The Association between Maternal Endocrine-Disrupting Chemical Exposure during Pregnancy and the Incidence of Male Urogenital Defects: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Eligibility Criteria
2.2. Literature Search and Screening
2.3. Data Extraction
2.4. Risk of Bias
2.5. Statistical Analysis
3. Results
3.1. Search Results
3.2. Characteristics of the Included Studies
3.3. Quality Assessment
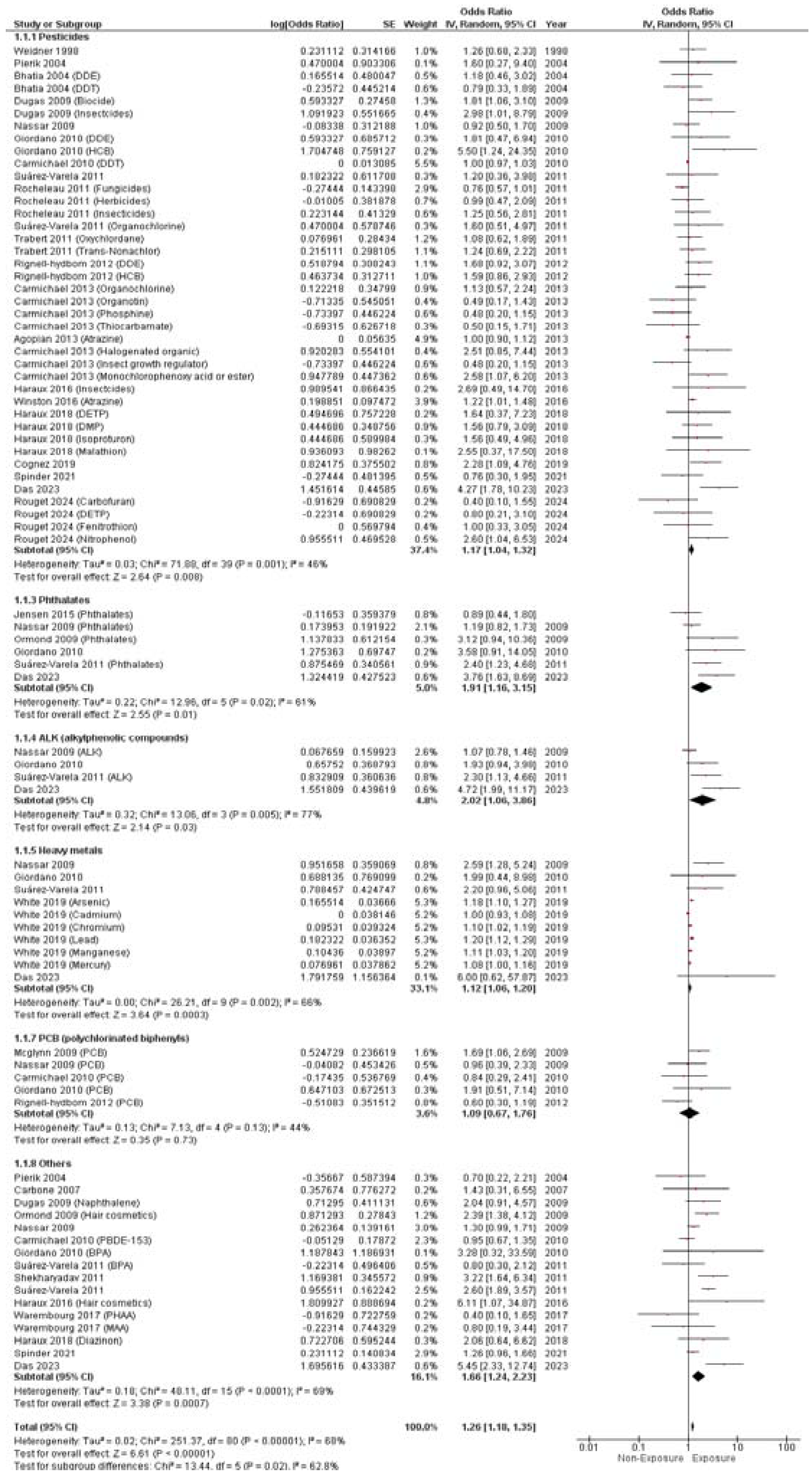
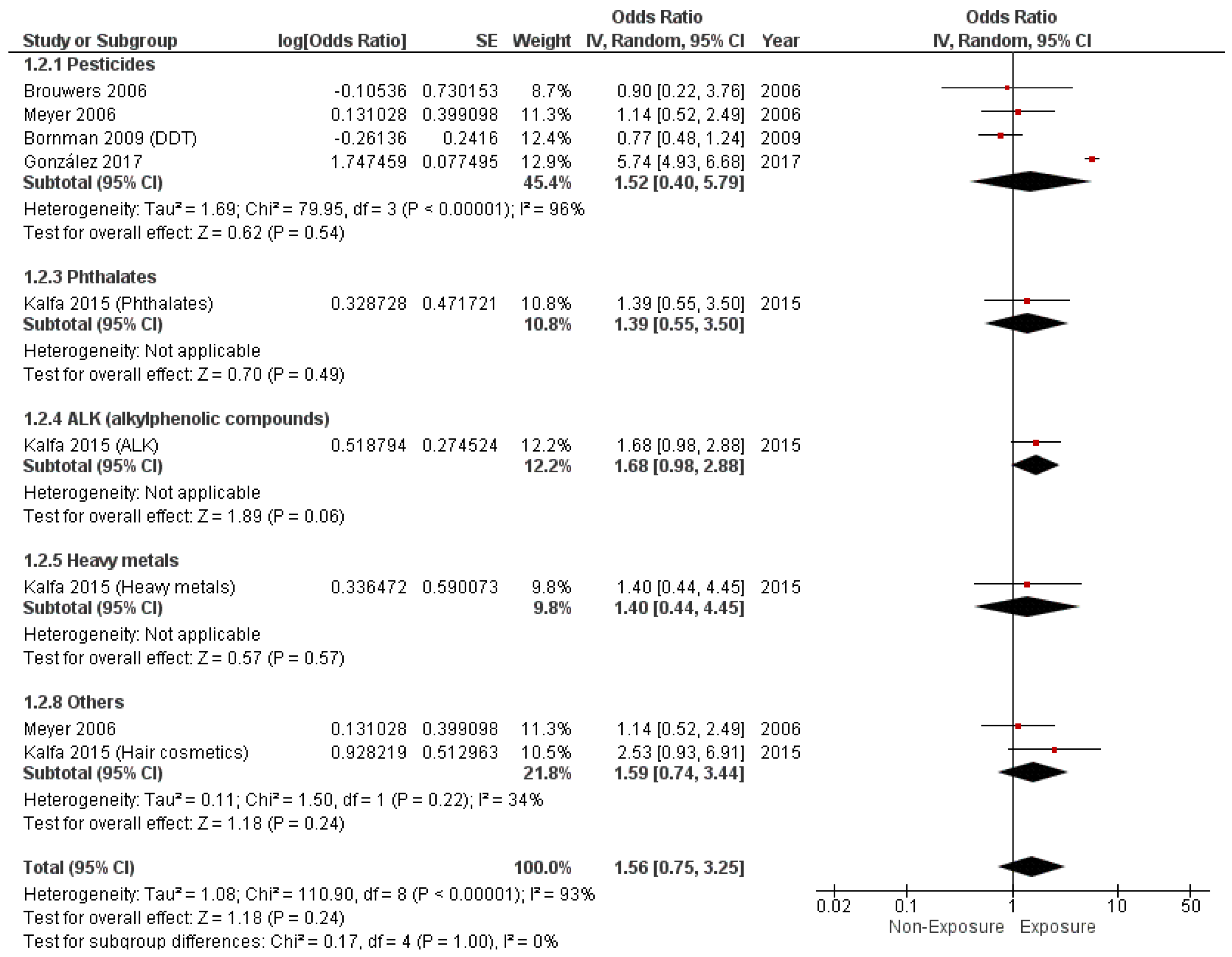
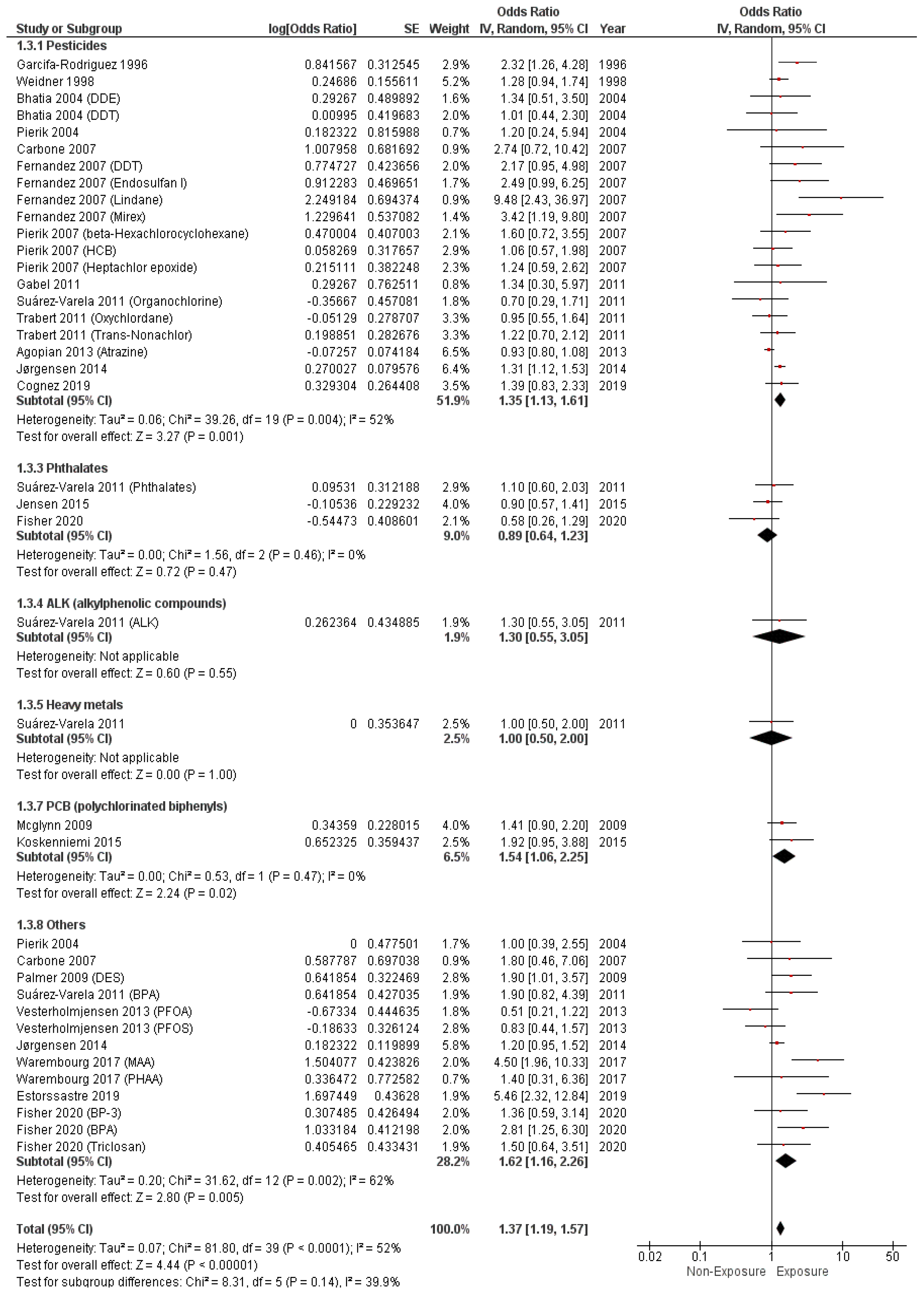
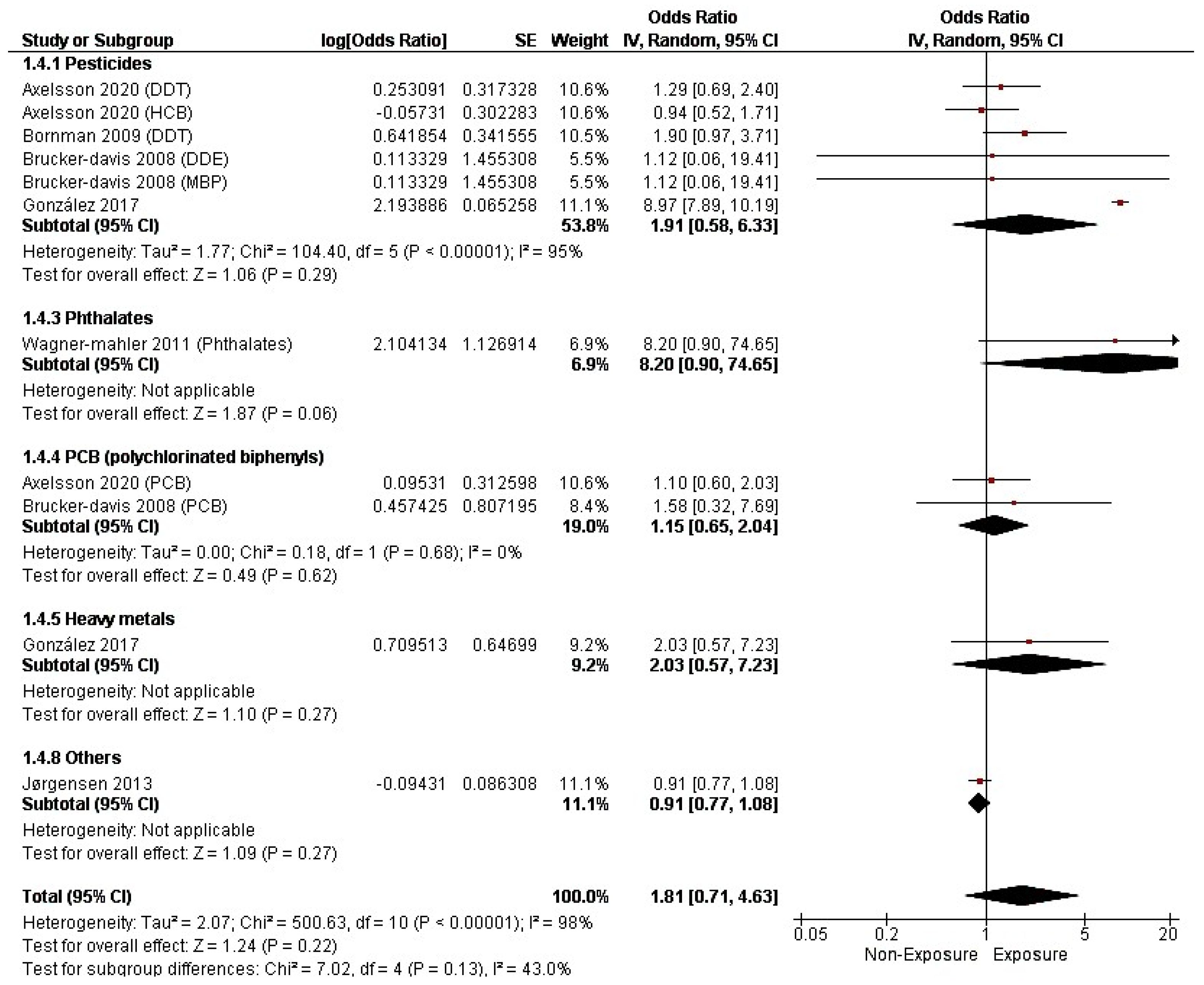
3.4. Study Outcomes
3.4.1. Hypospadias
3.4.2. Cryptorchidism
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kalfa, N.; Philibert, P.; Baskin, L.; Sultan, C. Hypospadias: Interactions between environment and genetics. Mol. Cell. Endocrinol. 2011, 335, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Thorup, J.; Nordenskjöld, A.; Hutson, J.M. Genetic and environmental origins of hypospadias. Curr. Opin. Endocrinol. Diabetes Obes. 2014, 21, 227–232. [Google Scholar] [CrossRef]
- Kalfa, N.; Cassorla, F.; Audran, F.; Oulad Abdennabi, I.; Philibert, P.; Béroud, C.; Guys, J.M.; Reynaud, R.; Alessandrini, P.; Wagner, K.; et al. Polymorphisms of MAMLD1 gene in hypospadias. J. Pediatr. Urol. 2011, 7, 585–591. [Google Scholar] [CrossRef]
