A System Biology Approach Reveals New Targets for Human Thyroid Gland Toxicity in Embryos and Adult Individuals
Abstract
1. Introduction
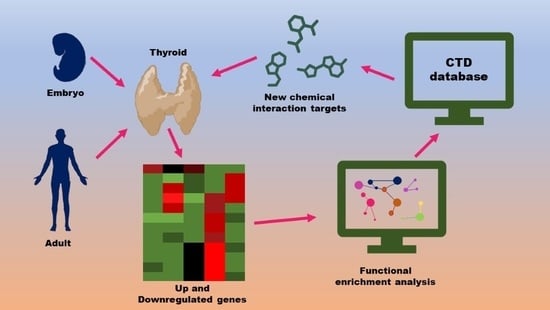
2. Materials and Methods
2.1. Dataset Selection
2.2. Gene Set Enrichment Analysis
2.3. Chemical Interaction Discovery
3. Results
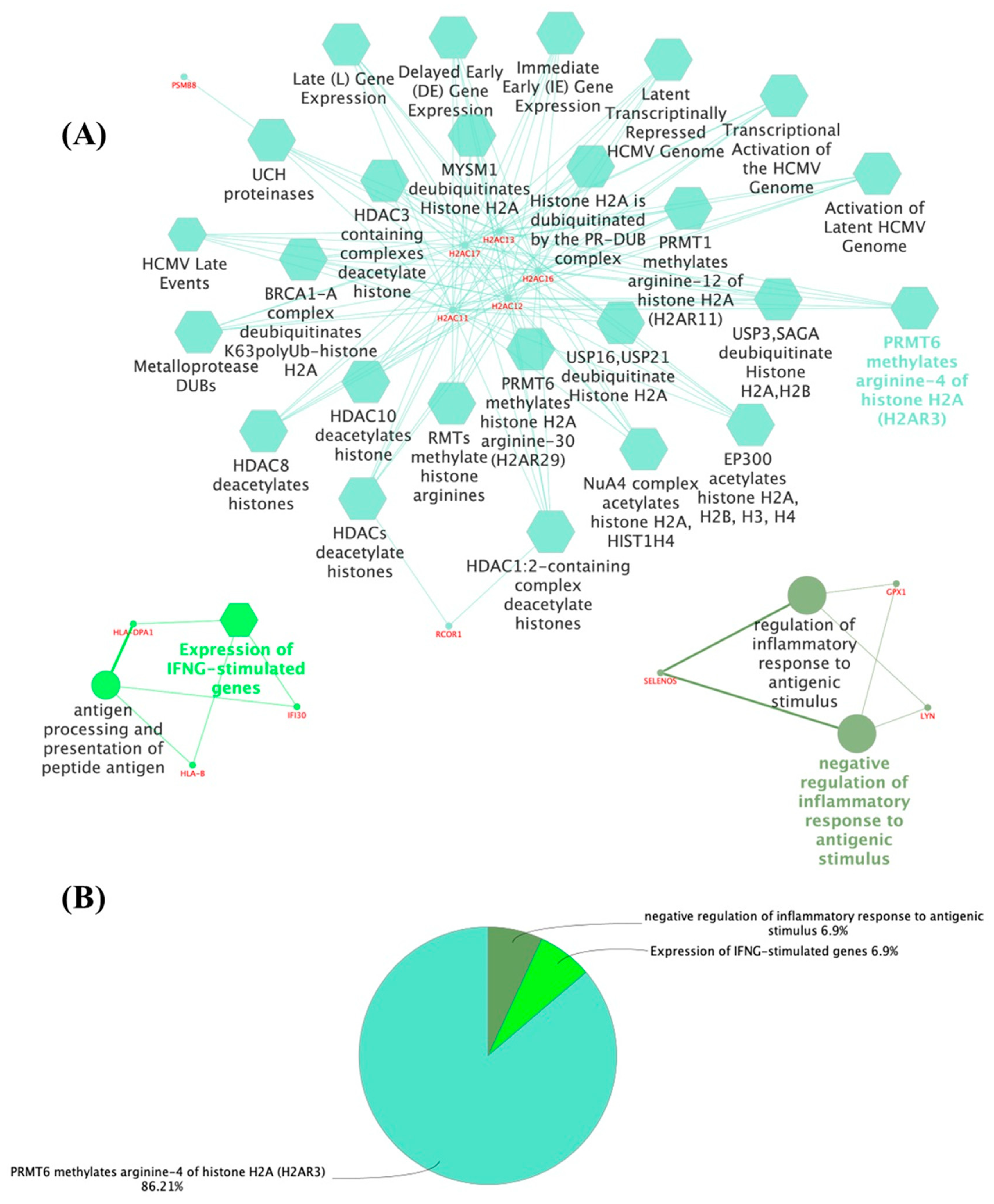
3.1. The Thyroid Embryonic Development Period as a Target for EDCs—A System Biology Approach
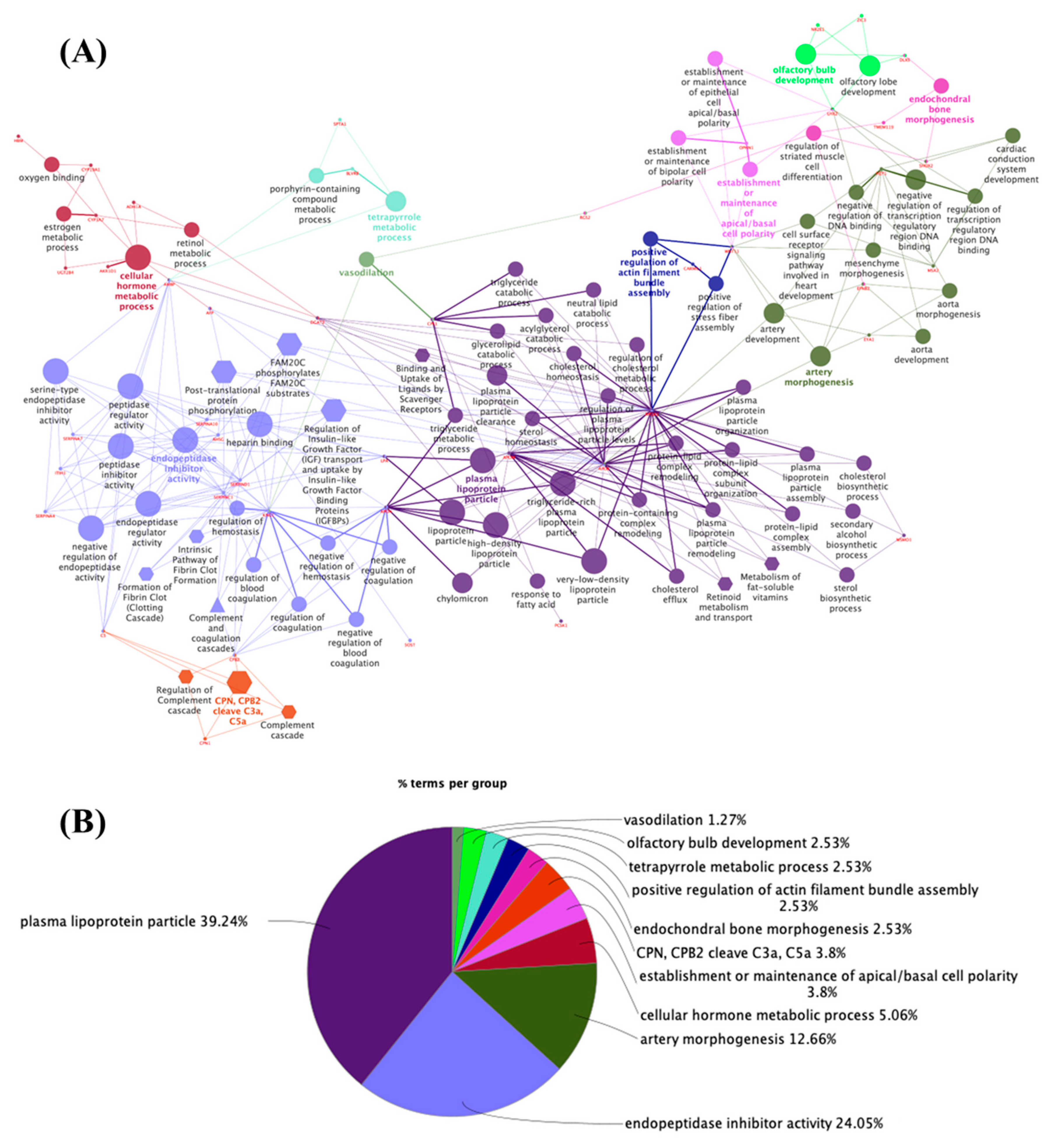
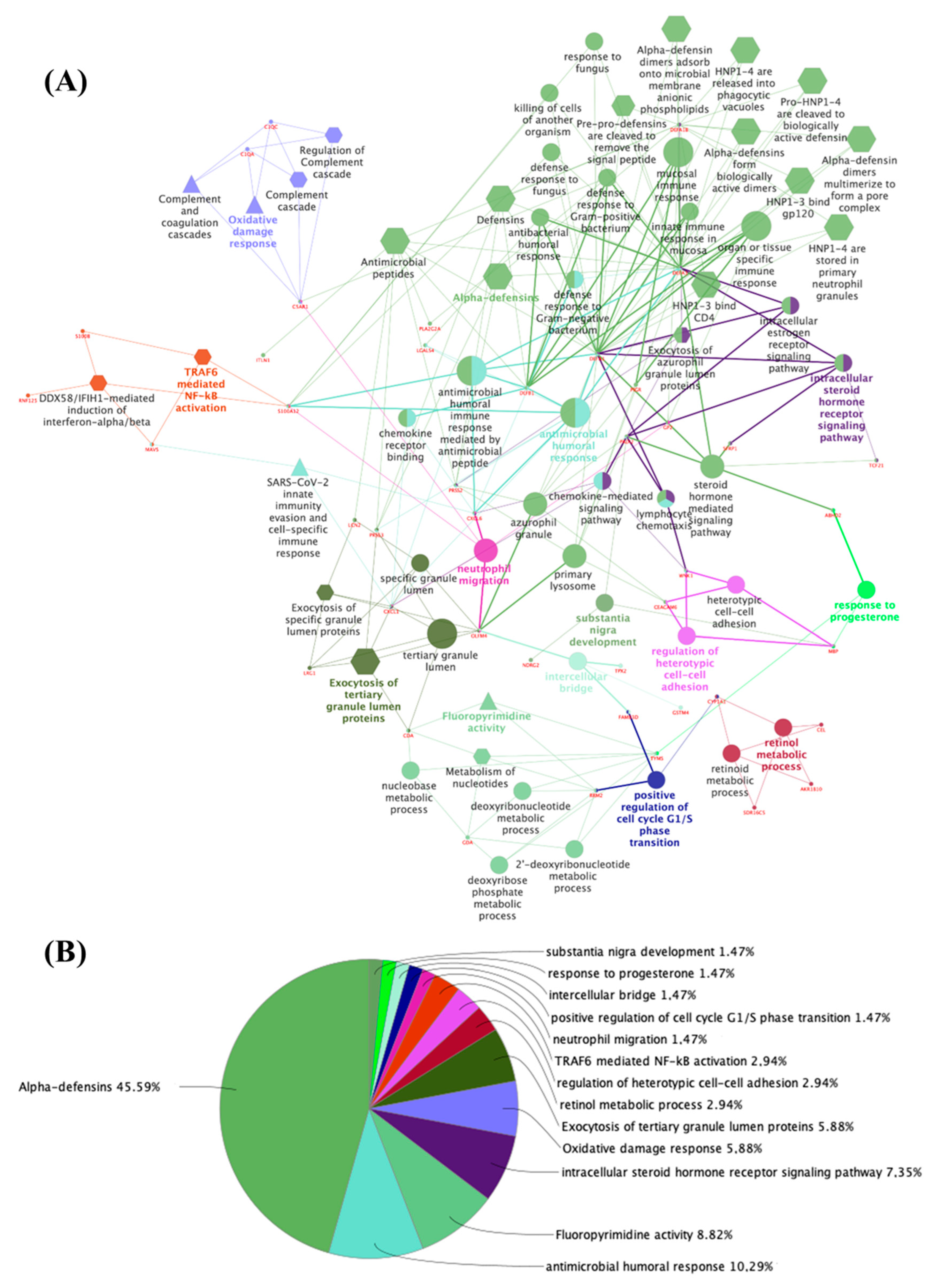
3.2. Potential Targets for EDCs in the Adult Thyroid Gland—A System Biology Approach
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chi, H.C.; Chen, C.Y.; Tsai, M.M.; Tsai, C.Y.; Lin, K.H. Molecular Functions of Thyroid Hormones and Their Clinical Significance in Liver-Related Diseases. Biomed Res. Int. 2013, 2013, 16. [Google Scholar] [CrossRef] [PubMed]
- Leemans, M.; Couderq, S.; Demeneix, B.; Fini, J.-B. Pesticides With Potential Thyroid Hormone-Disrupting Effects: A Review of Recent Data. Front. Endocrinol. 2019, 10, 468622. [Google Scholar] [CrossRef] [PubMed]
- Ortiga-Carvalho, T.M.; Chiamolera, M.I.; Pazos-Moura, C.C.; Wondisford, F.E. Hypothalamus-Pituitary-Thyroid Axis. In Comprehensive Physiology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; pp. 1387–1428. [Google Scholar]
