaks.

Table 2.
Comparison of peaks from 3 hop samples by GC–MS and GC×GC–MS.
The tentative identification was performed using the NIST 11 database, and the highest probability compound identity was determined, considering the MS RMatch (>700) and retention indices (±20 units) based on the van den Dool and Kratz equation (Table S3 and Figures S3–S5). A total of 137 compounds were identified with GC×GC–MS analysis of five hops and a total of 73 compounds for GC–MS analysis of three hops (n = 3, Table S4).
The final list of compounds tentatively identified by GC×GC–MS (Table 3) includes their molecular formula, CAS identifier, retention time, retention indices from literature and experiment, the relative chromatographic area of the peaks in each sample, and compound chemical class. Analysis of selected standards was performed for specific compound identification confirmation. The main classes of volatiles identified and the number of compounds were as follows: alcohols (3), aldehydes (4), esters (47), hydrocarbons (7), ketones (9), monoterpene hydrocarbons (17), oxygenated monoterpenes (12), sesquiterpene hydrocarbons (32), and oxygenated sesquiterpenes (3). The total amount of volatiles is expressed in the last line of the table, demonstrating that there remains a small fraction of unknown compounds, from 3.1% to 12.4% of the total area, which could not be identified using the reported criteria. However, this is an improvement in the identified or tentatively identified molecules over previous studies of essential oils since studies such as Wong et al. [33] reported 50.8–90.0% of the total area of the sample by GC×GC.

Table 3.
The composition of hop samples determined by using HS–SPME–GC×GC–MS.
The most abundant compounds detected using the SPME sampling method were related to the sesquiterpene hydrocarbons humulene (108) and caryophyllene (103), and the monoterpene hydrocarbon β-myrcene (22). These three compounds are reported in the literature as the major components present in hop, being responsible for up to 90% of the composition of hop essential oils [4]. All these substances are formed from precursors obtained through the methyl-D-erythritol 4-phosphate (MEP) pathway followed by the transformation by prenyltransferases into geranyl diphosphate (GPP) [51,52]. β-Myrcene is formed by GPP during hop growth by a monoterpene synthase (MTS2) [52,53] and has the odour characteristics of peppery, spicy, balsam, plastic, and terpene [24]. For the samples studied, the highest area of β-myrcene was reported for the hop ENIG (37.82%), followed by ZAPP (17.06%) and CASC (5.93%). Caryophyllene, or β-caryophyllene, is an isomer of humulene, also identified as α-humulene or α-caryophyllene, and both compounds are produced by sesquiterpene synthase 1 (HlSTS1) from the precursor β-farnesene formed after GPP [52,53]. Humulene is a metabolite that originated in the final stages of hop cone maturation and was reported in the samples with the highest area of 54.07% for the sample LORA, 41.36% for AZAC, and 36.44% for CASC; lowest humulene abundances, although still with a considerable percentage, were 16.04% for ENIG and 10.05% for ZAPP. Caryophyllene showed lower concentrations than humulene in the samples, except for ZAPP, which had twice the caryophyllene area than humulene. Table 3 lists the major compounds in the five hop samples. Another observation noted here and confirmed from the literature was the relation between β-myrcene and humulene, where β-myrcene has an inverse trend in concentration compared to humulene, described by a common intermediate in their biosynthesis via α-acids and β-acids [4,54].
Some compounds have been identified in only one of the samples (i.e., unique to a single hop), with possible use as chemical markers including those in Table 4.

Table 4.
Suggested unique marker compounds in each of the hop samples.
The elucidation of the GC×GC distribution of compounds tentatively identified can be expressed by the apex plot in Figure 3a, which was constructed considering all the identified molecules present in Table 3. The classes can be seen as different markers, where the location of peaks shows a region for various classes. For instance, esters (orange circles) persist over the full range of 1tR but cluster between 2tR = 1.0 and 2.0 s, and are shown above the hydrocarbons (dark blue circle). The terpenoid compounds, Figure 3b, demonstrate the power of separation of GC×GC through a clear distinction between the classes of MHyd, OM, SHyd, and OS.