- Kalfa, N.; Fukami, M.; Philibert, P.; Audran, F.; Pienkowski, C.; Weill, J.; Pinto, G.; Manouvrier, S.; Polak, M.; Ogata, T.; et al. Screening of MAMLD1 Mutations in 70 Children with 46, XY DSD: Identification and Functional Analysis of Two New Mutations. PLoS ONE 2012, 7, e32505. [Google Scholar] [CrossRef] [PubMed]
- Kalfa, N.; Philibert, P.; Werner, R.; Audran, F.; Bashamboo, A.; Lehors, H.; Haddad, M.; Guys, J.M.; Reynaud, R.; Alessandrini, P.; et al. Minor Hypospadias: The “Tip of the Iceberg” of the Partial Androgen Insensitivity Syndrome. PLoS ONE 2013, 8, e61824. [Google Scholar] [CrossRef] [PubMed]
- Wohlfahrt-Veje, C.; Boisen, K.A.; Boas, M.; Damgaard, I.N.; Kai, C.M.; Schmidt, I.M.; Chellakooty, M.; Suomi, A.; Toppari, J.; Skakkebæk, N.E.; et al. Acquired cryptorchidism is frequent in infancy and childhood. Int. J. Androl. 2009, 32, 423–428. [Google Scholar] [CrossRef]
- Virtanen, H.E.; Bjerknes, R.; Cortes, D.; Jørgensen, N.; Meyts, E.R.; Thorsson, A.V.; Thorup, J.; Main, K.M. Cryptorchidism: Classification, prevalence and long-term consequences. Acta Paediatr. 2007, 96, 611–616. [Google Scholar] [CrossRef]
- Nieschlag, E.; Behre, H.M.; Nieschlag, S. Andrology: Male Reproductive Health and Dysfunction; Springer: Dordrecht, The Netherlands, 2010; Available online: https://link.springer.com/book/10.1007/978-3-540-78355-8 (accessed on 13 May 2024).