- Sinha, R.A.; Singh, B.K.; Yen, P.M. Direct Effects of Thyroid Hormones on Hepatic Lipid Metabolism. Nat. Rev. Endocrinol. 2018, 14, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Graceli, J.B.; Dettogni, R.S.; Merlo, E.; Niño, O.; da Costa, C.S.; Zanol, J.F.; Ríos Morris, E.A.; Miranda-Alves, L.; Denicol, A.C. The Impact of Endocrine-Disrupting Chemical Exposure in the Mammalian Hypothalamic-Pituitary Axis. Mol. Cell. Endocrinol. 2020, 518, 110997. [Google Scholar] [CrossRef] [PubMed]
- Santos-Silva, A.P.; Andrade, M.N.; Pereira-Rodrigues, P.; Paiva-Melo, F.D.; Soares, P.; Graceli, J.B.; Dias, G.R.M.; Ferreira, A.C.F.; de Carvalho, D.P.; Miranda-Alves, L. Frontiers in Endocrine Disruption: Impacts of Organotin on the Hypothalamus-Pituitary-Thyroid Axis. Mol. Cell. Endocrinol. 2018, 460, 246–257. [Google Scholar] [CrossRef] [PubMed]
- Kavlock, R.J.; Daston, G.P.; DeRosa, C.; Fenner-Crisp, P.; Gray, L.E.; Kaattari, S.; Lucier, G.; Luster, M.; Mac, M.J.; Maczka, C.; et al. Research Needs for the Risk Assessment of Health and Environmental Effects of Endocrine Disrupters: A Report of the U.S. EPA-Sponsored Workshop. Environ. Health Perspect. 1996, 104, 715–740. [Google Scholar] [PubMed]
- Bertram, M.G.; Gore, A.C.; Tyler, C.R.; Brodin, T. Endocrine-Disrupting Chemicals. Curr. Biol. 2022, 32, R727–R730. [Google Scholar] [CrossRef] [PubMed]
- Lymperi, S.; Giwercman, A. Endocrine Disruptors and Testicular Function. Metab. Clin. Exp. 2018, 86, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Combarnous, Y.; Nguyen, T.M.D. Comparative Overview of the Mechanisms of Action of Hormones and Endocrine Disruptor Compounds. Toxics 2019, 7, 5. [Google Scholar] [CrossRef]