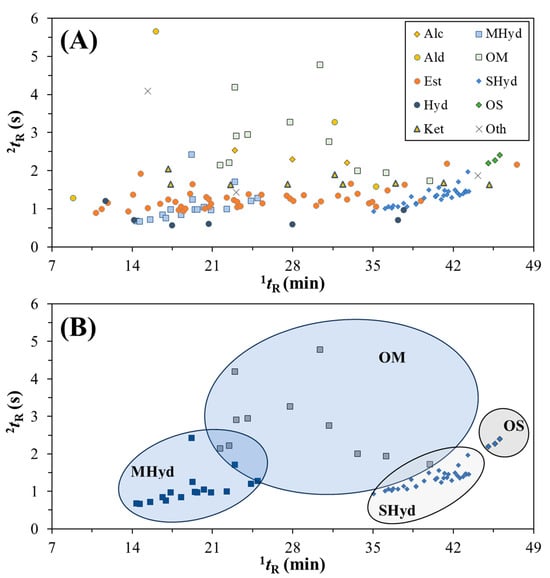
Figure 3.
(A) Apex plot of the compounds present in the hop samples AZAC, CASC, ENIG, LORA, and ZAPP showing the classes of compounds (refer to Table 3 for abbreviations). (B) Apex plot showing clustering of MHyd, OM, SHyd, and OS classes.
The classes of compounds represented in this study may be considered descriptors of the composition of the hop, displaying the metabolite formation and the identity of the cone. Figure 4 represents the compositional profile of each hop according to the percentage obtained by our methodology (subject to the limitations of reporting peak areas by using SPME), by a clear distinction of the samples. According to Figure 4, all the hops expressed the highest concentration of sesquiterpene hydrocarbons as the major class present, as expected based on the presence of caryophyllene (103) and humulene (108), with the largest amounts for AZAC, LORA, and CASC, respectively. The hop ENIG and ZAPP expressed a considerable concentration of monoterpene hydrocarbons, which differentiates these from the other hop; this characteristic can be related to the high concentration of β-myrcene (22). Furthermore, β-myrcene has a direct relationship with the amount of essential oil produced by the hop with regards to its biosynthetic pathway, giving a different complexity for the hop [4].
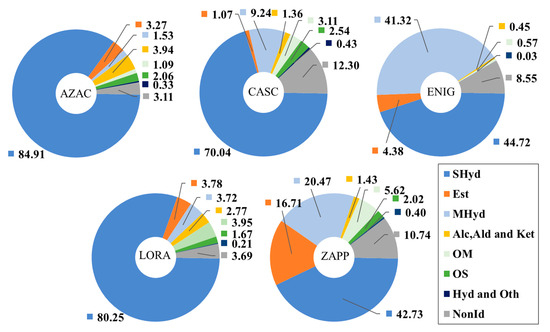
Figure 4.
Compositional plots for the hop samples AZAC, CASC, ENIG, LORA, and ZAPP based on the classes of compounds expressed in area percentage (%), according to the SPME methodology employed.
The amount of data provided by GC×GC illustrates the ease in profiling and describing the sample composition, which should be translated into multivariate statistics, a powerful tool to aid this data interpretation. Considering the data in Table 3, a principal component analysis (PCA) interpretation was applied to the compounds present. For the PCA, the model was built with the relative peak area (%) related to each compound with the selection of autoscale as preprocessing and 3 PCs representing 81.70% of the variance with 34.19% for PC1, 24.24% for PC2, and 23.27% for PC3. The biplot graphs, shown in Figure 5, represent (A) PC1×PC2 and (B) PC1×PC3, where the samples (scores) are represented by red triangles and the compounds (loadings) by blue squares. To improve the view of the compounds related to each sample, coloured regions were drawn for AZAC (blue), CASC (pink), ENIG (yellow), LORA (green), and ZAPP (grey). Regarding this result, a diversity of substances is related to the samples and, as expected, the complexity of compounds formed during hop metabolism. The distribution of compounds can be seen with the ZAPP hop related to the positive part of PC1, while ENIG is present in the negative part of PC2; moreover, AZAC is present in the positive part of PC1 and the negative part of PC2, while PC3 shows LORA in the positive section and CASC in the negative.