- Berman, T.; Levine, H.; Gamzu, R.; Grotto, I. Trends in reproductive health in Israel: Implications for environmental health policy. Isr. J. Health Policy Res. 2012, 1, 34. [Google Scholar] [CrossRef]
- Lund, L.; Engebjerg, M.C.; Pedersen, L.; Ehrenstein, V.; Nørgaard, M.; Sørensen, H.T. Prevalence of Hypospadias in Danish Boys: A Longitudinal Study, 1977–2005. Eur. Urol. 2009, 55, 1022–1026. [Google Scholar] [CrossRef]
- Fisch, H.; Lambert, S.M.; Hensle, T.W.; Hyun, G. Hypospadias Rates in New York State are Not Increasing. J. Urol. 2009, 181, 2291–2294. [Google Scholar] [CrossRef]
- Paulozzi, L.J. International trends in rates of hypospadias and cryptorchidism. Environ. Health Perspect. 1999, 107, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Acerini, C.L.; Miles, H.L.; Dunger, D.B.; Ong, K.K.; A Hughes, I. The descriptive epidemiology of congenital and acquired cryptorchidism in a UK infant cohort. Arch. Dis. Child. 2009, 94, 868–872. [Google Scholar] [CrossRef] [PubMed]
- Boisen, K.; Kaleva, M.; Main, K.; Virtanen, H.; Haavisto, A.-M.; Schmidt, I.; Chellakooty, M.; Damgaard, I.; Mau, C.; Reunanen, M.; et al. Difference in prevalence of congenital cryptorchidism in infants between two Nordic countries. Lancet 2004, 363, 1264–1269. [Google Scholar] [CrossRef]
- Conlon, J.L. Diethylstilbestrol: Potential health risks for women exposed in utero and their offspring. J. Am. Acad. Physician Assist. 2017, 30, 49–52. [Google Scholar] [CrossRef]
- Colborn, T.; Clement, C. Chemically Induced Alterations in Sexual and Functional Development: The Wildlife/Human Connection; Princeton Scientific Publishing: Princeton, NJ, USA, 1992; p. 21. [Google Scholar]
- Welsh, M.; Saunders, P.T.; Fisken, M.; Scott, H.M.; Hutchison, G.R.; Smith, L.B.; Sharpe, R.M. Identification in rats of a programming window for reproductive tract masculinization, disruption of which leads to hypospadias and cryptorchidism. J. Clin. Investig. 2008, 118, 1479–1490. [Google Scholar] [CrossRef]
- Sharpe, R.; Skakkebaek, N. Are oestrogens involved in falling sperm counts and disorders of the male reproductive tract? Lancet 1993, 341, 1392–1396. [Google Scholar] [CrossRef] [PubMed]
- Van Tongeren, M.; Nieuwenhuijsen, M.J.; Gardiner, K.; Armstrong, B.; Vrijheid, M.; Dolk, H.; Botting, B. A Job–Exposure Matrix for Potential Endocrine-disrupting Chemicals Developed for a Study into the Association between Maternal Occupational Exposure and Hypospadias. Ann. Occup. Hyg. 2002, 46, 465–477. [Google Scholar] [CrossRef][Green Version]
- Edwards, T.M.; Moore, B.C.; Guillette, L.J. Reproductive dysgenesis in wildlife: A comparative view. Int. J. Androl. 2006, 29, 109–121. [Google Scholar] [CrossRef]
- Nurminen, T. The Epidemiologic Study of Birth Defects and Pesticides. Epidemiology 2001, 12, 145–146. [Google Scholar] [CrossRef]
- Kristensen, P.; Irgens, L.M.; Andersen, A.; Bye, A.S.; Sundheim, L. Birth Defects among Offspring of Norwegian Farmers, 1967–1991. Epidemiology 1997, 8, 537–544. [Google Scholar] [CrossRef]
- Rocheleau, C.M.; Romitti, P.A.; Dennis, L.K. Pesticides and hypospadias: A meta-analysis. J. Pediatr. Urol. 2009, 5, 17–24. [Google Scholar] [CrossRef]
- Yu, C.; Lu, J.; Zhao, J.; Zhao, T.; Long, C.; Lin, T.; Wu, S.; Wen, S.; Wei, G. Maternal phthalate exposure during pregnancy and male reproductive disorders: A systematic review and meta-analysis. Turk. J. Pediatr. 2022, 64, 187–209. [Google Scholar] [CrossRef]
- Higgins, J.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.; Welch, V. Cochrane Handbook for Systematic Reviews and Interventions; Cochrane Training: London, UK, 2023; Available online: http://training.cochrane.org/handbook (accessed on 13 May 2024).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef]
- Wells, G.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle–Ottawa Scale (NOS) for Assessing the Quality of Non-Randomized Studies in Meta-Analysis. 2000. Available online: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 15 March 2024).