- Ravichandran, G.; Lakshmanan, D.K.; Raju, K.; Elangovan, A.; Nambirajan, G.; Devanesan, A.A.; Thilagar, S. Food Advanced Glycation End Products as Potential Endocrine Disruptors: An Emerging Threat to Contemporary and Future Generation. Environ. Int. 2019, 123, 486–500. [Google Scholar] [CrossRef]
- Romano, M.A.; Martino-Andrade, A.J.; Mathias, P.C.D.F.; Barella, L.F.; Romano, R.M. Editorial: Endocrine Disruption in Light of Dohad: The Challenges of Contaminants of Emerging Concern in Food and Water. Front. Endocrinol. 2022, 13, 898736. [Google Scholar] [CrossRef]
- Lauretta, R.; Sansone, A.; Sansone, M.; Romanelli, F.; Appetecchia, M. Endocrine Disrupting Chemicals: Effects on Endocrine Glands. Front. Endocrinol. 2019, 10, 178. [Google Scholar] [CrossRef]
- Matoso, V.; Bargi-Souza, P.; Ivanski, F.; Romano, M.A.M.A.; Romano, R.M.R.M. Acrylamide: A Review about Its Toxic Effects in the Light of Developmental Origin of Health and Disease (DOHaD) Concept. Food Chem. 2019, 283, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Jazwiec, P.A.; Sloboda, D.M. Nutritional Adversity, Sex and Reproduction: 30 Years of DOHaD and What Have We Learned? J. Endocrinol. 2019, 242, T51–T68. [Google Scholar] [CrossRef] [PubMed]
- Safi-Stibler, S.; Gabory, A. Epigenetics and the Developmental Origins of Health and Disease: Parental Environment Signalling to the Epigenome, Critical Time Windows and Sculpting the Adult Phenotype. Semin. Cell Dev. Biol. 2020, 97, 172–180. [Google Scholar] [CrossRef]
- Suzuki, K. The Developing World of DOHaD. J. Dev. Orig. Health Dis. 2018, 9, 266–269. [Google Scholar] [CrossRef]