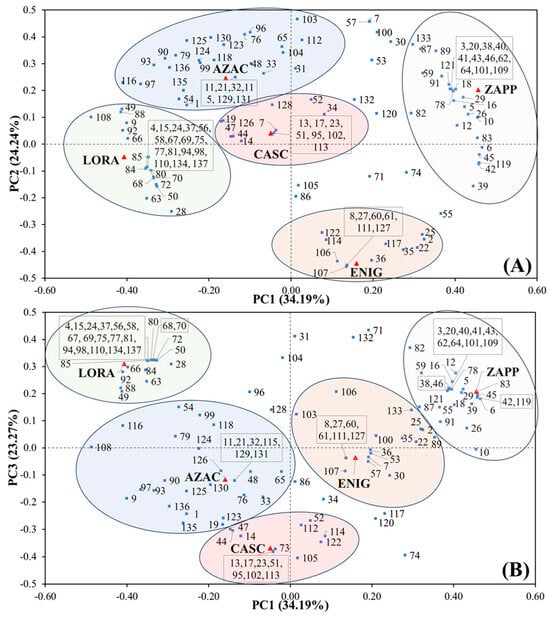
Figure 5.
Biplot graph for the principal component analysis (PCA), representing (A) PC1×PC2 and (B) PC1×PC3. The circles represent the hop samples as AZAC (blue), CASC (pink), ENIG (yellow), LORA (green), and ZAPP (grey).
Characterising some of the compounds, attention may be called to the unique compounds in each of the samples with the presence of clusters close to their respective red triangles shown in Figure 5. Characterising each hop variety, AZAC shows the influence of compounds hexanal (1), p-cymene (33), the sesquiterpene hydrocarbons δ-cadinene (124), calamenene (125) and α-calacorene (130), and the ketones 2-nonanone (48), 2-decanone (65), 2-undecanone (79), 2-dodecanone (96) and 2-tridecanone (116). This shows a significant connection between AZAC and the ketone formed in its metabolism. Regarding reports from the literature [55], the hop AZAC was studied by GC–MS and a method for the fraction of hydrodistilled essential oil followed by HS-SPME. This study also showed a high concentration of caryophyllene and a high percentage of humulene as reported by results in Table 3.
For the hop CASC, the monoterpene hydrocarbons camphene (14) and perillene (52) and the oxygenated monoterpenes geraniol (73), cis-linalool oxide (44), and trans-linalool oxide (47) were related by PCA analysis. This shows that CASC has a relation with the auto-oxidation of myrcene [56,57] by the formation of geraniol, an important floral odorant in the hop essential oil, as the presence of camphene and linalool oxide in the two isomeric forms, related to the “European hop aroma” [17,57]. The hop CASC is widely studied in the literature, as in the GC×GC-TOFMS study from Yan et al. [34], and by GC–MS from other studies [55]. In the results of Yan et al., the essential oil fraction of CASC was hydrodistilled and as a result also displayed the presence of geraniol and perillene, proving certain similarities. When compared to the composition of humulene (36.44%) and β-myrcene (5.93%) (noting limitations due to the SPME sampling), this paper shows a contrary behaviour compared to the literature [34,55,58], where the concentration of β-myrcene is higher than humulene, which is explained as a distinct sample with a probable cause of low content of β-myrcene and high content of humulene related to the ripening period, more specifically an early harvest or a not-well-ripe hop [4,54].
Regarding ENIG, three classes were significant: monoterpene hydrocarbons comprising β-myrcene (22), β-ocimene (35), and trans-β-ocimene (36), the two sesquiterpene hydrocarbons (E)-β-farnesene (107) and β-eudesmene (117), and the esters isobutyl 2-methylbutanoate (25) and 2-methylbutyl octanoate (106). Low concentrations of β-farnesene has been reported in low concentrations in hop [4,54], and this can be one of the differentiating components of ENIG hop, while the presence of the monoterpene β-myrcene showed the highest concentration, followed by the two forms of β-ocimene.
LORA composition noted the esters isoamyl isobutanoate (28), ethyl heptanoate (49), hexyl isobutyrate (63), ethyl octanoate (66), trans-geranic acid methyl ester (84), isobutyric acid 1-methyl-octyl ester (88), and ethyl cis-4-decenoate (92). As reported, the major group responsible for the PCA separation was the esters, even in the specific compounds expressed only by the hop LORA. Nevertheless, compounds such as fenchol (58), α-terpineol (67), perillaldehyde (77), and α-citral (75) were important monoterpene alcohols and aldehydes for the chemical composition of this hop.
Finally, ZAPP showed two compounds within the cluster of specific compounds: prenyl isobutyrate (38) and methyl 6-methyl heptanoate (46); relations with the esters 2-methylbutyl acetate (5), isobutyl isobutyrate (6), isobutyl butyrate (12), 2-methylbutyl propionate (16), 2-methylbutyl isobutyrate (29), methyl 8-methylnonanoate (78), methyl decanoate (83), and methyl 3,6-dodecadienoate (119); and the monoterpene hydrocarbons γ-terpinene (42), isoterpinolene (45), and α-muurolene (118). ZAPP was similar to the LORA sample, where the ester class had the capacity to represent the separation of samples by PCA.