- Agopian, A.; Lupo, P.J.; Canfield, M.A.; Langlois, P.H. Case–Control Study of Maternal Residential Atrazine Exposure and Male Genital Malformations. Am. J. Med. Genet. Part A 2013, 161, 977–982. [Google Scholar] [CrossRef]
- Axelsson, J.; Scott, K.; Dillner, J.; Lindh, C.H.; Zhang, H.; Rylander, L.; Rignell-Hydbom, A. Exposure to polychlorinated compounds and cryptorchidism; A nested case-control study. PLoS ONE 2020, 15, e0236394. [Google Scholar] [CrossRef]
- Bhatia, R.; Shiau, R.; Petreas, M.; Weintraub, J.M.; Farhang, L.; Eskenazi, B. Organochlorine Pesticides and Male Genital Anomalies in the Child Health and Development Studies. Environ. Health Perspect. 2005, 113, 220–224. [Google Scholar] [CrossRef]
- Bornman, R.; De Jager, C.; Worku, Z.; Farias, P.; Reif, S. DDT and urogenital malformations in newborn boys in a malarial area. BJU Int. 2010, 106, 405–411. [Google Scholar] [CrossRef]
- Bougnères, P.; Porcher, R.; Esterle, L.; Baker, D.; de la Vaissière, A.; Meurisse, S.; Valtat, S.; Castell, A.-L.; Mouriquand, P.; Valleron, A.-J. Exploring the risk of hypospadias in children born from mothers living close to a vineyard. PLoS ONE 2021, 16, e0249800. [Google Scholar] [CrossRef]
- Brouwers, M.M.; Feitz, W.F.J.; Roelofs, L.A.J.; Kiemeney, L.A.L.M.; de Gier, R.P.E.; Roeleveld, N. Risk factors for hypospadias. Eur. J. Pediatr. 2007, 166, 671–678. [Google Scholar] [CrossRef]
- Brucker-Davis, F.; Wagner-Mahler, K.; Delattre, I.; Ducot, B.; Ferrari, P.; Bongain, A.; Kurzenne, J.-Y.; Mas, J.-C.; Fénichel, P.; The Cryptorchidism Study Group from Nice Area. Cryptorchidism at birth in Nice area (France) is associated with higher prenatal exposure to PCBs and DDE, as assessed by colostrum concentrations. Hum. Reprod. 2008, 23, 1708–1718. [Google Scholar] [CrossRef]
- Carbone, P.; Giordano, F.; Nori, F.; Mantovani, A.; Taruscio, D.; Lauria, L.; Figà-Talamanca, I. The possible role of endocrine disrupting chemicals in the aetiology of cryptorchidism and hypospadias: A population-based case–control study in rural Sicily. Int. J. Androl. 2007, 30, 3–13. [Google Scholar] [CrossRef]
- Carmichael, S.L.; Herring, A.H.; Sjödin, A.; Jones, R.; Needham, L.; Ma, C.; Ding, K.; Shaw, G.M. Hypospadias and halogenated organic pollutant levels in maternal mid-pregnancy serum samples. Chemosphere 2010, 80, 641–646. [Google Scholar] [CrossRef]
- Carmichael, S.L.; Yang, W.; Roberts, E.M.; Kegley, S.E.; Wolff, C.; Guo, L.; Lammer, E.J.; English, P.; Shaw, G.M. Hypospadias and Residential Proximity to Pesticide Applications. Pediatrics 2013, 132, e1216–e1226. [Google Scholar] [CrossRef]
- Cognez, N.; Warembourg, C.; Zaros, C.; Metten, M.-A.; Bouvier, G.; Garlantézec, R.; Charles, M.-A.; Béranger, R.; Chevrier, C. Residential sources of pesticide exposure during pregnancy and the risks of hypospadias and cryptorchidism: The French ELFE birth cohort. Occup. Environ. Med. 2019, 76, 672–679. [Google Scholar] [CrossRef]
- Das, D.; Dutta, H.K.; Borbora, D.; Brahma, R.C.; Das, J.M. Assessing the relationship between hypospadias risk and parental occupational exposure to potential endocrine-disrupting chemicals. Occup. Environ. Med. 2023, 80, 93–96. [Google Scholar] [CrossRef]
- Dugas, J.; Nieuwenhuijsen, M.J.; Martinez, D.; Iszatt, N.; Nelson, P.; Elliott, P. Use of biocides and insect repellents and risk of hypospadias. Occup. Environ. Med. 2010, 67, 196–200. [Google Scholar] [CrossRef]
- Fernandez, M.F.; Olmos, B.; Granada, A.; López-Espinosa, M.J.; Molina-Molina, J.-M.; Fernandez, J.M.; Cruz, M.; Olea-Serrano, F.; Olea, N. Human Exposure to Endocrine-Disrupting Chemicals and Prenatal Risk Factors for Cryptorchidism and Hypospadias: A Nested Case–Control Study. Environ. Health Perspect. 2007, 115 (Suppl. S1), 8–14. [Google Scholar] [CrossRef]
- Fernández, M.F.; Arrebola, J.P.; Jiménez-Díaz, I.; Sáenz, J.M.; Molina-Molina, J.M.; Ballesteros, O.; Kortenkamp, A.; Olea, N. Bisphenol A and other phenols in human placenta from children with cryptorchidism or hypospadias. Reprod. Toxicol. 2015, 59, 89–95. [Google Scholar] [CrossRef]
- Fisher, B.G.; Thankamony, A.; Mendiola, J.; Petry, C.J.; Frederiksen, H.; Andersson, A.M.; Juul, A.; Ong, K.K.; Dunger, D.B.; Hughes, A.I.; et al. Maternal serum concentrations of bisphenol A and propyl paraben in early pregnancy are associated with male infant genital development. Hum. Reprod. 2020, 35, 913–928. [Google Scholar] [CrossRef]
- Gabel, P.; Jensen, M.S.; Andersen, H.R.; Baelum, J.; Thulstrup, A.M.; Bonde, J.P.; Toft, G. The risk of cryptorchidism among sons of women working in horticulture in Denmark: A cohort study. Environ. Health 2011, 10, 100. [Google Scholar] [CrossRef]
- García-Rodríguez, J.; García-Martín, M.; Nogueras-Ocaña, M.; Luna-Del-Castillo, J.D.D.; García, M.E.; Olea, N.; Lardelli-Claret, P. Exposure to pesticides and cryptorchidism: Geographical evidence of a possible association. Environ. Health Perspect. 1996, 104, 1090–1095. [Google Scholar] [CrossRef][Green Version]
- Giordano, F.; Abballe, A.; De Felip, E.; di Domenico, A.; Ferro, F.; Grammatico, P.; Ingelido, A.M.; Marra, V.; Marrocco, G.; Vallasciani, S.; et al. Maternal exposures to endocrine disrupting chemicals and hypospadias in offspring. Birth Defects Res. Part A Clin. Mol. Teratol. 2010, 88, 241–250. [Google Scholar] [CrossRef]
- García, J.; Ventura, M.I.; Requena, M.; Hernández, A.F.; Parrón, T.; Alarcón, R. Association of reproductive disorders and male congenital anomalies with environmental exposure to endocrine active pesticides. Reprod. Toxicol. 2017, 74, 91–99. [Google Scholar] [CrossRef]
- Haraux, E.; Braun, K.; Buisson, P.; Stéphan-Blanchard, E.; Devauchelle, C.; Ricard, J.; Boudailliez, B.; Tourneux, P.; Gouron, R.; Chardon, K. Maternal Exposure to Domestic Hair Cosmetics and Occupational Endocrine Disruptors Is Associated with a Higher Risk of Hypospadias in the Offspring. Int. J. Environ. Res. Public Health 2016, 14, 27. [Google Scholar] [CrossRef]
- Haraux, E.; Tourneux, P.; Kouakam, C.; Stephan-Blanchard, E.; Boudailliez, B.; Leke, A.; Klein, C.; Chardon, K. Isolated hypospadias: The impact of prenatal exposure to pesticides, as determined by meconium analysis. Environ. Int. 2018, 119, 20–25. [Google Scholar] [CrossRef]
- Jensen, M.S.; Anand-Ivell, R.; Nørgaard-Pedersen, B.; Jönsson, B.A.G.; Bonde, J.P.; Hougaard, D.M.; Cohen, A.; Lindh, C.H.; Ivell, R.; Toft, G. Amniotic Fluid Phthalate Levels and Male Fetal Gonad Function. Epidemiology 2015, 26, 91–99. [Google Scholar] [CrossRef]
- Jørgensen, K.T.; Jensen, M.S.; Toft, G.V.; Larsen, A.D.; Bonde, J.P.; Hougaard, K.S. Risk of cryptorchidism and hypospadias among boys of maternal hairdressers—A Danish population-based cohort study. Scand. J. Work. Environ. Health 2012, 39, 302–309. [Google Scholar] [CrossRef]
- Jørgensen, K.T.; Jensen, M.S.; Toft, G.V.; Larsen, A.D.; Bonde, J.P.; Hougaard, K.S. Risk of cryptorchidism among sons of horticultural workers and farmers in Denmark. Scand. J. Work. Environ. Health 2013, 40, 323–330. [Google Scholar] [CrossRef]