- Adedara, I.A.; Omole, O.; Okpara, E.S.; Fasina, O.B.; Ayeni, M.F.; Ajayi, O.M.; Busari, E.O.; Farombi, E.O. Impact of Prepubertal Exposure to Dietary Protocatechuic Acid on the Hypothalamic-Pituitary-Testicular Axis in Rats. Chem. Biol. Interact. 2018, 290, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, B.; Terekeci, H.; Sandal, S.; Kelestimur, F. Endocrine Disrupting Chemicals: Exposure, Effects on Human Health, Mechanism of Action, Models for Testing and Strategies for Prevention. Rev. Endocr. Metab. Disord. 2020, 21, 127–147. [Google Scholar] [CrossRef] [PubMed]
- Romano, R.M.; de Oliveira, J.M.; de Oliveira, V.M.; de Oliveira, I.M.; Torres, Y.R.; Bargi-Souza, P.; Martino Andrade, A.J.; Romano, M.A. Could Glyphosate and Glyphosate-Based Herbicides Be Associated With Increased Thyroid Diseases Worldwide? Front. Endocrinol. 2021, 12, 627167. [Google Scholar] [CrossRef]
- Muñoz, J.P.; Bleak, T.C.; Calaf, G.M. Glyphosate and the Key Characteristics of an Endocrine Disruptor: A Review. Chemosphere 2021, 270, 128619. [Google Scholar] [CrossRef]
- Dom, G.; Dmitriev, P.; Lambot, M.A.; Van Vliet, G.; Glinoer, D.; Libert, F.; Lefort, A.; Dumont, J.E.; Maenhaut, C. Transcriptomic Signature of Human Embryonic Thyroid Reveals Transition From Differentiation to Functional Maturation. Front. Cell Dev. Biol. 2021, 9, 1481. [Google Scholar] [CrossRef] [PubMed]
- Home-GEO-NCBI. Available online: https://www.ncbi.nlm.nih.gov/geo/ (accessed on 12 April 2023).