Based on all the information described above and noting some patterns in the PCA (Figure 5), the classes of compounds were also studied according to PCA and HCA. For the PCA, the model was developed with the sum of the relative chromatographic areas (%) of each class using the selection of autoscale as preprocessing and 3 PCs representing 90.58% of variance displaying the variance of 39.78% for PC1, 36.72% for PC2, and 14.08% for PC3. Regarding HCA, autoscale was used as preprocessing, Ward’s method was applied as an algorithm, and PCA was used to choose three PCs. The PCA graphs are expressed as biplot graphs, as shown in Figure 6A (PC1×PC2) and Figure 6B (PC1×PC3), while HCA is represented in Figure 6C.
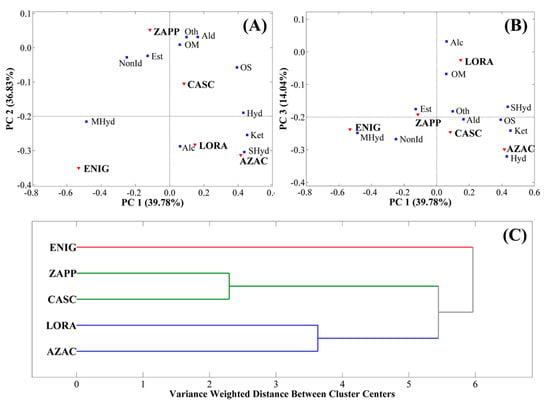
Figure 6.
Multivariate statistical analysis of PCA expressed by the biplot graph of (A) PC1×PC2 and (B) PC1×PC3, and (C) HCA for the hops AZAC, CASC, ENIG, LORA, and ZAPP regarding the classes of compounds.
The PCA for the classes of compounds separates the ZAPP sample by PC2, LORA by PC3, and ENIG by PC1. As explained previously, this represents that the sample ZAPP was most influenced by esters, LORA by alcohols, and ENIG by monoterpene hydrocarbons, with the influence of ketones over the AZAC hop. HCA gave the highest similarity between classes of ZAPP and CASC, followed by LORA and AZAC, which means that compared to the results shown by PCA, the first two classes were largely influenced by esters, aldehydes, oxygenated monoterpenes, and sesquiterpenes, while the second two were more affected by sesquiterpene hydrocarbons and ketones.
4. Conclusions
In this paper, a method for HS-SPME-GC×GC–MS using hop samples was developed, applied, and demonstrated to be a powerful technique to identify metabolites in the samples. By suitable experimental design of the method, we believe this to be a “hop-timal” analysis strategy for total VOC composition in the headspace of the hop cone. The identification of compounds as an untargeted study through this methodology provides considerable coverage of volatile metabolite expression, and it is possible to describe both major and minor compounds, including those most likely not readily measured by single-dimension GC separations since substantially fewer compounds are reported in GC–MS analysis. Thus, the identification of 137 substances in five diverse hop samples is described, with the potential of separating 471 peaks. This of course also highlights that in terms of metabolite identification, just having the ability to separate individual compounds is not the same as being able to unambiguously identify them. In this regard, in comparison with the conventional technique of GC–MS, GC×GC–MS improves the separation through an increase of over 300% in the number of peaks recognised as discrete components. This study reports the comprehensive study of hop through detailed chemical class assignment compounds, which can largely be separated in the 2D space of GC×GC analysis. Multivariate statistics analysis proved a similarity between the samples ZAPP and CASC, and the samples AZAC and LORA. The use of HS-SPME-GC×GC–MS is a bright light in understanding the metabolites present in hop.
Supplementary Materials
The following supporting information (abbreviated titles here) can be downloaded at: https://www.mdpi.com/article/10.3390/metabo14040237/s1. Table S1: Hop samples information; Table S2: Intra-day and inter-day precision of selected peaks; Figure S1: A comparison between two chromatographic settings for GC×GC; Table S3: n-Alkanes the series C8-C21 and their respective retention times; Figure S2: Retention time vs n-alkanes series (Cn) plot; Figure S3: GC×GC–MS chromatogram for the HS–SPME of the series of n-alkanes (C8-C15); Figure S4: GC×GC–MS chromatogram for the HS–SPME of the series of n-alkanes (C16-C21); Figure S5: Comparison of chromatograms obtained for the Enigma (ENIG) hop by HS–SPME; Figure S6: Comparison of chromatograms obtained for the Zappa (ZAPP) hop by HS-SPME; Table S4: Information in the hop composition profile using HS-SPME-GC–MS; Figure S7: GC×GC-MS chromatogram for the HS-SPME of Azacca (AZAC) hop; Figure S8: GC×GC-MS chromatogram for the HS-SPME of Loral (LORA) hop.