- Kalfa, N.; Paris, F.; Philibert, P.; Orsini, M.; Broussous, S.; Fauconnet-Servant, N.; Audran, F.; Gaspari, L.; Lehors, H.; Haddad, M.; et al. Is Hypospadias Associated with Prenatal Exposure to Endocrine Disruptors? A French Collaborative Controlled Study of a Cohort of 300 Consecutive Children Without Genetic Defect. Eur. Urol. 2015, 68, 1023–1030. [Google Scholar] [CrossRef]
- Koskenniemi, J.J.; Virtanen, H.E.; Kiviranta, H.; Damgaard, I.N.; Matomäki, J.; Thorup, J.M.; Hurme, T.; Skakkebaek, N.E.; Main, K.M.; Toppari, J. Association between levels of persistent organic pollutants in adipose tissue and cryptorchidism in early childhood: A case–control study. Environ. Health 2015, 14, 78. [Google Scholar] [CrossRef]
- McGlynn, K.A.; Guo, X.; Graubard, B.I.; Brock, J.W.; Klebanoff, M.A.; Longnecker, M.P. Maternal Pregnancy Levels of Polychlorinated Biphenyls and Risk of Hypospadias and Cryptorchidism in Male Offspring. Environ. Health Perspect. 2009, 117, 1472–1476. [Google Scholar] [CrossRef]
- Meyer, K.J.; Reif, J.S.; Veeramachaneni, D.R.; Luben, T.J.; Mosley, B.S.; Nuckols, J.R. Agricultural Pesticide Use and Hypospadias in Eastern Arkansas. Environ. Health Perspect. 2006, 114, 1589–1595. [Google Scholar] [CrossRef]
- Nassar, N.; Abeywardana, P.; Barker, A.; Bower, C. Parental occupational exposure to potential endocrine disrupting chemicals and risk of hypospadias in infants. Occup. Environ. Med. 2010, 67, 585–589. [Google Scholar] [CrossRef]
- Ormond, G.; Nieuwenhuijsen, M.J.; Nelson, P.; Toledano, M.B.; Iszatt, N.; Geneletti, S.; Elliott, P. Endocrine Disruptors in the Workplace, Hair Spray, Folate Supplementation, and Risk of Hypospadias: Case–Control Study. Environ. Health Perspect. 2009, 117, 303–307. [Google Scholar] [CrossRef]
- Palmer, J.R.; Herbst, A.L.; Noller, K.L.; A Boggs, D.; Troisi, R.; Titus-Ernstoff, L.; E Hatch, E.; A Wise, L.; Strohsnitter, W.C.; Hoover, R.N. Urogenital abnormalities in men exposed to diethylstilbestrol in utero: A cohort study. Environ. Health 2009, 8, 37. [Google Scholar] [CrossRef]
- Pierik, F.H.; Burdorf, A.; Deddens, J.A.; Juttmann, R.E.; Weber, R.F. Maternal and Paternal Risk Factors for Cryptorchidism and Hypospadias: A Case–Control Study in Newborn Boys. Environ. Health Perspect. 2004, 112, 1570–1576. [Google Scholar] [CrossRef]
- Pierik, F.H.; Klebanoff, M.A.; Brock, J.W.; Longnecker, M.P. Maternal pregnancy serum level of heptachlor epoxide, hexachlorobenzene, and β-hexachlorocyclohexane and risk of cryptorchidism in offspring. Environ. Res. 2007, 105, 364–369. [Google Scholar] [CrossRef]
- Rignell-Hydbom, A.; Lindh, C.H.; Dillner, J.; Jönsson, B.A.G.; Rylander, L. A Nested Case-Control Study of Intrauterine Exposure to Persistent Organochlorine Pollutants and the Risk of Hypospadias. PLoS ONE 2012, 7, e44767. [Google Scholar] [CrossRef]
- Rouget, F.; Bihannic, A.; Le Bot, B.; Mercier, F.; Gilles, E.; Garlantezec, R.; Multigner, L.; Cordier, S.; Arnaud, A.; Pladys, P.; et al. Meconium Concentrations of Pesticides and Risk of Hypospadias: A Case–Control Study in Brittany, France. Epidemiology 2024, 35, 185–195. [Google Scholar] [CrossRef]
- ShekharYadav, C.; Bajpai, M.; Kumar, V.; Ahmed, R.S.; Gupta, P.; Banerjee, B.D. Polymorphism in CYP1A1, GSTMI, GSTT1 genes and organochlorine pesticides in the etiology of hypospadias. Hum. Exp. Toxicol. 2011, 30, 1464–1474. [Google Scholar] [CrossRef]
- Spinder, N.; Bergman, J.E.H.; van Tongeren, M.; Boezen, H.M.; Kromhout, H.; Walle, H.E.K.d. Maternal occupational exposure to endocrine-disrupting chemicals and urogenital anomalies in the offspring. Hum. Reprod. 2021, 37, 142–151. [Google Scholar] [CrossRef]
- Morales-Suárez-Varela, M.M.; Toft, G.V.; Jensen, M.S.; Ramlau-Hansen, C.; Kaerlev, L.; Thulstrup, A.-M.; Llopis-González, A.; Olsen, J.; Bonde, J.P. Parental occupational exposure to endocrine disrupting chemicals and male genital malformations: A study in the danish national birth cohort study. Environ. Health 2011, 10, 3. [Google Scholar] [CrossRef]
- Trabert, B.; Longnecker, M.P.; Brock, J.W.; Klebanoff, M.A.; McGlynn, K.A. Maternal Pregnancy Levels of trans-Nonachlor and Oxychlordane and Prevalence of Cryptorchidism and Hypospadias in Boys. Environ. Health Perspect. 2012, 120, 478–482. [Google Scholar] [CrossRef]
- Jensen, D.V.; Christensen, J.; E Virtanen, H.; E Skakkebæk, N.; Main, K.M.; Toppari, J.; Veje, C.W.; Andersson, A.-M.; Nielsen, F.; Grandjean, P.; et al. No association between exposure to perfluorinated compounds and congenital cryptorchidism: A nested case–control study among 215 boys from Denmark and Finland. Reproduction 2014, 147, 411–417. [Google Scholar] [CrossRef]
- Wagner-Mahler, K.; Kurzenne, J.-Y.; Delattre, I.; Bérard, E.; Mas, J.-C.; Bornebush, L.; Tommasi, C.; Boda-Buccino, M.; Ducot, B.; Boullé, C.; et al. Prospective study on the prevalence and associated risk factors of cryptorchidism in 6246 newborn boys from Nice area, France. Int. J. Androl. 2011, 34, e499–e510. [Google Scholar] [CrossRef]
- Warembourg, C.; Botton, J.; Lelong, N.; Rouget, F.; Khoshnood, B.; Le Gléau, F.; Monfort, C.; Labat, L.; Pierre, F.; Heude, B.; et al. Prenatal exposure to glycol ethers and cryptorchidism and hypospadias: A nested case–control study. Occup. Environ. Med. 2018, 75, 59–65. [Google Scholar] [CrossRef]
- Weidner, I.S.; Møller, H.; Jensen, T.K.; E Skakkebaek, N. Cryptorchidism and hypospadias in sons of gardeners and farmers. Environ. Health Perspect. 1998, 106, 793–796. [Google Scholar] [CrossRef]
- White, J.T.; Kovar, E.; Chambers, T.M.; Sheth, K.R.; Peckham-Gregory, E.C.; O’neill, M.; Langlois, P.H.; Jorgez, C.J.; Lupo, P.J.; Seth, A. Hypospadias Risk from Maternal Residential Exposure to Heavy Metal Hazardous Air Pollutants. Int. J. Environ. Res. Public Health 2019, 16, 930. [Google Scholar] [CrossRef]
- Winston, J.J.; the National Birth Defects Prevention Study; Emch, M.; Meyer, R.E.; Langlois, P.; Weyer, P.; Mosley, B.; Olshan, A.F.; Band, L.E.; Luben, T.J. Hypospadias and maternal exposure to atrazine via drinking water in the National Birth Defects Prevention study. Environ. Health 2016, 15, 76. [Google Scholar] [CrossRef]
- Rocheleau, C.M.; Romitti, P.A.; Sanderson, W.T.; Sun, L.; Lawson, C.C.; Waters, M.A.; Stewart, P.A.; Olney, R.S.; Reefhuis, J. Maternal occupational pesticide exposure and risk of hypospadias in the national birth defects prevention study. Birth Defects Res. Part A Clin. Mol. Teratol. 2011, 91, 927–936. [Google Scholar] [CrossRef]
- Sastre, B.E.; Artero, C.C.; Ruiz, Y.G.; Atuan, R.F.; Rodríguez, P.B.; Juan, G.F.; Romero, J.G. Occupational exposure to endocrine-disrupting chemicals and other parental risk factors in hypospadias and cryptorchidism development: A case–control study. J. Pediatr. Urol. 2019, 15, 520.e1–520.e8. [Google Scholar] [CrossRef]
- Zhu, J.L.; Hjollund, N.H.; Andersen, A.-M.N.; Olsen, J. Occupational Exposure to Pesticides and Pregnancy Outcomes in Gardeners and Farmers: A Study Within the Danish National Birth Cohort. J. Occup. Environ. Med. 2006, 48, 347–352. [Google Scholar] [CrossRef]