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Bindea, G.; Mlecnik, B.; Hackl, H.; Charoentong, P.; Tosolini, M.; Kirilovsky, A.; Fridman, W.H.; Pagès, F.; Trajanoski, Z.; Galon, J. ClueGO: A Cytoscape Plug-in to Decipher Functionally Grouped Gene Ontology and Pathway Annotation Networks. Bioinformatics 2009, 25, 1091–1093. [Google Scholar] [CrossRef]
- Bindea, G.; Galon, J.; Mlecnik, B. CluePedia Cytoscape Plugin: Pathway Insights Using Integrated Experimental and in Silico Data. Bioinformatics 2013, 29, 661–663. [Google Scholar] [CrossRef]
- Davis, A.P.; Wiegers, T.C.; Johnson, R.J.; Sciaky, D.; Wiegers, J.; Mattingly, C.J. Comparative Toxicogenomics Database (CTD): Update 2023. Nucleic Acids Res. 2023, 51, D1257–D1262. [Google Scholar] [CrossRef] [PubMed]
- The Comparative Toxicogenomics Database|CTD. Available online: https://ctdbase.org/ (accessed on 11 April 2024).
- PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/ (accessed on 11 April 2024).
- PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 11 April 2024).
- Costa-e-Sousa, R.H.; Hollenberg, A.N. Minireview: The Neural Regulation of the Hypothalamic-Pituitary-Thyroid Axis. Endocrinology 2012, 153, 4128–4135. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, S.E.; von Oettingen, J.E.; Deladoëy, J. Normal Thyroid Development and Function in the Fetus and Neonate. In Maternal-Fetal and Neonatal Endocrinology; Academic Press: Cambridge, MA, USA, 2020; pp. 563–571. [Google Scholar] [CrossRef]
- Policeni, B.A.; Smoker, W.R.K.; Reede, D.L. Anatomy and Embryology of the Thyroid and Parathyroid Glands. Semin. Ultrasound CT MRI 2012, 33, 104–114. [Google Scholar] [CrossRef]
- Polak, M. Human Fetal Thyroid Function. Endocr. Dev. 2014, 26, 17–25. [Google Scholar] [CrossRef]
- López-Márquez, A.; Carrasco-López, C.; Fernández-Méndez, C.; Santisteban, P. Unraveling the Complex Interplay Between Transcription Factors and Signaling Molecules in Thyroid Differentiation and Function, From Embryos to Adults. Front. Endocrinol. 2021, 12, 654569. [Google Scholar] [CrossRef]
- Hyllus, D.; Stein, C.; Schnabel, K.; Schiltz, E.; Imhof, A.; Dou, Y.; Hsieh, J.; Bauer, U.M. PRMT6-Mediated Methylation of R2 in Histone H3 Antagonizes H3 K4 Trimethylation. Genes Dev. 2007, 21, 3369–3380. [Google Scholar] [CrossRef]
- Wei, X.; Orr, H.T. Differential Expression of HLA-E, HLA-F, and HLA-G Transcripts in Human Tissue. Hum. Immunol. 1990, 29, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Álvarez, B.; Rodriguez, R.M.; Calvanese, V.; Blanco-Gelaz, M.A.; Suhr, S.T.; Ortega, F.; Otero, J.; Cibelli, J.B.; Moore, H.; Fraga, M.F.; et al. Epigenetic Mechanisms Regulate MHC and Antigen Processing Molecules in Human Embryonic and Induced Pluripotent Stem Cells. PLoS ONE 2010, 5, e10192. [Google Scholar] [CrossRef]
- Wang, X.; Ge, X.; Qin, Y.; Liu, D.; Chen, C. Ifi30 Is Required for Sprouting Angiogenesis During Caudal Vein Plexus Formation in Zebrafish. Front. Physiol. 2022, 13, 919579. [Google Scholar] [CrossRef] [PubMed]
- Miyai, K.; Mizuta, H.; Nose, O.; Fukunishi, T.; Hirai, T.; Matsuda, S.; Tsuruhara, T. Increased Frequency of HLA-Aw24 in Congenital Hypothyroidism in Japan. N. Engl. J. Med. 1980, 303, 226. [Google Scholar] [CrossRef]
- Karp, M.; Kott, E.; Shapira, M.; Assa, S.; Brautbar, C.; Gurewitz, R.; Laron, Z. Unusual Association of Insulin-Dependent Diabetes Mellitus with Congenital Myasthenia Gravis and Autoimmune Thyroid Disease. Isr. J. Med. Sci. 1984, 20, 1078–1081. [Google Scholar]
- Chien, C.H.; Lee, J.S.; Tsai, W.Y.; Wang, T.R. Wiedemann-Beckwith Syndrome with Congenital Central Hypothyroidism in One of Monozygotic Twins. J. Formos. Med. Assoc. 1990, 89, 132–136. [Google Scholar] [PubMed]