Author Contributions
Conceptualisation: G.A.P.R. and P.J.M.; writing—original draft preparation: G.A.P.R., M.S.S.A. and P.J.M.; writing—review and editing: G.A.P.R., M.S.S.A., P.J.M. and B.G.B.; visualisation: G.A.P.R. and M.S.S.A.; supervision: P.J.M. and B.G.B.; funding acquisition: P.J.M. and B.G.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
The authors would like to thank the Brazilian government agencies CNPq, FAPEMIG, and CAPES for financial support. GAPR acknowledges CNPq for the PhD scholarship (project 155078/2019-4). This study was partially funded by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Brasil—Finance Code 001. Carlton & United Breweries are thanked for the provision of some hop samples.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- Moir, M. Hops—A millennium review. J. Am. Soc. Brew. Chem. 2018, 58, 131–146. [Google Scholar] [CrossRef]
- Steenackers, B.; De Cooman, L.; De Vos, D. Chemical transformations of characteristic hop secondary metabolites in relation to beer properties and the brewing process: A review. Food Chem. 2015, 172, 742–756. [Google Scholar] [CrossRef] [PubMed]
- Research, M. Global Hops Market—2023–2030. Available online: https://www.marketresearch.com/DataM-Intelligence-4Market-Research-LLP-v4207/Global-Hops-34833012/ (accessed on 25 September 2023).
- Almaguer, C.; Schönberger, C.; Gastl, M.; Arendt, E.K.; Becker, T. Humulus lupulus—A story that begs to be told. A review. J. Inst. Brew. 2014, 120, 289–314. [Google Scholar] [CrossRef]
- Cattoor, K.; Dresel, M.; De Bock, L.; Boussery, K.; Van Bocxlaer, J.; Remon, J.-P.; De Keukeleire, D.; Deforce, D.; Hofmann, T.; Heyerick, A. Metabolism of hop-derived bitter acids. J. Agric. Food Chem. 2013, 61, 7916–7924. [Google Scholar] [CrossRef] [PubMed]
- Knez Hrnčič, M.; Španinger, E.; Košir, I.J.; Knez, Ž.; Bren, U. Hop compounds: Extraction techniques, chemical analyses, antioxidative, antimicrobial, and anticarcinogenic effects. Nutrients 2019, 11, 257. [Google Scholar] [CrossRef] [PubMed]
- Rettberg, N.; Biendl, M.; Garbe, L.-A. Hop aroma and hoppy beer flavor: Chemical backgrounds and analytical tools—A review. J. Am. Soc. Brew. Chem. 2018, 76, 1–20. [Google Scholar] [CrossRef]
- King, A.J.; Dickinson, J.R. Biotransformation of hop aroma terpenoids by ale and lager yeasts. FEMS Yeast Res. 2003, 3, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, B.P.; Nascimento, P.G.B.D.; Ghesti, G.F. Intellectual property and plant variety protection: Prospective study on hop (Humulus lupulus L.) cultivars. World Patent Inf. 2021, 65, 102041. [Google Scholar] [CrossRef]
- Afendi, F.M.; Okada, T.; Yamazaki, M.; Hirai-Morita, A.; Nakamura, Y.; Nakamura, K.; Ikeda, S.; Takahashi, H.; Altaf-Ul-Amin, M.; Darusman, L.K.; et al. KNApSAcK family databases: Integrated metabolite-plant species databases for multifaceted plant research. Plant Cell Physiol. 2012, 53, e1. [Google Scholar] [CrossRef]
- Nezi, P.; Cicaloni, V.; Tinti, L.; Salvini, L.; Iannone, M.; Vitalini, S.; Garzoli, S. Metabolomic and proteomic profile of dried hop Inflorescences (Humulus lupulus L. cv. Chinook and cv. Cascade) by SPME-GC-MS and UPLC-MS-MS. Separations 2022, 9, 204. [Google Scholar] [CrossRef]
- Fiehn, O.; Kopka, J.; Dormann, P.; Altmann, T.; Trethewey, R.N.; Willmitzer, L. Metabolite profiling for plant functional genomics. Nat. Biotech. 2000, 18, 1157–1161. [Google Scholar] [CrossRef] [PubMed]
- Hughey, C.A.; McMinn, C.M.; Phung, J. Beeromics: From quality control to identification of differentially expressed compounds in beer. Metabolomics 2016, 12, 11. [Google Scholar] [CrossRef]