- Damgaard, I.N.; Skakkebæk, N.E.; Toppari, J.; Virtanen, H.E.; Shen, H.; Schramm, K.-W.; Petersen, J.H.; Jensen, T.K.; Main, K.M.; The Nordic Cryptorchidism Study Group; et al. Persistent Pesticides in Human Breast Milk and Cryptorchidism. Environ. Health Perspect. 2006, 114, 1133–1138. [Google Scholar] [CrossRef]


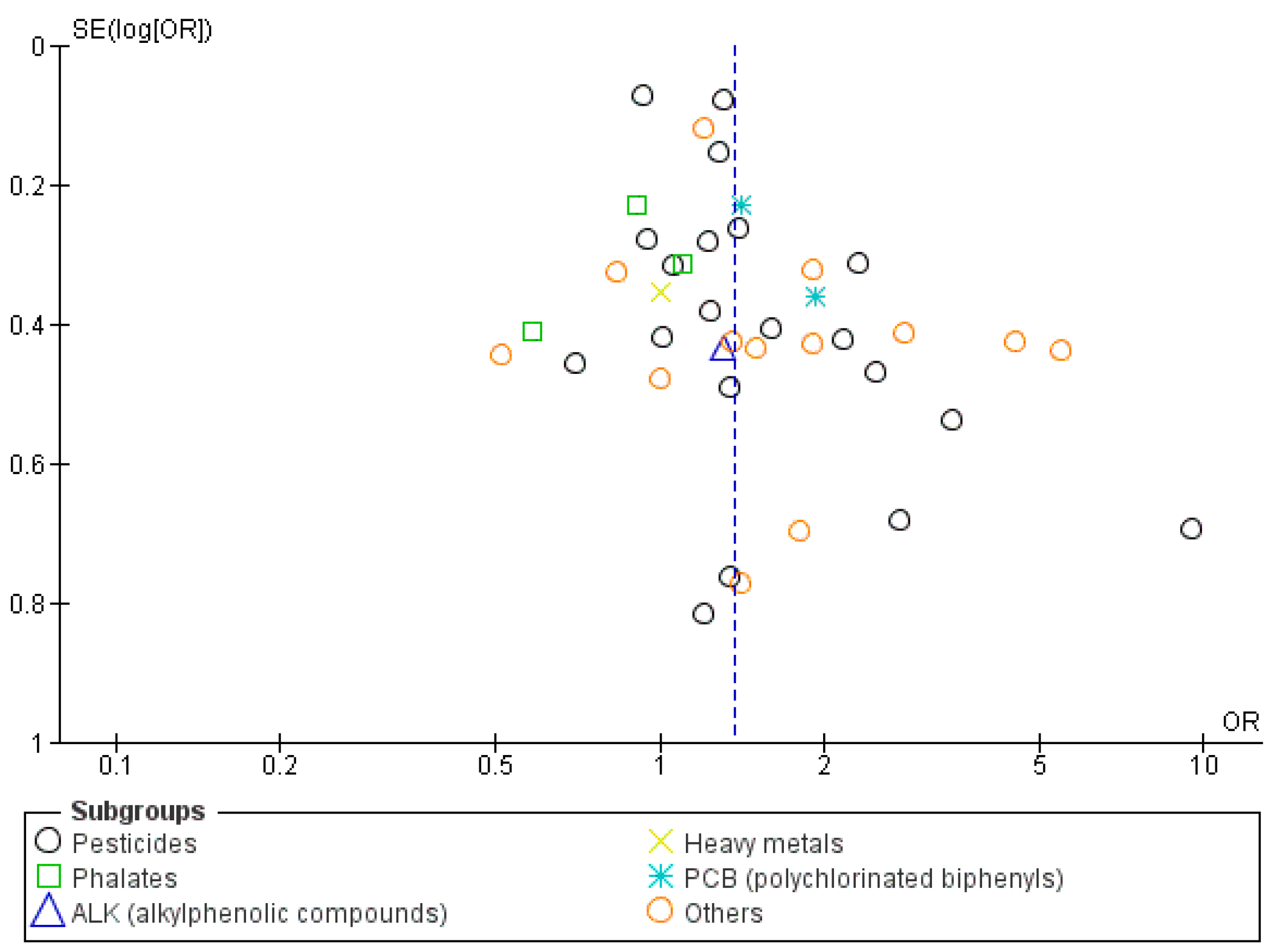
| Study ID | Year of Publication | Study Design | Location | Number of Hypospadias Cases | Number of Cryptorchidism Cases | Number of Controls | Exposure Assessment | Maternal Characteristics | Main Chemicals | Conclusions | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age, Mean Cases/Control (in Years) | Previous Pregnancy, N Cases/Control | Smoking, N Cases/Control | ||||||||||
| Garcifa-Martin [45] | 1996 | Case-control | Spain | 131 | NA | 243 | Geographical destination-based | NA | NA | NA | Pesticides | “Our results are compatible with a hypothetical association between exposure to hormone-disruptive chemicals and the induction of cryptorchidism.” |
| Weidner [71] | 1998 | Case-control | Denmark | 1345 | 6177 | 23,273 | Occupation-based | NA | NA | NA | NR | “The increased risk of cryptorchidism among sons of female gardeners could suggest an association with prenatal exposure to occupationally related chemicals.” |
| Bhatia [30] | 2004 | Case-control | USA | 66 | 75 | 283 | Serum sample-based | 27/26.6 | NA | 59/117 | DDT | “This study does not support an association of DDT or DDE and hypospadias or cryptorchidism.” |
| DDE | ||||||||||||
| Pierik [60] | 2004 | Case-control | The Netherlands | 56 | 78 | 313 | Survey-based | NA | NA | 51/98 | EDC | “This study suggests that paternal environmental exposures may increase the risk of cryptorchidism and hypospadias in newborn boys, which may indicate an effect on the paternal germline.” |
| Pesticides | ||||||||||||
| Solvents | ||||||||||||
| Brouwers [33] | 2006 | Case-control | The Netherlands | 583 | NA | 251 | Survey-based | NA | 35/6 | 133/35 | NR | “The associations found in this study support the hypothesis that genetic predisposition, placental insufficiency, and substances that interfere with natural hormones play a role in the etiology of hypospadias.” |
| Meyer [56] | 2006 | Case-control | USA | 354 | NA | 727 | Geographical destination-based | 25.2/24.7 | NA | NA | EDC | “Except for diclofop-methyl, we did not find evidence that estimated exposure to pesticides known to have reproductive, developmental, or endocrine disrupting effects increases the risk of hypospadias. Further research on the potential effects of exposure to diclofop-methyl is recommended.” |
| Atrazine | ||||||||||||
| Alachlor | ||||||||||||
| Bifenthrin | ||||||||||||
| Bromoxynil | ||||||||||||
| Carbaryl Dicamba | ||||||||||||
| Carbone [35] | 2007 | Case-control | Italy | 43 | 48 | 203 | Survey-based | NA | NA | 12-Jul | Pesticides | “The study provides only limited support to the hypothesis of a possible association between the risk of cryptorchidism and hypospadias and the occupational exposure to EDC and agricultural work.” |
| Fernandez [41] | 2007 | Case-control | Spain | 50 | 114 | Placenta tissue sample | NA | 4-Jun | Nov-35 | DDT | “We found an increased risk for male urogenital malformations related to the combined effect of environmental estrogens in the placenta.” | |
| Endosulfan I-Lindane-Mirex | ||||||||||||
| Pierik [61] | 2007 | Case-control | USA | NA | 219 | 564 | Serum sample-based | 24/22 | NA | NA | HCB | “These results provide little support for an association of cryptorchidism with exposure to low levels of HCE or HCB. For b-HCCH, the findings were somewhat suggestive of an association but inconclusive.” |
| Heptachlor epoxide-b-Hexachlorocyclohexane | ||||||||||||
| Brucker-davis [34] | 2008 | Case-control | France | NA | 78 | 86 | Colostrum sample-based | 30/30 | 41/44 | NA | PCB | “Our results support an association between congenital cryptorchidism and fetal exposure to PCBs and possibly DDE. Higher concentrations in milk could be a marker of higher exposure or for an impaired detoxification pattern in genetically predisposed individuals.” |
| DDE-mBP | ||||||||||||
| Bornman [31] | 2009 | Cross-sectional | Mexico | 171 | 70 | 216 | Survey-based | 25/25 | NA | NA | DDT | “Maternal exposure to DDT by living in a DDT-sprayed village was associated with having male offspring with one or more UGBDs.” |
| Dugas [40] | 2009 | Case-control | England | 471 | NA | 490 | Survey-based | NA | NA | NA | Biocide | “The authors found an association between the use of insect repellent and total biocide score and risk of hypospadias. In particular, the use of insect repellent warrants further investigation, specifically in relation to type, content, and frequency of use since this information was missing in the current study.” |
| Naphthalene | ||||||||||||
| Insect repellent | ||||||||||||
| Mcglynn [55] | 2009 | Case-control | USA | 201 | 230 | 593 | Serum sample-based | NA | NA | NA | PCB | “Given the large number of associations examined, these findings do not strongly support the hypothesis that PCBs are associated with cryptorchidism or hypospadias. Because population serum PCB levels at the time of sample collection were considerably higher than at present, it is unlikely that current PCB exposure is related to the development of either anomaly.” |
| Nassar [57] | 2009 | Case-control | Australia | 1202 | NA | 2583 | Survey-based | 28.2/27.9 | NA | NA | EDC | “Our findings provide preliminary evidence of an association between exposure to EDCs with oestrogenic or antiandrogenic properties and increased risk of hypospadias.” |
| Pesticides-POC-ALK-BPC-Heavy metals-Phthalates. | ||||||||||||
| Ormond [58] | 2009 | Case-control | England | 471 | NA | 490 | Survey-based | NA | NA | 113/88 | Hair spray | “Excess risks of hypospadias associated with occupational exposures to phthalates and hair spray suggest that antiandrogenic EDCs may play a role in hypospadias. Folate supplementation in early pregnancy may be protective.” |
| Cleaning agents | ||||||||||||
| Printing ink | ||||||||||||
| Exhaust fumes | ||||||||||||
| Palmer [59] | 2009 | Cohort | USA | NA | 38 | NA | Survey-based | NA | NA | NA | diethylstilbestrol | “These results indicate that prenatal exposure to DES increases the risk of male urogenital abnormalities and that the association is strongest for exposure that occurs early in gestation. The findings support the hypothesis that endocrine disrupting chemicals may cause the increased prevalence of cryptorchidism that has been seen in recent years.” |
| Carmichael [36] | 2010 | Case-control | USA | 20 | NA | 28 | Serum sample-based | NA | NA | NA | PCB | “Levels of the PBDEs and PCBs were not statistically significantly different, but the sample size was small. The current study adds to a relatively limited knowledge base regarding the potential association of specific contaminants with hypospadias or other birth defects.” |
| PBDE | ||||||||||||