- Cimino, P.; Banks, R.; Maclaren, N.; Rosenbloom, E.; Riley, W.; Rosenbloom, A.; Root, A. HLA and Congenital Hypothyroidism. N. Engl. J. Med. 1980, 303, 1177–1178. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, B.B.; Brandt, N.J.; Svejgaard, A. HLA Typing and Congenital, Primary Hypothyroidism. Pediatr. Res. 1981, 15, 1568. [Google Scholar] [CrossRef][Green Version]
- Jacobsen, B.B.; Brandt, N.J.; Svejgaard, A. Congenital Primary Hypothyroidism and HLA. Acta Paediatr. Scand. 1982, 71, 919–922. [Google Scholar] [CrossRef]
- De Farias, C.R.; Cardoso, B.R.; De Oliveira, G.M.B.; De Mello Guazzelli, I.C.; Catarino, R.M.; Chammas, M.C.; Cozzolino, S.M.F.; Knobel, M. A Randomized-Controlled, Double-Blind Study of the Impact of Selenium Supplementation on Thyroid Autoimmunity and Inflammation with Focus on the GPx1 Genotypes. J. Endocrinol. Investig. 2015, 38, 1065–1074. [Google Scholar] [CrossRef]
- Beckett, G.J.; Arthur, J.R. Selenium and Endocrine Systems. J. Endocrinol. 2005, 184, 455–465. [Google Scholar] [CrossRef]
- Hofstee, P.; Bartho, L.A.; McKeating, D.R.; Radenkovic, F.; McEnroe, G.; Fisher, J.J.; Holland, O.J.; Vanderlelie, J.J.; Perkins, A.V.; Cuffe, J.S.M. Maternal Selenium Deficiency during Pregnancy in Mice Increases Thyroid Hormone Concentrations, Alters Placental Function and Reduces Fetal Growth. J. Physiol. 2019, 597, 5597–5617. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Zhou, L.; Xu, J.; Liu, Z.; Liu, J.; Yan, C. Prenatal Maternal Low Selenium, High Thyrotropin, and Low Birth Weights. Biol. Trace Elem. Res. 2021, 199, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Mavromati, M.; Jornayvaz, F.R. Hypothyroidism-Associated Dyslipidemia: Potential Molecular Mechanisms Leading to NAFLD. Int. J. Mol. Sci. 2021, 22, 12797. [Google Scholar] [CrossRef] [PubMed]
- Clark, D.P.; Pazdernik, N.J.; McGehee, M.R. Protein Structure and Function. In Molecular Biology, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 445–483. [Google Scholar] [CrossRef]
- Soni, U.K.; Roychoudhury, K.; Hegde, R.S. The Eyes Absent Proteins in Development and in Developmental Disorders. Biochem. Soc. Trans. 2021, 49, 1397. [Google Scholar] [CrossRef] [PubMed]
- Babula, P.; Masarik, M.; Adam, V.; Eckschlager, T.; Stiborova, M.; Trnkova, L.; Skutkova, H.; Provaznik, I.; Hubalek, J.; Kizek, R. Mammalian Metallothioneins: Properties and Functions. Metallomics 2012, 4, 739–750. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Rais, A.; Sandhu, R.; Nel, W.; Ebadi, M. Clinical Significance of Metallothioneins in Cell Therapy and Nanomedicine. Int. J. Nanomed. 2013, 8, 1477–1488. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, J.; Otsuka, F.; Takeda, M.; Inagaki, K.; Miyoshi, T.; Mimura, Y.; Ogura, T.; Doihara, H.; Makino, H. Functional Roles of the Bone Morphogenetic Protein System in Thyrotropin Signaling in Porcine Thyroid Cells. Biochem. Biophys. Res. Commun. 2005, 327, 1124–1130. [Google Scholar] [CrossRef] [PubMed]
- Lademann, F.; Weidner, H.; Tsourdi, E.; Kumar, R.; Rijntjes, E.; Köhrle, J.; Hofbauer, L.C.; Rauner, M. Disruption of BMP Signaling Prevents Hyperthyroidism-Induced Bone Loss in Male Mice. J. Bone Miner. Res. 2020, 35, 2058–2069. [Google Scholar] [CrossRef]
- Deng, T.; Zhang, W.; Zhang, Y.; Zhang, M.; Huan, Z.; Yu, C.; Zhang, X.; Wang, Y.; Xu, J. Thyroid-Stimulating Hormone Decreases the Risk of Osteoporosis by Regulating Osteoblast Proliferation and Differentiation. BMC Endocr. Disord. 2021, 21, 49. [Google Scholar] [CrossRef]
- Thiel, G.; Cibelli, G. Regulation of Life and Death by the Zinc Finger Transcription Factor Egr-1. J. Cell. Physiol. 2002, 193, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Deleu, S.; Pirson, I.; Clermont, F.; Nakamura, T.; Dumont, J.E.; Maenhaut, C. Immediate Early Gene Expression in Dog Thyrocytes in Response to Growth, Proliferation, and Differentiation Stimuli. J. Cell. Physiol. 1999, 181, 342–354. [Google Scholar] [CrossRef]