- Ikhalaynen, Y.A.; Plyushchenko, I.V.; Rodin, I.A. Hopomics: Humulus lupulus brewing cultivars classification based on LC-MS profiling and nested feature selection. Metabolites 2022, 12, 945. [Google Scholar] [CrossRef] [PubMed]
- Brendel, R.; Schwolow, S.; Rohn, S.; Weller, P. Gas-phase volatilomic approaches for quality control of brewing hops based on simultaneous GC-MS-IMS and machine learning. Anal. Bioanal. Chem. 2020, 412, 7085–7097. [Google Scholar] [CrossRef] [PubMed]
- Fiehn, O. Metabolomics by gas chromatography-mass spectrometry: Combined targeted and untargeted profiling. Curr. Protocols Mol. Biol. 2016, 114, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Eyres, G.; Dufour, J.-P. Hop essential oil: Analysis, chemical composition and odor characteristics. In Beer in Health and Disease Prevention; Preedy, V.R., Ed.; Academic Press: Burlington, MA, USA, 2009; pp. 239–254. [Google Scholar]
- Aberl, A.; Coelhan, M. Determination of volatile compounds in different hop varieties by headspace-trap GC/MS—In comparison with conventional hop essential oil analysis. J. Agric. Food Chem. 2012, 60, 2785–2792. [Google Scholar] [CrossRef] [PubMed]
- Jorge, K.; Trugo, L.C. Discrimination of different hop varieties using headspace gas chromatographic data. J. Brazil. Chem. Soc. 2003, 14, 411–415. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, L.; Liu, Y. Rapid differentiation of Chinese hop varieties (Humulus lupulus) using volatile fingerprinting by HS-SPME-GC-MS combined with multivariate statistical analysis. J. Sci. Food Agric. 2018, 98, 3758–3766. [Google Scholar] [CrossRef] [PubMed]
- Eri, S.; Khoo, B.K.; Lech, J.; Hartman, T.G. Direct thermal desorption-gas chromatography and gas chromatography-mass spectrometry profiling of hop (Humulus lupulus L.) essential oils in support of varietal characterization. J. Agric. Food Chem. 2000, 48, 1140–1149. [Google Scholar] [CrossRef] [PubMed]
- Kovačevič, M.; Kač, M. Solid-phase microextraction of hop volatiles. Potential use for determination and verification of hop varieties. J. Chromatogr. A 2001, 918, 159–167. [Google Scholar] [CrossRef]
- Kishimoto, T.; Teramoto, S.; Fujita, A.; Yamada, O. Principal component analysis of hop-derived odorants identified by stir bar sorptive extraction method. J. Am. Soc. Brew. Chem. 2020, 79, 272–280. [Google Scholar] [CrossRef]
- Inui, T.; Tsuchiya, F.; Ishimaru, M.; Oka, K.; Komura, H. Different beers with different hops. Relevant compounds for their aroma characteristics. J. Agric. Food Chem. 2013, 61, 4758–4764. [Google Scholar] [CrossRef] [PubMed]
- Richter, T.M.; Eyres, G.T.; Silcock, P.; Bremer, P.J. Comparison of four extraction methods for analysis of volatile hop-derived aroma compounds in beer. J. Sep. Sci. 2017, 40, 4366–4376. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Wang, X.; Yuan, A.; Liu, J.; Li, Z.; Xie, D.; Zhang, H.; Luo, W.; Xu, H.; Liu, J.; et al. Chemical constituents and bioactivities of hops (Humulus lupulus L.) and their effects on beer-related microorganisms. Food Energ. Sec. 2022, 11, e367. [Google Scholar] [CrossRef]
- Rubiolo, P.; Sgorbini, B.; Liberto, E.; Cordero, C.; Bicchi, C. Essential oils and volatiles: Sample preparation and analysis. A review. Flav. Fragr. J. 2010, 25, 282–290. [Google Scholar] [CrossRef]
- Abolghasemi, M.M.; Piryaei, M. Development of direct microwave desorption/gas chromatography mass spectrometry system for rapid analysis of volatile components in medicinal plants. J. Sep. Sci. 2020, 43, 782–787. [Google Scholar] [CrossRef] [PubMed]
- Priest, M.A.; Boersma, J.A.; Bronczyk, S.A. Effects of aging on hops and liquid CO2 hop extracts. J. Am. Soc. Brew. Chem. 2018, 49, 98–100. [Google Scholar] [CrossRef]