| Giordano [46] | 2010 | Case-control | Italy | 80 | NA | 80 | Serum sample-based | NA | NA | NA | EDC | “This study, although based on a limited number of cases, for the first time provides evidence of an association between maternal exposure to EDCs, in particular elevated plasma hexachlorobenzene concentration, and the development of hypospadias in the offspring.” |
| Polychlorinated organic compounds | ||||||||||||
| ALK | ||||||||||||
| Biphenolic compounds | ||||||||||||
| Heavy metals | ||||||||||||
| Gabel [44] | 2011 | Cohort | Denmark | 11 | 17 | 477 | Survey-based | NA | NA | NA | NR | “The data are compatible with a slightly increased risk of cryptorchidism in sons of women exposed to pesticides by working in horticulture.” |
| Rocheleau [74] | 2011 | Case-control | USA | 647 | NA | 1496 | Survey-based | NA | NA | NA | Insecticides | “Using broad classes of insecticides, herbicides, and fungicides, we found no evidence that low intensity maternal periconceptional occupational pesticide exposure was a risk factor for hypospadias.” |
| Herbicides | ||||||||||||
| Fungicides | ||||||||||||
| Shekharyadav [64] | 2011 | Case-control | India | 80 | NA | 120 | Survey-based | NA | NA | NA | EDC | “Our study suggests irrespective of genetic predisposition, higher level of some OCPs may be associated with increased risk of hypospadias.” |
| Suarez-Varela [66] | 2011 | Cohort | Spain | 262 | 1002 | NA | Survey-based | NA | NA | NA | EDC | “The study provides some but limited evidence that occupational exposure to possible endocrine disrupting chemicals during pregnancy increases the risk of hypospadias.” |
| Pesticides | ||||||||||||
| Organochlorine | ||||||||||||
| compounds | ||||||||||||
| Phthalate esters | ||||||||||||
| ALK | ||||||||||||
| Heavy metals | ||||||||||||
| Bis-phenols | ||||||||||||
| Trabert [67] | 2011 | Case-control | USA | 197 | 217 | 557 | Serum sample-based | 24/22 | NA | 210/250 | trans-Nonachlor-Oxychlordane | “The results do not support an association between chlordane levels and cryptorchidism or hypospadias. It is unlikely that current chlordane exposure is related to the development of either anomaly, given that serum chlordane levels at the time of sample collection, the early 1960s, were considerably higher than levels at present.” |
| Wagner-mahler [69] | 2011 | Case-control | France | NA | 95 | 188 | Survey-based | NA | NA | NA | Phthalates | “Our results suggest that maternal exposure to anti-rust or phthalates could be a risk factor, whereas eating fruits daily seemed somewhat protective. The prevalence of cryptorchidism in our area is on the lower bracket compared with other countries and is associated with familial and environmental risk factors.” |
| Heavy metals | ||||||||||||
| Rignell-hydbom [62] | 2012 | Case-control | Sweden | 237 | NA | 237 | Serum sample-based | NA | NA | NA | PCB | “The present study suggests that fetal exposure to HCB and p,p’-DDE may be a risk factor for hypospadias.” |
| DDE | ||||||||||||
| HCB | ||||||||||||
| Agopian [28] | 2013 | Case-control | USA | 8909 | 4324 | 16,433 | Survey-based | NA | NA | 1025/989 | Atrazine | “In summary, we report on modest, consistent, inverted U-shaped associations between estimated maternal residential exposure to atrazine and several genital malformations in male offspring.” |
| Carmichael [37] | 2013 | Case-control | USA | 690 | NA | 2195 | Survey-based | NA | 382/1411 | NA | Insect growth regulator | “Most pesticides were not associated with elevated hypospadias risk. For the associated few, results should be interpreted with caution until replicated in other study populations.” |
| Halogenated organic | ||||||||||||
| monochlorophenoxy | ||||||||||||
| acid or ester | ||||||||||||
| Organochlorine-Organotin | ||||||||||||
| Phosphine-Thiocarbamate | ||||||||||||
| Jorgensen [51] | 2013 | Cohort | Denmark | 33 | 134 | NA | Occupation-based | NA | NA | NA | NR | “Our nationwide cohort study shows that, despite exposure to a complex chemical milieu, hairdressers do not have an increased risk of having boys with cryptorchidism and hypospadias.” |
| Vesterholmjensen [68] | 2013 | Case-control | Denmark-Finland | NA | 215 | 108 | Cord sample-based | NA | NA | NA | PFOA | “Our data indicate that women in Denmark and Finland are generally exposed to PFOA and PFOS, but there are differences in exposure levels between countries. We found no statistically significant association between cord blood PFOA and PFOS levels and congenital cryptorchidism; however, our study was small, and larger studies are warranted.” |
| PFOS | ||||||||||||
| Jorgensen [52] | 2014 | Cohort | Denmark | NA | 229 | NA | Occupation-based | NA | NA | NA | NR | “This nationwide cohort study found a slightly increased risk of cryptorchidism in sons of maternal horticultural workers and farmers. However, subgroup analyses indicated similar findings for paternal horticultural workers and no association for women likely working in the first trimester. The main findings should, therefore, be interpreted with caution.” |
| Fernandez [42] | 2015 | Case-control | Spain | 28 | 51 | Placenta tissue sample | 29/30 | 16-Apr | 17-May | Methyl-PB-ethyl-PB | “The multivariable regression analyses indicated a statistically significant association between exposure to BPA and propyl-PB and the risk of malformations.” | |
| Propyl-PB-butyl-PB | ||||||||||||
| Jensen [50] | 2015 | Case-control | Denmark | 75 | 270 | 300 | Amniotic fluid sample | NA | NA | NA | Phthalate | “Data on the DEHP metabolite indicate possible interference with human male fetal gonadal function. Considering the DiNP metabolite, we cannot exclude (nor statistically confirm) an association with hypospadias and, less strongly, with cryptorchidism.” |
| Kalfa [53] | 2015 | Case-control | France | 300 | NA | 302 | Survey-based | NA | NA | NA | EDC | “Our multi-institutional study showed that parental professional, occupational, and environmental exposures to chemical products increase the risk of hypospadias in children.” |
| Pesticides | ||||||||||||
| Cosmetics | ||||||||||||
| Herbicides | ||||||||||||
| Detergents | ||||||||||||
| ALK | ||||||||||||
| Phthalates | ||||||||||||
| Heavy metals | ||||||||||||
| Koskenniemi [54] | 2015 | Case-control | Turkey-Denmark-Finland | NA | 44 | 38 | Serum sample-based | NA | NA | NA | PCB | “Prenatal exposure to PCDD/Fs and PCDD/F-like PCBs may be associated with increased risk for cryptorchidism. Our finding does not exclude the possibility of an association between the exposure to PBDEs and cryptorchidism.” |
| PBDE | ||||||||||||
| Haraux [48] | 2016 | Case-control | France | 57 | NA | 162 | Survey-based | 29.7/28.7 | NA | NA | EDC | “Our results suggest that maternal occupational exposure to EDCs is a risk factor for hypospadias and suggests a possible influence of household use of hair cosmetics during early pregnancy on the incidence of hypospadias in the offspring.” |
| Hair cosmetic | ||||||||||||
| Insecticides | ||||||||||||