- Huang, X.F.; Luu-The, V. Molecular Characterization of a First Human 3(A→β)-Hydroxysteroid Epimerase. J. Biol. Chem. 2000, 275, 29452–29457. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.R.; Italiano, L.; Wilson, S.G.; Mullin, B.H.; Mead, R.; Dudbridge, F.; Watts, G.F.; Stuckey, B.G.A. Polymorphism in HSD17B6 Is Associated with Key Features of Polycystic Ovary Syndrome. Fertil. Steril. 2006, 86, 1438–1446. [Google Scholar] [CrossRef] [PubMed]
- Ke, L.; Che, Y.N.; Cao, Y.X.; Wu, X.K.; Hu, Y.L.; Sun, H.X.; Liang, F.J.; Sun, J.; Yi, L.; Wang, Y. Polymorphisms of the HSD17B6 and HSD17B5 Genes in Chinese Women with Polycystic Ovary Syndrome. J. Women’s Health 2010, 19, 2227–2232. [Google Scholar] [CrossRef] [PubMed]
- Ju, R.; Wu, W.; Fei, J.; Qin, Y.; Tang, Q.; Wu, D.; Xia, Y.; Wu, J.; Wang, X. Association Analysis between the Polymorphisms of HSD17B5 and HSD17B6 and Risk of Polycystic Ovary Syndrome in Chinese Population. Eur. J. Endocrinol. 2015, 172, 227–233. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fagerberg, L.; Hallström, B.M.; Oksvold, P.; Kampf, C.; Djureinovic, D.; Odeberg, J.; Habuka, M.; Tahmasebpoor, S.; Danielsson, A.; Edlund, K.; et al. Analysis of the Human Tissue-Specific Expression by Genome-Wide Integration of Transcriptomics and Antibody-Based Proteomics. Mol. Cell Proteomics. 2014, 13, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Joshi, B.; Ordonez-Ercan, D.; Dasgupta, P.; Chellappan, S. Induction of Human Metallothionein 1G Promoter by VEGF and Heavy Metals: Differential Involvement of E2F and Metal Transcription Factors. Oncogene 2005, 24, 2204–2217. [Google Scholar] [CrossRef] [PubMed]
- Bäck, C.M.; Stohr, S.; Schäfer, E.A.M.; Biebermann, H.; Boekhoff, I.; Breit, A.; Gudermann, T.; Büch, T.R.H. TSH Induces Metallothionein 1 in Thyrocytes via Gq/11- and PKC-Dependent Signaling. J. Mol. Endocrinol. 2013, 51, 79–90. [Google Scholar] [CrossRef]
- Liu, W.L.; Cao, Y.M.; Liao, T.; Qu, N.; Zhu, Y.X.; Wei, W.J. Multiple Lectin Assays in Detecting Glycol-Alteration Status of Serum NRG1 in Papillary Thyroid Cancer. Transl. Cancer Res. 2021, 10, 3218–3224. [Google Scholar] [CrossRef]
- Porcu, E.; Medici, M.; Pistis, G.; Volpato, C.B.; Wilson, S.G.; Cappola, A.R.; Bos, S.D.; Deelen, J.; den Heijer, M.; Freathy, R.M.; et al. A Meta-Analysis of Thyroid-Related Traits Reveals Novel Loci and Gender-Specific Differences in the Regulation of Thyroid Function. PLoS Genet. 2013, 9, e1003266. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.N.; Porcu, E.; Chew, S.; Campbell, P.J.; Traglia, M.; Brown, S.J.; Mullin, B.H.; Shihab, H.A.; Min, J.; Walter, K.; et al. Whole-Genome Sequence-Based Analysis of Thyroid Function. Nat. Commun. 2015, 6, 5681. [Google Scholar] [CrossRef]
- Rocnik, E.F.; Liu, P.; Sato, K.; Walsh, K.; Vaziri, C. The Novel SPARC Family Member SMOC-2 Potentiates Angiogenic Growth Factor Activity. J. Biol. Chem. 2006, 281, 22855–22864. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Choi, J.H.; Lee, J.Y.; Kang, J.H.; Myung, J.K.; Kim, W.H.; Jang, B.G. Downregulation of SMOC2 Expression in Papillary Thyroid Carcinoma and Its Prognostic Significance. Sci. Rep. 2020, 10, 4853. [Google Scholar] [CrossRef] [PubMed]
- Alkhateeb, A.; Marzouka, N.A.D.; Tashtoush, R. Variants in PTPN22 and SMOC2 Genes and the Risk of Thyroid Disease in the Jordanian Arab Population. Endocrine 2013, 44, 702–709. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Lu, W. Defensins: A Double-Edged Sword in Host Immunity. Front. Immunol. 2020, 11, 764. [Google Scholar] [CrossRef] [PubMed]
- Khong, J.J.; Wang, L.Y.; Smyth, G.K.; McNab, A.A.; Hardy, T.G.; Selva, D.; Llamas, B.; Jung, C.H.; Sharma, S.; Burdon, K.P.; et al. Differential Gene Expression Profiling of Orbital Adipose Tissue in Thyroid Orbitopathy. Investig. Ophthalmol. Vis. Sci. 2015, 56, 6438–6447. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.M.; Radisky, E.S.; Férec, C. Human Trypsins. Handb. Proteolytic Enzym. 2013, 3, 2600–2609. [Google Scholar] [CrossRef]