- Gonçalves, J.L.; Figueira, J.A.; Rodrigues, F.P.; Ornelas, L.P.; Branco, R.N.; Silva, C.L.; Camara, J.S. A powerful methodological approach combining headspace solid phase microextraction, mass spectrometry and multivariate analysis for profiling the volatile metabolomic pattern of beer starting raw materials. Food Chem. 2014, 160, 266–280. [Google Scholar] [CrossRef] [PubMed]
- Nolvachai, Y.; Amaral, M.S.S.; Herron, R.; Marriott, P.J. Solid phase microextraction for quantitative analysis—Expectations beyond design? Green Anal. Chem. 2023, 4, 100048. [Google Scholar] [CrossRef]
- Nolvachai, Y.; Amaral, M.S.S.; Marriott, P.J. Foods and contaminants analysis using multidimensional gas chromatography: An update of recent studies, technology, and applications. Anal. Chem. 2023, 95, 238–263. [Google Scholar] [CrossRef] [PubMed]
- Wong, Y.F.; Perlmutter, P.; Marriott, P.J. Untargeted metabolic profiling of Eucalyptus spp. leaf oils using comprehensive two-dimensional gas chromatography with high resolution mass spectrometry: Expanding the metabolic coverage. Metabolomics 2017, 13, 46. [Google Scholar] [CrossRef]
- Yan, D.; Wong, Y.F.; Tedone, L.; Shellie, R.A.; Marriott, P.J.; Whittock, S.P.; Koutoulis, A. Chemotyping of new hop (Humulus lupulus L.) genotypes using comprehensive two-dimensional gas chromatography with quadrupole accurate mass time-of-flight mass spectrometry. J. Chromatogr. A 2018, 1536, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Dufour, J.-P.; Marriott, P.J.; Reboul, E.; Leus, M.; Silcock, P. Quantitative analysis of complex flavour mixtures using comprehensive multidimensional gas chromatography. In Proceedings of the Flavour Research at the Dawn of the 21st Century, Beaune, France, 24–28 June 2002; Quéré, J.L., Étiévant, P.X., Eds.; Editions Tec & Doc: Paris, France, 2002; pp. 500–504. [Google Scholar]
- Yan, D.; Tedone, L.; Koutoulis, A.; Whittock, S.P.; Shellie, R.A. Parallel comprehensive two-dimensional gas chromatography. J. Chromatogr. A 2017, 1524, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Reglitz, K.; Lemke, N.; Steinhaus, M.; Hanke, S. On the behavior of the important hop odorant 4-mercapto-4-methylpentan-2-one (4MMP) during dry hopping and during Storage of dry hopped beer. Brew. Sci. 2018, 71, 96–99. [Google Scholar] [CrossRef]
- Eyres, G.T.; Marriott, P.J.; Dufour, J.-P. Comparison of odor-active compounds in the spicy fraction of hop (Humulus lupulus L.) essential oil from four different varieties. J. Agric. Food Chem. 2007, 55, 6252–6261. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, C.; Biendl, M.; Hanke, S.; Reglitz, K.; Steinhaus, M. Dry hopping potential of Eureka! A new hop variety. Brew. Sci. 2019, 72, 173–178. [Google Scholar]
- Yan, D.; Wong, Y.F.; Whittock, S.P.; Koutoulis, A.; Shellie, R.A.; Marriott, P.J. Sequential hybrid three-dimensional gas chromatography with accurate mass spectrometry: A novel tool for high-resolution characterization of multicomponent samples. Anal. Chem. 2018, 90, 5264–5271. [Google Scholar] [CrossRef] [PubMed]
- Reglitz, K.; Steinhaus, M. Quantitation of 4-Methyl-4-sulfanylpentan-2-one (4MSP) in hops by a stable isotope dilution assay in combination with GCxGC–TOFMS: Method development and application to study the influence of variety, Provenance, harvest year, and processing on 4MSP concentrations. J. Agric. Food Chem. 2017, 65, 2364–2372. [Google Scholar] [CrossRef] [PubMed]