| Winston [73] | 2016 | Case-control | USA | 343 | NA | 1422 | Geographical destination-based | NA | NA | NA | Atrazine | “While the association that we observed was weak, our results suggest that additional research into a possible association between atrazine and hypospadias occurrence, using a more sensitive exposure metric, would be useful.” |
| Gonzalez 2017 [47] | 2017 | Case-control | Spain | 678 | 963 | 587,142 | Geographical destination-based | NA | NA | NA | Pesticides | “Data on environmental exposure to pesticides and gestational disorders are scarce. A population-based case-control study estimated the risk of maternal-infant disorders. Prevalence and risk of reproductive disorders and congenital anomalies were estimated. Higher prevalences and risk were observed in areas of high exposure to pesticides. Environmental pesticides can be risk factors for developing maternal-infant disorder.” |
| Warembourg [70] | 2017 | Case-control | France | 15 | 14 | 86 | Urine sample-based | NA | NA | 19/9 | MAA | “In view of the toxicological plausibility of our results, this study, despite its small sample size, raises concern about the potential developmental toxicity of MAA on the male genital system and calls for thorough identification of current sources of exposure to MAA.” |
| PhAA | ||||||||||||
| Haraux [49] | 2018 | Case-control | France | 25 | NA | 58 | Meconium sample-based | 29/28.2 | NA | 21-Sep | Diazinon | “We conclude that prenatal exposure to these two herbicides (as assessed by meconium analysis) correlated with isolated hypospadias. The results of our case-control study (i) suggest that prenatal exposure to pesticides interferes with the development of the male genitalia, and (ii) emphasize the importance of preventing pregnant women from being exposed to EDCs in general and pesticides in particular.” |
| Malathion | ||||||||||||
| DETP | ||||||||||||
| DEP | ||||||||||||
| DMP | ||||||||||||
| Isoproturon | ||||||||||||
| Desmethylisoproturon | ||||||||||||
| MCPA | ||||||||||||
| Cognez [38] | 2019 | Case-control | France | 50 | 123 | 8199 | Survey-based | NA | NA | 30/1679 | Pesticides | “Our results suggest a possible increased risk of hypospadias associated with prenatal use of some domestic pesticide products, likely to contain insecticides, and of cryptorchidism with nearby orchard acreage (crops repeatedly sprayed with pesticides). This work is limited by its modest number of cases.” |
| Estors Sastre [75] | 2019 | Case-control | Spain | 210 | 210 | Survey-based | NA | NA | 33/29 | EDC | “Advanced age, some parental occupational exposure to EDCs, some drug consumption, smoking, and the father’s history of urological disorders may increase risk and predict the developments of these malformations. Studies with larger sample sizes are needed to assess associations between individual EDC occupational exposures and drugs and these malformations.” | |
| White [72] | 2019 | Case-control | USA | 8981 | NA | 89,806 | Geographical destination-based | NA | NA | 576/5280 | Heavy metals | “Using data from one of the world’s largest active surveillance birth defects registries, we identified significant associations between hypospadias and HMHAP exposures.” |
| Axelsson [29] | 2020 | Case-control | Sweden | NA | 165 | 165 | Serum sample-based | 29/28 | NA | 15/16 | PCB | “We found no evidence of an association between maternal levels of PCB or HCB during the pregnancy and the risk of having cryptorchidism in the sons.” |
| DDE | ||||||||||||
| HCB | ||||||||||||
| Fisher [43] | 2020 | Case-control | UK | NA | 30 | 275 | Serum sample-based | 32.98/33.54 | NA | 0/4 | Phthalates | “Our observational findings support experimental evidence that intrauterine exposure to BPA and n-PrP during early gestation may adversely affect male reproductive development. More evidence is required before specific public health recommendations can be made.” |
| BPA | ||||||||||||
| TCS | ||||||||||||
| BP-3 | ||||||||||||
| Bougneres [32] | 2021 | Case-control | France | 8766 | 13,105 | 43,830 | Geographical destination-based | NA | NA | NA | NR | “Our study supports that children born to mothers living close to a vineyard have a two-fold increased risk of H. For environmental research, using VC = provides an alternative to a classical case-control technique.” |
| Spinder [65] | 2021 | Case-control | The Netherlands | 364 | NA | 5602 | Survey-based | NA | NA | NA | EDC | “Women, their healthcare providers, and their employers need to be aware that occupational exposure to specific EDCs early in pregnancy may be associated with CAKUT in their offspring. An occupational hygienist should be consulted in order to take exposure to those specific EDCs into consideration when risk assessments are carried out at the workplace.” |
| Pesticides | ||||||||||||
| ALK | ||||||||||||
| Phthalates | ||||||||||||
| Benzophenones | ||||||||||||
| parabens-siloxanes | ||||||||||||
| Das [39] | 2023 | Case-control | India | 37 | NA | 146 | Survey-based | NA | NA | NA | Pesticides | “This study suggests that EDCs are a risk factor for hypospadias through occupational exposure during fetal life.” |
| Phthalates | ||||||||||||
| ALK | ||||||||||||
| Heavy metals | ||||||||||||
| Rouget [63] | 2024 | Case-control | France | 69 | NA | 135 | Meconium sample-based | NA | 35/39 | 21/36 | Nitrophenol-diethyl phosphate-Fenitrothion-Carbofuran | “Our small study provides a robust assessment of fetal exposure. Fenitrothion’s established antiandrogenic activities provide biological plausibility for our observations. Further studies are needed to confirm this hypothesis.” |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albadawi, E.A.; Alzaman, N.S.; Elhassan, Y.H.; Eltahir, H.M.; Abouzied, M.M.; Albadrani, M.S. The Association between Maternal Endocrine-Disrupting Chemical Exposure during Pregnancy and the Incidence of Male Urogenital Defects: A Systematic Review and Meta-Analysis. Metabolites 2024, 14, 477. https://doi.org/10.3390/metabo14090477
Albadawi EA, Alzaman NS, Elhassan YH, Eltahir HM, Abouzied MM, Albadrani MS. The Association between Maternal Endocrine-Disrupting Chemical Exposure during Pregnancy and the Incidence of Male Urogenital Defects: A Systematic Review and Meta-Analysis. Metabolites. 2024; 14(9):477. https://doi.org/10.3390/metabo14090477
Chicago/Turabian StyleAlbadawi, Emad Ali, Naweed SyedKhaleel Alzaman, Yasir Hassan Elhassan, Heba M. Eltahir, Mekky M. Abouzied, and Muayad Saud Albadrani. 2024. "The Association between Maternal Endocrine-Disrupting Chemical Exposure during Pregnancy and the Incidence of Male Urogenital Defects: A Systematic Review and Meta-Analysis" Metabolites 14, no. 9: 477. https://doi.org/10.3390/metabo14090477
APA StyleAlbadawi, E. A., Alzaman, N. S., Elhassan, Y. H., Eltahir, H. M., Abouzied, M. M., & Albadrani, M. S. (2024). The Association between Maternal Endocrine-Disrupting Chemical Exposure during Pregnancy and the Incidence of Male Urogenital Defects: A Systematic Review and Meta-Analysis. Metabolites, 14(9), 477. https://doi.org/10.3390/metabo14090477