- Stoytcheva, Z.R.; Berry, M.J. Transcriptional Regulation of Mammalian Selenoprotein Expression. Biochim. Biophys. Acta Gen. Subj. 2009, 1790, 1429–1440. [Google Scholar] [CrossRef]
- Di Marzo, N.; Chisci, E.; Giovannoni, R. The Role of Hydrogen Peroxide in Redox-Dependent Signaling: Homeostatic and Pathological Responses in Mammalian Cells. Cells 2018, 7, 156. [Google Scholar] [CrossRef]
- Rhee, S.G. Redox Signaling: Hydrogen Peroxide as Intracellular Messenger. Exp. Mol. Med. 1999, 31, 53–59. [Google Scholar] [CrossRef]
- Reed, J.C.; Torigoe, T.; Turner, B.C.; Merida, I.; Gaulton, G.; Saragovi, H.U.; Rapp, U.R.; Taichman, R. Protooncogene-Encoded Protein Kinases in Interleukin-2 Signal Transduction. Semin. Immunol. 1993, 5, 327–336. [Google Scholar] [CrossRef][Green Version]
- Connolly, R.M.; Nguyen, N.K.; Sukumar, S. Molecular Pathways: Current Role and Future Directions of the Retinoic Acid Pathway In Cancer Prevention and Treatment. Clin. Cancer Res. 2013, 19, 1651. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Privalsky, M.L. Heterodimers of Retinoic Acid Receptors and Thyroid Hormone Receptors Display Unique Combinatorial Regulatory Properties. Mol. Endocrinol. 2005, 19, 863–878. [Google Scholar] [CrossRef] [PubMed]
- Bernard, P.; Scior, T.; Do, Q.T. Modulating Testosterone Pathway: A New Strategy to Tackle Male Skin Aging? Clin. Interv. Aging 2012, 7, 351–361. [Google Scholar] [CrossRef][Green Version]
- Wang, J.; Li, X.; Wang, C.; Li, Y.; Wang, J.; Fang, R.; Wang, J.; Chen, J.; Dong, J. Exposure to Di-(2-Ethylhexyl) Phthalate Reduces Secretion of GDNF via Interfering with Estrogen Pathway and Downregulating ERK/c-Fos Signaling Pathway in Astrocytes. Food Chem. Toxicol. 2021, 158, 112592. [Google Scholar] [CrossRef]
- Zhang, B.; Li, Y.; Liu, M.; Duan, X.H.; Hu, K.L.; Li, L.N.; Yu, X.; Chang, H.S. Antidepressant-like Mechanism of Honokiol in a Rodent Model of Corticosterone-Induced Depression. J. Integr. Neurosci. 2020, 19, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Jung, U.J. Honokiol Improves Insulin Resistance, Hepatic Steatosis, and Inflammation in Type 2 Diabetic Db/Db Mice. Int. J. Mol. Sci. 2019, 20, 2303. [Google Scholar] [CrossRef]
- Chang, Z.; Qin, W.; Zheng, H.; Schegg, K.; Han, L.; Liu, X.; Wang, Y.; Wang, Z.; McSwiggin, H.; Peng, H.; et al. Triptonide Is a Reversible Non-Hormonal Male Contraceptive Agent in Mice and Non-Human Primates. Nat. Commun. 2021, 12, 1253. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, J.M.; Zenzeluk, J.; Serrano-Nascimento, C.; Romano, M.A.; Romano, R.M. A System Biology Approach Reveals New Targets for Human Thyroid Gland Toxicity in Embryos and Adult Individuals. Metabolites 2024, 14, 226. https://doi.org/10.3390/metabo14040226
Oliveira JM, Zenzeluk J, Serrano-Nascimento C, Romano MA, Romano RM. A System Biology Approach Reveals New Targets for Human Thyroid Gland Toxicity in Embryos and Adult Individuals. Metabolites. 2024; 14(4):226. https://doi.org/10.3390/metabo14040226
Chicago/Turabian StyleOliveira, Jeane Maria, Jamilli Zenzeluk, Caroline Serrano-Nascimento, Marco Aurelio Romano, and Renata Marino Romano. 2024. "A System Biology Approach Reveals New Targets for Human Thyroid Gland Toxicity in Embryos and Adult Individuals" Metabolites 14, no. 4: 226. https://doi.org/10.3390/metabo14040226
APA StyleOliveira, J. M., Zenzeluk, J., Serrano-Nascimento, C., Romano, M. A., & Romano, R. M. (2024). A System Biology Approach Reveals New Targets for Human Thyroid Gland Toxicity in Embryos and Adult Individuals. Metabolites, 14(4), 226. https://doi.org/10.3390/metabo14040226