- Inui, T.; Matsui, H.; Hosoya, T.; Kumazawa, S.; Fukui, N.; Oka, K. Effect of harvest time and pruning date on aroma characteristics of hop teas and related compounds of Saaz hops. J. Am. Soc. Brew. Chem. 2016, 74, 231–241. [Google Scholar] [CrossRef]
- Lusk, L.T.; Kay, S.B.; Porubcan, A.; Ryder, D.S. Key olfactory cues for beer oxidation. J. Am. Soc. Brew. Chem. 2012, 70, 257–261. [Google Scholar] [CrossRef]
- Eyres, G.; Marriott, P.J.; Dufour, J.-P. The combination of gas chromatography-olfactometry and multidimensional gas chromatography for the characterisation of essential oils. J. Chromatogr. A 2007, 1150, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.T.; Dufour, J.P.; Lewis, A.C. Application of comprehensive multidimensional gas chromatography combined with time-of-flight mass spectrometry (GC x GC-TOFMS) for high resolution analysis of hop essential oil. J. Sep. Sci. 2004, 27, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Yin, Y. Aroma characterization of regional Cascade and Chinook hops (Humulus lupulus L.). Food Chem. 2021, 364, 130410. [Google Scholar] [CrossRef] [PubMed]
- Amaral, M.S.S.; Hearn, M.T.W.; Marriott, P.J. Lipase-catalysed changes in essential oils revealed by comprehensive two-dimensional gas chromatography. Anal. Bioanal. Chem. 2023, 415, 3189–3199. [Google Scholar] [CrossRef] [PubMed]
- Marriott, P.J.; Massil, T.; Hugel, H. Molecular structure retention relationships in comprehensive two-dimensional gas chromatography. J. Sep. Sci. 2004, 27, 1273–1284. [Google Scholar] [CrossRef] [PubMed]
- Marriott, P.J.; Resende, G.A.P. GC×GC technology for non-targeted analysis of volatile compounds It just makes sense. Wiley Analyt. Sci. Mag. 2023, 3, 29–34. [Google Scholar]
- Wong, Y.F.; Marriott, P.J. Approaches and challenges for analysis of flavor and fragrance volatiles. J. Agric. Food Chem. 2017, 65, 7305–7307. [Google Scholar] [CrossRef]
- Champagne, A.; Boutry, M. A comprehensive proteome map of glandular trichomes of hop (Humulus lupulus L.) female cones: Identification of biosynthetic pathways of the major terpenoid-related compounds and possible transport proteins. Proteomics 2017, 17, 1600411. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, R.L.; Padgitt-Cobb, L.K.; Townsend, M.S.; Henning, J.A. Gene expression for secondary metabolite biosynthesis in hop (Humulus lupulus L.) leaf lupulin glands exposed to heat and low-water stress. Sci. Rep. 2021, 11, 5138. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Tian, L.; Aziz, N.; Broun, P.; Dai, X.; He, J.; King, A.; Zhao, P.X.; Dixon, R.A. Terpene biosynthesis in glandular trichomes of hop. Plant Physiol. 2008, 148, 1254–1266. [Google Scholar] [CrossRef]
- Howard, G.A.; Slater, C.A. Evaluation of hops VII. Composition of the essential oil of hops. J. Inst. Brew. 1957, 63, 491–506. [Google Scholar] [CrossRef]
- Móricz, Á.M.; Bartoszek, M.; Polak, J.; Marczewska, P.; Knaś, M.; Böszörményi, A.; Fodor, J.; Kowalska, T.; Sajewicz, M. A comparison of quantitative composition and bioactivity of oils derived from seven North American varieties of hops (Humulus lupulus L.). Separations 2023, 10, 402. [Google Scholar] [CrossRef]
- Stevens, R. The chemistry of hop constituents. Chem. Rev. 1967, 67, 19–71. [Google Scholar] [CrossRef]
- Rutnik, K.; Knez Hrnčič, M.; Jože Košir, I. Hop essential oil: Chemical composition, extraction, analysis, and applications. Food Rev. Int. 2021, 38, 529–551. [Google Scholar] [CrossRef]
- Buglass, A.J.; Caven-Quantrill, D.J. Applications of natural ingredients in alcoholic drinks. In Natural Food Additives, Ingredients and Flavourings; Baines, D., Seal, R., Eds.; Woodhead Publishing: Cambridge, UK, 2012; pp. 358–416. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).